Abstract
Fungal insecticides developed from filamentous pathogens of insects are notorious for their slow killing action through cuticle penetration, depressing commercial interest and practical application. Genetic engineering may accelerate their killing action but cause ecological risk. Here we show that a Beauveria bassiana formulation, HV8 (BbHV8), engineered for fast per os killing of caterpillars by an insect midgut-acting toxin (Vip3Aa1) overexpressed in conidia has both high field efficacy and safety in full-season protection of cabbage from the damage of an insect pest complex dominated by Pieris rapae larvae, followed by Plutella xylostella larvae and aphids. In two fields repeatedly sprayed during summer, BbHV8 resulted in overall mean efficacies of killing of 71% and 75%, which were similar or close to the 70% and 83% efficacies achieved by commercially recommended emamectin benzoate but much higher than the 31% and 48% efficacies achieved by the same formulation of the parental wild-type strain (WT). Both BbHV8 and WT sprays exerted no adverse effect on a nontarget spider community during the trials, and the sprays did not influence saprophytic fungi in soil samples taken from the field plots during 4 months after the last spray. Strikingly, BbHV8 and the WT showed low fitness when they were released into the environment because both were decreasingly recovered from the field lacking native B. bassiana strains (undetectable 5 months after the spray), and the recovered isolates became much less tolerant to high temperature and UV-B irradiation. Our results highlight for the first time that a rationally engineered fungal insecticide can compete with a chemical counterpart to combat insect pests at an affordable cost and with low ecological risk.
INTRODUCTION
Fungal insecticides are alternative solutions for insect pests highly resistant to chemical insecticides and genetically modified crops (1), but their commercialization is often restrained by a notoriously slow killing action. Vegetable foliage feeders that cause severe leaf damage within a short period cannot be killed by their ingesting formulated conidia on sprayed plants, because insect-pathogenic fungi, such as Beauveria bassiana, infect their hosts by means of the cuticle instead of the digestive track, for which virulence factors are absent (2). This limitation can be alleviated by recent progress in engineering fungal candidates for improved virulence and stress tolerance (3). For instance, fungal virulence was enhanced by overexpressing exogenous chitinase, hybrid chitinase, or Pr1A in B. bassiana (4–6). Integration of a scorpion neurotoxin into fungal entomopathogens resulted in a great increase of toxicity to several lepidoteran pests (7–9) and malaria parasites within mosquitoes (10). Transgenic strains expressing exogenous tyrosinase, thioredoxin, and endogenous manganese superoxide dismutase (MnSOD) became more tolerant to oxidation, UV irradiation, and high temperature (11–13). While such progress facilitates the development of more-efficacious mycoinsecticides by making use of the normal infection mode of cuticle penetration, we were successful in the acquisition of fungal per os virulence by integrating Vip3Aa1, a vegetative insecticidal protein of Bacillus thuringiensis, into a wild-type B. bassiana strain (WT) (14). Its virulence was further enhanced by driving Vip3Aa1 expression specifically during conidiation with an optimized endogenous promoter, and a new B. bassiana transgenic strain, HV8 (BbHV8), under the control of a promoter showed extremely high per os toxicity to Spodoptera litura larvae of all instars as well as normal virulence through cuticle infection (15).
Despite the rapid progress in genetic modification of insect-pathogenic fungi, both the field efficacy and the ecological risk of a transgenic mycoinsecticide are of public concern (16). A recent study to evaluate the ecological risk of wild-type and engineered Metarhizium strains released into a grassland has revealed that genetic improvement may be acceptable for the fungal agents that lack postrelease gene flow and virulence evolution (17). However, little effort has been made to assess both the field efficacy and the safety of a transgenic mycoinsecticide under real conditions. Still, no mycoinsecticide has been developed to compete with a chemical insecticide to seasonally control an insect pest complex infesting a crop.
Numerous B. bassiana insecticides against global arthropod pests have been developed, with no safety problems being documented (18, 19). Field release of transgenic crops expressing Vip3A toxins has been permitted (20) due to the safety of those toxins to vertebrates (21, 22). For these two reasons, a B. bassiana strain integrated with a Vip3A toxin for high per os virulence to caterpillars may be of low ecological risk after being applied for more effective pest control in the field. This study sought to test this hypothesis in repeated field trials by evaluating both the field efficacy and the safety of our transgenic strain against a cabbage pest complex dominated by the cabbage butterfly (CBF), Pieris rapae, followed by the diamond-back moth (DBM), Plutella xylostella, and the green peach aphid (GPA), Myzus persicae. We found that the emulsifiable formulation of transgenic BbHV8 conidia provided full-season cabbage protection similar or close to that achieved by emamectin benzoate (EB), a chemical insecticide recommended for commercial use, and that it exerted no adverse effect on the communities of spiders and soil fungi in the field in which it was released.
MATERIALS AND METHODS
Production and formulation of aerial conidia.
Fungal strains used in the study were wild-type Bb2860, which is highly virulent to piercing pests (23, 24), and its transgenic strain BbHV8, which acquired high per os virulence to S. litura larvae by abundant Vip3Aa1 expression in conidia (15). The two strains were cultured for 7 days on Sabouraud dextrose agar plus 1% yeast extract (SDAY) at 25°C. The resultant conidia were suspended in liquid medium (agar-free SDAY) for propagation by two-stage fermentation. The liquid cultures were mixed with steamed shell-free barley grains at a 1:10 ratio and then uploaded into an upright multitray conidiation chamber (60 by 60 by 200 mm) for 7-day solid fermentation around 25°C (25), followed by drying at 30 to 33°C under ventilation. Powder of aerial conidia was harvested from the dried cultures using a self-designed cyclone spore separator and vacuum dried to ∼5% water content at ambient temperature, followed by storage at 4°C to warrant ≥90% viability within 1 year. The conidial powder was suspended in an oil (paraffin) carrier containing 8% emulsifier (26) and standardized to the concentration of 1010 conidia/ml, which formed an emulsifiable formulation of each strain for use.
Field trials for control of cabbage pest complex.
Two fields (∼0.2 ha each) surrounded by rice and/or corn crops were located for trial 1 (N29°01′, E119°37′) and trial 2 (N29°03′, E119°38′) near Sumeng, Jinhua, Zhejiang, and transplanted with cabbage seedlings (Brassica oleracea var. capitata cv. Jingfeng no. 1), with 50 by 50 cm between plants, on 2 and 12 April 2012, respectively, to attract heavy infestation of lepidopteran pests in early summer. From then on, CBF, DBM, and GPA populations were monitored by making in situ counts of all individuals on nine plants randomly located in each plot every 5 days. The two fields were first sprayed on 22 April and 5 May due to the density of CBF larvae exceeding the economic injury level of 0.5 larva per plant (27, 28). Both fields received repeated sprays until cabbage heads were ready for harvest. Each trial included four treatments of four plots (6 by 11 m); treatments 1 and 2 consisted of BbHV8 and Bb2860 sprayed at the rate of 1013 conidia/ha, treatment 3 consisted of 5% emamectin benzoate (EB) water-dispersible granules (WDG) sprayed at the labeled rate of 2.1 g active ingredients per hectare, and treatment 4 consisted of the oil carrier sprayed at a commensurate rate as a blank control.
All the plots were separated by a 2-m-wide buffer area and were arranged in each trial via a randomized block design and sprayed four times at sunset every 5 to 15 days (varied with pest density) during a 35-day period. Each spray was performed at the volume rate of 150 liters/ha using a knapsack electronic sprayer at uniform working pressure. For the fungal treatments, four glass slides (75 by 25 mm) were diagonally placed in each plot to collect deposited conidia under the first spray. Deposits on each slide were then estimated as the number of conidia per square millimeter from five microscopic view fields (0.2165 mm2 per field). An electronic hydrothermometer was placed ∼20 cm above the soil surface at the center of each field to take hourly records of temperature and relative humidity (RH), which spanned from 12.8 to 36.3°C and 15.5% to 100%, respectively, during the field trials. Daily values from the records were averaged as 22.3°C (18.5 to 25.3°C) and 81.5% RH (58.6 to 99.2% RH) in trial 1 and 22.9°C (18.5 to 26.3°C) and 83.5% RH (58.6 to 100% RH) in trial 2.
Since CBF larvae dominating the pest complex could transfer from severely damaged plants to those less damaged, all visited plants on each sampling occasion were graded by foliage losses of <5% (grade 0), 5 to 20% (grade 1), 20 to 40% (grade 2), 40 to 60% (grade 3), and >60% (grade 4) in order to reflect the real damage level.
A supplementary experiment of four replicates was conducted in a cabbage field adjacent to the trial 2 field to assay Bb2860 and BbHV8 virulence to fourth-instar CBF larvae. Each replicate included three cupping plants (∼12 leaves), with each harboring 10 to 15 larvae. The located plants were labeled and sprayed with a 100-fold aqueous dilution of each fungal formulation (treatment) or the oil carrier (control) in the two field trials, followed by daily examination of larval mortalities for 8 days under the field conditions. Deposited conidia under each fungal spray were assessed as described above using a slide placed on each plant for spore collection.
Assessment of ecological risk after fungal spray.
During the field trials, spiders appearing on visited plants were counted in situ on each sampling occasion. To assess the effect of the fungal release on the soil fungal community, five soil samples (5 cm below the surface) were randomly taken from each plot of the control and fungal-treatment fields of trial 1 the day after the last spray, every 10 days for the first month, and every month for the subsequent 6 months and stored at −20°C for immediate analysis. For each sample, four 100-μl aliquots of 10% (wt/vol) soil suspension in sterile water were evenly spread onto the plates of CTC medium selective for fungal entomopathogens (29) and of Martin's selection medium (MSM) for the growth of saprophytic fungi. CFU grown on each plate were counted after an 8-day incubation on CTC medium at 25°C or after a 5-day incubation on MSM at 28°C.
Since BbHV8 was constructed by transforming the Via3Aa1 gene into Bb2860, 36 B. bassiana isolates were randomly taken from those recovered from the BbHV8 or Bb2860 soil samples 30 to 90 days after the last spray and grown on SDAY for 5 days at 25°C, followed by DNA extraction. The target gene was then amplified from the DNAs via PCR with specific primers (15) to determine if the isolates were recovered from the plots treated with BbHV8 or Bb2860.
Two isolates, i.e., rHV8 and rWT, recovered, respectively, from BbHV8 and Bb2860 isolates 3 months after the spray, were compared with the parental BbHV8 and Bb2860 strains in stress assays of four replicates. The thermotolerance of their conidia produced on SDAY was assayed by exposing 5-ml aliquots of 107 conidia/ml suspension in glass tubes to a hot-water bath at 45°C for up to 150 min (30). During the exposure, 100-μl aliquots were pipetted from each tube every 15 min and released into 1 ml of germination broth (GB; 2% sucrose and 0.5% peptone in 0.02% Tween 80) for 24 h with shaking at 25°C. Percent germination was then assessed using microscopic counting with a hemocytometer. Conidial UV-B resistance was assayed using our previously described method (31). Briefly, 10-μl aliquots of 106 conidia/ml GB were smeared onto central marked areas of the glass slides and exposed to the irradiation of a weighted UV-B wavelength (312 nm; range, 280 to 320 nm) at the gradient doses of 0.1 to 0.7 J/cm2 in a Bio-Sun++ chamber (Vilber Lourmat, Marne-la-Vallée, France). The slides were then incubated for 24 h at 25°C under saturated humidity and stained with cotton blue for estimating percent germination under the microscope.
Data analysis.
The relative efficacy (percentage) of each treatment against the pest complex in each field trial was calculated as [(DSc − DSt)/DSc] × 100, where DSc and DSt denote the damage scores of a blank control and a given treatment, respectively, on a sampling occasion after a spray. One-way (treatment) analysis of variance (ANOVA) was performed to differentiate all the log-transformed counts of insect pests or spiders per plant and the square roots of arcsine values of percent efficacies among the treatments over the sampling occasions of each trial, while damage grades in the treatments were differentiated during each trial via the nonparametric Kruskal-Wallis test. Survival trends of postspray CBF larvae from the field bioassays of BbHV8 andBb2860 were subjected to Kaplan-Meier survivorship analysis with GraphPad Prism 5, yielding a median survival time (ST50) for each fungal strain. The trends of a conidial survival index, Is (i.e., relative germination under a given stress versus that of an unstressed control) over the time of the heat stress (T) and the dose of UV-B irradiation (D) were fitted to the equation Is = 1/[1 + exp(a + bx)], where x is T or D (30, 31). Solving the fitted equations at an Is of 0.5 gave median lethal responses of conidia to the heat (LT50) and UV-B (LD50). The solutions indicative of conidial thermotolerance and UV-B resistance were compared among the tested strains via one-way ANOVA.
RESULTS AND DISCUSSION
Arthropod populations during two field trials.
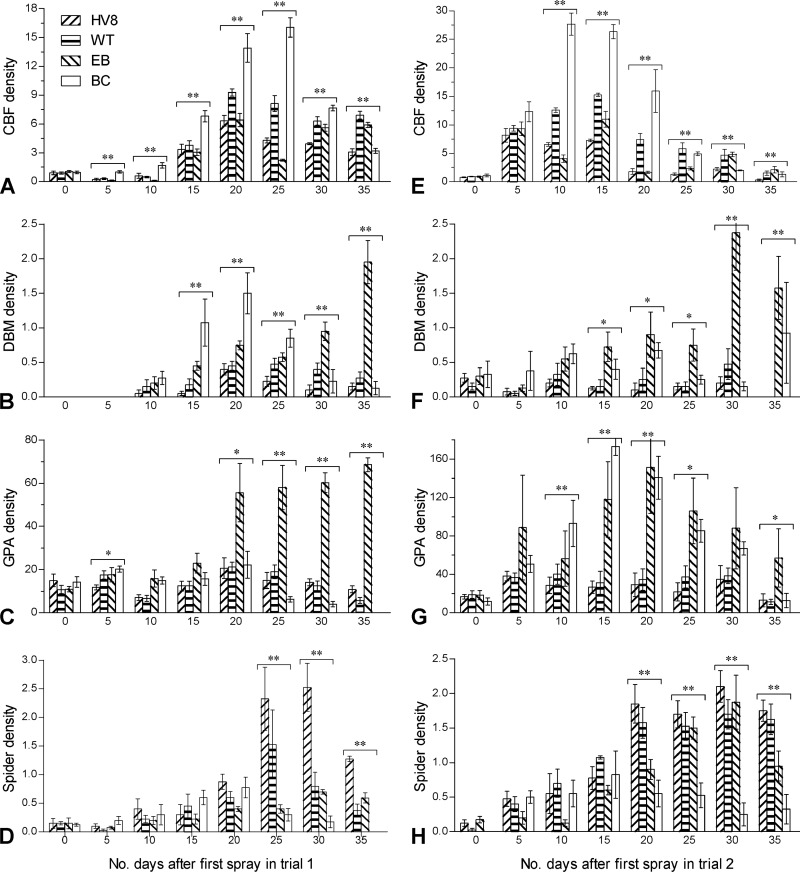
Cabbage grown in two fields surrounded by nonvegetable crops functioned just like a pest trap during early summer and was most attractive to CBFs, followed by DBMs and GPAs. Soon after the cabbage was transplanted, CBFs were attracted to lay eggs on plants, resulting in mean (± standard error of the mean [SEM]) densities (numbers of larvae per plant) of 0.94 ± 0.06 and 0.93 ± 0.05, which exceeded the economic injury level of 0.5 CBF larva per cabbage plant (27, 28) and necessitated the first spray in the two fields on 22 April 2012 and 5 May 2012, respectively. In trial 1, the control plots were infested by the peak populations of 16.1 ± 1.0 CBFs, 1.5 ± 0.1 DBMs, and 22.2 ± 6.2 GPAs per plant in mid-May (Fig. 1A to C). The cabbage transplanted later for trial 2 was more heavily infested by CBFs than the other pests during the early stage and subsequently by GPAs but less infested by DBMs (Fig. 1E to G). In the control plots of this trial, CBF and GPA densities reached 22.3 ± 3.5 and 175.4 ± 8.2, respectively, on 20 May, in contrast to the low DBM density of ∼0.5 during the trial. The population densities of the three pests differed significantly among the four treatments on most sampling occasions (P < 0.05 or 0.01 in the F tests of one-way ANOVA). In both trials, EB and BbHV8 controlled the CBF population significantly better than the formulated wild-type strain (Bb2860), while the fungal treatments suppressed both DBM and GPA populations more than EB, which was ineffective for DBM or GPA control and even caused their rebound at a later stage.
Fig 1.
Field population densities (number of individuals per cabbage plant) of P. rapae (CBF), P. xylostella (DBM), M. persicae (GPA), and spiders during trials 1 (A to D) and 2 (E to H). Sprays of transgenic (HV8) and WT B. bassiana formulations, emamectin benzoate (EB), and a blank control (BC) were provided on days 0, 10, 20, and 30 during trial 1 and on days 0, 5, 15, and 30 during trial 2, respectively. One or two asterisks marked on the bars grouped on a given sampling occasion indicate significant differences among the treatments at P levels of <0.05 or <0.01, respectively, in a one-way ANOVA. Error bars indicate SEMs from four plots of 6 by 11 m.
As common predators of DBM larvae and aphids, the spiders Pardosa astrigera, Erigonidium graminicola, and Oedothorax feminea occurred at the pooled densities of 0.2 to 0.8 individual per plant in the control plots of trials 1 (Fig. 1D) and 2 (Fig. 1H). Notably, the spiders developed significantly larger populations in the fungal-treatment plots than in the control plots (Tukey's honestly significant difference [HSD] test, P < 0.05) after the spray, suggesting no adverse effect of either the BbHV8 or the Bb2860 formulation on the spider community.
Differential damage grades and control efficacies.
The first BbHV8 and Bb2860 sprays resulted in the respective mean deposits of 940 ± 57 and 835 ± 43 conidia/mm2 in trial 1 (Student's t28 = 1.48, P = 0.15) and of 789 ± 55 and 908 ± 81 conidia/mm2 in trial 2 (t28 = 1.21, P = 0.24). The subsequent three sprays in each trial were performed in the same manner, but conidial deposits were not further assessed.
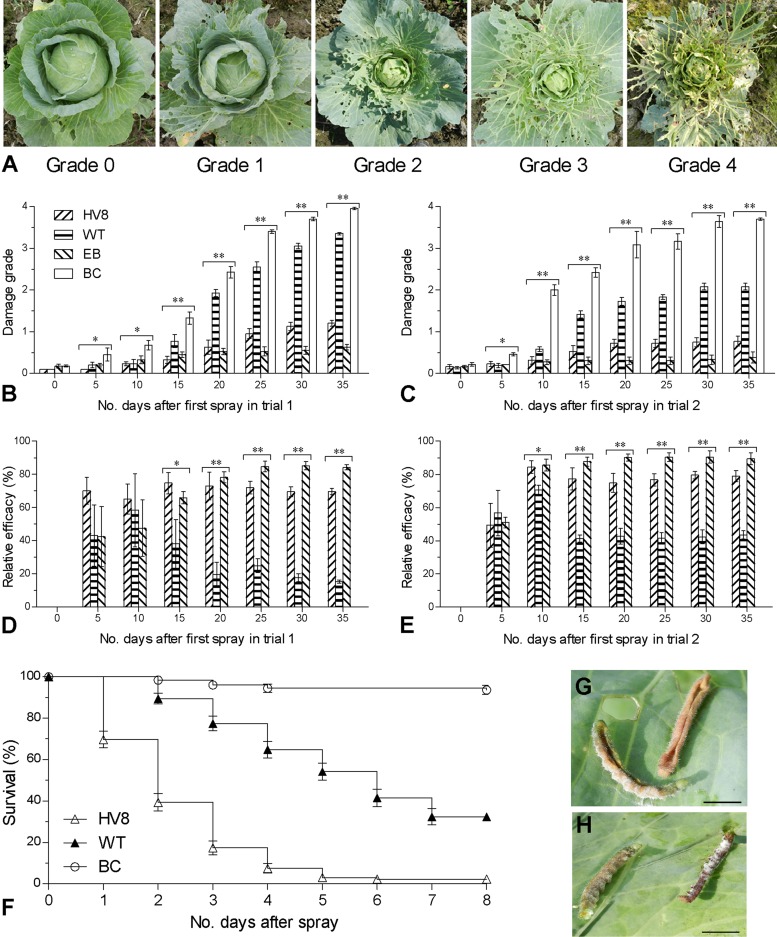
In both trials, cabbage damage caused by the foliage feeder CBF overwhelmed the damage of DBMs and GPAs and thus was graded on the basis of foliage loss (Fig. 2A) to quantify the full-season protection of each treatment from the pest damage. As a result, cabbage damage trends in the fields differed significantly among the treatments on most sampling occasions (P < 0.05 by Kruskal-Wallis [KW] tests for the KW statistic). After two and three sprays within 15 days, the cupping-stage damage grades after the EB and BbHV8 treatments on day 20 were, respectively, reduced to 0.63 ± 0.17 and 0.53 ± 0.08 in trial 1 (Fig. 2B) and 0.72 ± 0.10 and 0.31 ± 0.09 in trial 2 (Fig. 2C) from the control grades of 2.43 ± 0.14 and 3.08 ± 0.32. The corresponding damage grades after two and three sprays in the Bb2860 plots were 1.93 ± 0.09 and 1.72 ± 0.10, respectively, which were significantly lower than those in the control plots of both trials but higher than those in the plots treated with EB and BbHV8. In both trials, the fourth spray on day 30 resulted in the final damage grades of 1.20 ± 0.07 and 0.78 ± 0.12 in the BbHV8-treated field, 3.35 ± 0.03 and 2.08 ± 0.08 in the Bb2860-treated field, 0.63 ± 0.06 and 0.39 ± 0.13 in the EB-treated field, and 3.95 ± 0.03 and 3.69 ± 0.03 in the control field, respectively.
Fig 2.
Cabbage damage and control efficacy in field trials. (A) Images of five leaf damage grades. (B to E) Damage grades and relative efficacies in different treatments against cabbage pest complex during trials 1 and 2 (HVB and WT, transgenic and wild-type B. bassiana formulations; EB, emamectin benzoate recommended for cabbage pest control; BC, blank control). One or two asterisks marked on the bars grouped on a given sampling occasion denote significant differences among the treatments at P levels of <0.05 or <0.01, respectively, in a Kruskal-Wallis test (for damage grades) or one-way ANOVA (for efficacies). (F) Postspray survival trends of fourth-instar P. rapae larvae in the field bioassays of HV8 and WT versus BC. (G and H) Symptoms of P. rapae larvae that died of HV8 and WT, respectively. Error bars indicate SEMs from four plots (B to E) or four replicates (F).
Based on the damage grades, relative control efficacies resulting from the sprays of BbHV8, Bb2860, and EB fell in the respective ranges of 65 to 72%, 15 to 58%, and 42 to 85% during trial 1 (Fig. 2D) and of 42 to 70%, 41 to 70%, and 51 to 90% during trial 2 (Fig. 2E). Overall means of the percent efficacies in trials 1 and 2 were, respectively, 70.6 ± 1.6 and 74.5 ± 4.3 with BbHV8, 30.9 ± 6.1 and 48.4 ± 4.2 with Bb2860, and 69.7 ± 6.9 and 83.5 ± 5.5 with EB. These estimates differed significantly between the Bb2860 and BbHV8 or EB treatments but insignificantly between the BbHV8 and EB treatments on most sampling occasions of both trials (Tukey's HSD, P > 0.05). Taken together, the full-season cabbage protection provided by the transgenic BbHV8 formulation was similar or close to that given by the commercially recommended insecticide EB under conditions of heavy pest infestation from early to middle summer. The wild-type Bb2860 formulation provided a significant level of pest control but was not sufficient to protect cabbage from pest damage in either trial. Notably, the modified schedules for the second and third sprays in trial 2 resulted in better pest control than those in trial 1.
Virulence of BbHV8 and Bb2860 to CBF larvae.
The two fungal formulations were assayed for their virulence to fourth-instar CBF larvae on labeled field cabbage plants. The sprays of formulated BbHV8 and Bb2860 resulted in similar deposits of 836 ± 75 and 934 ± 113 conidia (Student's t22 = 0.74, P = 0.47) per mm2 of the plants. As illustrated in Fig. 2F, postspray CBF survival trends differed significantly between the fungal treatments or between either fungal treatment and the control by the log-rank (Mantel-Cox) test (χ2 = 276.3, df = 2, P < 0.0001). The median survival time (ST50) was estimated as 2 days for BbHV8 and 6 days for Bb2860 but undefined for the control. In other words, BbHV8 killed 50% of the CBF larvae 4 days faster than Bb2860. Moreover, the larvae that died of BbHV8 showed the same symptoms of shrinkage and palsy (Fig. 2G) that were observed previously with S. litura larvae killed by the same strain (15), whereas those killed by Bb2860 displayed typical mycosis syndrome (Fig. 2H). Thus, the faster killing action of BbHV8 was attributed to the ingestion of sprayed cabbage leaves by the larvae because BbHV8 conidia were confirmed to harbor a large quantity of Vip3Aal molecules and to release them into the midgut environment after ingestion (15).
Field safety of fungal release.
Apart from the in situ spider counts during the field trials, the counts of B. bassiana CFU from soil samples (Fig. 3A) decreased significantly over sampling occasions (F6,60 = 4.6, P = 0.01) among the treatments of trial 1 (F2,60 = 28.6, P < 0.01) but insignificantly between the two fungal treatments within 20 days after the last spray (Tukey's HSD, P ≥ 0.23). Compared to the count of ∼2.5 × 104 CFU/g soil the day after the last spray on 23 May, the CFU counts in the BbHV8 and Bb2860 plots decreased by 84.4% and 86.1% 10 days later, 98.9% and 96.8% 2 months later, and 99.8% and 99.5% 4 months later, respectively. The soil samples subsequently taken from the plots of either fungal treatment had very few CFU grown on selective CTC medium (29). Interestingly, a few B. bassiana CFU appeared in the control soil samples taken only on days 10 and 20 (69 and 34 CFU/g) after the spray. In contrast, CFU counts of saprophytic fungi (Fig. 3B) grown on MSM did not differ significantly between the control and either fungal treatment (F2,60 = 0.8, P = 0.49) despite seasonal fluctuation over the sampling period (F6,60 = 60.9, P < 0.01). Each gram of samples taken within 30 days of the spray harbored significantly fewer CFU of saprophytes (∼7.5 × 104) than each gram of samples taken at the ends of July, August, and September (∼25.8 × 104, ∼18.6 × 104, and ∼17.6 × 104, respectively).
Fig 3.
CFU counts of B. bassiana (A) and saprophytic fungi (B) in soil samples taken from plots treated with HV8, the WT, and the control (BC) after the last spray of trial 1. An asterisk marked on bars grouped by a given sampling occasion denotes a significant difference among the treatments at the P level of <0.05 in a one-way ANOVA. Error bars indicate SEMs from four plots.
Seventy-two B. bassiana isolates recovered from the soil samples, which were taken on days 30, 60, and 90 after the last spray, were examined for the presence or absence of the Vip3Aa1 gene in their DNA. The target gene was present in 33 of the 36 BbHV8 isolates and in 2 of the 36 Bb2860 isolates (Fig. 4). The occasional cross-presence of the target gene occurred only in the earlier soil samples. Since B. bassiana was also occasionally recovered from the control plots (Fig. 3A), our data indicated that the cabbage field lacked native B. bassiana strains and that the fungal sprays caused a low frequency of the strains crossing between the treatments, perhaps due to frequent light rains during the period.
Fig 4.
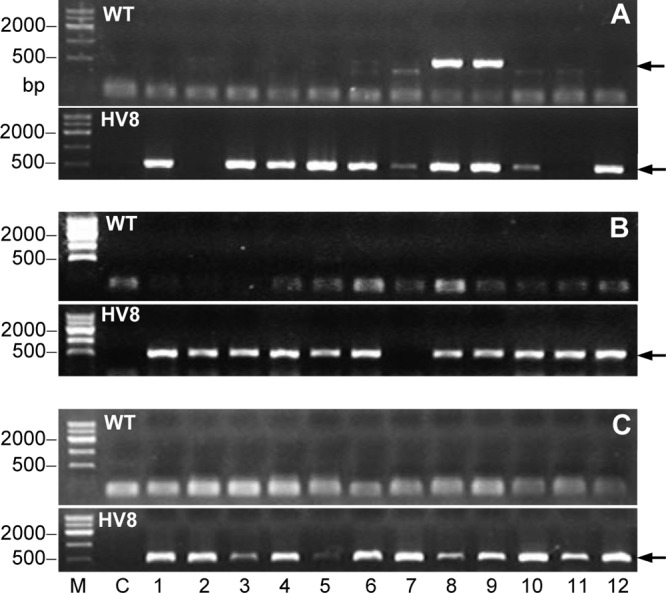
PCR detection of the Vip3Aa1 toxin gene (arrows) in the B. bassiana isolates recovered from the HV8 and WT treatment plots on days 30 (A), 60 (B), and 90 (C) after the last spray of trial 1.
To give insight into the trends of declining B. bassiana CFU counts after field release, rHV8 and rWT recovered from the soil samples taken 90 days after spray were assayed together with the parental BbHV8 and Bb2860 isolates for their responses to wet-heat stress at 45°C and UV-B irradiation in the laboratory. Modeling analyses of conidial survival trends of the tested strains after the intensity of either stress (fitness test, r2 ≥ 0.98; P < 0.0001) generated median times to lethality (LT50s) of 38 to 71 min under the heat stress (Fig. 5A) and median lethal doses (LD50s) of 0.16 to 0.30 J/cm2 under the UV-B stress (Fig. 5B). There were significant differences among the estimated LT50s (F3,9 = 327, P < 0.0001) or LD50s (F3,9 = 364, P < 0.0001) of different strains. As a result, Bb2860 was 12.5% and 19.2% more tolerant to the heat and UV-B than BbHV8 and also 26.7% and 33.6% more tolerant to the two stresses than rWT. The thermotolerance and UV-B resistance of the recovered rHV8 strain decreased by 38.9% and 32.5% compared to those estimated for BbHV8. These data indicate the low fitness of both Bb2860 and BbHV8 to the released field, and the fitness of the engineered strain was even lower.
Fig 5.
Trends of conidial survival indices (Is) of four B. bassiana strains over the intensities of wet-heat stress at 45°C (A) and UV-B irradiation (B). rHV8 and rWT were recovered from the HV8 and WT treatment plots 90 days after the last spray of trial 1. The inset bar charts illustrate their LT50s (min) and LD50s (J/cm2) under the heat and UV-B stresses. Different lowercase letters on the bars denote significant differences (Tukey's HSD, P < 0.05). Error bars indicate SEMs from four repeated assays.
Conclusive remarks.
The results of this study demonstrate for the first time that a transgenic mycoinsecticide can break through a bottleneck of commercial limitation notorious for conventional fungal formulations and that the ecological risk of its field release is very low and affordable if caution is taken to select genetically stable candidate strains, such as Bb2860, and transformed genes, such as those in the Vip3A family. Our transgenic mycoinsecticide not only provided full-season cabbage protection similar to that given by a commercial chemical but had no adverse effect on field spider populations. More importantly, both the transgenic and wild-type mycoinsecticides were recovered from the field lacking native B. bassiana strains at a rapid rate of decline and became undetectable from the fifth month onward. Our findings support a previous conclusion of low risk for the Metarhizium strains released in grassland (17) and largely alleviate public concerns of environmental safety with regard to the application of genetically engineered or nonengineered fungal agents for arthropod pest control (32). Our study has shed light upon bright prospects for the development and application of engineered fungal insecticides against major lepidoptera pests, such as CBFs and DBMs.
ACKNOWLEDGMENTS
Funding of this work was supported by the Ministry of Science and Technology of China (grant 2011AA10A204) and the Natural Science Foundation of China (grants 30930018 and 31021003).
Footnotes
Published ahead of print 16 August 2013
REFERENCES
- 1.Bates SL, Zhao JZ, Roush RT, Shelton AM. 2005. Insect resistance management in GM crops: past, present and future. Nat. Biotechnol. 23:57–62 [DOI] [PubMed] [Google Scholar]
- 2.St Leger RJ, Joshi L, Bidochka MJ, Roberts DW. 1996. Construction of an improved mycoinsecticide overexpressing a toxic protease. Proc. Natl. Acad. Sci. U. S. A. 93:6349–6354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang WG, St Leger RJ. 2012. Enhanced UV resistance and improved killing of malaria mosquitoes by photolyase transgenic entomopathogenic fungi. PLoS One 7:e43069. 10.1371/journal.pone.0043069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan YH, Fang WG, Guo SJ, Pei XQ, Zhang YJ, Xiao YH, Li DM, Jin K, Bidochka MJ, Pei Y. 2007. Increased insect virulence in Beauveria bassiana strains overexpressing an engineered chitinase. Appl. Environ. Microbiol. 73:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang WG, Leng B, Xiao YH, Jin K, Ma JC, Fan YH, Feng J, Yang XY, Zhang YJ, Pei Y. 2005. Cloning of Beauveria bassiana chitinase gene Bbchit1 and its application to improve fungal strain virulence. Appl. Environ. Microbiol. 71:363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang WG, Feng J, Fan YH, Zhang YJ, Bidochka MJ, St Leger RJ, Pei Y. 2009. Expressing a fusion protein with protease and chitinase activities increases the virulence of the insect pathogen Beauveria bassiana. J. Invertebr. Pathol. 102:155–159 [DOI] [PubMed] [Google Scholar]
- 7.Wang CS, St Leger RJ. 2007. A scorpion neurotoxin increases the potency of a fungal insecticide. Nat. Biotechnol. 25:1455–1456 [DOI] [PubMed] [Google Scholar]
- 8.Lu DD, Pava-Ripoll M, Li ZZ, Wang CS. 2008. Insecticidal evaluation of Beauveria bassiana engineered to express a scorpion neurotoxin and a cuticle degrading protease. Appl. Microbiol. Biotechnol. 81:515–522 [DOI] [PubMed] [Google Scholar]
- 9.Pava-Ripoll M, Posada FJ, Momen B, Wang C, St Leger RJ. 2008. Increased pathogenicity against coffee berry borer, Hypothenemus hampei (Coleoptera: Curculionidae) by Metarhizium anisopliae expressing the scorpion toxin (AaIT) gene. J. Invertebr. Pathol. 99:220–226 [DOI] [PubMed] [Google Scholar]
- 10.Fang WG, Vega-Rodríguez J, Ghosh AK, Jacobs-Lorena M, Kang A, St Leger RJ. 2011. Development of transgenic fungi that kill human malaria parasites in mosquitoes. Science 331:1074–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie XQ, Wang J, Huang BF, Ying SH, Feng MG. 2010. A new manganese superoxide dismutase identified from Beauveria bassiana enhances virulence and stress tolerance when overexpressed in the fungal pathogen. Appl. Microbiol. Biotechnol. 86:1543–1553 [DOI] [PubMed] [Google Scholar]
- 12.Ying SH, Feng MG. 2011. Integration of Escherichia coli thioredoxin (trxA) into Beauveria bassiana enhances the fungal tolerance to the stresses of oxidation, heat and UV-B irradiation. Biol. Control 59:255–260 [Google Scholar]
- 13.Shang YF, Duan ZB, Huang W, Gao Q, Wang CS. 2012. Improving UV resistance and virulence of Beauveria bassiana by genetic engineering with an exogenous tyrosinase gene. J. Invertebr. Pathol. 109:105–109 [DOI] [PubMed] [Google Scholar]
- 14.Qin Y, Ying SH, Chen Y, Shen ZC, Feng MG. 2010. Integration of insecticidal protein Vip3Aa1 into Beauveria bassiana enhances fungal virulence to Spodoptera litura larvae by cuticle and per os infection. Appl. Environ. Microbiol. 76:4611–4618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang ZL, Ying SH, Feng MG. 2013. Recognition of a core fragment of Beauveria bassiana hydrophobin gene promoter (Phyd1) and its special use in improving fungal biocontrol potential. Microb. Biotechnol. 6:27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paraiso O, Kairo MTK, Hight S, Leppla N, Cuda J, Owens M, Olexa MT. 2013. Opportunities for improving risk communication during the permitting process for entomophagous biological control agents: a review of current systems. BioControl 58:1–15 [Google Scholar]
- 17.Wang SB, O'brien TR, Pava-Ripoll M, St Leger RJ. 2011. Local adaptation of an introduced transgenic insect fungal pathogen due to new beneficial mutations. Proc. Natl. Acad. Sci. U. S. A. 108:20449–20454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Faria MR, Wraight SP. 2007. Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 43:237–256 [Google Scholar]
- 19.Zimmermann G. 2007. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci. Technol. 17:553–596 [Google Scholar]
- 20.Llewellyn DJ, Mares CL, Fitt GP. 2007. Field performance and seasonal changes in the efficacy against Helicoverpa armigera (Hübner) of transgenic cotton expressing the insecticidal protein vip3A. Agric. For. Entomol. 9:93–101 [Google Scholar]
- 21.Brake J, Faust M, Stein J. 2005. Evaluation of transgenic hybrid corn (Vip3A) in broiler chickens. Poult. Sci. 84:503–512 [DOI] [PubMed] [Google Scholar]
- 22.Peng DH, Chen SW, Ruan LF, Li L, Yu ZN, Sun M. 2007. Safety assessment of transgenic Bacillus thuringiensis with Vip insecticidal protein gene by feeding studies. Food Chem. Toxicol. 45:1179–1185 [DOI] [PubMed] [Google Scholar]
- 23.Feng MG, Chen B, Ying SH. 2004. Trials of Beauveria bassiana, Paecilomyces fumosoroseus and imidacloprid for management of Trialeurodes vaporariorum (Homoptera: Aleyrodidae) on greenhouse grown lettuce. Biocontrol Sci. Technol. 14:531–544 [Google Scholar]
- 24.Ye SD, Dun YH, Feng MG. 2005. Time and concentration dependent interactions of Beauveria bassiana with sublethal rates of imidacloprid against the aphid pests Macrosiphoniella sanborni and Myzus persicae. Ann. Appl. Biol. 146:459–468 [Google Scholar]
- 25.Ye SD, Ying SH, Chen C, Feng MG. 2006. New solid-state fermentation chamber for bulk production of aerial conidia of fungal biocontrol agents on rice. Biotechnol. Lett. 28:799–804 [DOI] [PubMed] [Google Scholar]
- 26.Shi WB, Feng MG. 2006. Field efficacy of application of Beauveria bassiana formulation and low rate pyridaben for sustainable control of citrus red mite Panonychus citri (Acari: Tetranychidae) in orchards. Biol. Control 39:210–217 [Google Scholar]
- 27.Shelton A, Andaloro J, Barnards J. 1982. Effects of cabbage looper, imported cabbageworm, and diamondback moth on fresh market and processing cabbage. J. Econ. Entomol. 75:742–745 [Google Scholar]
- 28.Maltais P, Nuckle J, Leblanc P. 1998. Economic threshold for three lepidopterous larval pests of fresh-market cabbage in southeastern New Brunswick. J. Econ. Entomol. 91:699–707 [Google Scholar]
- 29.Fernandes EKK, Keyser CA, Rangel DEN, Foster RN, Roberts DW. 2010. CTC medium: a novel dodine-free selective medium for isolating entomopathogenic fungi, especially Metarhizium acridum, from soil. Biol. Control 54:197–205 [Google Scholar]
- 30.Li J, Feng MG. 2009. Intraspecific tolerance of Metarhizium anisopliae conidia to the upper thermal limits of summer with a description of a quantitative assay system. Mycol. Res. 113:93–99 [DOI] [PubMed] [Google Scholar]
- 31.Huang BF, Feng MG. 2009. Comparative tolerances of various Beauveria bassiana isolates to UV-B irradiation with a description of a modeling method to assess lethal dose. Mycopathologia 168:145–152 [DOI] [PubMed] [Google Scholar]
- 32.Jaronski ST. 2010. Ecological factors in the inundative use of fungal entomopathogens. BioControl 55:159–185 [Google Scholar]