Abstract
Shiga toxin-producing Escherichia coli (STEC) belonging to certain serogroups (e.g., O157 and O26) can cause serious conditions like hemolytic-uremic syndrome (HUS), but other strains might be equally pathogenic. While virulence factors, like stx and eae, have been well studied, little is known about the prevalence of the E. coli hemolysin genes (hlyA, ehxA, e-hlyA, and sheA) in association with these factors. Hemolysins are potential virulence factors, and ehxA and hlyA have been associated with human illness, but the significance of sheA is unknown. Hence, 435 E. coli strains belonging to 62 different O serogroups were characterized to investigate gene presence and phenotypic expression of hemolysis. We further investigated ehxA subtype patterns in E. coli isolates from clinical, animal, and food sources. While sheA and ehxA were widely distributed, e-hlyA and hlyA were rarely found. Most strains (86.7%) were hemolytic, and significantly more hemolytic (95%) than nonhemolytic strains (49%) carried stx and/or eae (P < 0.0001). ehxA subtyping, as performed by using PCR in combination with restriction fragment length polymorphism analysis, resulted in six closely related subtypes (>94.2%), with subtypes A/D being eae-negative STECs and subtypes B, C, E, and F eae positive. Unexpectedly, ehxA subtype patterns differed significantly between isolates collected from different sources (P < 0.0001), suggesting that simple linear models of exposure and transmission need modification; animal isolates carried mostly subtypes A/C (39.3%/42.9%), food isolates carried mainly subtype A (81.9%), and clinical isolates carried mainly subtype C (66.4%). Certain O serogroups correlated with particular ehxA subtypes: subtype A with O104, O113, and O8; B exclusively with O157; C with O26, O111, and O121.
INTRODUCTION
Non-O157 Shiga toxin-producing Escherichia coli (STEC) became a focus of attention based on the continuously increasing numbers of non-O157-related illnesses reported worldwide (1–5). Over 100 different O serogroups are associated with Shiga toxin production, although not all STEC strains are pathogenic to humans (2, 6). However, some STEC strains are as virulent as O157:H7 and have caused outbreaks of severe illness (7, 8), including hemorrhagic colitis (HC), hemolytic-uremic syndrome (HUS), and even death (8–10). Relatively few organisms of these strains are needed to cause disease, and the consumption of contaminated foods or drinking water or close contact with STEC-infected animals have been shown to be important transmission routes for STEC infections in humans (11–13). Domestic and wild ruminants, especially cattle, are natural reservoirs of STEC that shed the bacteria with their feces in the environment (12). While STEC-infected animals usually do not show signs of disease and can be included in food production, STEC from these animals can be highly virulent to humans. Consumption of products of animal origin, such as meat, therefore presents a risk factor for STEC infection. Furthermore, most fresh produce is grown in close contact with the ground and is vulnerable to E. coli contamination from livestock area runoff, manure when used as fertilizer, and field intrusion of wildlife (5, 11, 14, 15).
While O157:H7 is well characterized and widely accepted as a human pathogen, non-O157 E. coli strains are very heterogenous in their virulence attributes. Certain serogroups are known to cause HC and HUS (e.g., O26 and O111), and O45 is associated with HC (1, 2); however, other serogroups (O91, O113, and O128) may cause HC and HUS but are less commonly isolated (1, 6). Specific virulence markers that could definitely characterize STEC isolates as pathogenic have not been universally agreed upon. Shiga toxin (stx1 and stx2), its variants, and the protein intimin (encoded by the eae allele), which is involved in attachment of the organisms to and effacing of gut mucosal cells, are only a few examples of established virulence factors (16–21). Also, several different types of hemolysin genes have been identified in various bacterial species and are often regarded as major virulence factors (22, 23). Four types of hemolysin have been identified in E. coli: alpha-hemolysin (hlyA), plasmid- and phage-carried enterohemolysin (ehxA, e-hlyA), and silent hemolysin (sheA). The plasmid-carried ehxA and hlyA belong to the RTX (repeat in toxin) family, which are widespread among Gram-negative pathogens and lyse erythrocytes from different mammalian species (24–26). The presence of enterohemolysin is only detected on washed sheep blood agar after overnight incubation at 37°C, as opposed to alpha-hemolysin, which is detectable after 3 to 6 h of incubation on standard blood agar plates (25, 27, 28). hlyA, found on the pathogenicity island (29), is produced by many E. coli strains that are associated with urinary tract infections (30–32). The ehxA gene, located on the large enterohemorrhagic E. coli virulence plasmid (28, 33), is frequently associated with diarrheal disease and HUS (34, 35). Since the detection of enterohemolysin is relatively easy due to its hemolytic activity, and its presence correlates with that of Shiga toxin, it has been suggested as an epidemiological marker for the rapid and simple detection of STEC strains (28, 36). Six genetically distinct ehxA subtypes (A to F) have been identified in E. coli by using PCR and restriction fragment length polymorphism (RFLP) analysis (37).
The bacteriophage-associated enterohemolysin (e-hlyA) is genetically unrelated to the plasmid-carried ehxA (38–40). It was suggested that the e-hlyA determinant does not encode a hemolysin, per se, as reported in earlier studies (38, 39), but instead causes the release of the silent hemolysin by lysis of the bacterial cells (40). The silent hemolysin (sheA), also called cytolysin A, encodes a pore-forming toxin (41, 42) not related to the RTX family (43, 44). It was first found in E. coli strain K-12 (45, 46), but it is also present in E. coli O157:H7 (47), as well as in other enteropathogenic E. coli strains (48). In cultured mammalian cells, cytotoxicity and inducement of macrophage apoptosis through the action of the silent hemolysin have been reported (49, 50). These findings indicate that sheA might contribute to the pathogenicity of E. coli strains, although its presence in correlation with the appearance of extraintestinal infections has yet not been clarified. Hemolytic activity due to the silent hemolysin can be only detected under certain conditions, for example, if sheA is present on high-copy-number plasmids or when certain regulator genes are overexpressed (51, 52).
The objective of this study was to investigate the prevalence of hemolytic phenotypes and/or genotypes (ehxA, e-hlyA, sheA, and hlyA) in correlation with the potential pathogenicity of various E. coli strains, as judged by the presence or absence of stx and eae. Furthermore, this study aimed to investigate the distribution of ehxA subtypes within and between isolates from clinical, food, and animal sources, as well as different O serogroups from different geographical areas. By including isolates from ground beef and fresh produce, food products which have been important transmission vehicles of E. coli infections in humans in the past (3–5, 11, 13, 53), we were able to investigate, by using ehxA subtype occurrence, a potential linear flow of infection originating from farm animals and food to ill individuals.
MATERIALS AND METHODS
Bacterial strains.
A total of 435 E. coli isolates from different sources (154 clinical, 200 food, 58 animal, nine environmental, and 14 unidentified source) and geographical areas were investigated. The strains belonged to 62 different O serogroups, including the 10 most clinically relevant STEC serogroups (O26, O103, O111, O145, O157, O91, O113, O128, O45, and O121). The 435 strains used in this study were kindly provided by the following (see also Table S1 in the supplemental material): 46 E. coli strains from various sources (water, animal, and clinical) from the E. coli Reference Center at Pennsylvania State University (54), 67 ground beef isolates from Mick Bosilevac (USDA U.S. Meat Animal Research Center, Clay Center, NE) (55), 101 agricultural food isolates from Shanker Reddy (USDA AMS Microbiological Data Program [MDP]), 32 food isolates from an FDA Center for Food Safety and Applied Nutrition (CFSAN)-sponsored produce survey, 99 isolates of different sources from the STEC Center at Michigan State University (www.shigatox.net), five strains of different sources obtained through Lydia V. Rump (University of Maryland, Department of Nutrition and Food Science, College Park, MD), and seven bovine isolates, five clinical strains, and strain AD4001-1B were provided by Andrew Lin (FDA San Francisco District Laboratory, Alameda, CA). Of the clinical isolates, 32 were obtained from Alifiya Ghadiali (FDA CFSAN, College Park, MD), and 40 were provided by Nancy Strockbine (Centers for Disease Control and Prevention, Atlanta, GA). Potential duplicate strains (e.g., strains originating from the same outbreak) were removed according to the available information, such as clinical outcome, host, geographical location, date of isolation, pulsed-field gel electrophoresis (PFGE) pattern, and the virulence gene pattern confirmed in this study.
Preparation of template DNA and culture conditions for hemolysin expression.
Bacterial strains were grown aerobically for 18 to 24 h on tryptic soy agar (TSA) at 37°C. One colony was transferred into 1.5 ml of tryptic soy broth and incubated for another 18 to 24 h at 37°C in a shaking incubator. DNA was extracted using the DNeasy blood and tissue kit (Qiagen Inc., Valencia, CA) as per the manufacturer's instructions for Gram-negative bacteria. Template DNA was stored at −20°C until use.
Hemolytic activity was verified on STEC heart infusion washed blood agar with mitomycin C (SHIBAM) as described previously (56). A single colony of each strain was inoculated from TSA onto SHIBAM and incubated for 6 and 24 h at 37°C. The agar plates were examined after 6 h for indications of alpha-hemolysin effects and after 24 h for all hemolysin types.
Detection of established and putative virulence factors.
Each strain was screened for the targets stx1, stx2, eae, uidA, ehxA, hlyA, e-hlyA, and sheA by using PCR (C1000 thermal cycler; Bio-Rad Laboratories Inc., Hercules, CA). One microliter of DNA template was added to a 24-μl PCR mixture containing 0.6 units HotStarTaq DNA polymerase (Qiagen Inc.), 2.5 μl 10× PCR buffer with 15 mM MgCl2 (Qiagen Inc.), 0.2 mM deoxynucleoside triphosphates (Invitrogen Life Technologies, Grand Island, NY), and 4 μM of each primer (IDT, Inc., Coralville, IA, manufactured with standard desalting) (Table 1). The template was denatured for 15 min at 95°C, followed by 25 cycles of denaturation at 95°C for 1 min, annealing at 54°C for 1 min, and extension at 72°C for 1 min, and a final extension step at 72°C for 5 min. All products were electrophoresed on precast 2% agarose gels containing ethidium bromide (E-Gel; Invitrogen Life Technologies) and visualized on a UV transilluminator (G:Box; Imgen Technologies, Alexandria, VA).
Table 1.
PCR Primers used in this study for detection of target genes and sequencing analysis
| Target | Primer name | Sequence (5′–3′) | Amplicon size (bp) | Reference or source |
|---|---|---|---|---|
| Shiga toxin 1 | stx1 F | GACTTCTCGACTGCAAAGAC | 306 | Son et al.a |
| stx1 R | TGTAACCGCTGTTGTACCTG | |||
| Shiga toxin 2 | stx2 F | CCCGGGAGTTTACGATAGAC | 482 | Son et al. |
| stx2 R | ACGCAGAACTGCTCTGGATG | |||
| Intiminb | eae F | GCGCGTTACATTGACTCCCG | 245 | Son et al. |
| eae R | CCATTTGCTGGGCGCTCATC | |||
| uidA | uidA F | GGTCACTCATTACGGCAAAG | 379 | Son et al. |
| uidA R | CAGTTCAACGCTGACATCAC | |||
| Enterohemolysin (plasmid) | ehxA F | TCTGTATCTGCGGGAGTTAG | 136 | Son et al. |
| ehxA R | CAACGTGCTCAAACATAGCC | |||
| Silent hemolysin | sheA F | GAGGCGAATGATTATGACTG | 920 | 74 |
| sheA R | ACTTCAGGTACCTCAAAGAG | |||
| Alpha-hemolysin | hlyA F | GTCTGCAAAGCAATCCGCTGCAAATAAA | 561 | 74 |
| hlyA R | CTGTGTCCACGAGTTGGTTGATTAG | |||
| Enterohemolysin (phage) | ehlyA F | TCGCAATCACATCACAACC | 810 | 74 |
| ehlyA R | CCAGCAGTTCGTCATCATCTGAA | |||
| Complete ehxA gene for RFLP and sequencing | ehxA ext. F | CAGGCAATACCATCATGAAC | 3,166 | Present study |
| ehxA ext. R | GTGCATACAGACTATTATGAG | |||
| Complete ehxA gene for subtype D | ehxARFLP F | ATGACAGTAAATAAAATAAAGAAC | 2,997 | 37 |
| ehxARFLP R | TCAGACAGTTGTCGTTAAAGTTG | |||
| Internal sequencing primers for complete ehxA gene | ehxA Seq R1 | TGAGCCAAGCTGGTTAAGCT | Present study | |
| ehxA Seq F2 | GGCTCTTGATGAATTGCTGAG | 633 | Present study | |
| ehxA Seq R2 | ATTGTTGTCAGGGCTGCATC | |||
| ehxA Seq F3 | GTCTGATCACATCGGCTGTT | 642 | Present study | |
| ehxA Seq R3 | GTGTAAACTCCTTCGGTTGA | |||
| ehxA Seq F4 | GGGATGAGAAGATCGGTGAAC | 625 | Present study | |
| ehxA Seq R4 | CCTACTGACACCTCCTGTTC | |||
| ehxA Seq F5 | CCGTATCTTATAATAAGACGG | 670 | Present study | |
| ehxA Seq R5 | CCTCCTTCATCTGCAATTG | |||
| ehxA Seq F6 | CTGACAGGAGGAAGCGGTAATG | Present study |
I. Son, R. Binet, A. Lin, T. S. Hammack, and J. A. Kase, submitted for publication.
The eae primers were tested to include 15 eae variants.
O serogroup identification.
Serotype information for all 435 E. coli isolates used in this study was provided by the contributors of the strains. However, serogroups O26, O45, O91, O103, O104, O111, O113, O121, O128, O145, and O157 were confirmed via a Luminex microbead-based suspension array with the Bio-Plex 200 instrument (Luminex Corporation, Austin, TX) as described previously (57). Antisera from Statens Serum Institut (MiraVista, Indianapolis, IN) were used to confirm the Bio-Plex results (58) for non-O157 E. coli (except serogroup O104), and the E. coli O157:H7 latex test kit (Remel, Lenexa, KS) was used to confirm O157.
Molecular characterization of ehxA subtypes.
ehxA subtype identification via RFLP analysis was conducted for all E. coli strains that were ehxA PCR positive. PCR amplification of the complete ehxA gene was performed with 1 μl template DNA and 0.6 μM of each primer (ehxA ext. F/R) (Table 1). Amplification of the ehxA gene for ehxA subtype D strains did not result in PCR products; hence, the full-length ehxA gene of subtype D strains was amplified using the primers (ehxA RFLP F/R) described by Cookson et al. (37) (Table 1). Platinum PCR SuperMix High Fidelity (Invitrogen Life Technologies) was added to a total of 20 μl. Amplification and digestion with TaqI (Invitrogen Life Technologies) was conducted as described previously (37) with the following adjustments: an initial denaturing step at 95°C for 2 min and polymerization for 3.5 min at 72°C. Products were then electrophoresed and visualized on a UV transilluminator as described previously. For better amplicon separation and exact size determination, 1-μl aliquots of the digested PCR products were analyzed on an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA).
Sequencing of the ehxA gene.
The full-length ehxA gene of 12 ehxA-positive isolates—two of each of the six ehxA subtypes—was sequenced via standard DNA sequencing through a commercial facility (MCLAB, South San Francisco, CA). The ehxA gene was amplified using the primers described earlier (ehxA ext. F/R and ehxA RFLP F/R for subtype D strains) (Table 1). PCR products were purified using the QIAquick PCR purification kit (Qiagen Inc.) as per the manufacturer's instruction using a microcentrifuge. Sequencing was performed with 10 internally designed primers (Table 1), as well as the two primers flanking the ehxA gene.
Sequencing and phylogenetic analysis.
CLC Main Workbench 6.7.2 (CLC bio, Cambridge, MA) was used for editing DNA sequences. The phylogenetic tree was compiled using MEGA 4.0.2 (59, 60) with the neighbor-joining method (61) and the maximum composite likelihood method for determination of evolutionary distances (62). Sequences were aligned in MEGA 4.0.2 using ClustalW. The percent nucleotide sequence identity of the ehxA sequences was calculated using CLC Main Workbench 6.7.2.
Statistical analysis.
The significance of observed differences in the pattern of ehxA subtypes among ehxA-positive isolates obtained from different sources (clinical, food, and animal), as well as observed differences in frequencies of isolates carrying one or more virulence factors (stx1, stx2, or eae) among hemolytic versus nonhemolytic isolates were evaluated by using Fischer's exact test. Differences in virulence factor carriage were evaluated both overall and with respect to each source type separately. All calculations were performed using the R programming language (63). An α level of 0.05 was used as the criterion for statistical significance.
RESULTS
ehxA PCR-RFLP subtyping.
ehxA-positive E. coli strains were subtyped by PCR-RFLP. Overall, 301 (69.2%) of the 435 E. coli isolates investigated were ehxA positive (Table 2). The majority (85.0%; 96/113) of the ehxA-positive clinical isolates carried stx and eae, while almost the same proportion (84.5%; 131/155) of the ehxA-positive food isolates carried only stx. The majority of the ehxA-positive strains isolated from animal sources carried stx and eae (60.7%; 17/28) or stx only (35.7%; 10/28) (Table 3). The five ehxA-positive E. coli strains isolated from unidentified sources were all positive for stx and eae, except for one strain which was only eae positive.
Table 2.
Prevalence of ehxA gene among E. coli strains from clinical, food, animal, and unidentified sources
| ehxA genotype | Total no. (%) of strains with genotype | No. (%) of strains with ehxA genotype isolated from source |
|||
|---|---|---|---|---|---|
| Clinical | Food | Animal | Unidentified | ||
| Positive | 301 (69.2) | 113 (73.4) | 155 (77.5) | 28 (48.3) | 5 (35.7) |
| Negative | 134 (30.8)a | 41 (26.6) | 45 (22.5) | 30 (51.7) | 9 (64.3) |
Nine ehxA-negative water isolates were included in the analysis, to represent the total of 435 E. coli isolates investigated in the study.
Table 3.
Virulence factors (stx only, stx and eae, or eae only) associated with ehxA subtypes of E. coli strains from clinical, food, animal, and unidentified sources
| ehxA subtype | No. (%) of strains with subtype | No. of strainsa with virulence factor(s) in isolates from source |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical |
Food |
Animal |
Unidentified |
||||||||||
| stx | stx/eae | eae | stx | stx/eae | eae | stx | stx/eae | eae | stx | stx/eae | eae | ||
| A | 153 (50.8) | 15 | 127 | 10 | 1 | ||||||||
| B | 9 (3.0) | 5 | 2 | 1 | 1 | ||||||||
| C | 109 (36.2) | 73 | 2 | 19 | 11 | 1 | 3 | ||||||
| D | 4 (1.3) | 4 | |||||||||||
| E | 2 (0.7) | 2 | |||||||||||
| F | 24 (8.0) | 16 | 3 | 4 | 1 | ||||||||
The values listed in bold correspond to the predominant/unique ehxA subtype(s) in each category of isolate.
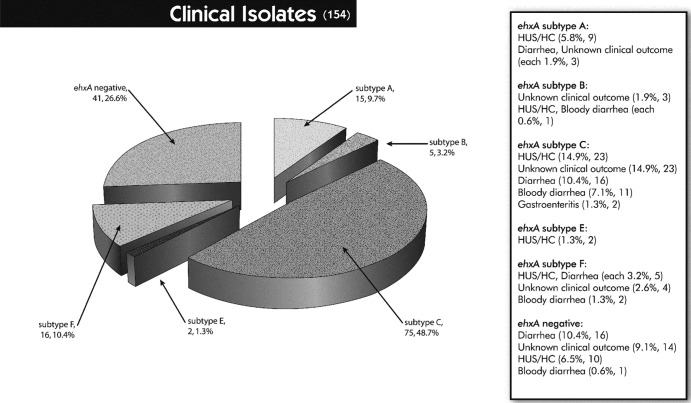
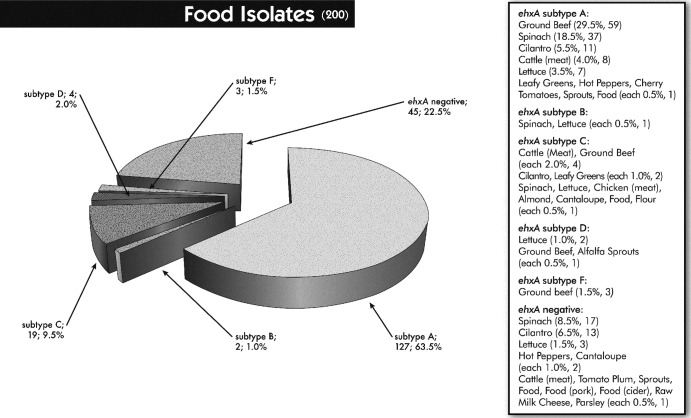
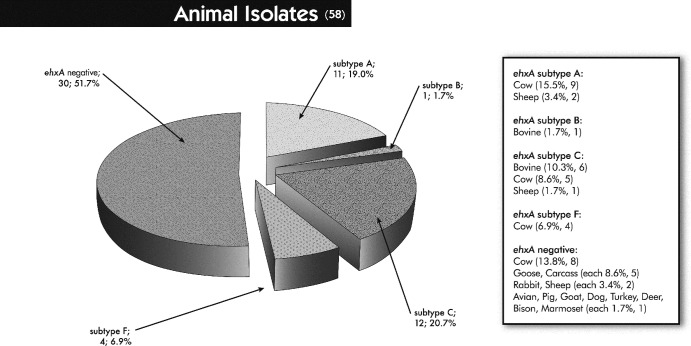
For the PCR step, an attempt was made to use the primers described by Cookson and colleagues (37), but not all ehxA-positive isolates investigated in this study were detected. Hence, a new primer pair located just outside the ehxA gene was designed, yet the ehxA RFLP profiles obtained appeared as described previously (37). Six distinct (A, B, C, D, E, and F) ehxA subtypes were obtained by PCR-RFLP. Among a total of 301 ehxA-positive E. coli strains, the majority were represented by ehxA subtype A (50.8%), followed by subtypes C (36.2%) and F (8.0%). ehxA subtypes B (3.0%), D (1.3%), and E (0.7%) were detected less frequently (Table 3). The six ehxA subtypes were subdivided into eae-negative and eae-positive STEC strains; subtypes A and D were eae negative, except for one subtype A strain (an animal isolate) that was eae positive, and subtypes B, C, E, and F were eae-positive strains. Three subtype C strains (two clinical and one animal isolate) and one subtype B strain (unidentified source) were non-STEC strains but eae positive. ehxA subtype patterns differed significantly between isolates collected from different sources (P < 0.0001). While the animal isolates could be divided into subtypes A (39.3%; 11) and C (42.9%; 12) and the food isolates were mostly associated with subtype A (81.9%; 127), the majority of the ehxA-positive clinical isolates belonged to subtype C (66.4%; 75) (Table 3). Overall, all subtypes were found more or less in all categories of isolates, except for subtype D (2.6%, 4), which was exclusively found in food isolates (alfalfa sprouts [1], lettuce [2], and ground beef [1]), and subtype E (1.8%; 2), which was found only in E. coli strains isolated from ill individuals (Table 3). Furthermore, specific ehxA subtypes were not related to particular animal hosts, food products, or a specific clinical outcome (Fig. 1, 2, and 3).
Fig 1.
Distribution of clinical isolates to ehxA subtypes and ehxA-negative samples in association with the available clinical data. Values in parentheses are the total numbers of strains; percentages were calculated based on the total number of clinical isolates (n = 154).
Fig 2.
Distribution of food isolates to ehxA subtypes and ehxA-negative samples in association with different food products. Values in parentheses are the total numbers of strains; percentages were calculated based on the total number of food isolates (n = 200).
Fig 3.
Distribution of animal isolates to ehxA subtypes and ehxA-negative samples in association with different animal hosts. Values in parentheses are the total numbers of strains; percentages were calculated based on the total number of animal isolates (n = 58).
Distribution of O serogroups and association with ehxA subtypes.
Overall, 259 (59.5%) of the 435 E. coli isolates used in this study were serotyped as O26 (30 isolates), O45 (11), O91 (17), O103 (29), O104 (50), O111 (29), O113 (19), O121 (17), O128 (10), O145 (22), O157 (9) or O8 (16). The O serogroup for 62 (14.3%) strains was not determined or not provided. Of the remaining 114 (26.2%) strains, five each were of serogroups O1, O163, and O168, and six each were of serogroups O22, O116, and O174; the rest belonged to different serogroups associated with less than five strains. In this study, 130 (84.4%) of the 154 clinical and 52 (89.7%) of the 58 animal isolates, but only 40 (20.0%) of the 200 food isolates, were of the clinically important serogroups (O26, O45, etc.). Certain O serogroups were associated with particular ehxA subtypes. All ehxA-positive isolates of serogroups O26, O111, and O121 carried ehxA subtype C regardless of source, whereas all ehxA-positive isolates of serogroups O104, O113, and O8 were of ehxA subtype A. ehxA subtype B exclusively corresponded to E. coli strains of serogroup O157, and ehxA subtype F was found in all ehxA-positive isolates of serogroup O45. Other serogroups corresponded to different ehxA subtypes, with serogroup O91 being of ehxA subtypes A and C, serogroup O145 being of subtypes C and E, and O103 being of subtypes F and C.
Sequencing analysis and phylogenetic profiling of ehxA subtypes.
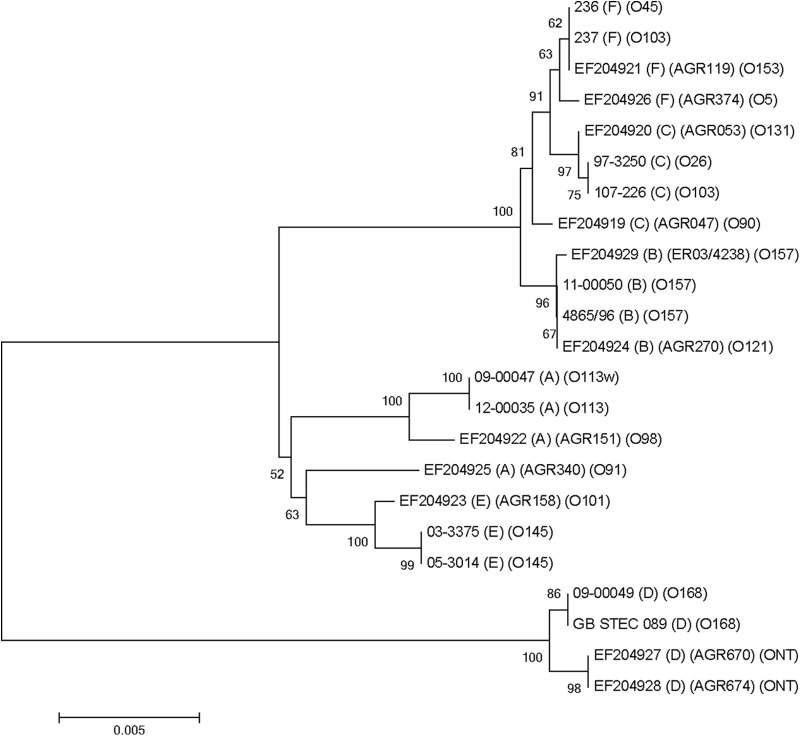
Twelve ehxA genes, two of each of the six ehxA subtypes, were fully sequenced and aligned along with 11 ehxA sequences published by Cookson et al. (37). Based on the 23 ehxA sequences, a phylogenetic tree was generated using the neighbor-joining method with MEGA 4.0. Phylogenetic profiling showed a close relationship between eae-positive ehxA subtype B, C, and F strains, while eae-negative subtype A strains formed a distinct group with subtype E strains. The latter were associated with eae-positive STEC isolates in our study, but they can be eae negative as well (37). ehxA subtype D strains (eae negative) formed the most divergent subdivision, one well separated from the other subtypes (Fig. 4). Pairwise comparison analysis of the 23 ehxA sequences revealed a percent nucleotide sequence identity of 94.2% to 96.4% between ehxA subtype D and the remaining five ehxA subtypes. The sequence similarity of non-subtype D ehxA subtypes was ≥97.3%.
Fig 4.
Phylogenetic relationships of E. coli ehxA sequences from this study, in comparison with sequencing results reported by Cookson et al. (EF204919 to EF204929) (37). The phylogenetic tree was generated using the neighbor-joining method with MEGA 4.0. Bootstrap values of ≥50% are shown at branch points.
Hemolysin gene prevalence and correlation with hemolytic expression.
The ability of certain E. coli strains to lyse erythrocytes of mammalian species is termed hemolysis (27), and the use of SHIBAM proved to be an excellent medium for hemolysis detection for all types of hemolysins (56, 64). The degree of hemolysis varied by strain, from large and clear zones to turbid and smaller zones of hemolysis surrounding single colonies, as noted by others (25, 37, 65). Largely, the different degrees of hemolysis could not be linked to a particular hemolysin type or ehxA subtype, except for the alpha-hemolytic strains, which characteristically showed strong lysis and visible clearing on SHIBAM (27). Overall, 301 (69.2%) of the 435 E. coli strains were positive for ehxA, 408 (93.8%) were positive for sheA, 22 (5.1%) were positive for e-hlyA, and 12 (2.8%) were alpha-hemolytic. sheA was present mainly in correlation with other hemolysins, but 63 (16.7%) of the hemolytic strains carried sheA only. The phage-carried enterohemolysin (e-hlyA) was found in less than 6% of the strains and was mainly associated with the presence of ehxA and sheA. Generally, hemolytic activity could be attributed to the different types of hemolysins investigated in this study; all 377 hemolytic E. coli strains carried at least one hemolysin gene. Of the 57 nonhemolytic strains, two carried ehxA, but no hemolytic phenotype was detected.
Hemolytic expression in association with virulence factors.
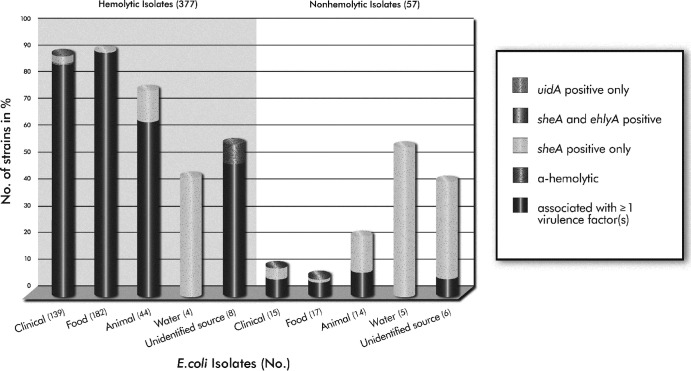
Overall, 377 (86.7%) of the 435 E. coli strains investigated in this study showed hemolytic activity on washed sheep blood agar after 24 h of incubation at 37°C. Significantly more hemolytic strains (95.0%; 358/377) were associated with stx and/or eae positivity than among nonhemolytic strains (49.1%; 28/57) (P < 0.0001) (Table 4; Fig. 5). Of the 19 hemolytic strains (5.0%) that lacked the presence of Shiga toxin and/or intimin, two strains were alpha-hemolytic and sheA positive, 16 carried only sheA, and one carried sheA and e-hlyA. Also noteworthy was one strain, isolated from food, that did not grow on SHIBAM but carried the stx1, stx2, eae, ehxA, and sheA genes. Of the 154 clinical isolates, 95.7% (133/139) of the hemolytic strains carried stx and/or eae, as opposed to 53.3% (8/15) of the nonhemolytic strains (P < 0.0001). Of the 200 food isolates, 98.9% (180/182) of the hemolytic strains were stx and/or eae positive, while 76.5% (13/17) of the nonhemolytic strains harbored stx only (P < 0.001). About 86% (38/44) of the hemolytic strains and only 24% (6/14) of the nonhemolytic strains isolated from animals were associated with stx and/or eae (P < 0.05). The nine E. coli strains isolated from water were all positive for sheA only, and four strains showed hemolytic activity. Fourteen strains were isolated from unidentified sources, and seven of the eight hemolytic strains were positive for stx and/or eae. Of the six nonhemolytic strains, one strain carried stx2 (Table 4; Fig. 5).
Table 4.
Virulence factors in hemolytic and nonhemolytic E. coli strains from clinical, food, animal, and unidentified sources
| Virulence factor(s) | No. of H and NH strainsa associated with virulence factor(s) that were isolated from indicated source |
|||||||
|---|---|---|---|---|---|---|---|---|
| Clinical (n = 154) |
Food (n = 199)b |
Animal (n = 58) |
Unidentified (n = 14) |
|||||
| H | NH | H | NH | H | NH | H | NH | |
| stx only | 19 | 3 | 157 | 13 | 16 | 4 | 1 | 1 |
| stx and eae | 103 | 3 | 23 | 0 | 18 | 1 | 5 | 0 |
| eae only | 11 | 2 | 0 | 0 | 4 | 1 | 1 | 0 |
| stx and eae negative | 6 | 7 | 2 | 4 | 6 | 8 | 1 | 5 |
H, hemolytic; NH, nonhemolytic.
One strain failed to grow on SHIBAM.
Fig 5.
Proportional distribution of hemolytic and nonhemolytic phenotypes within the different categories of isolates, in relation to the presence of at least one/or no virulence factor (stx1, stx2, eae). Values in parentheses are the total numbers of isolates investigated. (Note: One food isolate failed to grow on SHIBAM.)
DISCUSSION
Certain E. coli virulence factors, such as Shiga toxin and intimin, have been well investigated, but the role of hemolysin genes (i.e., hlyA, ehxA, and sheA) in E. coli pathogenicity and the association between these potential virulence factors and stx and eae are largely unknown. Others have noted that the presence of enterohemolysin (ehxA) is a good epidemiological marker for the presence of Shiga toxin (28, 36) and that at least six different genetic ehxA subtypes exist in E. coli (37). To our knowledge, the investigation of hemolysin gene prevalence, as well as ehxA subtype distribution in food isolates compared to clinical and animal isolates from various geographical locations, has not been previously documented.
One interesting finding from the current study was the variation in ehxA subtype patterns observed between the different categories of isolates (P < 0.0001). The majority of food isolates contained ehxA subtype A (64%); however, approximately 20% did not carry the ehxA gene. Nearly 10% of the food isolates were associated with ehxA subtype C. In contrast, the clinical isolates more often carried ehxA subtype C (50%) versus A (10%), while approximately 25% were ehxA negative. Alternately, the animal isolates were nearly equally related to ehxA subtypes A and C (each 20%), but about 50% lacked ehxA (Fig. 1 to 3). Importantly, 60% of the ehxA-negative animal isolates were non-STEC.
Another remarkable finding was the stark contrast in intimin prevalence rates between food and clinical isolates; the majority of the food isolates (85%) were associated with eae-negative STEC strains, while nearly the same proportion (78%) of clinical isolates carried eae (Table 3). The association of eae-positive clinical STEC isolates and severe diarrhea and HUS has been noted (2, 19, 66–68). However, several eae-negative non-O157 STEC strains have been associated with severe human disease and HUS outbreaks, e.g., O104:H21 (USA, 1994), O113:H21 (Australia, 1998), and O104:H4 (Germany, 2011). It is surprising that the overall prevalence patterns for eae and ehxA subtypes obtained from the food isolates were generally not reflected in the examined clinical isolates. Although the food isolates investigated in this study did encompass a variety of fresh produce types (e.g., spinach, lettuce, sprouts) as well as ground beef, they did not represent a survey of all food products available. In the United States, leafy greens (e.g., lettuce and spinach) and ground beef have been important transmission vehicles for human E. coli infections involving E. coli O157:H7, as well as non-O157 STEC (3–5, 13, 53). While the USDA recommends that ground beef be cooked to 160°F to destroy pathogens that might be present, including harmful E. coli, leafy greens are usually consumed raw and possibly with little cleaning. Thus, any enteric pathogens acquired during growth in an open agricultural environment might be still present and infectious (11, 14). However, the observation of mostly eae-positive clinical isolates and eae-negative food isolates has been noted in several studies and therefore seems to be a general trend (18, 75–78). It might be that STEC strains carrying ehxA subtype A, strains which lack the eae gene, generally do not cause severe illness in infected patients or are mainly associated with sporadic cases; thus, we have not found this subtype as frequently in clinical isolates, when often a patient is not ill enough to seek medical care.
Another reason for the minority of ehxA subtypes C and F strains among isolates from food might be the limitation of the isolation method used. For example, the 67 ground beef isolates used in this study were isolated by the use of phenotypic characteristics (55). As shown in this study and supported by others, hemolytic activity as observed on washed sheep blood agar is a good indicator to identify ehxA-positive STEC (28, 35, 36, 64). However, in our study, two ehxA-positive isolates, one of which carried ehxA subtype C and one that carried ehxA subtype A, did not express hemolytic activity on SHIBAM. In addition, one eae-, ehxA-positive STEC isolate of ehxA subtype C isolated from food failed to grow on SHIBAM. Because of their nonhemolytic activity, such colonies might have gone unrecognized in the Bosilevac et al. study (55). In fact, Bosilevac and colleagues noted an inconsistency between the screening of the enrichment and the culture results; significantly fewer STEC isolates were obtained from eae- and stx-positive samples than from samples that were only stx positive (69). This result may explain the higher frequency of eae-negative isolates in food samples. Based on these findings, it is possible that more eae-positive STEC isolates and correspondingly more subtype C and F strains will be identified in food samples, as improved isolation methods and selective agars for non-O157 STEC are developed.
Three previous studies investigated ehxA subtypes. The study by Boerlin et al. mainly focused on the evolutionary lineage of ehxA and noted that ehxA sequences cluster into two main groups corresponding to the presence or absence of eae, suggesting that this is due to the evolution of two different virulence plasmids associated with these strains (70). Newton et al. confirmed the ehxA phylogeny division into two major clusters and showed that, in fact, eae-negative strains carry quite a different virulence plasmid than that found in many eae-positive strains (71). We intend to investigate these findings further by using ehxA-positive isolates identified in this study. Along with elucidating the finer structure in the ehxA phylogeny by using RFLP, Cookson and colleagues identified six ehxA subtypes that clustered into groups of eae-negative (subtype A) and eae-positive (subtype E) strains, eae-positive strains (subtypes B, C, and F), and a third cluster of eae-negative subtype D strains (37). Sequencing analysis and phylogenetic and pairwise comparison of the ehxA gene in the present study confirmed the Cookson et al. findings (Fig. 4).
Cookson et al. also investigated the ehxA subtype prevalence mainly in sheep and cattle isolates, as well as in 25 clinical isolates, all of which originated from New Zealand (37). Comparably, we investigated the ehxA subtype prevalence in a much larger data set: 154 clinical isolates, 200 food isolates, and 58 animal isolates from various U.S. geographical areas and beyond. While the study by Cookson and colleagues revealed that clinical isolates carry subtypes C, A, and B in decreasing numbers, the present study additionally identified subtypes E and F, which had not been previously reported (37). The overall predominance of ehxA subtypes A and C, as well as the strong link between subtypes and eae presence or absence, were confirmed in the current study (Table 3). We furthermore validated that ehxA subtypes were closely tied to particular O serogroups (37). For example, ehxA subtype A strains were closely associated with O8, O22, O88, O91, O113, O116, O163, and O174 strains, which are less commonly isolated during outbreaks (1, 6) but represented the most frequently isolated STEC isolates from food samples in our study. Importantly, O serogroups O91 and O113 have been linked to human illness in the past (1, 6, 7, 72), and their presence in the U.S. food supply is of concern. In contrast, the six serogroups (O26, O45, O103, O111, O121, and O145) that are most frequently isolated due to outbreaks (1, 2, 73) and accounted for the majority of the clinical isolates (64%) in our study were closely associated with ehxA subtypes C and F. Subtype B was exclusively associated with strains of O serogroup O157. These findings confirmed associations that may help with the identification of clinically relevant STEC without the need for laborious and expensive serotyping methods. Of note, Cookson at al. identified a single O121 strain of ehxA subtype B, indicating that subtype B might not be exclusively associated with O157 STEC. This was not confirmed in our study; all O121 strains were of subtype C.
Most of the strains (87%) analyzed in this study were hemolytic, as observed on SHIBAM, and almost all (95%) carried stx and/or eae. Hemolytic strains carried at least one hemolysin gene; while e-hlyA (5%) and hlyA (3%) were found less frequently, the silent hemolysin was widely distributed (94%) among E. coli isolates, as noted previously (48, 74). The majority of the sheA-positive strains carried other hemolysin genes, mainly ehxA; however, 63 hemolytic strains carried only sheA, suggesting that sheA might be responsible for hemolytic expression. In fact, previous studies have revealed that sheA is not totally silent in some E. coli strains, and construction of sheA deletion mutants uncovered a complete loss of hemolytic activity, indicating a sheA-dependent hemolytic phenotype (22, 40, 48, 74). The addition of mitomycin C into the SHIBAM agar used in this study (56) may have further contributed to an increased release of sheA, presumably resulting in detectable levels of hemolytic activity (40). Additionally, anaerobic conditions during incubation of blood agar plates resulted in increased hemolytic expression due to sheA, and strains that were nonhemolytic under aerobic conditions expressed hemolytic activity when incubated anaerobically (22). These findings were not confirmed in our study; most of the strains either did not grow anaerobically, or hemolytic activity was decreased or completely absent compared to aerobic conditions (unpublished data). The significance of sheA in pathogenicity of E. coli strains remains largely unclear. Although sheA is present in highly pathogenic E. coli strains (e.g., O157:H7 and O104:H4 [Germany, 2011]) and previous studies have shown the ability of sheA to lyse erythrocytes and to exhibit cytotoxic and apoptotic activities toward cultured mammalian cells (41, 42, 46, 49, 50), sheA is also widely spread among many nonpathogenic E. coli strains. But as shown by Ludwig et al. (48), many strains harbor only mutant sheA derivatives or fragments therefore of nonfunctional sheA genes. Overall, more study is necessary to identify the exact role of the silent hemolysin in E. coli strains. Importantly, data generated in this study established that strains that express hemolytic activity, whether due to sheA or other hemolysin genes, are significantly more likely (95%) to be associated with highly deleterious virulence factors (stx, eae) than nonhemolytic strains (49%) (P < 0.0001) (Table 4; Fig. 5).
In summary, no apparent association was found between specific ehxA subtypes and a particular clinical outcome or food/animal source (Fig. 1 to 3). The widely different ehxA subtype prevalence rates between clinical, food, and animal isolates were surprising, given that farm animals are natural reservoirs of STEC and that food, especially fresh produce and ground beef, is an important transmission vehicle for E. coli infections in humans (3–5, 11, 12, 13, 53). One would expect similar ehxA subtype patterns between isolates from clinical, animal, and food sources. However, a linear flow of infection originating from farm animals and food to ill individuals persists, since we did find all ehxA subtypes more or less in all categories of isolates; also, not all isolates carried ehxA (Tables 2 and 3). Based on our findings, ehxA subtype C and F strains (eae positive), which were predominant in the clinical isolates (80%) and are associated with O serogroups such as O26, O45, O103, O111, O121, and O145, are possibly more frequently linked to severe clinical outcomes than ehxA subtype A strains (14%; eae negative). Overall, more clinical samples associated with bloody diarrhea, HC, and HUS, as well as a greater variety of food products, are needed to draw more exact conclusions.
The strong link between ehxA subtypes and eae in STEC makes it largely possible to distinguish between eae-positive/negative STEC/E. coli strains by ehxA subtype. Additionally, the close association between particular ehxA subtypes and certain O serogroups, especially O157 and O serogroups that are frequently isolated due to outbreaks, might further contribute to the detection of clinically important STEC. But further work is needed to refine ehxA RFLP primers in order to detect all subtypes reliably. Hemolytic activity, as observed on SHIBAM, shows that this medium generally represents an excellent medium for the isolation of stx- and/or eae-positive E. coli strains, regardless of the type of hemolysin gene expressed. In fact, significantly more hemolytic strains were associated with virulence factors (stx, eae) than nonhemolytic strains (P < 0.0001) (Table 4; Fig. 5). Overall, sheA and ehxA were widely distributed among the E. coli isolates tested, while e-hlyA and hlyA were rarely identified. Further studies are required to investigate the lack of hemolytic expression for the few ehxA-positive E. coli strains identified in this study.
Supplementary Material
ACKNOWLEDGMENTS
This project was supported by an appointment (S.C.L.) to the Research Fellowship Program for the Center for Food Safety and Applied Nutrition administered by the Oak Ridge Associated Universities through a contract with the FDA. This project is further part of the PhD program (S.C.L.) at the University of Hamburg, Germany, under the supervision of Markus Fischer.
We are deeply grateful to Nancy Strockbine for her critical review of the manuscript. We also thank John C. Bowers (FDA CFSAN) for statistical assistance and interpretation of the data, as well as Aaron Heifetz (FDA CFSAN) for technical review of the manuscript.
Footnotes
Published ahead of print 9 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02200-13.
REFERENCES
- 1.Johnson KE, Thorpe CM, Sears CL. 2006. The emerging clinical importance of non-O157 Shiga toxin-producing Escherichia coli. Clin. Infect. Dis. 43:1587–1595 [DOI] [PubMed] [Google Scholar]
- 2.Brooks JT, Sowers EG, Wells JG, Greene KD, Griffin PM, Hoekstra RM, Strockbine NA. 2005. Non-O157 Shiga toxin-producing Escherichia coli infections in the United States, 1983–2002. J. Infect. Dis. 192:1422–1429 [DOI] [PubMed] [Google Scholar]
- 3.CDC 2010. Investigation update: multistate outbreak of human E. Coli O145 infections linked to shredded romaine lettuce from a single processing facility. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/ecoli/2010/ecoli_o145/index.html [Google Scholar]
- 4.CDC 2012. Multistate outbreak of Shiga toxin-producing Escherichia coli O157:H7 infections linked to organic spinach and spring mix blend. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/ecoli/2012/O157H7-11-12/index.html [Google Scholar]
- 5.Rangel JM, Sparling PH, Crowe C, Griffin PM, Swerdlow DL. 2005. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerg. Infect. Dis. 11:603–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bettelheim KA. 2007. The non-O157 Shiga-toxigenic (verocytotoxigenic) Escherichia coli: under-rated pathogens. Crit. Rev. Microbiol. 33:67–87 [DOI] [PubMed] [Google Scholar]
- 7.Paton AW, Woodrow MC, Doyle RM, Lanser JA, Paton JC. 1999. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J. Clin. Microbiol. 37:3357–3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beutin L, Martin A. 2012. Outbreak of Shiga toxin-producing Escherichia coli (STEC) O104:H4 infection in Germany causes a paradigm shift with regard to human pathogenicity of STEC strains. J. Food Prot. 75:408–418 [DOI] [PubMed] [Google Scholar]
- 9.Gyles CL. 2007. Shiga toxin-producing Escherichia coli: an overview. J. Anim. Sci. 85:E45–E62 [DOI] [PubMed] [Google Scholar]
- 10.Levine MM, Xu J-G, Kaper JB, Lior H, Prado V, Tall B, Nataro J, Karch H, Wachsmuth K. 1987. A DNA probe to identify enterohemorrhagic Escherichia coli of O157:H7 and other serotypes that cause hemorrhagic colitis and hemolytic uremic syndrome. J. Infect. Dis. 156:175–182 [DOI] [PubMed] [Google Scholar]
- 11.Tauxe R, Kruse H, Hedberg C, Potter M, Madden J, Wachsmuth K. 1997. Microbial hazards and emerging issues associated with produce: a preliminary report to the National Advisory Committee on Microbiologic Criteria for Foods. J. Food Prot. 60:1400–1408 [DOI] [PubMed] [Google Scholar]
- 12.Caprioli A, Morabito S, Brugère H, Oswald E. 2005. Enterohaemorrhagic Escherichia coli: emerging issues on virulence and modes of transmission. Vet. Res. 36:289–311 [DOI] [PubMed] [Google Scholar]
- 13.Erickson MC, Doyle MP. 2007. Food as a vehicle for transmission of Shiga toxin-producing Escherichia coli. J. Food Prot. 70:2426–2449 [DOI] [PubMed] [Google Scholar]
- 14.Beutin L. 2006. Emerging enterohaemorrhagic Escherichia coli, causes and effects of the rise of a human pathogen. J. Vet. Med. B Infect. Dis. Vet. Public Health 53:299–305 [DOI] [PubMed] [Google Scholar]
- 15.Karch H, Bielaszewska M, Bitzan M, Schmidt H. 1999. Epidemiology and diagnosis of Shiga toxin-producing Escherichia coli infections. Diagn. Microbiol. Infect. Dis. 34:229–243 [DOI] [PubMed] [Google Scholar]
- 16.Konowalchuk J, Speirs JI, Stavric S. 1977. Vero response to a cytotoxin of Escherichia coli. Infect. Immun. 18:775–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. U. S. A. 92:1664–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paton AW, Paton JC. 1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36:598–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paton JC, Paton AW. 1998. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 11:450–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karmali M, Petric M, Steele B, Lim C. 1983. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet 321:619–620 [DOI] [PubMed] [Google Scholar]
- 21.Levine MM. 1987. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J. Infect. Dis. 155:377–389 [DOI] [PubMed] [Google Scholar]
- 22.Murase K, Ooka T, Iguchi A, Ogura Y, Nakayama K, Asadulghani M, Islam MR, Hiyoshi H, Kodama T, Beutin L, Hayashi T. 2012. Haemolysin E- and enterohaemolysin-derived haemolytic activity of O55/O157 strains and other Escherichia coli lineages. Microbiology 158:746–758 [DOI] [PubMed] [Google Scholar]
- 23.Alouf JE. 2001. Pore-forming bacterial protein toxins: an overview. Curr. Top. Microbiol. Immunol. 257:1–14 [PubMed] [Google Scholar]
- 24.Welch RA. 1991. Pore-forming cytolysins of Gram-negative bacteria. Mol. Microbiol. 5:521–528 [DOI] [PubMed] [Google Scholar]
- 25.Beutin L, Prada J, Zimmermann S, Stephan R, Ørskov I, Ørskov F. 1988. Enterohemolysin, a new type of hemolysin produced by some strains of enteropathogenic E. coli (EPEC). Zentralbl. Bakteriol. Mikrobiol. Hyg. A 267:576–588 [DOI] [PubMed] [Google Scholar]
- 26.Rennie RP, Arbuthnott JP. 1974. Partial characterisation of Escherichia coli haemolysin. J. Med. Microbiol. 7:179–188 [DOI] [PubMed] [Google Scholar]
- 27.Beutin L. 1991. The different hemolysins of Escherichia coli. Med. Microbiol. Immunol. 180:167–182 [DOI] [PubMed] [Google Scholar]
- 28.Beutin L, Montenegro MA, Ørskov I, Ørskov F, Prada J, Zimmermann S, Stephan R. 1989. Close association of verotoxin (Shiga-like toxin) production with enterohemolysin production in strains of Escherichia coli. J. Clin. Microbiol. 27:2559–2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao J-S, Stucker DM, Warren JW, Mobley HLT. 1997. Pathogenicity island sequences of pyelonephritogenic Escherichia coli CFT073 are associated with virulent uropathogenic strains. Infect. Immun. 65:2812–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson JR. 1991. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 4:80–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hacker J, Hughes C, Hof H, Goebel W. 1983. Cloned hemolysin genes from Escherichia coli that cause urinary tract infection determine different levels of toxicity in mice. Infect. Immun. 42:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welch RA, Dellinger EP, Minshew B, Falkow S. 1981. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature 294:665–667 [DOI] [PubMed] [Google Scholar]
- 33.Schmidt H, Karch H, Beutin L. 1994. The large-sized plasmids of enterohemorrhagic Escherichia coli O157 strains encode hemolysins which are presumably members of the E. coli alpha-hemolysin family. FEMS Microbiol. Lett. 117:189–196 [DOI] [PubMed] [Google Scholar]
- 34.Schmidt H, Beutin L, Karch H. 1995. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect. Immun. 63:1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt H, Karch H. 1996. Enterohemolytic phenotypes and genotypes of Shiga toxin-producing Escherichia coli O111 strains from patients with diarrhea and hemolytic-uremic syndrome. J. Clin. Microbiol. 34:2364–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beutin L, Aleksic S, Zimmermann S, Gleier K. 1994. Virulence factors and phenotypical traits of verotoxigenic strains of Escherichia coli isolated from human patients in Germany. Med. Microbiol. Immunol. 183:13–21 [DOI] [PubMed] [Google Scholar]
- 37.Cookson AL, Bennett J, Thomson-Carter F, Attwood GT. 2007. Molecular subtyping and genetic analysis of the enterohemolysin gene (ehxA) from Shiga toxin-producing Escherichia coli and atypical enteropathogenic E. coli. Appl. Environ. Microbiol. 73:6360–6369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beutin L, Bode L, Özel M, Stephan R. 1990. Enterohemolysin production is associated with a temperate bacteriophage in Escherichia coli serogroup O26 strains. J. Bacteriol. 172:6469–6475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stroeher UH, Bode L, Beutin L, Manning PA. 1993. Characterization and sequence of a 33-kDa enterohemolysin (Ehlyl)-associated protein in Escherichia coli. Gene 132:89–94 [DOI] [PubMed] [Google Scholar]
- 40.Oscarsson J, Westermark M, Beutin L, Uhlin BE. 2002. The bacteriophage-associated Ehly1 and Ehly2 determinants from Escherichia coli O26:H− strains do not encode enterohemolysins per se but cause release of the ClyA cytolysin. Int. J. Food Microbiol. 291:625–631 [DOI] [PubMed] [Google Scholar]
- 41.Ludwig A, Bauer S, Benz R, Bergmann B, Goebel W. 1999. Analysis of the SlyA-controlled expression, subcellular localization and pore-forming activity of a 34 kDa haemolysin (ClyA) from Escherichia coli K-12. Mol. Microbiol. 31:557–567 [DOI] [PubMed] [Google Scholar]
- 42.Wallace AJ, Stillman TJ, Atkins A, Jamieson SJ, Bullough PA, Green J, Artymiuk PJ. 2000. E. coli hemolysin E (HlyE, ClyA, SheA): X-ray crystal structure of the toxin and observation of membrane pores by electron microscopy. Cell 100:265–276 [DOI] [PubMed] [Google Scholar]
- 43.Eifler N, Vetsch M, Gregorini M, Ringler P, Chami M, Philippsen A, Fritz A, Müller SA, Glockshuber R, Engel A, Grauschopf U. 2006. Cytotoxin ClyA from Escherichia coli assembles to a 13-meric pore independent of its redox-state. EMBO J. 25:2652–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mueller M, Grauschopf U, Maier T, Glockshuber R, Ban N. 2009. The structure of a cytolytic α-helical toxin pore reveals its assembly mechanism. Nature 459:726–730 [DOI] [PubMed] [Google Scholar]
- 45.Ludwig A, Tengel C, Bauer S, Bubert A, Benz R, Mollenkopf H-J, Goebel W. 1995. SlyA, a regulatory protein from Salmonella typhimurium, induces a haemolytic and pore-forming protein in Escherichia coli. Mol. Gen. Genet. 249:474–486 [DOI] [PubMed] [Google Scholar]
- 46.Oscarsson J, Mizunoe Y, Uhlin BE, Haydon DJ. 1996. Induction of haemolytic activity in Escherichia coli by the slyA gene product. Mol. Microbiol. 20:191–199 [DOI] [PubMed] [Google Scholar]
- 47.del Castillo FJ, Moreno F, del Castillo I. 2000. Characterization of the genes encoding the SheA haemolysin in Escherichia coli O157:H7 and Shigella flexneri 2a. Res. Microbiol. 151:229–230 [DOI] [PubMed] [Google Scholar]
- 48.Ludwig A, von Rhein C, Bauer S, Hüttinger C, Goebel W. 2004. Molecular analysis of cytolysin A (ClyA) in pathogenic Escherichia coli strains. J. Bacteriol. 186:5311–5320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai X-H, Arencibia I, Johansson A, Wai SN, Oscarsson J, Kalfas S, Sundqvist K-G, Mizunoe Y, Sjöstedt A, Uhlin BE. 2000. Cytocidal and apoptotic effects of the ClyA protein from Escherichia coli on primary and cultured monocytes and macrophages. Infect. Immun. 68:4363–4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oscarsson J, Mizunoe Y, Li L, Lai X-H, Wieslander Å, Uhlin BE. 1999. Molecular analysis of the cytolytic protein ClyA (SheA) from Escherichia coli. Mol. Microbiol. 32:1226–1238 [DOI] [PubMed] [Google Scholar]
- 51.del Castillo FJ, Leal SC, Moreno F, del Castillo I. 1997. The Escherichia coli K-12 sheA gene encodes a 34-kDa secreted haemolysin. Mol. Microbiol. 25:107–115 [DOI] [PubMed] [Google Scholar]
- 52.Westermark M, Oscarsson J, Mizunoe Y, Urbonaviciene J, Uhlin BE. 2000. Silencing and activation of ClyA cytotoxin expression in Escherichia coli. J. Bacteriol. 182:6347–6357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hussein HS. 2007. Prevalence and pathogenicity of Shiga toxin-producing Escherichia coli in beef cattle and their products. J. Anim. Sci. 85:E63–E72 [DOI] [PubMed] [Google Scholar]
- 54.Rump LV, Bodeis-Jones S, Abbott J, Zhao S, Kase J, Lorenz S, Fischer M, Brown E, Meng J. 2012. Genetic characterization of Escherichia coli O104 isolates from different sources in the United States. Appl. Environ. Microbiol. 78:1615–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bosilevac JM, Koohmaraie M. 2011. Prevalence and characterization of non-O157 Shiga toxin-producing Escherichia coli isolates from commercial ground beef in the United States. Appl. Environ. Microbiol. 77:2103–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin A, Nguyen L, Clotilde LM, Kase JA, Son I, Lauzon CR. 2012. Isolation of Shiga toxin-producing Escherichia coli from fresh produce using STEC heart infusion washed blood agar with mitomycin C. J. Food Prot. 75:2028–2030 [DOI] [PubMed] [Google Scholar]
- 57.Lin A, Nguyen L, Lee T, Clotilde LM, Kase JA, Son I, Carter JM, Lauzon CR. 2011. Rapid O serogroup identification of the ten most clinically relevant STECs by Luminex microbead-based suspension array. J. Microbiol. Methods 87:105–110 [DOI] [PubMed] [Google Scholar]
- 58.Lin A, Sultan O, Lau HK, Wong E, Hartman G, Lauzon CR. 2011. O serogroup specific real time PCR assays for the detection and identification of nine clinically relevant non-O157 STECs. Food Microbiol. 28:478–483 [DOI] [PubMed] [Google Scholar]
- 59.Kumar S, Nei M, Dudley J, Tamura K. 2008. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinformatics 9:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 61.Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 62.Tamura K, Nei M, Kumar S. 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. U. S. A. 101:11030–11035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Core Team R 2013. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 64.Beutin L, Zimmermann S, Gleier K. 1996. Rapid detection and isolation of Shiga-like toxin (verocytotoxin)-producing Escherichia coli by direct testing of individual enterohemolytic colonies from washed sheep blood agar plates in the VTEC-RPLA assay. J. Clin. Microbiol. 34:2812–2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taneike I, Zhang H-M, Wakisaka-Saito N, Yamamoto T. 2002. Enterohemolysin operon of Shiga toxin-producing Escherichia coli: a virulence function of inflammatory cytokine production from human monocytes. FEBS Lett. 524:219–224 [DOI] [PubMed] [Google Scholar]
- 66.Karmali MA. 1989. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 2:15–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beutin L, Krause G, Zimmermann S, Kaulfuss S, Gleier K. 2004. Characterization of Shiga toxin-producing Escherichia coli strains isolated from human patients in Germany over a 3-year period. J. Clin. Microbiol. 42:1099–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oswald E, Schmidt H, Morabito S, Karch H, Marchès O, Caprioli A. 2000. Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infect. Immun. 68:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bosilevac JM, Koohmaraie M. 2012. Predicting the presence of non-O157 Shiga toxin-producing Escherichia coli in ground beef by using molecular tests for Shiga toxins, intimin, and O serogroups. Appl. Environ. Microbiol. 78:7152–7155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boerlin P, Chen S, Colbourne JK, Johnson R, Grandis SD, Gyles C. 1998. Evolution of enterohemorrhagic Escherichia coli hemolysin plasmids and the locus for enterocyte effacement in Shiga toxin-producing E. coli. Infect. Immun. 66:2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Newton HJ, Sloan J, Bulach DM, Seemann T, Allison CC, Tauschek M, Robins-Browne RM, Paton JC, Whittam TS, Paton AW, Hartland EL. 2009. Shiga toxin-producing Escherichia coli strains negative for locus of enterocyte effacement. Emerg. Infect. Dis. 15:372–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mellmann A, Fruth A, Friedrich AW, Wieler LH, Harmsen D, Werber D, Middendorf B, Bielaszewska M, Karch H. 2009. Phylogeny and disease association of Shiga toxin-producing Escherichia coli O91. Emerg. Infect. Dis. 15:1474–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karmali MA, Mascarenhas M, Shen S, Ziebell K, Johnson S, Reid-Smith R, Isaac-Renton J, Clark C, Rahn K, Kaper JB. 2003. Association of genomic O island 122 of Escherichia coli EDL 933 with verocytotoxin-producing Escherichia coli seropathotypes that are linked to epidemic and/or serious disease. J. Clin. Microbiol. 41:4930–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kerényi M, Allison HE, Bátai I, Sonnevend Á, Emödy L, Plaveczky N, Pál T. 2005. Occurrence of hlyA and sheA genes in extraintestinal Escherichia coli strains. J. Clin. Microbiol. 43:2965–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beutin L, Miko A, Krause G, Pries K, Haby S, Steege K, Albrecht N. 2007. Identification of human-pathogenic strains of Shiga toxin-producing Escherichia coli from food by a combination of serotyping and molecular typing of Shiga toxin genes. Appl. Environ. Microbiol. 73:4769–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bugarel M, Beutin L, Martin A, Gill A, Fach P. 2010. Micro-array for the identification of Shiga toxin-producing Escherichia coli (STEC) seropathotypes associated with hemorrhagic colitis and hemolytic uremic syndrome in humans. Int. J. Food Microbiol. 142:318–329 [DOI] [PubMed] [Google Scholar]
- 77.Eklund M, Scheutz F, Siitonen A. 2001. Clinical isolates of non-O157 Shiga toxin-producing Escherichia coli: serotypes, virulence characteristics, and molecular profiles of strains of the same serotype. J. Clin. Microbiol. 39:2829–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meng J, Zhao S, Doyle MP. 1998. Virulence genes of Shiga toxin-producing Escherichia coli isolated from food, animals and humans. Int. J. Food Microbiol. 45:229–235 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.