Abstract
SUMMARY
Varicella-zoster virus (VZV) was once thought to be a fairly innocuous pathogen. That view is no longer tenable. The morbidity and mortality due to the primary and secondary diseases that VZV causes, varicella and herpes zoster (HZ), are significant. Fortunately, modern advances, including an available vaccine to prevent varicella, a therapeutic vaccine to diminish the incidence and ameliorate sequelae of HZ, effective antiviral drugs, a better understanding of VZV pathogenesis, and advances in diagnostic virology have made it possible to control VZV in the United States. Occult forms of VZV-induced disease have been recognized, including zoster sine herpete and enteric zoster, which have expanded the field. Future progress should include development of more effective vaccines to prevent HZ and a more complete understanding of the consequences of VZV latency in the enteric nervous system.
INTRODUCTION
For many years, little serious medical attention was paid to infections due to varicella-zoster virus (VZV) and its primary infection, varicella (chickenpox), was considered to be little more than a rite of childhood. At worst, varicella was regarded as an irritant, not a serious disease like poliomyelitis or smallpox. Even the name chickenpox, possibly a reference to the appearance of the skin of an infected host, which looks somewhat like that of a defeathered chicken, connotes triviality. Although varicella was recognized to be milder than smallpox, which it certainly is, mild cases of smallpox and severe varicella could be confused, and it was helpful to remember that the distribution of the rash of smallpox was concentrated on the small parts (peripheral portions of the limbs). That history now is ancient; smallpox has been eradicated, and VZV has been recognized to be a herpesvirus, not, like smallpox, a poxvirus (1, 2). Interestingly, however, recent analyses have indicated that VZV evolved with primates and emerged with early hominids from Africa about 7 million years ago. Like that of smallpox, therefore, the relationship of VZV with humanity is a very old one (3).
VZV disease resembles poliomyelitis in that serious disease is at least a partial function of the advancement of civilization in the developed world. The medically forgettable nature of varicella changed radically when Sydney Farber introduced effective chemotherapy and began, for the first time in the history of humankind, to cure childhood cancers (4–8). The joy of that success, however, was unexpectedly turned to despair when a child who was surviving treatment for malignancy was killed by varicella, which can be deadly in an immunocompromised host. After the use of drugs that compromised immunity entered common usage, varicella could no longer be considered a benign rite of childhood. Instead, it was a disease to be feared and avoided. Thomas Weller took the first steps toward the solution of the conundrum that accompanied the iatrogenic introduction of an immunocompromised set of childhood hosts for VZV. He cultured VZV successfully, a feat that was a prerequisite to the development of an effective varicella vaccine. Although clinical evidence had suggested that varicella and herpes zoster (HZ) (shingles) were caused by the same agent, Weller and his colleagues were the first prove this scientifically (1, 2). Weller and his colleagues realized that in order for VZV to give rise to HZ, VZV had to persist in the body asymptomatically after chickenpox and then to reactivate from latency at a later date to cause HZ; this pattern is similar to that of herpes simplex virus (HSV), which also reactivates from latency to give rise to a recurrent disease (9).
Within a few years of Farber's remarkable introduction of treatment for childhood cancer, VZV's ability to kill became apparent when Weller and his colleagues reported the startling deaths of two children from varicella (10). One had been receiving chemotherapy for neuroblastoma, while the other received high-dose steroid therapy for acute rheumatic fever (10). Varicella in these children presented with a hemorrhagic vesicular rash, similar to smallpox in its extent if not the individual lesions, with severe symptoms of a disseminated viral infection that proved fatal. The nature of these cases of varicella was completely different from that of the mild disease that led to neighborhood chickenpox parties. During the 20 to 30 years that followed the pioneering work of Farber, cancer chemotherapy became more common, complex, and intense. Organ and bone marrow transplantation came into use, while steroids and antimetabolites were employed to treat many autoimmune diseases. The resulting spread of immunodeficiency meant that not only varicella but also HZ became a significant clinical problem. Medical successes in all age groups caused longevity in the United States to increase. Again, VZV tempered the benefits of advances of medical progress; the incidence of HZ and its complications increased in tandem with age. It thus became necessary to control VZV, which had become an increasingly dangerous virus. Control was accomplished first with a vaccine to prevent varicella, then with antiviral therapy, and finally with a vaccine to prevent HZ. Not surprisingly, control of VZV was associated with the acquisition of knowledge of the viral life cycle, its cell biology, and finally the pathogenesis of VZV-induced disease.
GROWTH OF VZV IN CELL CULTURE
VZV is known to utilize a variety of binding sites and receptors to gain access to cells and to spread from an infected cell to an uninfected neighbor. Cell-free VZV binds to heparin sulfate proteoglycan, while the large cation-independent mannose 6-phosphate (Man 6-P) receptor (MPR) evidently binds to Man 6-P moieties on glycoproteins of the viral envelope and is required for viral entry (11–14). Heparin and Man 6-P, therefore, are able to compete with cell-free VZV in vitro and protect cells from infection. In contrast, neither heparin nor mannose 6-phosphate interferes with the cell-to-cell spread of cell-associated VZV. That process requires the insulin-degrading enzyme (IDE), which is cytosolic but is expressed near cell surfaces and can interact with gE (15, 16).
During lytic infection, each of the 72 genes (open reading frames [ORFs]) of VZV is expressed in a relatively orderly cascade. These include immediate early (regulatory), early, and late (structural) genes. At least 9 glycoproteins (gps) are encoded in the VZV genome. Major gps include gE, which forms a complex with gI, the gH/gL complex, gB, and gC. Most of these gps play a role in infectivity of the virus, enabling the agent to spread from an infected cell to a naive one. The gps also stimulate immune responses in an infected host. Although the precise natures of protective humoral and cellular immune responses are unclear, gE is likely to be an important antigen because it is the major gp that VZV expresses (17).
In cell culture and as it spreads within the body, VZV is a highly cell-associated virus, because of the exposed Man 6-P moieties of the N-linked gps of the viral envelope (18). The MPR on the viral envelope enables VZV to bind to intracellular MPRs, which are present in almost every cell of the body. After VZV has acquired an envelope, therefore, the newly enveloped virions follow the itinerary of MPRs. Like the VZV gps, newly synthesized lysosomal enzymes also express Man 6-P and encounter MPRs in the trans-Golgi network (TGN). MPRs divert lysosomal enzymes to late endosomes, where MPRs dissociate from lysosomal enzymes in the acidic environment that prevails in these organelles, enabling the enzymes to traffic to lysosomes. The MPRs travel from late endosomes, normally without bound ligand, to the plasma membrane, where, as noted above, they can facilitate viral entry. Newly assembled VZV acquires its envelope and thus also its gps in the TGN, where virions also interact with MPRs (19). Newly enveloped virions then follow the itinerary of MPRs and traffic, like lysosomal enzymes, to late endosomes. The acidic environment of late endosomes degrades virions, which are acid labile, the morphology of virions becomes distorted, and the virus loses infectivity. When finally released from cells, therefore, the postexocytotic virions are no longer infectious. Cell-to-cell spread, therefore, requires the fusion of infected cells with their neighbors, which is a slow process, both in vitro and in vivo. The evolutionary value of this process to VZV is likely to be that the mechanism prevents highly infectious viral particles from being disseminated within the body. As a result, a newly infected host survives and is not overwhelmed by VZV before the host can mount an adaptive immune response to control the virus. In turn, the virus can become latent within the surviving host and remain there until some time in the future, when a new group of susceptible subjects has developed and is ready to receive VZV. Reactivation of VZV from latency provides a continuing source of VZV to infect a regenerated host pool. The mechanism, however, requires that there be a site somewhere in the body where VZV can escape diversion to late endosomes, form infectious virions, and gain access to the outside to transmit infection to new hosts. At least one such site is the epidermis, which is a target of disease in both varicella and HZ (11). The superficial cells of the suprabasal epidermis downregulate MPRs as the cells mature into squames. This enables VZV to be secreted constitutively by default. As a result the virions in cutaneous vesicles are morphologically intact and highly infectious. VZV can thus spread from the skin through the normal process of desquamation. As the clouds of dead squames slough from the skin and waft on drafts of air, infectious particles of VZV can waft with them and, when inhaled by an unsuspecting and susceptible host, start a new cycle of varicella, latency, and HZ.
GROWTH, MULTIPLICATION, AND CLINICAL MANIFESTATIONS OF VZV WITHIN ITS HOSTS
VZV is remarkably host specific. Its only naturally occurring reservoir is in humans; although small animal models of apparent latent infection, such as Wistar rats and cotton rats, have been reported, reactivation of VZV has not been achieved in these models (20, 21). Guinea pigs can be infected with VZV and have even developed latent infection, although it has not been possible to reproduce varicella in these animals (22, 23). A guinea pig model of latent VZV infection was developed in the Gershon laboratory and is interesting because it may provide insight into the pathogenesis of human infection (22, 24). Neurons can be isolated from the guinea pig enteric nervous system (ENS). Intrinsic primary afferent neurons are found in the ENS, and VZV is known to establish latency in the primary afferent neurons of dorsal root ganglia (DRG) and cranial nerve ganglia (CNG). Although it was anticipated that VZV might preferentially infect intrinsic primary afferent neurons in cultures of enteric ganglia, that turned out not to be the case; VZV infected neurons from the guinea pig ENS in vitro but showed no preference for type. When the neurons were exposed to cell-free VZV, the virions infected neurons but established latency. Neurons survived indefinitely, only latency-associated VZV gene products were expressed, and these were restricted to the cytoplasm. In contrast, when fibroblasts, guinea pig or human, were present at the time enteric neurons were exposed to VZV, infection was lytic. Neurons died within 48 to 72 h, immediate early proteins translocated to the nucleus, and gps were expressed. Viral particles were detected electron microscopically within infected enteric neurons, and infection was passed to cocultured MeWo cells. Addition of fibroblasts did not reactivate VZV from latency in infected enteric neurons; however, reactivation was induced when the nonstructural protein ORF61p was expressed in latently infected neurons (22, 24). This in vitro system is the only latency model thus far to demonstrate reactivation of VZV from its latent state.
Because VZV was found to infect enteric neurons of guinea pigs in vitro, attempts were made to determine whether latent infection could also be established in the guinea pig ENS in vivo. Three methods were tested, and each was able to establish latent infection in the guinea pig ENS (22). These were intracutaneous injection of VZV-infected human embryonic lung fibroblasts (HELF), direct injection of VZV-infected HELF into the gut, and intravenous (i.v.) injection of VZV-infected human or guinea pig peripheral blood mononuclear cells (PBMC) (among which most of the infected cells were T lymphocytes). The intravenous route led to the establishment of VZV latency in virtually every neuron of the guinea pig gut and also to latency in neurons of dorsal route ganglia. Preliminary experiments, reported at the Colorado Alphaherpesvirus Latency Symposium in Vail, CO (2013), suggest that a combination of stress and immunodeficiency may reactivate VZV in guinea pigs bearing latent infection.
The means that the highly cell-associated VZV evolved to gain host-to-host access have generated, if not controversy, some level of disagreement. It was assumed for years that VZV spreads from the respiratory tract, but there is no actual evidence that this occurs (25, 26). In contrast, the fluid in the epidermal vesicles that characterize the rash of varicella and HZ is highly infectious and, as noted above, is filled with well-formed intact virions (11). The downregulation of MPRs that accompanies maturation of keratinocytes into squames allows the default pathway of secretion to transport enveloped VZV to the extracellular space. That space becomes continuous with vesicles, and desquamation does the rest (11). The highly infectious VZV particles aerosolize and drift away from the skin of infected patients, ready for anyone nearby to inhale them (11, 27). VZV thus spreads by the airborne route (28, 29). VZV does not appear to spread from one person to another if cutaneous lesions are absent, and when such lesions are present, the degree of contagion is directly related to the number of cutaneous lesions that are present (27, 30). VZV does not spread by coughing or sneezing, although VZV DNA is present in saliva during infection (31). Infectious VZV is labile, and infectious virus does not persist for significant periods of time on surfaces or clothing, although its DNA may be detectable for long periods in room dust (32).
Following transmission of VZV to a varicella-susceptible individual, the respiratory mucosa becomes infected, leading to invasion of the epithelium of the tonsils, where there may be some production of cell-free VZV that can potentially be neutralized by passive immunization (33, 34). Subsequently, during the incubation period of varicella, VZV infects CD4+ and CD8+ T lymphocytes (33). During VZV viremia, which may last for some days, VZV-infected immune cells, which home to the skin, carry infection to keratinocytes. This viremia may also infect other cells and tissues in the body. Innate immunity, involving production of alpha interferon, transiently controls the multiplication of VZV in the skin, but eventually innate resistance in skin is overcome, resulting in the development of cutaneous lesions (35, 36). The initial resistance that innate immunity provides has been postulated to slow viral multiplication, thereby providing time for the development of adaptive immunity, which finally controls multiplication of the virus (33). The ability of innate immunity to slow the spread of VZV backs up the diversion of VZV to late endosomes during envelopment in infected cells, which ensures slow cell-to-cell transmission, and thus provides a fail-safe mechanism to prevent VZV from overwhelming its host. These mechanisms, which promote host survival, thus confer an evolutionary advantage to the virus (11). The relatively long incubation period of 2 to 3 weeks may also facilitate the ability of vaccination to prevent clinically manifest disease. The cell-to-cell spread of VZV, which eliminates extracellular circulation of virions, explains why CD4 and CD8 T lymphocytes are more critical for host defense than specific antibodies. Patients who are deficient in innate (37–40) and/or adaptive (41–45) cell-mediated host responses are subject to severe VZV infections, which may even be caused by the live attenuated vaccine strain of the virus (vOka). Laboratory studies of VZV cell-mediated immunity (CMI) in adults suffering from varicella have demonstrated that disease severity is positively correlated with viral load and negatively correlated with virus-specific responses of T lymphocytes (46).
There is only one serotype of VZV, although there are at least 7 viral clades (47), which have been identified in different geographic areas of the world such as Europe, Africa, Australia, and Asia. Analysis of clades has been useful in interpretation of epidemiologic information on VZV (48).
CLINICAL ILLNESSES CAUSED BY VZV
In typical varicella there is a generalized rash, with a concentration of skin vesicles on the head, including the scalp and trunk (Fig. 1 A). There are fewer skin lesions on the extremities; the distribution of the rash is a diagnostic clue and may be used to differentiate varicella from smallpox. The rash evolves over a few days from maculopapular lesions to vesicles, pustules, and scabs. In contrast to the case for smallpox, in any one area of skin, vesicles, pustules, and scabs may be present at the same time. Contagion is highest during the phase when the rash is vesicular; most of the transmissible VZV comes from the skin lesions (11, 26, 27, 49). It is difficult to rule out the possibility of respiratory spread of VZV in the preeruptive phase; it is usually assumed that the illness is transmissible 48 h before rash onset, but the evidence for this is limited. After the pustular stage is reached, transmissibility is no longer occurring. Other symptoms associated with varicella are malaise and fever. Varicella usually lasts 5 to 7 days in immunologically normal hosts and is represented by a spectrum of illness, ranging from mild symptoms and scant rash (which may be overlooked or forgotten) to severe illness with over 1,000 vesicles. The occurrence of varicella without an accompanying rash is rare (50). Varicella is 25 times more likely to be severe (and may even be fatal) in adults than in children (51). Adults with varicella are especially at risk to develop primary varicella pneumonia, a dreaded complication but one that usually responds to prompt antiviral therapy.
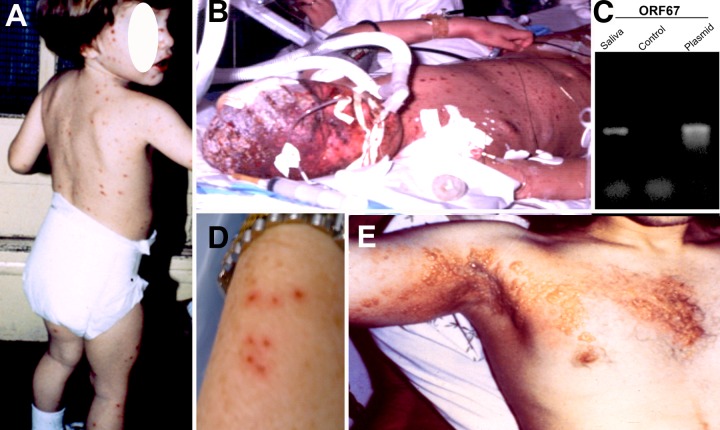
Fig 1.
The clinical spectra of VZV infection. (A) An otherwise healthy 2-year-old child with typical varicella (primary infection). (B) A child with underlying malignant disease receiving chemotherapy who died from disseminated varicella with pneumonia. (C) Salivary VZV DNA was demonstrated in a 72-year-old patient with severe unilateral neuropathic pain, which cleared on valacyclovir. There was no rash; VZV DNA was not detected in his saliva following recovery from pain. The diagnosis was zoster sine herpete. (D) Skin of wrist of a 71-year-old otherwise healthy woman with 7 tiny vesicles that caused severe itch but no pain. This was the extent of the rash, which resembled bites from a small insect. VZV DNA was demonstrated by PCR in skin vesicles and transiently in saliva. The diagnosis was mild HZ in an elderly woman. (E) Severe, disseminated HZ in a 35-year-old man with lymphoma on anticancer therapy, with severe pain, despite antiviral therapy.
The cutaneous vesicles that arise during varicella are usually pruritic; scratching is common and may contribute to aerosolizing VZV with subsequent spread to others (28, 29). Shedding of squames, however, is sufficiently common and profuse that even if scratching is prevented, VZV may still aerosolize from the skin and transmit disease. Scratching, however, may promote superinfection with bacteria such as streptococci and staphylococci. As a result, bacterial cellulitis, pneumonia, and/or sepsis, requiring hospitalization and intravenous antimicrobial therapy, are complications of varicella (52). Two forms of central nervous system (CNS) complications may occur in varicella. One is the usually self-limited cerebellar ataxia (1 in 4,000 cases); the other is the more serious encephalitis (1 in 10,000 cases), which can be severe or fatal (53). Immunocompromised patients who contract varicella may experience severe or fatal illness with a prolonged course, including high fever, extensive rash that may become hemorrhagic, pneumonia, hepatitis, and encephalitis (53). Usually one attack of varicella provides lifelong immunity, but reinfections have been reported (54–57).
A congenital varicella syndrome occurs in about 2% of the offspring of women who develop varicella between the 8th and the 26th weeks of pregnancy (58). Affected infants manifest a variety of problems, including scarring of the skin, severe damage to the central and autonomic nervous systems, eye involvement, and limb abnormalities. Children born with the congenital varicella syndrome often do not survive infancy, and if they do, they are highly likely to develop HZ early in life. The introduction of the routine use of varicella vaccine in the United States has caused this syndrome to become extremely rare in the United States. Because of the resistance to vaccination in other countries and the absence of herd immunity to VZV in those locations, the congenital varicella syndrome has not totally vanished; it might also be imported as a result of international travel.
HZ is a secondary VZV disease; it is due to the reactivation of VZV that was acquired during varicella and remained latent thereafter (Fig. 1D and E). VZV reactivates from latency despite the presence of circulating antibodies to VZV, which are often present at high titer when HZ occurs. The rash of HZ is typically unilateral and dermatomal; it is also vesicular, painful, and/or pruritic. There is a spectrum of illness with HZ, ranging from pain with no rash to mild rash to severe rash with dissemination (Fig. 1C, D, and E).
Individuals with HZ who have a vesicular rash may transmit VZV to susceptible subjects. When host-to-host transmission occurs, the resulting illness in susceptible individuals is not HZ, however, but varicella, the primary VZV-induced disease. HZ can occasionally occur in the absence of a rash. When it does it is known as zoster sine herpete and is manifested as a unilateral, dermatome-restricted pain syndrome, encephalitis or other neurologic manifestation, or a gastrointestinal disturbance (59–62).
Particularly in immunocompromised individuals such as those with HIV infection, HZ may present as loss of vision, due to progressive outer retinal necrosis (PORN) (63, 64), an extremely serious infection, which despite antiviral therapy usually progresses to blindness. HZ affecting the trigeminal nerve may also lead to ophthalmitis with keratitis (65, 66). Such infections have been reported in immunocompromised patients (67). Diagnosis may be made by PCR (68). There is one report of retinal necrosis in a vaccinated immunocompromised young adult that was identified as caused by vaccine type (Oka) VZV (69).
A VZV-induced vasculopathy, which may accompany HZ or occur without a rash, mimics giant cell arteritis of cerebral arteries (70). This is a serious condition that may present as a cerebrovascular accident, disturbed vision or blindness, and/or transient ischemic attacks. Diagnosis can be made if the temporal artery is biopsied; VZV DNA can be detected, and immunocytochemistry can reveal viral proteins if the condition is due to VZV. Measurement of antibodies to VZV, including calculation of serum/cerebrospinal fluid (CSF) specific IgG ratios, may also be used to implicate VZV in this neurologic disease (70, 71).
HZ typically occurs in people who are more than 50 years old, but it may also occur in younger individuals, including children (53). People who manifest HZ usually acquire wild-type (WT) VZV through an episode of varicella; however, HZ has also been reported to occur in children who have received the varicella vaccine, although HZ is less likely to occur after vaccination than after natural infection (53). Interestingly, when HZ occurs in vaccinated individuals, about 1/3 of the childhood cases are due to WT VZV (72, 73). Oka HZ has not yet been reported in vaccinated adults. The exact reason why WT HZ occurs in a vaccinated individual is uncertain. One explanation may be that WT VZV may asymptomatically displace vOka; alternatively, some subjects who receive the varicella vaccine may previously have been infected asymptomatically with WT VZV (49). Other possibilities are that WT strains may be better suited for reactivation than Oka strains, and it also may be that one ganglion may harbor latent infection with both types of VZV. Immunocompromised individuals are at greater risk to develop HZ than are immunologically normal persons (53).
Postherpetic neuralgia (PHN) is a dreaded sequel of HZ; it is a neuropathic pain syndrome that occurs a month or so after the onset of HZ and is often severe. The incidence of PHN increases as a function of age, becoming extremely common after the age of 80 (74–76). The pain of HZ is distinguished from the acute pain that is associated with rash at disease onset. PHN involves chronic pain that appears or persists after the rash of HZ has healed. Acute pain often responds to antiviral therapy, but PHN is generally thought not to do so (76), although the case of a patient with PHN in which 5 trials of valacyclovir were each associated with a reduction in pain and the disappearance of VZV DNA from circulating mononuclear cells has been reported (77). There is also some evidence that famciclovir can reduce the duration of pain during HZ and the incidence of PHN if it is given early enough in HZ (78). There is little evidence, however, that early treatment with antivirals invariably prevents PHN (76). The problem in prevention may lie in the difficulty of reaching a diagnosis early enough to abort HZ soon enough to prevent PHN. The cause of PHN is unknown, although there are published data that suggest that prolonged VZV multiplication and viremia may be involved (79). Vaccination against HZ with the Oka strain prevented 66% of PHN in the double-blind vaccine study involving Zostavax (80).
DIAGNOSIS
The diagnosis of VZV infection is usually made clinically because the signs and symptoms of varicella and HZ are characteristic and obvious. Laboratory diagnosis becomes important when the presentation of disease is confusing, for infection control, in immunocompromised individuals in whom drug-resistant VZV is suspected, or in vaccine-modified breakthrough cases of possible varicella. A number of approaches to laboratory diagnosis are available. The “gold standard” for diagnosis of VZV infections was once virus isolation. This requires the use of fresh vesicular fluid, which is inoculated immediately onto monolayers of susceptible cells, such as human embryonic lung fibroblasts (HELF), and incubated at 35 to 37°C for 3 to 7 days; cultures are then examined microscopically for cytopathic effect. This technique is costly and slow. Worse, because VZV is a labile virus, cultures can fail to reveal infection even when VZV is present. The slow growth of VZV in cell culture is also a serious handicap for patient care, which often involves decisions that have to be made quickly. Virus isolation is thus not much used for diagnosis today, although it is still useful in the determination of whether a putative VZV infection is resistant to antiviral drugs.
The diagnostic procedure of choice today is to use PCR to amplify VZV DNA. This assay can be carried out rapidly, within a day or two. It is highly accurate and sensitive and costs about the same as viral culture (81). In addition many laboratories, such as the National VZV Laboratory at the Centers for Disease Control and Prevention (CDC) and the Merck Worldwide Adverse Experience System (WAES), with laboratories located at Columbia University, perform this assay without charge (82). WAES studies potential adverse events associated with VZV vaccines [hotline, (800) 672-6372].
PCR can be used to detect VZV in vesicular fluid, scabs from vesicles, skin swabs, throat swabs, cerebrospinal fluid (CSF), tissues from biopsies or autopsies, blood, and saliva (31, 48, 81, 83, 84). Demonstration of VZV DNA in saliva deserves special mention; it is an attractive test because collection of the specimen is noninvasive. The occurrence of VZV DNA in saliva was first demonstrated to follow space travel transiently in 1/3 of asymptomatic astronauts (85). The phenomenon might represent the reactivation of VZV in response to the extreme stress of space flight, which could have transiently reduced cell-mediated immunity (86). Similarly, 17% of stressed hospitalized children in intensive care units transiently have VZV DNA in their saliva (84). The presence of VZV DNA in saliva as the result of VZV reactivation has subsequently been translated into diagnostic utility in patients with HZ (87–89) (Fig. 1C and D). This test is particularly useful for diagnosis of those VZV infections that are not accompanied by cutaneous lesions, such as zoster sine herpete or enteric zoster (87, 88), but further research on the accuracy and sensitivity of identifying VZV DNA in saliva as a routine diagnostic test is necessary. For example, it will be important to distinguish asymptomatic viral reactivation putatively due to stress from VZV-induced disease.
It is possible to use restriction enzyme treatment of PCR products to differentiate DNA encoding vOka from DNA encoding WT VZV gene products in saliva (90, 91). Infectious VZV itself has only rarely been identified in saliva, and transmission of VZV from saliva has not been observed (92). In contrast to the case for VZV, asymptomatic shedding of infectious HSV in bodily secretions, including saliva, is common (93). HSV DNA has also been found in saliva in stressed individuals (84).
Performance of quantitative PCR (qPCR) has indicated thus far that there is considerable variation in amounts of VZV DNA in saliva among patients with HZ, stressed astronauts, and stressed children. Because these studies were performed in different laboratories, quantitative comparisons cannot be formally made. In general, the largest reported amounts of VZV occurred in patients with acute HZ, whose levels ranged from 10 to 10 million DNA copies/ml of saliva (89). There was considerable variability among recovering HZ patients, with some having no VZV DNA detected in saliva and others having up to 1,000 copies/ml (94). Astronauts following space flight had levels ranging from 0 to 6,000 copies/ml, but only ∼30% had detectable VZV DNA in saliva (85). Possibly the lowest levels of VZV DNA were seen in very stressed children hospitalized in intensive care units, but again this testing was not standardized, so comparisons are subject to possible inaccuracies (84). Attempts are being made to standardize salivary testing through an ongoing project conducted by the CDC involving several laboratories where this assay is being carried out.
In addition to its utility in diagnosing whether or not an infection is due to VZV, PCR is useful in distinguishing WT VZV from vOka. This distinction is critical in the evaluation of the safety of the varicella and HZ vaccines. Symptoms that occur after vaccination are often attributed to these vaccines because of the perceived temporal relationship. Determination of the identity of the responsible virus is thus necessary. Restriction endonuclease analysis of PCR products can be used to identify differences in nucleotide sequences between the two kinds of virus. For example, ORF38 of WT VZV has a PstI restriction site that is lacking in ORF38 of vOka. A simple screening test, therefore, is to determine whether this restriction site is present in the sample of VZV DNA under analysis (90). Most of the ORF38 genes from WT VZVs worldwide contain the PstI site and thus are “PstI positive.” Unfortunately, ORF38 in some WT VZV lacks this restriction site; therefore, “PstI-negative” strains of VZV are not invariably vOka, especially in Asia. To be certain that one is dealing with vOka, more complex analyses must be employed. Four loci in VZV ORF 62 (105705,106262, 107252, and 108111) are recommended to distinguish a putative vOka strain from WT VZV (91). Restriction enzyme analysis of ORF62 with SmaI and sequencing of ORF62 can be used to make a definitive identification of vOka. Because a few recombinants between WT VZV and vOka have now been identified, it may be difficult to distinguish WT from vOka in rare isolates (91).
In addition to PCR, indirect immunofluorescence is an excellent method to use to identify VZV in skin vesicles for diagnostic purposes. Immunofluorescence is more sensitive than culture and can be carried out far more rapidly. Immunofluorescence, however, is less sensitive than PCR (81, 95, 96) and cannot be employed to distinguish vOka from WT VZV. VZV infections may also be diagnosed serologically. Acute- and convalescent-phase sera can be examined to determine whether VZV antibodies increase in association with illness. This method is obviously slow, requiring 10 to 14 days. It may also be inaccurate because some patients with HSV infections sometimes exhibit an increase in antibodies to VZV (81). This may occur because antibodies to subsets of antigens in HSV and VZV cross-react. Alternatively, it is possible that HSV infection reactivates VZV, which has been suspected but not yet definitively demonstrated. Serology is also sometimes used as an indicator to judge whether individuals are protected against varicella; however, most serologic tests of immunity to VZV are insufficiently sensitive to be used for this purpose (97). WT VZV cannot be distinguished serologically from vOka. The utility of the serologic diagnosis of VZV is thus limited and imperfect.
Antibodies to VZV can be measured by a variety of techniques. The most commonly used is the enzyme-linked immunosorbent assay (ELISA); many commercial tests are available, and these vary somewhat in their sensitivity and specificity. As noted above, the most common problem with all of these tests is a lack of sensitivity. Individuals vaccinated successfully against varicella, for example, are often found with commercial ELISAs to lack antibodies to VZV (81). ELISAs may also yield false-positive results for as many as 10% of varicella-susceptible individuals (98). A more specialized gp ELISA has been devised to enhance sensitivity, but this test is actually too sensitive. It detects very low levels of antibodies and may thus mistakenly identify a varicella-susceptible individual with insufficient protection as immune to varicella (99).
A method that detects immunity to varicella more reliably than ELISA is the fluorescent antibody to membrane antigen (FAMA) assay (100). Clinical validation over many years has proven this assay to be an excellent surrogate for protection against varicella in healthy individuals (81, 99, 101). In healthy persons with a FAMA titer of ≥1:4 (n = 131), fewer than 2% developed (mild) varicella after a household exposure to VZV; the attack rate in similar individuals with a titer of <1:4 was 59% (n = 68) (99). The FAMA assay as a correlate of immunity was employed in the early studies of the efficacy of varicella vaccine (101–103). FAMA does not perform quite as well in immunocompromised patients, some of whom may develop mild varicella upon exposure even if FAMA reveals that they have detectable antibodies to VZV (101). The main problem with the FAMA assay is that it requires a specifically trained technician and thus is not generally available. FAMA today is used mainly for research purposes.
VZV disease may be identified by a 4-fold rise in antibody titer in acute- and convalescent-phase sera. The diagnostic use of demonstration of VZV IgM in one serum sample, however, is risky. False-negative and false-positive IgM reactions have been reported to occur; therefore, the use of this assay for clinical diagnosis is discouraged (97).
In stark contrast to the case for varicella, specific antibodies to VZV are not protective against HZ. Humoral immunity does not prevent reactivation of VZV; individuals who develop HZ invariably have detectable and often high levels of antibodies to VZV. Therefore, measuring antibodies to VZV in sera of patients is not useful to determine if they are at risk to develop HZ. Clinical and laboratory evidence has shown that CMI is essential to prevent disease caused by the reactivation of VZV (104, 105). Interestingly, while infiltrating CD8 T cells surrounding neurons that are latently infected with HSV have been identified, similar cells have not been observed around neurons that are latently infected with VZV (106). The mechanism by which CMI controls the reactivation of VZV requires further study.
Individuals who develop HZ have demonstrable low CMI to VZV (104, 105). CMI to VZV begins to decrease, presumably due to aging, after about 50 years of age, which is also precisely the same age at which the incidence of HZ begins to increase (107, 108). Immunocompromised patients, in whom CMI responses to VZV are lower than normal, are at a greater risk to develop HZ than are similarly aged healthy persons (17). Children and adolescents who develop HZ may have transient decreases in CMI to VZV, possibly due to preceding episodes of asymptomatic viral infection or stress (85, 109). HZ has also been reported to follow trauma to nervous tissues (110).
VZV reactivation usually, but not always, presents with a rash (60, 62, 111). In the absence of a rash, demonstration VZV DNA in saliva (111) or cerebrospinal fluid (CSF) (60) may be diagnostic. The presence of antibodies to VZV in CSF, with calculation of serum/CSF VZV-specific IgG ratios, may also be diagnostic of VZV reactivation in the CNS, which can occur in the absence of an accompanying rash (60).
THERAPY OF VZV INFECTIONS
For many years, except in immunocompromised patients, VZV infections were treated symptomatically, with acetaminophen and medications to decrease itching. Today, antiviral drugs, which are effective, well tolerated, and able to be administered orally, are commonly used. Specific antiviral therapy for VZV became available in the mid-1980s in the form of the nucleoside analog acyclovir (ACV), an inhibitor of DNA polymerase and a DNA chain terminator (112). ACV may be given orally or may be given intravenously (i.v.) if high drug levels are needed in severely VZV-infected patients. Following development of ACV, prodrugs of ACV, i.e., valacyclovir and famciclovir, were introduced. These medications, which are given orally several times a day, lead to high levels of ACV in the blood, which approach those seen after intravenous administration of acyclovir. Antiviral therapies directed against varicella and zoster are similar. Early intravenous therapy with ACV should be instituted for VZV-infected patients at high risk for development of severe infections, such as leukemic children and those who have undergone transplantation, to prevent viral dissemination (113, 114). This therapy may be lifesaving in immunocompromised patients; moreover, it also decreases morbidity. Fortunately, resistance of VZV to ACV and its analogs is unusual, but it does occur; disease due to the uncommon resistant strains of VZV can be treated with foscarnet, which is a second choice because it is more toxic than ACV.
Early therapy of HZ (within 3 days of onset), usually with valacyclovir or famciclovir, appears to result in the most satisfactory outcomes (76). As noted above, famciclovir given to elderly patients with zoster early in the course of infection was reported to decrease the duration of PHN, if not necessarily its incidence (78). Details about antiviral therapy for VZV infections, including drug doses and length of therapy, have been reviewed elsewhere (115).
LATENCY AND REACTIVATION OF VZV
VZV becomes latent in neurons of the dorsal root ganglia (DRG), cranial nerve ganglia (CNG), and autonomic ganglia, including enteric (gastrointestinal) ganglia (Fig. 2). VZV latency occurs after chickenpox and/or vaccination. Expression of transcripts and/or proteins of at least 6 of the 71 VZV genes (ORF4, -21, -29, -62, -63, and -66) have been reported in neurons with latent VZV infection (22, 116, 117). Late proteins, such as gE, are not detected. There is some controversy in the literature, however, concerning whether proteins as well as transcripts are actually expressed. Recently, human neurons from patients with blood group A1 have been found to express that antigen in the Golgi region (118). Ascites fluid and sera from animals may contain endogenous antibodies that cross-react with human blood group A1 antigens (118, 119). Neuronal VZV protein expression detected with ascites-derived monoclonal antibodies or rabbit sera may thus be misinterpreted. The antibodies might react with neurons from donors whose blood type is A1, not because they contain VZV proteins but because they contain human blood group A1 antigens. The actual protein expression in human neurons must therefore be reevaluated using either monoclonal antibodies produced in tissue culture, which lack endogenous antibodies to human blood group A1 antigens, or antibodies that have been absorbed with red blood cells from type A donors or donors who do not have type A blood. It is curious, however, that reports of VZV protein expression in human ganglia have failed to detect late proteins, such as gE, even when the reagents used are ascites-derived monoclonal antibodies or polyclonal antibodies from rabbits. Antibodies to gE should be just as subject to cross-reaction with human blood group A1 antigens in neurons as antibodies to the ORF62 or ORF63 proteins; moreover, it is also interesting that the proteins that have been detected immunocytochemically are limited to those for which the corresponding transcripts have also been found to be expressed. It is possible that discrepancies found in the literature on VZV protein expression in human neurons are due not only to potential interference from human blood group A1 antigens but also to the time between death and autopsy as well as effects of age and disease prior to death.
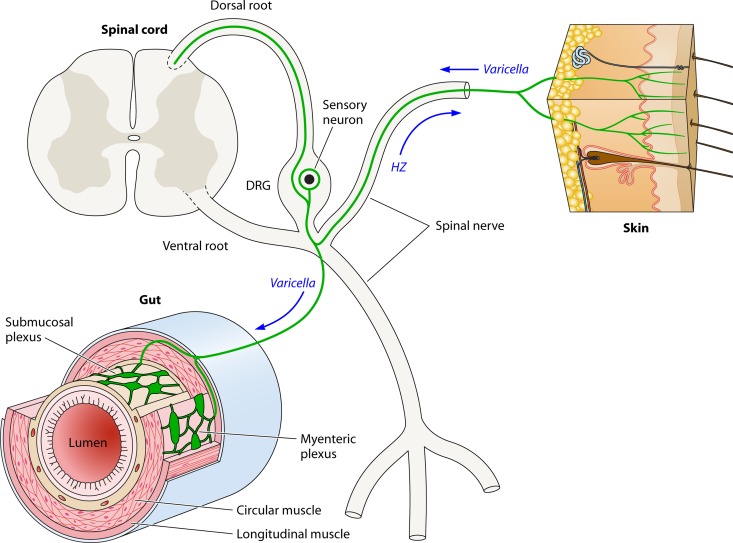
Fig 2.
Potential routes taken by VZV during its life cycle. Dorsal (sensory) and ventral (motor) roots carrying axons leave the spinal cord and fuse to form a mixed spinal nerve. A dorsal root ganglion (DRG) is present within the dorsal root. The DRG contains pseudounipolar sensory neurons (red) that extend a central process in the dorsal root to the posterior spinal cord and a peripheral process that reaches its targets of innervation via the spinal nerve. The skin is one such target, and sensory nerve fibers (red) ramify within the epidermis. Visceral sensory neurons also within the DRG send their peripheral process to the gut and terminate within the ganglia of the enteric nervous system (ENS) (submucosal and myenteric) or within other layers of the bowel wall. There is evidence that rare DRG neurons extend peripheral processes both to the skin and to the gut (indicated in the diagram). During varicella, retrograde transport from the skin can enable VZV to reach sensory neurons in the DRG and, from there, the neurons of the ENS (green arrows). Following the reactivation of VZV in a sensory DRG neuron, VZV can travel via anterograde transport in spinal nerve fibers to return to the skin and infect the epidermis in a restricted dermatomal distribution. Reactivation of VZV within neurons of the ENS affects the targets that these cells innervate and gives rise to local enteric disease, such as gastric ulceration (not illustrated) (see the text).
VZV may establish latency by either of two mechanisms (Fig. 2). Retrograde axonal transport from the skin may transmit VZV directly from cutaneous lesions to neuronal cell bodies in DRG or CNG (120). Latency may also be established during a viremia, as shown in animal models (22). Transfer during latency, however, implies that the T lymphocytes that carry VZV during a viremia (33) are able to establish latent infection in neurons. That has been suggested from studies of human ganglia transplanted to mice with severe combined immunodeficiency disease (Hu-SCID) (121–123); however, the initial infection of grafted neurons appeared to be lytic. Viral persistence and latency were eventually found to occur in grafted neurons, but the mechanism by which this occurred was not determined. It seems unlikely that a neuron in which VZV replicates could survive to support a transition from the lytic to the latent state. In vitro, guinea pig neurons that manifest lytic infection die within 48 to 72 h, and neurons that survive with latent infection die rapidly when ORF61 is expressed and VZV reactivates (124). Cell-free VZV may be necessary for the establishment of latency in neurons. If so, it is possible that neurons that become infected are able to release cell-free VZV, which establishes latency in neighboring neurons. Studies of vaccinated children who have died have supported the importance of viremia in leading to latency in DRG and CNG (116). These children were vaccinated in the deltoid region of an arm; nevertheless, VZV was found to be latent in the trigeminal ganglion and in contralateral lumbar DRG, which do not project to the vaccinated region. Although the presence of virus in distant regions implies viremic spread, it is not clear that the T lymphocytes carrying VZV transferred it to neurons in distant DRG and CNG. Asymptomatic infection of the skin might occur in distant regions with the consequent acquisition of latency in neurons via retrograde axonal transport. T lymphocytes have been reported to secrete filterable VZV (125); however, this assertion has been challenged as a result of experiments in which the size of the pores in filters was selected to exclude the transfilter passage of cells (126). Humans who were found to develop HZ due to WT VZV months to years after varicella vaccination, furthermore, must have experienced an asymptomatic WT infection that led to latency (22, 116). WT VZV infection could have occurred prior to vaccination, or WT VZV might have replaced vOka after vaccination. In either case, the WT VZV infection was asymptomatic. Whether the skin is affected when VZV infections are asymptomatic is unknown. It is clear, however, that symptomatic infection, viremic and/or cutaneous, is not necessary for the establishment of latency in DRG and CNG. In 1/3 of cases of HZ in children who received varicella vaccine without breakthrough varicella disease, the virus in the HZ lesions was shown to be WT VZV (72, 73). Viremia has been postulated to be important in the establishment of simian varicella virus (SVV) latency in neurons in monkeys (127). SVV has been studied as a model that is similar, albeit not identical, to VZV. The ability, discussed above, of infected human or guinea pig PBMC to establish latency in guinea pig DRG and enteric neurons following intravenous injection (22) is consistent with transfer of virus from VZV-infected T lymphocytes to lymphocytes. Still, even in this case, the possibility that PBMC infect a third cell, such as a keratinocyte, which then releases the cell-free VZV that establishes latency in neurons cannot be ruled out.
Transcripts encoding latency-associated WT VZV and vOka gene products have been demonstrated in enteric neurons in fresh human surgical specimens in 12/13 pediatric subjects who either had a history of varicella or were vaccinated against it (22). In contrast, no transcripts or DNA encoding VZV gene products was demonstrable in 7 control specimens, which were obtained from gut surgically removed from infants and children less than 1 year old without a history of varicella or vaccination. These findings in children are consistent with an earlier study that found transcripts encoding at least one immediate early VZV gene product in 88% of 30 specimens of adult gut removed at surgery and the immunocytochemical detection of ORF29 and ORF62 proteins in myenteric and submucosal neurons in 2/2 patients (124). These observations in both pediatric and adult bowel specimens are consistent with the possibility that VZV is latent in the ENS of virtually everyone who has been exposed to VZV through natural varicella or vaccination; moreover, the studies also suggest that, with the exception of the congenital varicella syndrome, VZV is not acquired congenitally and that latency is established when an individual experiences varicella or receives the varicella vaccine. When VZV becomes latent in the gut during childhood, furthermore, viral latency persists in enteric neurons into adult life. Demonstration of VZV RNA transcripts in fresh, surgically removed intestinal tissues (22) avoids the confounding possibility of reactivation of VZV after death, which has been postulated to complicate analyses of autopsy specimens (128).
Latency of VZV in enteric neurons suggests that local reactivation in the bowel may be causally related to a number of gastrointestinal diseases clinically attributed to VZV. These conditions include achalasia, gastric ulcers, and colonic pseudo-obstruction (Oglivie's syndrome) (59, 129–135). VZV may also give rise to enteric disease that had not previously been associated with the virus, such as idiopathic gastroparesis, irritable bowel syndrome, and inflammatory bowel disease (111). A 16-year old male patient, for example was subjected to a partial gastrectomy because of severe gastric ulceration with necrosis and perforation. VZV DNA was found in his saliva and the surgical specimen; moreover, the cells surrounding the ulcers contained the immunoreactivities of ORF63 and gE. DNA disappeared from saliva, and VZV gene products were no longer detected in a biopsy specimen of the stomach following recovery. The boy had received 2 doses of varicella vaccine, and when typed, the VZV DNA in the patient's saliva was found to have been derived from vOka. Because the virus was vaccine type, the ulcer must have been due to reactivation of latent virus, most probably in the ENS. No ganglia were found to be present in the surgically removed tissue, and the patient was left with achalasia following his recovery. There were no cutaneous manifestations of disease in this patient. VZV was suspected because VZV DNA was detected in saliva. This is thus a case of enteric zoster, and it suggests that a number of gastrointestinal disturbances, the etiology of which is currently unclear, might well be caused by VZV, which because of the absence of a concurrent rash is occult and goes unsuspected (87).
PREVENTION OF VZV INFECTIONS BY IMMUNIZATION
The vOka strain of VZV was attenuated by Takahashi and colleagues in 1974 by ∼30 passages in cell culture, including some early passages in guinea pig cells. Live attenuated vaccines (vOka) were then developed for VZV, against varicella in 1974 (136) and against herpes zoster (HZ) in 2005 (80). These vaccines are quite effective in preventing varicella, although somewhat less so in preventing HZ (80, 105, 137–143). VZV vaccines were licensed for routine use against varicella in 1995 (144) and against HZ in 2006 (145) in the United States.
Varicella Vaccine
In addition to the United States, live attenuated varicella vaccine is licensed for routine use in many countries worldwide, including Australia, Brazil, Canada, China, Germany, Greece, Israel, Italy, Japan, Uruguay, Qatar, South Korea, Spain, and Taiwan. In the United States, one dose was initially recommended for immunization of children under 12 years of age (144). Because the FAMA test indicated that seroconversion did not occur after 1 dose of varicella vaccine in roughly 20% of children (99) and many outbreaks of varicella were reported in day care facilities and schools among the immunized (53), a two-dose regimen was recommended by the Centers for Disease Control and Prevention (CDC) in 2006 (146). One dose protected about 85% of children (96, 143), which increased to 98% following a second dose (143) (Fig. 3). Additional and more recent studies have indicated improved protection after 2 doses (138, 141). There appears to be little waning of immunity to VZV with time, even after as many as 14 years postvaccination (137, 147). Waning immunity is a significant concern, because if it occurred, varicella might be postponed to adulthood when the condition may be severe. Evidence that might be interpreted in favor of waning immunity to varicella after vaccination is questionable (148, 149) because, as noted above, primary vaccine failure is relatively common after a single dose of vaccine. As the numbers of subjects in the population who lack protection against varicella due to primary vaccine failure accumulate, there would be an apparent time-dependent increase in occurrences of varicella in vaccinated subjects, even if immunity in successful vaccinees does not wane. The experience with 2 doses of vaccine now suggests that at least after 14 years in the United States, immunity does not wane significantly (137).
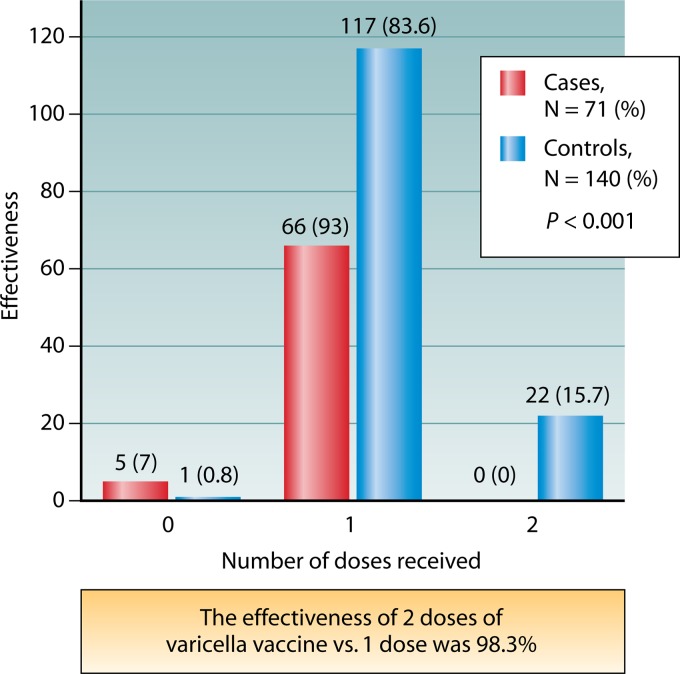
Fig 3.
A case-control study shows that two doses of varicella vaccine provide better protection than a single dose (based on data from reference 143). Between July 2006 and January 2010, 71 case subjects (children with PCR-verified varicella) and 140 matched healthy controls were enrolled in a study of vaccine efficacy. None of the cases (0%) but 22 controls (15.7%) were found to be children who received 2 doses of varicella vaccine. Sixty-six cases (93.0%) but 117 controls (83.6%) were found to be children who received 1 dose. Five cases (7.0%) but 1 control (0.7%) were found to be children who did not receive varicella vaccine (P < 0.001). The effectiveness of 2 doses of the vaccine was calculated to be 98.3% (P < 0.001). The matched odds ratio for 2 doses against a single dose of the vaccine was 0.053 (P < 0.001). The effectiveness of 2 doses of varicella vaccine is thus excellent. The odds of acquiring varicella appear to be 95% lower in children who received 2 doses of varicella vaccine than in children who received only a single dose.
Varicella-susceptible adults may be safely and successfully immunized with varicella vaccine. Two doses at least 1 month apart should be given. While some loss of specific antibodies has been reported with time, loss of immunity has not been demonstrated with time. While adults born and raised in the continental United States may report that they have never had varicella, they are likely to be immune. On the other hand adult immigrants from countries with tropical climates are likely to be susceptible, because VZV spreads poorly in the tropics. CDC criteria for immunity to varicella (as indicated on their website) are history of varicella (preferably provided by a health care provider), epidemiologic data (such as household exposure to other children with varicella), birth in the United States before 1980, and laboratory evidence of immunity. A reliable history of HZ is also an indication of immunity to varicella. When there is doubt and the individual is healthy and not immunocompromised, 2 doses of monovalent varicella vaccine may be given, 1 month apart.
The incidence of HZ after vaccination, in both immunocompromised and healthy vaccinees, has consistently been lower than that in patients who experience natural infection (105, 137, 150, 151). The rate of HZ in young adults who were vaccinated as long as 20 years previously is extremely low, 0.9/1,000 person-years (P-Y) of observation. This rate is less than would be expected after natural infection (140). The rate of HZ in healthy vaccinated children has been reported in two studies to be 0.33/1,000 P-Y (151) and 0.45/1,000 P-Y (137). No cases of HZ were reported after varicella vaccination of HIV-infected children, most of whom were receiving highly active antiretroviral therapy (HAART) (150). The incidence of HZ also decreased with introduction of HAART in HIV-infected adults who had natural varicella prior to HIV infection (152).
WT VZV, rather than vOka, has been found to be the cause of HZ in about 33% of vaccinated children, although clinical breakthrough varicella may not have occurred (72, 73). As noted above, the WT VZV, therefore, must have been acquired as a result of asymptomatic infection with the WT virus, either prior to or after vaccination. There are several possible explanations for the 33% rate of WT HZ after vaccination. Because vOka normally establishes latency following vaccination, it is possible that WT VZV can displace vOka. Alternatively vOka may have less propensity for reactivation, or possibly latent infection is less extensive after vaccination than it is following WT VZV infection. Although vOka is an attenuated virus, no difference between vOka and WT VZV in regard to the severity of the HZ they cause in children has been observed (73).
Prior to widespread vaccination in the United States, there were 100 to 150 annual deaths from varicella, mostly in children and adults who were healthy before they became infected (53). Even when only 1 dose of vaccine was given to children, the incidence of varicella declined dramatically in the United States, as did also the rate of hospitalizations and deaths from varicella in the years between 1995 and 2007 (53, 142, 153). Now that 2 doses of vaccine have become standard, one would expect the morbidity and mortality from varicella caused by WT VZV to decrease even more.
Varicella vaccine has been shown to be extremely safe. Most vaccinees experience no particular symptoms after vaccination; about 5% develop a mild transient rash, either generalized or at the injection site or both, about a month after vaccination (72, 82, 139, 154–156). Extensive rashes, with occasional pneumonia or neurologic symptoms, have rarely been reported in immunocompromised children who were not recognized to be immunodeficient when they were vaccinated (39, 44, 45, 157). These have included a group of fewer than 20 children worldwide, who had undiagnosed AIDS (45), invariant natural killer (INK) cell deficiency (37, 39), or other immunodeficiency diseases (43) and those receiving high doses of steroids for an underlying disease such as asthma (111). Two vaccinated children subsequently developed neuroblastoma followed by severe HZ, which was accompanied by meningitis (158, 159). With one exception, they were treated successfully with antiviral therapy; one vaccinated child died from the vOka strain, a 4-year old girl in Germany with underlying leukemia who was vaccinated when she was in remission for less than 5 months (53, 160).
Possible phenomena that contribute to the success of varicella vaccine regarding safety and efficacy are as follows: (i) vOka is attenuated and very safe, even in selected immunocompromised patients, and severe adverse reactions are very rare in healthy vaccinees; (ii) vOka is highly immunogenic, especially after 2 doses, and waning immunity has not been identified as a problem; (iii) there is an available immune surrogate, the FAMA test, which was immensely helpful in first evaluating vaccine efficacy; (iv) it is possible to differentiate WT VZV from vOka in vaccine recipients to determine whether disease that is temporally related to vaccination was actually caused by the vaccine virus; (v) the potential of vOka to give rise to HZ is lower than that of WT VZV; (vi) vOka has very limited transmissibility and has not reverted clinically to virulence; (vii) vOka is susceptible to treatment with standard antiviral drugs (acyclovir, valacyclovir, and famciclovir); (viii) immunity to VZV may be boosted by asymptomatic or mild reactivations of WT VZV or vOka from latency, helping to maintain long-term immunity against varicella; and (ix) immunity for those whose CMI is waning due to aging can be boosted to prevent HZ and PHN with a vOka vaccine that is stronger than that used to prevent varicella.
If a vOka rash develops in vaccinees, transmission of the vaccine virus to susceptible hosts might potentially occur. Transmission of vOka, however, is extremely rare when healthy individuals are vaccinated; only 10 instances of transmission have been reported since the 1995 vaccine licensure in the United States. An estimated 60 million children have been vaccinated during the ensuing period of time (72, 82, 154, 161). While transmission thus is possible, it does not appear to be a significant threat. An interesting observation of possible spread was the inadvertent spraying of the vaccine into the atmosphere at the time of vaccination (162). Aerosolization of vOka was reported to be a likely cause of transmission to a newborn by a provider who was preparing a syringe for vaccination of a susceptible mother while the infant was in the same room.
Nine cases of meningitis associated with varicella vaccine have been reported. Most occurred in previously healthy American children in whom HZ due to vOka was accompanied by meningitis (and in one case also mild encephalitis). PCR was used to amplify VZV in the CSF in each of these cases (72, 83, 154, 158, 159, 163–167) (Table 1). All of the children received antiviral therapy, and all recovered (83). HZ complicated by meningitis has also been reported to be caused by WT VZV (60, 168–170). The incidence of this complication of HZ is not known, but meningitis in vaccinees appears to be rare. Transient cerebellar ataxia has been reported (72, 82, 154), but the association with vaccination is unproven because vOka DNA has not been demonstrated in CSF in any of the children in which it occurred.
Table 1.
Meningitis caused by the Oka strain of VZV in healthy children vaccinated at ∼1 year of age, diagnosed by PCR on CSF indicating the presence of VZV DNA
| Case | Medical condition | Report yr | First author(s) | Reference(s) | ORF(s) tested | Age of patient | Location of zoster rash |
|---|---|---|---|---|---|---|---|
| 1 | Neuroblastomaa | 2003 | Levin | 159 | 62 | 1 yr | Right thigh |
| 2 | Neuroblastomaa | 2008 | Bryan | 158 | 62 | 21 mob | Hands, right leg, abdomen |
| 3 | Leukemia chemotherapya | 2008 | Chavez, Galea | 154, 72 | 38, 54 | 4 yr | Arm |
| 4 | Otherwise healthy | 2008 | Chavez | 154 | 38, 54 | 4 yrc | Right arm |
| 5 | Otherwise healthy | 2008 | Levin | 167 | 38, 54, 62d | 8 yr | Left shoulder |
| 6 | Otherwise healthy | 2009 | Iyer | 166 | 38, 54, 62 | 9 yr | Left arm |
| 7 | Otherwise healthy | 2010 | Chouliaras, Goulleret | 163, 164 | 62 | 3 yr | Face (trigeminal)e |
| 8 | Otherwise healthy | 2011 | Han | 165 | 38, 54, 62 | 7 yr | Right arm, shoulder |
| 9 | Otherwise healthy | 2011 | Pahud | 83 | 38, 54, 62d | 12 yr | Neck |
Healthy when immunized.
Immunized at 20 months.
Immunized at 32 months.
And other ORFs.
Also had encephalitis.
Zoster Vaccine
Following the successful development of a preventive varicella vaccine, interest in a therapeutic vaccine against HZ emerged. This live attenuated vaccine is also composed of vOka, but at a dose ∼14 times greater than that used in the varicella vaccine (53). Early open-label dose-finding experiments were initially carried out to determine the concentration of VZV needed to restore the CMI response to VZV in the aged (171). It is interesting that the dose of virus needed to reconstitute CMI in the elderly is so much greater than that needed to stimulate a primary immune response in the young.
An impressive multicenter, double-blind, placebo-controlled clinical trial of zoster vaccine involved a cohort of ∼38,500 relatively healthy individuals over age 60 years and took 5 years to complete (80). This trial indicated that the HZ vaccine is roughly 60% effective in preventing HZ and PHN in the age group tested (80). This vaccine has now been recommended in the United States for individuals over the age of 50, in whom it is 70% effective (172). Like the varicella vaccine, the HZ vaccine is extremely safe, and reports of serious adverse events from it are essentially nonexistent. Perhaps because the adult vaccinees who receive the HZ vaccine have almost always encountered VZV previously and have high antibody titers at the time of vaccination, they rarely exhibit a vaccine-associated rash; HZ due to vOka in HZ vaccine recipients has yet to be reported.
Undoubtedly more vaccines against HZ will be developed in the future. A subunit HZ vaccine composed of VZV gE with an adjuvant was recently developed in Europe and appeared to be highly immunogenic in phase 2 studies (173). This subunit vaccine may be more immunogenic than the live attenuated vaccine, but only CD4 T lymphocytes seem to respond to it, as with the live attenuated HZ vaccine. The relevance of this phenomenon is not known. This subunit vaccine could potentially be used to protect immunocompromised individuals because it is not infectious. It is currently in phase 3 clinical trials, but as yet there are no data available regarding its safety or efficacy. (http://clinicaltrials.gov/ct2/show/NCT01165203)
The issue of whether or not decreased exposure to WT VZV will lead to an increase in the incidence of HZ in the middle-aged unvaccinated population has been debated for some years (174, 175). Varicella vaccine has not been licensed for healthy children in the United Kingdom. This failure may reflect the cost of the vaccine, which in the United Kingdom is borne by the National Health Service; however, it is also justified by a hypothetical fear of zoster in the elderly, which in the United Kingdom evidently transcends in importance the fear of varicella in the young and in individuals who are immunocompromised. The hypothesis, backed by computer models, is that periodic exposure to WT VZV, derived from children with varicella, is essential to maintain long-term immunity to VZV (176, 177). In essence, the idea is to preserve varicella in children as a kind of natural vaccination against HZ in adults. While some might question the wisdom of this idea due to the collateral damage resulting from the unchecked spread of WT VZV, there is good reason to believe that the underlying hypothesis is wrong. Computer models reflect the assumptions that underlie them. If periodic exposure to children with varicella does not reduce the incidence of HZ, there is no reason to force children and their parents to cope with varicella or to risk the damage that varicella can do to immunocompromised hosts. In a French study of isolated middle-aged individuals living in a convent and a monastery without exposure to children, the incidence of HZ was not higher than that of the population living with children in the neighboring village (178). Although the incidence of HZ is increasing in the United States, the increase began long before the varicella vaccine was licensed in 1995 (179, 180). In fact, the rate of HZ increased significantly between 1945 to 1949 and 1955 and almost doubled by 1992 (181); moreover, the increase in the incidence of HZ since 1945 to 1949 has been almost linear and does not seem to have been influenced by the introduction of the varicella vaccine. The increase in HZ incidence is undoubtedly multifactorial and probably includes improved ascertainment and diagnosis, as well as increases in the proportion of the American population that is immunocompromised or elderly. Therapies of many conditions, including cancer, involve the use of treatments that reduce CMI, and the populations of most developed countries, including that of the United States, are aging.
Reactivation from latency, which might be subclinical or mild enough not to be recognized, is a possible mechanism that could maintain long-term immunity to VZV. This possibility was suspected years ago (182, 183). Luby and his colleagues found frequent increases of VZV antibodies in asymptomatic renal transplant patients that were not believed to be due to exogenous exposure to VZV. The use of PCR in diagnostic virology has provided evidence that subclinical reactivation of VZV occurs in both immunocompromised and immunocompetent individuals (85, 184–187). The manifestations of the reactivation of VZV form a spectrum that ranges from subclinical occurrences to zoster sine herpete (61, 111, 188) to localized unilateral dermatomal vesicular cutaneous lesions and finally to disseminated HZ, which involves widespread cutaneous lesions that resemble those of varicella. Subclinical reactivation may generate an immune response to VZV that stimulates long-term immunity. The fact that VZV establishes latency in enteric neurons may be particularly important from the standpoint of the maintenance of immunity. The gut is the largest immune organ of the body; therefore, repetitive mild or asymptomatic reactivation of VZV in the ENS could result in significant restimulation of protective immune responses. If reactivation of VZV from latency indeed occurs at intervals, long-term immunity could be ensured. That remains to be proven, for both WT VZV and vOka; however, if immunity following vaccination does not wane, it would be unnecessary as well as immoral to hold children hostage to varicella in order to protect the elderly from HZ.
In summary, VZV vaccines have proven to be remarkably effective and safe (53, 80, 189). The use of varicella vaccine has dramatically decreased the incidence of varicella and its complications in the United States. Viral transmission has decreased, and the United States has acquired a significant protective blanket of herd immunity (53). Over 90% of young children have been immunized in the United States; moreover, hospitalizations and deaths from varicella have fallen (142, 190). HZ and PHN remain, but advances against each are being made, mainly as a result of the use of the varicella and HZ vaccines, both of which contribute to vaccine-mediated prevention. Interestingly, this success with VZV vaccines has occurred although the vaccines do not achieve the ideal of “sterilizing” immunity. It seems unlikely that VZV can ever be eliminated due to the phenomenon of latency and reactivation, which may actually confer the advantage of long-term maintenance of immunity. Reactivation, however, will perpetually return virus to the population. There seems little question that the observed decrease in viral transmission, which has led to marked decreases in varicella, represents a great achievement for American public health. The ability to prevent HZ is useful but imperfect and provides a field for further vaccine development.
ACKNOWLEDGMENT
This work was supported by NIH grant R01 DK093094.
Biographies

Anne A. Gershon is the Driscoll Professor of Pediatrics at Columbia University College of Physicians and Surgeons. She is a graduate of Cornell Medical School. Her research over the past 40 years has included epidemiology, diagnosis, immunology, latency, prevention, and treatment of varicella and zoster. Her studies with varicella vaccine were critical for its licensure in the United States. She is continuing to study the safety and efficacy of varicella vaccine in the “vaccine era.” She has also focused on HIV infection in children, particularly opportunistic infections. She has received research funding from NIH for the past 40 years. Dr. Gershon has served on numerous national and international medical committees. She was President of the Infectious Diseases Society of America in 2009. She has received many professional awards, including the Gold Medal of the Sabin Vaccine Institute. She is the author of over 300 publications and has edited 11 books.

Michael D. Gershon received his M.D. in 1963 from Cornell University Medical College and was Chairman of Anatomy and Cell Biology at Columbia University for almost 30 years; he is now Professor of Pathology at Columbia. Dr. Gershon has been called the “father of neurogastroenterology”; in addition to his seminal work on neuronal control of gastrointestinal (GI) behavior and development of the enteric nervous system (ENS), his classic trade book, The Second Brain, has made physicians, scientists, and the lay public aware of the significance of the unique ability of the ENS to regulate GI activity in the absence of input from the brain and spinal cord. He has published almost 400 peer-reviewed papers. With Anne Gershon, he demonstrated that varicella-zoster virus (VZV) infects, becomes latent, and reactivates in enteric neurons, including those of humans, and may cause “enteric zoster” upon local reactivation, in the absence of skin rash.
REFERENCES
- 1.Weller T, Stoddard MB. 1952. Intranuclear inclusion bodies in cultures of human tissue inoculated with varicella vesicle fluid. J. Immunol. 68:311–319 [PubMed] [Google Scholar]
- 2.Weller TH. 1953. Serial propagation in vitro of agents producing inclusion bodies derived from varicella and herpes zoster. Proc. Soc. Exp. Biol. Med. 83:340–346 [DOI] [PubMed] [Google Scholar]
- 3.Wagenaar TR, Chow VT, Buranathai C, Thawatsupha P, Grose C. 2003. The out of Africa model of varicella-zoster virus evolution: single nucleotide polymorphisms and private alleles distinguish Asian clades from European/North American clades. Vaccine 21:1072–1081 [DOI] [PubMed] [Google Scholar]
- 4.Djerassi I, Farber S, Abir E, Neikirk W. 1967. Continuous infusion of methotrexate in children with acute leukemia. Cancer 20:233–242 [DOI] [PubMed] [Google Scholar]
- 5.Evans AE, Farber S, Brunet S, Mariano PJ. 1963. Vincristine in the treatment of acute leukemia in children. Cancer 16:1302–1306 [DOI] [PubMed] [Google Scholar]
- 6.Farber S. 1950. Chemotherapeutic studies of tumors, including leukemia, in children. Am. J. Dis. Child. 79:961–962 [PubMed] [Google Scholar]
- 7.Farber S. 1966. Chemotherapy in the treatment of leukemia and Wilms' tumor. JAMA 198:826–836 [PubMed] [Google Scholar]
- 8.Farber S. 1956. The treatment of acute leukemia. J. Chronic Dis. 3:455–457 [DOI] [PubMed] [Google Scholar]
- 9.Garland J. 1943. Varicella following exposure to herpes zoster. N. Engl. J. Med. 228:336–337 [Google Scholar]
- 10.Cheatham WJ, Weller TH, Dolan TF, Dower JC. 1956. Varicella: report of two fatal cases with necropsy, virus isolation, and serologic studies. Am. J. Pathol. 32:1015–1035 [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JJ, Zhu Z, Gershon AA, Gershon MD. 2004. Mannose 6-phosphate receptor dependence of varicella zoster virus infection in vitro and in the epidermis during varicella and zoster. Cell 119:915–926 [DOI] [PubMed] [Google Scholar]
- 12.Zhu Z, Gershon MD, Ambron R, Gabel C, Gershon AA. 1995. Infection of cells by varicella zoster virus: inhibition of viral entry by mannose 6-phosphate and heparin. Proc. Natl. Acad. Sci. U. S. A. 92:3546–3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Z, Gershon MD, Gabel C, Sherman D, Ambron R, Gershon AA. 1995. Entry and egress of VZV: role of mannose 6-phosphate, heparan sulfate proteoglycan, and signal sequences in targeting virions and viral glycoproteins. Neurology 45:S15–S17 [DOI] [PubMed] [Google Scholar]
- 14.Zhu Z, Gershon MD, Hao Y, Ambron RT, Gabel CA, Gershon AA. 1995. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J. Virol. 69:7951–7959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Ali MA, Cohen JI. 2006. Insulin degrading enzyme is a cellular receptor mediating varicella-zoster virus infection and cell-to-cell spread. Cell 127:305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q, Ali MA, Wang K, Sayre D, Hamel FG, Fischer ER, Bennett RG, Cohen JI. 2010. Insulin degrading enzyme induces a conformational change in varicella-zoster virus gE, and enhances virus infectivity and stability. PLoS One 5:e11327. 10.1371/journal.pone.0011327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen JI, Straus SE, Arvin A. 2007. Varicella-zoster virus: replication, pathogenesis, and management, p 2773–2818 In Knipe DM, Howley PM. (ed), Fields virology, 5th ed. Lippincott Williams and Wilkins, Philadelphia, PA [Google Scholar]
- 18.Gabel C, Dubey L, Steinberg S, Gershon M, Gershon A. 1989. Varicella-zoster virus glycoproteins are phosphorylated during posttranslational maturation. J. Virol. 63:4264–4276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gershon AA, Sherman DL, Zhu Z, Gabel CA, Ambron RT, Gershon MD. 1994. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J. Virol. 68:6372–6390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadzot-Delvaux C, Merville-Louis M-P, Delree P, Marc P, Moonen G, Rentier B. 1990. An in vivo model of varicella-zoster virus latent infection of dorsal root ganglia. J. Neurosci. Res. 26:83–89 [DOI] [PubMed] [Google Scholar]
- 21.Sato H, Pesnicak L, Cohen JI. 2003. Use of a rodent model to show that varicella-zoster virus ORF61 is dispensable for establishment of latency. J. Med. Virol. 70(Suppl 1):S79–S81 [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Gershon AA, Li Z, Cowles RA, GMD 2011. Varicella zoster virus (VZV) infects and establishes latency in enteric neurons. J. Neurovirol. 17:578–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers M, Connelly BL. 1992. Animal models of varicella. J. Infect. Dis. 166:S48–S50 [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Gershon A, Silverstein SJ, Li ZS, Lungu O, Gershon MD. 2003. Latent and lytic infection of isolated guinea pig enteric and dorsal root ganglia by varicella zoster virus. J. Med. Virol. 70:S71–78 [DOI] [PubMed] [Google Scholar]
- 25.Brunell PA. 1989. Transmission of chickenpox in a school setting prior to the observed exanthem. Am. J. Dis. Child. 143:1451–1452 [DOI] [PubMed] [Google Scholar]
- 26.Gold E. 1966. Serologic and virus-isolation studies of patients with varicella or herpes zoster infection. N. Engl. J. Med. 274:181–185 [DOI] [PubMed] [Google Scholar]
- 27.Tsolia M, Gershon A, Steinberg S, Gelb L. 1990. Live attenuated varicella vaccine: evidence that the virus is attenuated and the importance of skin lesions in transmission of varicella-zoster virus. J. Pediatr. 116:184–189 [DOI] [PubMed] [Google Scholar]
- 28.Gustafson TL, Lavely GB, Brauner ER, Hutcheson RH, Wright P, Schaffner W. 1982. An outbreak of airbirne nosocomial varicella. Pediatrics 70:550–556 [PubMed] [Google Scholar]
- 29.Leclair JM, Zaia J, Levin MJ, Congdon RG, Goldmann D. 1980. Airborne transmission of chickenpox in a hospital. N. Engl. J. Med. 302:450–453 [DOI] [PubMed] [Google Scholar]
- 30.Seward JF, Zhang JX, Maupin TJ, Mascola L, Jumaan AO. 2004. Contagiousness of varicella in vaccinated cases: a household contact study. JAMA 292:704–708 [DOI] [PubMed] [Google Scholar]
- 31.Leung J, Harpaz R, Baughman AL, Heath K, Loparev V, Vazquez M, Watson BM, Schmid DS. 2010. Evaluation of laboratory methods for diagnosis of varicella. Clin. Infect. Dis. 51:23–32 [DOI] [PubMed] [Google Scholar]
- 32.Sawyer M, Chamberlin C, Wu Y, Aintablian N, Wallace M. 1994. Detection of varicella-zoster virus DNA in air samples from hospital rooms. J. Infect. Dis. 169:91–94 [DOI] [PubMed] [Google Scholar]
- 33.Arvin AM, Moffat JF, Sommer M, Oliver S, Che X, Vleck S, Zerboni L, Ku CC. 2010. Varicella-zoster virus T cell tropism and the pathogenesis of skin infection. Curr. Top. Microbiol. Immunol. 342:189–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gershon A, Steinberg S, Brunell P. 1974. Zoster immune globulin: a further assessment. N. Engl. J. Med. 290:243–245 [DOI] [PubMed] [Google Scholar]
- 35.Ku CC, Besser J, Abendroth A, Grose C, Arvin AM. 2005. Varicella-zoster virus pathogenesis and immunobiology: new concepts emerging from investigations with the SCIDhu mouse model. J. Virol. 79:2651–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ku CC, Zerboni L, Ito H, Graham BS, Wallace M, Arvin AM. 2004. Varicella-zoster virus transfer to skin by T cells and modulation of viral replication by epidermal cell interferon-α. J. Exp. Med. 200:917–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banovic T, Yanilla M, Simmons R, Robertson I, Schroder WA, Raffelt NC, Wilson YA, Hill GR, Hogan P, Nourse CB. 2011. Disseminated varicella infection caused by varicella vaccine strain in a child with low invariant natural killer T cells and diminished CD1d expression. J. Infect. Dis. 204:1893–1901 [DOI] [PubMed] [Google Scholar]
- 38.Etzioni A, Eidenschenk C, Katz R, Beck R, Casanova JL, Pollack S. 2005. Fatal varicella associated with selective natural killer cell deficiency. J. Pediatr. 146:423–425 [DOI] [PubMed] [Google Scholar]
- 39.Levy O, Orange JS, Hibberd P, Steinberg S, LaRussa P, Weinberg A, Wilson SB, Shaulov A, Fleisher G, Geha RS, Bonilla FA, Exley M. 2003. Disseminated varicella infection due to vaccine (Oka) strain varicella-zoster virus in a patient with a novel deficiency in natural killer cells. J. Infect. Dis. 188:948–953 [DOI] [PubMed] [Google Scholar]
- 40.Novakova L, Lehuen A, Novak J. 2011. Low numbers and altered phenotype of invariant natural killer T cells in recurrent varicella zoster virus infection. Cell. Immunol. 269:78–81 [DOI] [PubMed] [Google Scholar]
- 41.Chilek K, Routhouska S, Tamburro J. 2010. Disseminated varicella zoster virus in an immunized child as the acquired immunodeficiency syndrome-defining illness. Pediatr. Dermatol. 27:192–194 [DOI] [PubMed] [Google Scholar]
- 42.Feldman S, Hughes W, Daniel C. 1975. Varicella in children with cancer: 77 cases. Pediatrics 80:388–397 [PubMed] [Google Scholar]
- 43.Ghaffar F, Carrick K, Rogers BB, Margraf LR, Krisher K, Ramilo O. 2000. Disseminated infection with varicella-zoster virus vaccine strain presenting as hepatitis in a child with adenosine deaminase deficiency. Pediatr. Infect. Dis. J. 19:764–766 [DOI] [PubMed] [Google Scholar]
- 44.Jean-Philippe P, Freedman A, Chang MW, Steinberg SP, Gershon AA, LaRussa PS, Borkowsky W. 2007. Severe varicella caused by varicella-vaccine strain in a child with significant T-cell dysfunction. Pediatrics 120:e1345–1349 [DOI] [PubMed] [Google Scholar]
- 45.Kramer JM, LaRussa P, Tsai WC, Carney P, Leber SM, Gahagan S, Steinberg S, Blackwood RA. 2001. Disseminated vaccine strain varicella as the acquired immunodeficiency syndrome-defining illness in a previously undiagnosed child. Pediatrics 108:E39. [DOI] [PubMed] [Google Scholar]
- 46.Malavige GN, Jones L, Kamaladasa SD, Wijewickrama A, Seneviratne SL, Black AP, Ogg GS. 2008. Viral load, clinical disease severity and cellular immune responses in primary varicella zoster virus infection in Sri Lanka. PLoS One 3:e3789. 10.1371/journal.pone.0003789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Breuer J, Grose C, Norberg P, Tipples G, Schmid DS. 2010. A proposal for a common nomenclature for viral clades that form the species varicella-zoster virus: summary of VZV Nomenclature Meeting 2008, Barts and the London School of Medicine and Dentistry, 24-25 July 2008. J. Gen. Virol. 91:821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quinlivan M, Sengupta N, Papaevangelou V, Sauerbrei A, Grillner L, Rousseva R, Hague R, Lutsar I, Jogi P, Leca A, Grytchol R, Alain S, Breuer J. 2013. Use of oral fluid to examine the molecular epidemiology of varicella zoster virus in the United kingdom and continental europe. J. Infect. Dis. 207:588–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gershon AA. 2013. Varicella zoster vaccines and their implications for development of HSV vaccines. Virology 435:29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ross AH, Lencher E, Reitman G. 1962. Modification of chickenpox in family contacts by administration of gamma globulin. N. Engl. J. Med. 267:369–376 [DOI] [PubMed] [Google Scholar]
- 51.Preblud SR. 1981. Age-specific risks of varicella complications. Pediatrics 68:14–17 [PubMed] [Google Scholar]
- 52.Ziebold C, von Kries R, Lang R, Weigl J, Schmitt H. 2001. Severe complications of varicella in previously healthy children in Germany. Pediatrics 108:1194–1195 [PubMed] [Google Scholar]
- 53.Gershon A, Takahashi M, Seward JF. 2013. Live attenuated varicella vaccine, p 837–869 In Plotkin S, Orenstein W, Offit P. (ed), Vaccines, 6th ed. W.B. Saunders, Philadelphia, PA [Google Scholar]
- 54.Gershon AA, Steinberg S, Gelb L, NIAID Collaborative Varicella Vaccine Study Group 1984. Clinical reinfection with varicella-zoster virus. J. Infect. Dis. 149:137–142 [DOI] [PubMed] [Google Scholar]
- 55.Junker AK, Angus E, Thomas E. 1991. Recurrent varicella-zoster virus infections in apparently immunocompetent children. Ped. Infect. Dis. J. 10:569–575 [DOI] [PubMed] [Google Scholar]
- 56.Junker AK, Tilley P. 1994. Varicella-zoster virus antibody avidity and IgG-subclass patterns in children with recurrent chickenpox. J. Med. Virol. 43:119–124 [DOI] [PubMed] [Google Scholar]
- 57.Junker K, Avnstorp C, Nielsen C, Hansen N. 1989. Reinfection with varicella-zoster virus in immunocompromised patients. Curr. Probl. Dermatol. 18:152–157 [DOI] [PubMed] [Google Scholar]
- 58.Gershon A. 2011. Chickenpox, measles, and mumps, p 661–705 In Remington J, Klein J, Wilson C, Nizet V, Maldonato Y. (ed), Infections of the fetus and newborn infant, 7th ed. Saunders, Philadelphia, PA [Google Scholar]
- 59.Edelman DA, Antaki F, Basson MD, Salwen WA, Gruber SA, Losanoff JE. 2010. Ogilvie syndrome and herpes zoster: case report and review of the literature. J. Emerg. Med. 39:696–700 [DOI] [PubMed] [Google Scholar]
- 60.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. 2010. Neurological disease produced by varicella zoster virus reactivation without rash. Curr. Top. Microbiol. Immunol. 342:243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gilden D, Dueland A, Devlin M, Mahlingham R, Cohrs R. 1992. Varicella-zoster virus reactivation without rash. J. Infect. Dis. 166:S30–S34 [DOI] [PubMed] [Google Scholar]
- 62.Gilden DH, Wright R, Schneck S, Gwaltney JM, Mahalingam R. 1994. Zoster sine herpete, a clinical variant. Ann. Neurol. 35:530–533 [DOI] [PubMed] [Google Scholar]
- 63.Gore DM, Gore SK, Visser L. 2012. Progressive outer retinal necrosis: outcomes in the intravitreal era. Arch. Ophthalmol. 130:700–706 [DOI] [PubMed] [Google Scholar]
- 64.Yin PD, Kurup SK, Fischer SH, Rhee HH, Byrnes GA, Levy-Clarke GA, Buggage RR, Nussenblatt RB, Mican JM, Wright ME. 2007. Progressive outer retinal necrosis in the era of highly active antiretroviral therapy: successful management with intravitreal injections and monitoring with quantitative PCR. J. Clin. Virol. 38:254–259 [DOI] [PubMed] [Google Scholar]
- 65.Pavan-Langston D. 2000. Ophthalmic zoster, p 276–298 In Arvin A, Gershon A. (ed), Varicella-zoster virus: virology and clinical management. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 66.Wilhelmus KR, Hamill MB, Jones DB. 1991. Varicella disciform stromal keratitis. Am. J. Ophthalmol. 111:575–580 [DOI] [PubMed] [Google Scholar]
- 67.Del Pozo JL, van de Beek D, Daly RC, Pulido JS, McGregor CG, Patel R. 2009. Incidence and clinical characteristics of ocular infections after heart transplantation: a retrospective cohort study. Clin. Transplant. 23:484–489 [DOI] [PubMed] [Google Scholar]
- 68.Sugita S, Ogawa M, Shimizu N, Morio T, Ohguro N, Nakai K, Maruyama K, Nagata K, Takeda A, Usui Y, Sonoda KH, Takeuchi M, Mochizuki M. 7 May 2013. Use of a comprehensive polymerase chain reaction system for diagnosis of ocular infectious diseases. Ophthalmology 10.1016/j.ophtha.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 69.Gonzales JA, Levison AL, Stewart JM, Acharya NR, Margolis TP. 2012. Retinal necrosis following varicella-zoster vaccination. Arch. Ophthalmol. 130:1355–1356 [DOI] [PubMed] [Google Scholar]
- 70.Nagel MA, Russman AN, Feit H, Traktinskiy I, Khmeleva N, Schmid DS, Skarf B, Gilden D. 2013. VZV ischemic optic neuropathy and subclinical temporal artery infection without rash. Neurology 80:220–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Silver B, Nagel MA, Mahalingam R, Cohrs R, Schmid DS, Gilden D. 2012. Varicella zoster virus vasculopathy: a treatable form of rapidly progressive multi-infarct dementia after 2years' duration. J. Neurol. Sci. 323:245–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galea SA, Sweet A, Beninger P, Steinberg SP, Larussa PS, Gershon AA, Sharrar RG. 2008. The safety profile of varicella vaccine: a 10-year review. J. Infect. Dis. 197(Suppl. 2):S165–S169 [DOI] [PubMed] [Google Scholar]
- 73.Vazquez M. 2012. Presented at the Pediatric Academic Specialties, Boston, MA [Google Scholar]
- 74.Drolet M, Brisson M, Levin MJ, Schmader KE, Oxman MN, Johnson RW, Camden S, Mansi JA. 2010. A prospective study of the herpes zoster severity of illness. Clin. J. Pain 26:656–666 [DOI] [PubMed] [Google Scholar]
- 75.Drolet M, Brisson M, Schmader K, Levin M, Johnson R, Oxman M, Patrick D, Camden S, Mansi JA. 2010. Predictors of postherpetic neuralgia among patients with herpes zoster: a prospective study. J. Pain 11:1211–1221 [DOI] [PubMed] [Google Scholar]
- 76.Johnson RW. 2010. Herpes zoster and postherpetic neuralgia. Exp. Rev. Vaccines 9:21–26 [DOI] [PubMed] [Google Scholar]
- 77.Gilden DH, Cohrs RJ, Hayward AR, Wellish M, Mahalingam R. 2003. Chronic varicella-zoster virus ganglionitis—a possible cause of postherpetic neuralgia. J. Neurovirol. 9:404–407 [DOI] [PubMed] [Google Scholar]
- 78.Tyring S, Barbarash RA, Nahlik JE, Cunningham A, Marley J, Heng M, Jones T, Rea T, Boon R, Saltzman R, Group CFS. 1995. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and post herpetic neuralgia. Ann. Int. Med. 123:89–96 [DOI] [PubMed] [Google Scholar]
- 79.Quinlivan ML, Ayres KL, Kelly PJ, Parker SP, Scott FT, Johnson RW, Maple C, Breuer J. 2011. Persistence of varicella-zoster virus viraemia in patients with herpes zoster. J. Clin. Virol. 50:130–135 [DOI] [PubMed] [Google Scholar]
- 80.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW, Jr, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh SS, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan IS, Wang WW, Annunziato PW, Silber JL. 2005. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 352:2271–2284 [DOI] [PubMed] [Google Scholar]
- 81.Gershon A, Chen J, LaRussa P, Steinberg S. 2007. Varicella-zoster virus, p 1537–1548 In Murray PR, Baron E, Jorgensen J, Landry M, Pfaller M. (ed), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC [Google Scholar]
- 82.Sharrar RG, LaRussa P, Galea S, Steinberg S, Sweet A, Keatley M, Wells M, Stephenson W, Gershon A. 2000. The postmarketing safety profile of varicella vaccine. Vaccine 19:916–923 [DOI] [PubMed] [Google Scholar]
- 83.Pahud BA, Glaser CA, Dekker CL, Arvin AM, Schmid DS. 2011. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J. Infect. Dis. 203:316–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Papaevangelou V, Quinlivan M, Lockwood J, Papaloukas O, Sideri G, Critselis E, Papassotiriou I, Papadatos J, Breuer J. 2013. Subclinical VZV reactivation in immunocompetent children hospitalized in the ICU associated with prolonged fever duration. Clin. Microbiol. Infect. 19:245–251 [DOI] [PubMed] [Google Scholar]
- 85.Mehta SK, Cohrs RJ, Forghani B, Zerbe G, Gilden DH, Pierson DL. 2004. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J. Med. Virol. 72:174–179 [DOI] [PubMed] [Google Scholar]
- 86.Mehta SK, Stowe RP, Feiveson AH, Tyring SK, Pierson DL. 2000. Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J. Infect. Dis. 182:1761–1764 [DOI] [PubMed] [Google Scholar]
- 87.Chen JJ, Gershon AA, Diacou A, Gershon MD. 2013. Enteric zoster: human occurrence and development of a guinea pig model. Gastroenterology 144(Suppl 1):S-904 [Google Scholar]
- 88.Gershon AA. 2011. The history and mystery of VZV in saliva. J. Infect. Dis. 204:815–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mehta SK, Tyring SK, Gilden DH, Cohrs RJ, Leal MJ, Castro VA, Feiveson AH, Ott CM, Pierson DL. 2008. Varicella-zoster virus in the saliva of patients with herpes zoster. J. Infect. Dis. 197:654–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.LaRussa P, Lungu O, Hardy I, Gershon A, Steinberg S, Silverstein S. 1992. Restriction fragment length polymorphism of polymerase chain reaction products from vaccine and wild-type varicella-zoster virus isolates. J. Virol. 66:1016–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Quinlivan ML, Jensen NJ, Radford KW, Schmid DS. 2012. Novel genetic variation identified at fixed loci in ORF62 of the Oka varicella vaccine and in a case of vaccine-associated herpes zoster. J. Clin. Microbiol. 50:1533–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cohrs RJ, Mehta SK, Schmid DS, Gilden DH, Pierson DL. 2008. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J. Med. Virol. 80:1116–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wald A, Zeh J, Selke S, Ashley RL, Corey L. 1995. Virologic characteristics of subclinical and symptomatic genital herpes infections. N. Engl. J. Med. 333:770–775 [DOI] [PubMed] [Google Scholar]
- 94.Nagel MA, Choe A, Cohrs RJ, Traktinskiy I, Sorensen K, Mehta SK, Pierson DL, Tyring SK, Haitz K, Digiorgio C, Lapolla W, Gilden D. 2011. Persistence of varicella zoster virus DNA in saliva after herpes zoster. J. Infect. Dis. 204:820–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coffin SE, Hodinka RL. 1995. Utility of direct immunofluorescence and virus culture for detection of varicella-zoster virus in skin lesions. J. Clin. Microbiol. 33:2792–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vazquez M, LaRussa P, Gershon A, Steinberg S, Freudigman K, Shapiro E. 2001. The effectiveness of the varicella vaccine in clinical practice. N. Engl. J. Med. 344:955–960 [DOI] [PubMed] [Google Scholar]
- 97.Forghani B. 2000. Laboratory diagnosis of infection, p 351–382 In Arvin A, Gershon A. (ed), Varicella-zoster virus: virology and clinical management. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 98.Saiman L, LaRussa P, Steinberg S, Zhu J, Baron K, Whittier S, Della Latta P, Gershon A. 2001. Persistence of immunity to varicella-zoster virus vaccination among health care workers. Infect. Control Hosp. Epidemiol. 22:279–283 [DOI] [PubMed] [Google Scholar]
- 99.Michalik DE, Steinberg SP, LaRussa PS, Edwards KM, Wright PF, Arvin AM, Gans HA, Gershon AA. 2008. Primary vaccine failure after 1 dose of varicella vaccine in healthy children. J. Infect. Dis. 197:944–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Williams V, Gershon A, Brunell P. 1974. Serologic response to varicella-zoster membrane antigens measured by indirect immunofluorescence. J. Infect. Dis. 130:669–672 [DOI] [PubMed] [Google Scholar]
- 101.Gershon AA, Steinberg S, Gelb L, NIAID Collaborative Varicella Vaccine Study Group 1984. Live attenuated varicella vaccine: efficacy for children with leukemia in remission. JAMA 252:355–362 [DOI] [PubMed] [Google Scholar]
- 102.Asano Y, Albrecht P, Vujcic LK, Quinnan GV, Jr, Kawakami K, Takahashi M. 1983. Five-year follow-up study of recipients of live varicella vaccine using enhanced neutralization and fluorescent antibody membrane antigen assays. Pediatrics 72:291–294 [PubMed] [Google Scholar]
- 103.Asano Y, Suga S, Yoshikawa T, Kobayashi H, Yazaki T, Shibata M, Tsuzuki K, Ito S. 1994. Experience and reason: twenty year follow up of protective immunity of the Oka live varicella vaccine. Pediatrics 94:524–526 [PubMed] [Google Scholar]
- 104.Arvin AM, Pollard RB, Rasmussen L, Merigan T. 1978. Selective impairment in lymphocyte reactivity to varicella-zoster antigen among untreated lymphoma patients. J. Infect. Dis. 137:531–540 [DOI] [PubMed] [Google Scholar]
- 105.Hardy I, Gershon AA, Steinberg SP, LaRussa P, et al. 1991. The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. Varicella Vaccine Collaborative Study Group. N. Engl. J. Med. 325:1545–1550 [DOI] [PubMed] [Google Scholar]
- 106.Verjans GM, Hintzen RQ, van Dun JM, Poot A, Milikan JC, Laman JD, Langerak AW, Kinchington PR, Osterhaus AD. 2007. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc. Natl. Acad. Sci. U. S. A. 104:3496–3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Berger TM, Caduff JH, Gebbers J-O. 2000. Fatal varicella-zoster virus antigen-positive giant cell arteritis of the central nervous system. Pediatr. Infect. Dis. J. 19:653–656 [DOI] [PubMed] [Google Scholar]
- 108.Burke BL, Steele RW, Beard OW, Wood JS, Cain TD, Marmer DJ. 1982. Immune responses to varicella-zoster in the aged. Arch. Intern. Med. 142:291–293 [PubMed] [Google Scholar]
- 109.Schmader K, Studenski S, MacMillan J, Grufferman S, Cohen SJ. 1990. Are stressful life events risk factors for herpes zoster? J. Am. Geriart. Soc. 38:1188–1194 [DOI] [PubMed] [Google Scholar]
- 110.Furuta Y, Ohtani F, Fukuda S, Inuyama Y, Nagashima K. 2000. Reactivation of varicella-zoster virus in delayed facial palsy after dental treatment and oro-facial surgery. J. Med. Virol. 62:42–45 [PubMed] [Google Scholar]
- 111.Cohrs RJ, Gilden D. 2012. 2012 Colorado alphaherpesvirus latency symposium. J. Neurovirol. 10.1007/s13365-012-0129-7 [DOI] [PubMed] [Google Scholar]
- 112.Whitley RJ, Middlebrooks M, Gnann JW. 1990. Acyclovir: the past ten years. Adv. Exp. Med. Biol. 278:243–253 [DOI] [PubMed] [Google Scholar]
- 113.Shepp DH, Dandliker PS, Meyers JD. 1986. Treatment of varicella-zoster virus infection in severely immunocompromised patients: a randomized comparison of acyclovir and vidarabine. N. Engl. J. Med. 314:208–212 [DOI] [PubMed] [Google Scholar]
- 114.Whitley RJ, Gnann JW. 1992. Acyclovir: a decade later. N. Engl. J. Med. 327:782–789 [DOI] [PubMed] [Google Scholar]
- 115.Aoki F, Hayden F, Dolin R. 2010. Antiviral drugs (other than antiretrovirals), p 565–610 In Mandell G, Bennett J, Dolin R. (ed), Principles and practice of infectious diseases, 7th ed. Churchill Livingstone Elsevier, Philadelphia, PA [Google Scholar]
- 116.Gershon AA, Chen J, Davis L, Krinsky C, Cowles R, Reichard R, Gershon M. 2012. Latency of varicella zoster virus in dorsal root, cranial, and enteric ganglia in vaccinated children. Trans. Am. Clin. Climatol. Assoc. 123:17–33(Discussion, 123:33–35.) [PMC free article] [PubMed] [Google Scholar]
- 117.Kennedy PG, Cohrs RJ. 2010. Varicella-zoster virus human ganglionic latency: a current summary. J. Neurovirol. 16:411–418 [DOI] [PubMed] [Google Scholar]
- 118.Zerboni L, Sobel RA, Lai M, Triglia R, Steain M, Abendroth A, Arvin A. 2012. Apparent expression of varicella-zoster virus proteins in latency resulting from reactivity of murine and rabbit antibodies with human blood group a determinants in sensory neurons. J. Virol. 86:578–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ouwendijk WJ, Flowerdew SE, Wick D, Horn AK, Sinicina I, Strupp M, Osterhaus AD, Verjans GM, Hufner K. 2012. Immunohistochemical detection of intra-neuronal VZV proteins in snap-frozen human ganglia is confounded by antibodies directed against blood group A1-associated antigens. J. Neurovirol. 18:172–180 [DOI] [PubMed] [Google Scholar]
- 120.Silverstein S, Straus SE. 2000. Pathogenesis of latency and reactivation, p 123–141 In Arvin A, Gershon A. (ed), Varicella-zoster virus: virology and clinical managment. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 121.Baiker A, Fabel K, Cozzio A, Zerboni L, Sommer M, Uchida N, He D, Weissman I, Arvin AM. 2004. Varicella-zoster virus infection of human neural cells in vivo. Proc. Natl. Acad. Sci. U. S. A. 101:10792–10797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reichelt M, Zerboni L, Arvin AM. 2008. Mechanisms of varicella-zoster virus neuropathogenesis in human dorsal root ganglia. J. Virol. 82:3971–3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zerboni L, Ku CC, Jones CD, Zehnder JL, Arvin AM. 2005. Varicella-zoster virus infection of human dorsal root ganglia in vivo. Proc. Natl. Acad. Sci. U. S. A. 102:6490–6495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gershon AA, Chen J, Gershon MD. 2008. A model of lytic, latent, and reactivating varicella-zoster virus infections in isolated enteric neurons. J. Infect. Dis. 197(Suppl. 2):S61–S65 [DOI] [PubMed] [Google Scholar]
- 125.Moffat JF, Stein MD, Kaneshima H, Arvin AM. 1995. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J. Virol. 69:5236–5242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Soong W, Schultz JC, Patera AC, Sommer MH, Cohen JI. 2000. Infection of human T lymphocytes with varicella-zoster virus: an analysis with viral mutants and clinical isolates. J. Virol. 74:1864–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ouwendijk WJ, Mahalingam R, Traina-Dorge V, van Amerongen G, Wellish M, Osterhaus AD, Gilden D, Verjans GM. 2012. Simian varicella virus infection of Chinese rhesus macaques produces ganglionic infection in the absence of rash. J. Neurovirol. 18:91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ouwendijk WJ, Choe A, Nagel MA, Gilden D, Osterhaus AD, Cohrs RJ, Verjans GM. 2012. Restricted VZV transcription in human trigeminal ganglia obtained early after death. J. Virol. 86:10203–10206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Castex F, Guillemot F, Talbodec N, Colombel JF, Paris JC, Cortot A. 1995. Association of an attack of varicella and an achalasia. Am. J. Gastroenterol. 90:1188–1189 [PubMed] [Google Scholar]
- 130.Milligan KL, Jain AK, Garrett JS, Knutsen AP. 2012. Gastric ulcers due to varicella-zoster reactivation. Pediatrics 130:e1377–1381 [DOI] [PubMed] [Google Scholar]
- 131.Pui JC, Furth EE, Minda J, Montone KT. 2001. Demonstration of varicella-zoster virus infection in the muscularis propria and myenteric plexi of the colon in an HIV-positive patient with herpes zoster and small bowel pseudo-obstruction (Ogilvie's syndrome). Am. J. Gastroenterol. 96:1627–1630 [DOI] [PubMed] [Google Scholar]
- 132.Robertson CS, Martin BA, Atkinson M. 1993. Varicella-zoster virus DNA in the oesophageal myenteric plexus in achalasia. Gut 34:299–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stratman E. 2002. Visceral zoster as the presenting feature of disseminated herpes zoster. J. Am. Acad. Dermatol. 46:771–774 [DOI] [PubMed] [Google Scholar]
- 134.Ugras M, Vitrinel A, Yilmaz G, Midilli K, Ozkan F. 2013. Varicella gastritis in an immunocompetent child. J. Clin. Virol. 56:153–155 [DOI] [PubMed] [Google Scholar]
- 135.Ussery XT, Annunziato P, Gershon A, Reid B, Lungu O, Langston C, Silverstein S, Lee K, Baker CJ. 1998. Congenital varicella-zoster infection and Barrett's esophagus. J. Infect. Dis. 178:539–543 [DOI] [PubMed] [Google Scholar]
- 136.Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T, Isomura S. 1974. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet ii:1288–1290 [DOI] [PubMed] [Google Scholar]
- 137.Baxter R, Ray P, Tran TN, Black S, Shinefield HR, Coplan PM, Lewis E, Fireman B, Saddier P. 2013. Long-term effectiveness of varicella vaccine: a 14-year, prospective cohort study. Pediatrics 131:1389–1396 [DOI] [PubMed] [Google Scholar]
- 138.Daly ER, Anderson L, Dreisig J, Dionne-Odom J. 27 March 2013. Decrease in varicella incidence following implementation of the 2-dose recommendation for varicella vaccine in New Hampshire. Pediatr. Infect. Dis. J. 10.1097/INF.0b013e318293308e [DOI] [PubMed] [Google Scholar]
- 139.Gershon AA, Gershon MD. 2010. Perspectives on vaccines against varicella-zoster virus infections. Curr. Top. Microbiol. Immunol. 342:359–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hambleton S, Steinberg SP, Larussa PS, Shapiro ED, Gershon AA. 2008. Risk of herpes zoster in adults immunized with varicella vaccine. J. Infect. Dis. 197(Suppl. 2):S196–S199 [DOI] [PubMed] [Google Scholar]
- 141.Kattan JA, Sosa LE, Bohnwagner HD, Hadler JL. 2011. Impact of 2-dose vaccination on varicella epidemiology: Connecticut—2005-2008. J. Infect. Dis. 203:509–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marin M, Zhang JX, Seward JF. 2011. Near elimination of varicella deaths in the US after implementation of the vaccination program. Pediatrics 128:214–220 [DOI] [PubMed] [Google Scholar]
- 143.Shapiro ED, Vazquez M, Esposito D, Holabird N, Steinberg SP, Dziura J, Larussa PS, Gershon AA. 2011. Effectiveness of 2 doses of varicella vaccine in children. J. Infect. Dis. 203:312–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Centers for Disease Control and Prevention 1996. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal Wkly. Rep. 45:1–36 [PubMed] [Google Scholar]
- 145.Mitka M. 2006. FDA approves shingles vaccine: herpes zoster vaccine targets older adults. JAMA 296:157–158 [DOI] [PubMed] [Google Scholar]
- 146.Centers for Disease Control Prevention 2007. Prevention of varicella. MMWR Morb. Mortal. Wkly. Rep. 56:1–5517218934 [Google Scholar]
- 147.Vazquez M, LaRussa PS, Gershon AA, Niccolai LM, Muehlenbein CE, Steinberg SP, Shapiro ED. 2004. Effectiveness over time of varicella vaccine. JAMA 291:851–855 [DOI] [PubMed] [Google Scholar]
- 148.Chaves SS, Gargiullo P, Zhang JX, Civen R, Guris D, Mascola L, Seward JF. 2007. Loss of vaccine-induced immunity to varicella over time. N. Engl. J. Med. 356:1121–1129 [DOI] [PubMed] [Google Scholar]
- 149.Gershon AA, Arvin AM, Shapiro E. 2007. Varicella vaccine. N. Engl. J. Med. 356:2648–2649 [DOI] [PubMed] [Google Scholar]
- 150.Son M, Shapiro ED, LaRussa P, Neu N, Michalik DE, Meglin M, Jurgrau A, Bitar W, Vasquez M, Flynn P, Gershon AA. 2010. Effectiveness of varicella vaccine in children infected with HIV. J. Infect. Dis. 201:1806–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tseng HF, Smith N, Marcy SM, Sy LS, Jacobsen SJ. 2009. Incidence of herpes zoster among children vaccinated with varicella vaccine in a prepaid health care plan in the United States, 2002–2008. Pediatr. Infect. Dis. J. 28:1069–1072 [DOI] [PubMed] [Google Scholar]
- 152.Moanna A, Rimland D. 2013. Decreasing incidence of herpes zoster in the highly active antiretroviral therapy era. Clin. Infect. Dis. 57:122–125 [DOI] [PubMed] [Google Scholar]
- 153.Marin M, Meissner HC, Seward JF. 2008. Varicella prevention in the United States: a review of successes and challenges. Pediatrics 122:e744–751 [DOI] [PubMed] [Google Scholar]
- 154.Chaves SS, Haber P, Walton K, Wise RP, Izurieta HS, Schmid DS, Seward JF. 2008. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995-2005. J. Infect. Dis. 197(Suppl 2):S170–S177 [DOI] [PubMed] [Google Scholar]
- 155.Gershon AA, LaRussa P, Steinberg S. 1991. Live attenuated varicella vaccine: current status and future uses. Semin. Pediatr. Infect. Dis. 2:171–177 [Google Scholar]
- 156.Gershon AA, Steinberg S, LaRussa P, Hammerschlag M, Ferrara A, NIAID Collaborative Varicella Vaccine Study Group. 1988. Immunization of healthy adults with live attenuated varicella vaccine. J. Infect. Dis. 158:132–137 [DOI] [PubMed] [Google Scholar]
- 157.Gershon AA. 2003. Varicella vaccine: rare serious problems—but the benefits still outweigh the risks. J. Infect. Dis. 188:945–947 [DOI] [PubMed] [Google Scholar]
- 158.Bryan CJ, Prichard MN, Daily S, Jefferson G, Hartline C, Cassady KA, Hilliard L, Shimamura M. 2008. Acyclovir-resistant chronic verrucous vaccine strain varicella in a patient with neuroblastoma. Pediatr. Infect. Dis. J. 27:946–948 [DOI] [PubMed] [Google Scholar]
- 159.Levin MJ, Dahl KM, Weinberg A, Giller R, Patel A. 2003. Development of resistance to acyclovir during chronic Oka strain varicella-zoster virus infection in an immunocompromised child. J. Infect. Dis. 188:954–959 [DOI] [PubMed] [Google Scholar]
- 160.Schrauder A, Henke-Gendo C, Seidemann K, Sasse M, Cario G, Moericke A, Schrappe M, Heim A, Wessel A. 2007. Varicella vaccination in a child with acute lymphoblastic leukaemia. Lancet 369:1232. [DOI] [PubMed] [Google Scholar]
- 161.Centers for Disease Control Prevention 2007. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 56:1–4017218934 [Google Scholar]
- 162.Kluthe M, Herrera A, Blanca H, Leung J, Bialek SR, Schmid DS. 2012. Neonatal vaccine-strain varicella-zoster virus infection 22 days after maternal postpartum vaccination. Pediatr. Infect. Dis. J. 31:977–979 [DOI] [PubMed] [Google Scholar]
- 163.Chouliaras G, Spoulou V, Quinlivan M, Breuer J, Theodoridou M. 2010. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child. Pediatrics 125:e969–972 [DOI] [PubMed] [Google Scholar]
- 164.Goulleret N, Mauvisseau E, Essevaz-Roulet M, Quinlivan M, Breuer J. 2010. Safety profile of live varicella virus vaccine (Oka/Merck): five-year results of the European Varicella Zoster Virus Identification Program (EU VZVIP). Vaccine 28:5878–5882 [DOI] [PubMed] [Google Scholar]
- 165.Han JY, Hanson DC, Way SS. 2011. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr. Infect. Dis. J. 30:266–268 [DOI] [PubMed] [Google Scholar]
- 166.Iyer S, Mittal MK, Hodinka RL. 2009. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann. Emerg. Med. 53:792–795 [DOI] [PubMed] [Google Scholar]
- 167.Levin MJ, DeBiasi RL, Bostik V, Schmid DS. 2008. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J. Infect. Dis. 198:1444–1447 [DOI] [PubMed] [Google Scholar]
- 168.De La Blanchardiere A, Rozenberg F, Caumes E, Picard O, Lionnet F, Livartowski J, Coste J, Sicard D, Lebon P, Salmon-Ceron D. 2000. Neurological complications of varicella-zoster virus infection in adults with human immunodeficiency virus infection. Scand. J. Infect. Dis. 32:263–269 [DOI] [PubMed] [Google Scholar]
- 169.Dueland AN, Devlin M, Martin JR, Mahalingam R, Cohrs R, Manz H, Trombley I, Gilden D. 1991. Fatal varicella-zoster virus meningoradiculitis without skin involvement. Ann. Neurol. 29:569–572 [DOI] [PubMed] [Google Scholar]
- 170.Spiegel R, Miron D, Lumelsky D, Horovitz Y. 2010. Severe meningoencephalitis due to late reactivation of Varicella-Zoster virus in an immunocompetent child. J. Child Neurol. 25:87–90 [DOI] [PubMed] [Google Scholar]
- 171.Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, Caulfield MJ, Irwin MR, Smith JG, Clair J, Chan IS, Williams H, Harbecke R, Marchese R, Straus SE, Gershon A, Weinberg A. 2008. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J. Infect. Dis. 197:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schmader KE, Levin MJ, Gnann JW, Jr, McNeil SA, Vesikari T, Betts RF, Keay S, Stek JE, Bundick ND, Su SC, Zhao Y, Li X, Chan IS, Annunziato PW, Parrino J. 2012. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin. Infect. Dis. 54:922–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Leroux-Roels I, Leroux-Roels G, Clement F, Vandepapeliere P, Vassilev V, Ledent E, Heineman TC. 2012. A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein E subunit vaccine candidate in young and older adults. J. Infect. Dis. 206:1280–1290 [DOI] [PubMed] [Google Scholar]
- 174.Crumpacker C. 2011. Absence of exposure to varicella does not increase the risk of zoster. Clin. Infect. Dis. 53:412–413 [DOI] [PubMed] [Google Scholar]
- 175.Goldman GS, King PG. 2013. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine 31:1680–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Brisson M, Gay N, WJdo Andrews NJ. 2002. Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine 20:2500–2507 [DOI] [PubMed] [Google Scholar]
- 177.Thomas S, Wheeler J, Hall AJ. 2002. Contacts with varicella or with children and protection against herpes zoster in adults: a case-control study. Lancet 360:678–682 [DOI] [PubMed] [Google Scholar]
- 178.Gaillat J, Gajdos V, Launay O, Malvy D, Demoures B, Lewden L, Pinchinat S, Derrough T, Sana C, Caulin E, Soubeyrand B. 2011. Does monastic life predispose to the risk of Saint Anthony's Fire (herpes zoster)? Clin. Infect. Dis. 53:405. [DOI] [PubMed] [Google Scholar]
- 179.Donahue JG, Choo PW, Manson JE, Platt R. 1995. The incidence of herpes zoster. Arch. Intern. Med. 155:1605–1609 [PubMed] [Google Scholar]
- 180.Ragozzino ME, Melton LF, Kurland L, Chu CP, Perry HO. 1982. Population-based study of herpes zoster and its sequelae. Medicine 61:310–316 [DOI] [PubMed] [Google Scholar]
- 181.Schmid DS, Jumaan AO. 2010. Impact of varicella vaccine on varicella-zoster virus dynamics. Clin. Microbiol. Rev. 23:202–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hope-Simpson RE. 1965. The nature of herpes zoster: a long term study and a new hypothesis. Proc. R. Soc. Med. 58:9–20 [PMC free article] [PubMed] [Google Scholar]
- 183.Luby J, Ramirez-Ronda C, Rinner S, Hull A, Vergne-Marini P. 1977. A longitudinal study of varicella zoster virus infections in renal transplant recipients. J. Infect. Dis. 135:659–663 [DOI] [PubMed] [Google Scholar]
- 184.Birlea M, Arendt G, Orhan E, Schmid DS, Bellini WJ, Schmidt C, Gilden D, Cohrs RJ. 2011. Subclinical reactivation of varicella zoster virus in all stages of HIV infection. J. Neurol. Sci. 304:22–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cinque P, Bossolasco S, Vago L, Fornara C, Lipari S, Racca S, Lazzarin A, Linde A. 1997. Varicella-zoster virus (VZV) DNA in cerebrospinal fluid of patients infected with human immunodeficiency virus: VZV disease of the central nervous system or subclinical reactivation of VZV infection? Clin. Infect. Dis. 25:634–639 [DOI] [PubMed] [Google Scholar]
- 186.Ljungman P, Lonnqvist B, Gahrton G, Ringden O, Sundqvist V-A, Wahren B. 1986. Clinical and subclinical reactivations of varicella-zoster virus in immunocompromised patients. J. Infect. Dis. 153:840–847 [DOI] [PubMed] [Google Scholar]
- 187.Wilson A, Sharp M, Koropchak CM, Ting SF, Arvin AM. 1992. Subclinical varicella-zoster virus viremia, herpes zoster, and T lymphocyte immunity to varicella-zoster viral antigens after bone marrow transplantation. J. Infect. Dis. 165:119–126 [DOI] [PubMed] [Google Scholar]
- 188.Blumenthal DT, Shacham-Shmueli E, Bokstein F, Schmid DS, Cohrs RJ, Nagel MA, Mahalingam R, Gilden D. 2011. Zoster sine herpete: virologic verification by detection of anti-VZV IgG antibody in CSF. Neurology 76:484–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schmader K, Oxman M, Levin M, Johnson G, Zhang J, Betts R, Morrison V, Gelb L, Guatelli J, Harbecke R, Pachucki C, Keay S, Menzies B, Griffin M, Kauffman C, Marques A, Toney J, Keller P, Li X, Chan I, Annunziato P. 2012. Persistence of the efficacy of zoster vaccine in the Shingles Prevention Study and the Short-Term Persistence Substudy. Clin. Infect. Dis. 55:1320–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shah SS, Wood SM, Luan X, Ratner AJ. 2010. Decline in varicella-related ambulatory visits and hospitalizations in the United States since routine immunization against varicella. Pediatr. Infect. Dis. J. 29:199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]