Abstract
SUMMARY
In the last 10 years, extended-spectrum β-lactamase-producing enterobacteria (ESBL-E) have become one of the main challenges for antibiotic treatment of enterobacterial infections, largely because of the current CTX-M enzyme pandemic. However, most studies have focused on hospitalized patients, though today it appears that the community is strongly affected as well. We therefore decided to devote our investigation to trends in ESBL-E fecal carriage rates and comprehensively reviewed data from studies conducted on healthy populations in various parts of the world. We show that (i) community ESBL-E fecal carriage, which was unknown before the turn of the millennium, has since increased significantly everywhere, with developing countries being the most affected; (ii) intercontinental travel may have emphasized and globalized the issue; and (iii) CTX-M enzymes, especially CTX-M-15, are the dominant type of ESBL. Altogether, these results suggest that CTX-M carriage is evolving toward a global pandemic but is still insufficiently described. Only a better knowledge of its dynamics and biology will lead to further development of appropriate control measures.
INTRODUCTION
The first strains of extended-spectrum beta-lactamase-producing enterobacteria (ESBL-E) were reported at the beginning of the 1980s (1), shortly after the release of broad-spectrum cephalosporins for clinical use (2). In the 1980s and -90s, ESBL were produced mostly by Klebsiella spp. and Enterobacter spp. and were encoded by genes derived through mutations of the ubiquitous plasmid-borne blaTEM and blaSHV wild-type penicillinase genes (3, 4). These early ESBL-E were observed almost exclusively in hospitals, first in Europe and subsequently in other parts of the world (5), especially in intensive care units (ICU), where they sometimes generated large-scale outbreaks (6).
Twenty years later, in the late 1990s, soon after the patents of extended-spectrum cephalosporins fell into the public domain and generics were flourishing, community-acquired infections due to ESBL-E emerged, mainly as urinary tract infections (UTI) (7). Among the very wide variety of enzymes exhibiting ESBL activity (8), class A beta-lactamases have had particular epidemiological success. These enzymes hydrolyze penicillins, oxyimino-cephalosporins, and aztreonam to various degrees, but they spare carbapenems and cephamycins. The emergence of community-acquired ESBL-E infections was associated with two major epidemiological changes. First, unlike ESBL-E previously isolated in hospitals, the strains responsible for community-acquired infections were mostly strains of Escherichia coli, a species which is both a normal intestinal commensal in humans and a major pathogen (9). Second, they produced CTX-M enzymes, a group of ESBL that are highly divergent from TEM- and SHV-derived mutants, initially named because of their particular affinity for cefotaxime (10). blaCTX-M genes, most probably mobilized from the chromosomes of environmental bacteria belonging to various species of the Kluyvera genus (11), have repeatedly moved to plasmids well adapted to E. coli (12). Currently, 136 CTX-M alleles have been identified. They are divided into 5 groups according to their different progenitor species. This can be compared to the greater diversity associated with the 208 and 173 mutants of TEM and SHV, respectively (http://www.lahey.org/Studies/; accessed 12 April 2013). Strikingly, CTX-M enzymes have rapidly supplanted TEM- and SHV-derived ESBL, even in hospitals (13), although what has endowed them with such an obvious epidemiological advantage is not yet understood. What is also worrisome is that ESBL-E often show multiple coresistance (13), complicating first-line treatment of many frequent community infections, such as UTI (14). For severe ESBL-E infections, carbapenems have become the drugs of choice (15), which is cumbersome because these antibiotics are for parenteral use only and thus are difficult to administer and often unavailable in low-resource countries, where the incidence of ESBL-E infections is particularly high (16).
The digestive tract is the main reservoir from which enterobacteria originate, whatever the type (community or hospital acquired) of infection (17, 18). It is also a melting pot where exchanges of resistance genes occur and antibiotic treatments select for the overgrowth of resistant bacteria (19). Fecal carriage of ESBL-E in the community was first reported in Spain and Poland, in 2001 and 2002, respectively (20, 21). Many other reports describing wide differences in carriage rates have since been published, suggesting dissimilarities in the levels and dynamics of ESBL-E epidemiology between geographic areas. However, as far as we know, the literature on ESBL-E community carriage rates has never been reviewed comprehensively, making it difficult to compose a global picture.
In this work, studies published in the English and French literature were grouped together according to World Health Organization (WHO) geographical areas (http://www.who.int/healthinfo/global_burden_disease/definition_regions/en/index.html; accessed 18 December 2012), and temporal trends were analyzed according to the year of sampling.
GLOBAL DISSEMINATION AND DISTRIBUTION
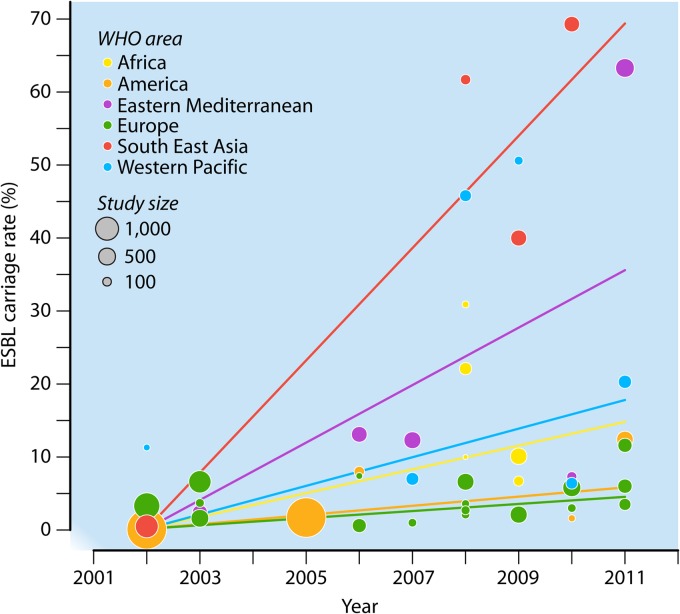
In all areas, the reported rates of ESBL-E community carriage were almost always under 10% before 2008 but often higher afterwards (Fig. 1). In 2008, the carriage rate skyrocketed to over 60% for the first time, in Thailand (22).
Fig 1.
ESBL carriage rates in the community, according to their geographical and temporal distribution. Each bubble area is proportional to the size of the corresponding study. The lines represent the evolution of ESBL-E carriage rates over time for each geographical area, as established by a weighted linear regression model using the values reported in the literature from 2002 to 2011. Over this period, ESBL-E carriage increased significantly in all regions, with differences within regions. In Europe, the ESBL-E carriage rate increased significantly by 0.5% per year from 2002 to 2011 (95% confidence interval [95% CI] = 0.04% to 0.90%; P = 0.03). Compared to the rise in Europe, the progression rate was not different in Africa (difference in annual progression compared to that in Europe, +1.1% [95% CI = −0.4% to 2.7%]; P = 0.1) or America (+0.1% [95% CI = −0.6% to 0.9%]; P = 0.7) but was significantly higher in Southeast Asia (+7.2% [95% CI = 5.1% to 9.2%]; P < 10−7), the Eastern Mediterranean region (+3.5% [95% CI = 2.0% to 4.9%]; P < 10−4), and the Western Pacific region (+1.5% [95% CI = 0.04% to 2.90%]; P = 0.04). The differences in rate increases between Southeast Asia, the Eastern Mediterranean region, and the Western Pacific region were all significant.
Although increasing over time everywhere, carriage of ESBL-E did not evolve with the same dynamics (Fig. 1). Large intra- and interregional variations have been observed. Reports from the Western Pacific, Eastern Mediterranean, and Southeast Asia regions showed the highest carriage rates and the most striking recent ascending trends. In contrast, rates reported in Europe never exceeded 10%, with the exception of a recent report of 11.6% observed in 2011 among patients upon admission to a geriatric unit in Belgium (23).
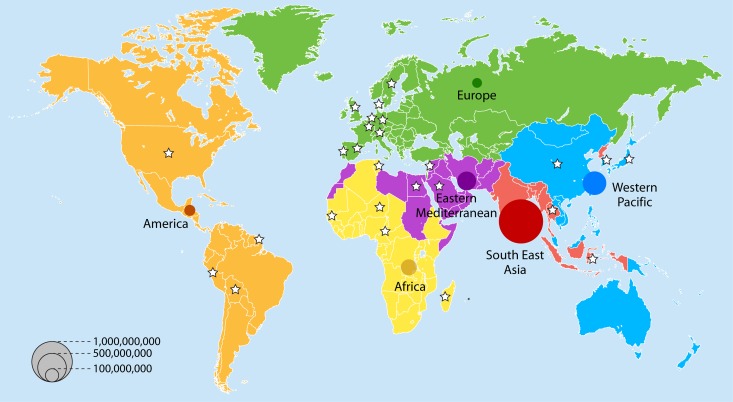
Because the countries where these data were obtained have populations that differ considerably, the data do not adequately reflect the magnitude of the problem, i.e., the number of carriers worldwide. Figure 2 shows the number of ESBL-E carriers estimated for 2010, according to the data analyzed in this review and the WHO 2010 population census (http://www.who.int/research; accessed 18 December 2012). Strikingly, over 1.1 billion ESBL-E carriers appear to be present in the community populations of Southeast Asia. The Western Pacific and Eastern Mediterranean regions rank second and third, with 280 and 180 million carriers, respectively, ahead of Africa, where 110 million carriers are estimated to be present. America and Europe appear to be far behind, with 48 and 35 million carriers, respectively (Fig. 2). This ranking suggests that poor access to drinking water, poverty, and a high population density are extremely efficient driving forces for ESBL-E dissemination, as is the case for any fecally-orally transmitted diseases. Indeed, the role of water pollution as a major reservoir for ESBL-E dissemination has been well documented. This has been the case not only for wastewater in China (24), the Czech Republic (25), Austria (26), India (27), Brazil (28), and Congo (29) but also for many rivers or aquatic ecosystems. ESBL-E have indeed been isolated from well water in Nicaragua (30) and from diverse other aquatic environments in Switzerland (31), the United Kingdom (32), China (33), South Korea (34), Portugal (35), and Tunisia (36). Even seawater from beaches in Algeria (37) and water from the Antarctic have been found to be positive for ESBL-E (38), suggesting that the current reservoir of these bacteria is in reality massive (Fig. 3). Human activities such as those associated with farming and food chain production may be at the root of ESBL-E dissemination, as recently reviewed (39) (Fig. 3). Surprisingly, the rates of colonization in Switzerland in 2012 were as high as 15% in pigs and 63% in chickens (40), despite the rather strict antibiotic policy in that country (41). E. coli was the predominant colonizing species, and CTX-M enzymes were the most frequent ESBL. The spread of ESBL strains from animals to humans via the food industry is now strongly suggested by genetic comparisons of strains from both settings (42, 43). Finally, pets are also involved, as suggested by reports from Portugal on healthy animal carriers (44) and by reports on infected animals in the United States (45), China (46), and Switzerland (47). Transmission from pets to humans is suggested when the genetic backgrounds of the strains and CTX-M alleles are compared (48, 49), but a human-to-pet route of transmission has also been proposed (50) (Fig. 3). In addition, the dissemination of some strains may be restricted to pets, as suggested by a recent study describing specific Klebsiella pneumoniae clones with their own genetic ESBL plasmids that have been isolated exclusively from companion animals (51).
Fig 2.
Number of ESBL carriers in the community in 2010, according to WHO region grouping. The 6 WHO regions are represented by different colors. WHO estimates of the population of each geographic area in 2010 (http://www.who.int/research; accessed 18 December 2012) were used to compute the number of ESBL-E carriers. ESBL carrier rates were established using the model presented in Fig. 1 for the year 2010. Stars represent countries with available data for modeling. Each bubble area is proportional to the estimated number of ESBL carriers in that region.
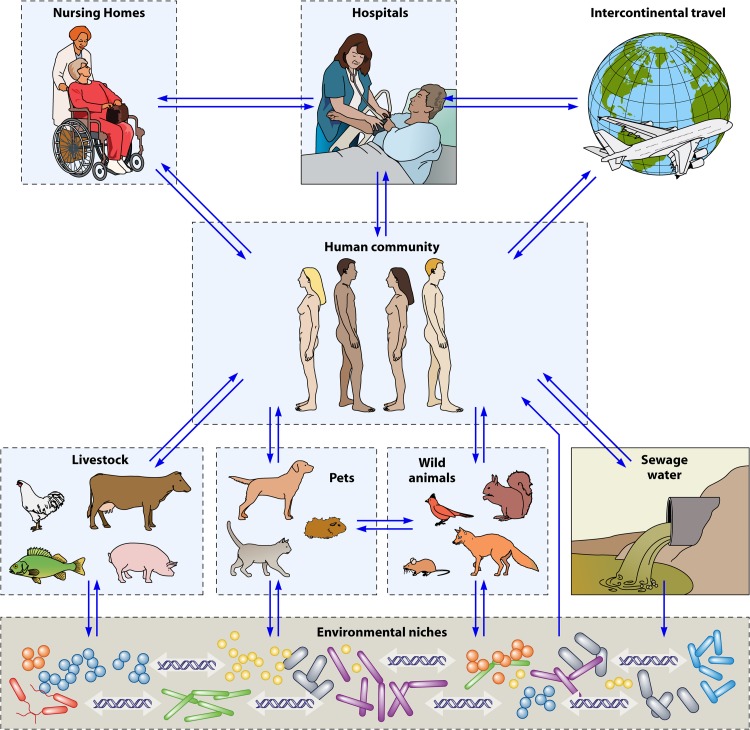
Fig 3.
Representation of the main digestive or environmental reservoirs of ESBL-E to which the worldwide human community belongs and is also exposed. Each independent reservoir is included in a dashed black outline, inside which cross-transmission may occur. Arrows show the flux of ESBL-E from one reservoir to another. Environmental niches comprise mainly water, soils, and plants, where genetic material exchanges between bacteria of digestive and/or environmental origin occur.
REGIONAL SPECIFICITIES
Europe
As mentioned previously, Europe is the area where community ESBL-E were first described, in 2001, in outpatients in Spain (21) and a cohort of healthy children in Poland (20). Fine characterization of ESBL types was not performed, but an association with E. coli was underlined in the Spanish study, which contrasted with the predominance of ESBL-producing K. pneumoniae strains in hospitals at that time. In the Polish study, MICs of cefotaxime for the strains were higher than those of ceftazidime, suggesting that the isolates were actually of the CTX-M type (52). Clearly, these features forecasted those that were going to be described later. Spanish teams were also the first to report the gradual rise in carriage rates in the community (53, 54) and to point out the role of the community as a reservoir possibly maintained by transfer from contaminated food (55, 56). In 2008, they reported the first evidence of dissemination of carriage between household members (57) and showed that contact with patients with ESBL-E UTI was a risk factor for carriage (58). Elsewhere in Europe, carriage rates were lower than those in Spain and remained below 5% (59–64). However, this may be changing, as suggested by recent studies performed in Switzerland and France (65, 66). Aging populations may also be at particular risk (23, 67). Differences in carriage rates have been reported between residents of the United Kingdom belonging to various ethnic groups (68), which might reflect differences in contacts with subjects from countries of high prevalence. The predominant allele appears to be CTX-M-15, as documented in France (67, 69), the United Kingdom (63), and Switzerland (65). However, the Spanish epidemiology seems to have specific characteristics, with a predominance of CTX-M-9 and CTX-M-14 alleles (Table 1). This could be linked to different migration trends.
Table 1.
Main results of the studies included in this work
| WHO areaa | Yr of studyb | Country | No. of individuals included | ESBL carriage rate (% [no. of ESBL carriers])c | % of carriers with CTX-M-type ESBL (no. of positive results/total no. of carriers)d | Species identified (no. with species/total no. of carriers)d,e,f | CTX-M allele(s) identified (no. of isolates with allele/total no. of carriers)d,g | Clonality rate (%) (detection method)d,h | ST identifiedd | Independent risk factor(s) identified (by multivariate model)d | Subjectse | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Europe | 1999 | France | 517 | 0 (0) | NA | NA | NA | NA | NP | NP | Army recruits | 61 |
| 2001–2002 | Spain | 1,321 | 3.3 (44) | 75 (33/44) | Ecol (42/44), Pmir, Eclo | 9 (18/33), 14, 15, 29, 1, 3, 34 | NP | NP | NP | Emergency patients | 56 | |
| 2003 | UK | 565 | 1.6 (9) | 100 (9/9) | Ecol (8/9), Salm spp. | 15 (4/9), 14, 9 | None (RAPD) | NP | NP | General practice patients | 63 | |
| 2003 | Spain | 108 | 3.7 (4) | 50 (2/4) | Ecol | 14, 2-like | None (PFGE) | NP | NP | Healthy individuals | 54 | |
| 2003 | Spain | 948 | 6.6 (63) | NP | Ecol (73/75), Kp, Ckos | NP | NP | NP | NP | Emergency patients | 55 | |
| 2005–2006 | Spain | 54 | 7.4 (4) | 50.0 (2/4) | Ecol | 14 | None (rep, PFGE) | NP | NP | Healthy individuals (controls of a case-control study) | 57 | |
| 2006 | France | 332 | 0.6 (2) | 0 (0/2) | NA | NA | NA | NA | NA | Healthy individuals | 62 | |
| 2007 | Spain | 105 | 6.7 (7) | 85.7 (6/7) | Ecol | 1 (2/6), 14a, 14b, 8, 32 | NP | NP | NP | Healthy individuals, only Ecol studied | 145 | |
| 2007–2008 | Portugal | 112 | 2.7 (3) | 33.3 (1/3) | Ecol | 1 | NP | NP | NP | Healthy children, only Ecol studied | 59 | |
| 2008 | Denmark | 84 | 3.6 (3) | 100 (3/3) | Ecol | 14a | 2/3 in 1 clone (ST1631) (MLST, PFGE) | 1631, 641 | None identified | Army recruits | 60 | |
| 2008 | Sweden | 96 | 2.1 (2) | 93.8 (15/16) | Ecol | 1 (11/15), 9 | None (rep, serotyping) | NP | None identified | Healthy individuals | 64 | |
| 2010 | Sweden | 100 | 3.0 (3) | Ecol | ||||||||
| 2008 | France | 500 | 6.6 (33) | 78.8 (26/33) | Ecol (21/26), Kp, Eclo | 15 (11/26), 1, 14, 65, 27 | 3/28 in 1 clone (rep) | NP | Previous carriage, antibiotic use during the last 3 months | Patients with first admission to hospital | 67 | |
| 2009 | France | 517 | 2.1 (11) | 90.1 (10/11) | Ecol (9/10), Kp | 15 (3/10), 61 (3/10), 2, 27, 14 | None (PFGE) | NP | NP | Army recruits | 61 | |
| 2010 | Switzerland | 586 | 5.8 (34) | 97.1 (33/34) | Ecol | 15 (14/33), 1, 14, 2 | 3*2 and 1*3 clones (serotyping) | NP | NP | Healthy individuals | 65 | |
| 2010–2011 | Belgium | 337 | 11.6 (39) | 71.8 (28/39) | Ecol (39/45), Kp, Ent spp., Cit spp. | Group 1 predominant | NP | NP | Multiple contacts with hospital over 1 year (long term) | Geriatric unit admission | 23 | |
| 2011 | Germany | 231 | 3.5 (8) | NP | Ecol | NP | NP | NP | Travel to Greece and Africa during the past 12 months, domestic animals | Participants in a symposium | 146 | |
| 2011 | France | 345 | 6 (21) | 86 (18/21) | Ecol | 15 (7/18), 1 (7/18), 14, 2 | 4/18 in 2 clones (361 and 131) (MLST, PFGE) | 156, 10, 167, 744, 34, 210, 216, 1125, 155, 95, 131, 141, 69, 120, 5211, 2439 | None | Healthy individuals | 69 | |
| Eastern Mediterranean | 2003 | Lebanon | 382 | 2.4 (9) | 100 (9/9) | Ecol | 15 | None (MLST) | NP | NP | Healthy students | 70 |
| 2006 | Saudi Arabia | 426 | 13.1 (56) | NP | Ecol (52/56), Kp | NP | NP | NP | NP | Healthy students, only Ecol and Kp were selected | 147 | |
| 2006–2007 | Saudi Arabia | 505 | 12.3 (62) | NP | Ecol (?), Kp | NP | NP | NP | NP | Healthy individuals | 148 | |
| 2009–2010 | Tunisia | 150 | 7.3 (11) | 91.0 (10/11) | Ecol | 1 | 3/10 in 1 clone (ST58) (PFGE, MLST) | 165, 57, 155, 58, 10, 48, 398, 219 | NP | Healthy individuals | 71 | |
| 2010–2011 | Egypt | 632 | 63.3 (400) | NP | Ecol (285/400), Kp, Ko, Ent spp. | NP | NP | NP | NP | Healthy individuals | 72 | |
| Africa | 2007–2008 | Niger | 55 | 30.9 (17) | 90.9 (20/22) | Ecol (13/20), Kp, Eclo, Easb | 15 | 3/17 in 1 Ecol clone (ST361) (rep, PFGE, MLST) | 361, 354, 5, 131, 10, 101, 68, 448, 196, 617, 410 | NP | Pediatric admission | 74 |
| 2008* | Senegal | 20 | 10.0 (2) | 100 (2/2) | Ecol | 15 | 100 (2/2) (rep) | NP | NP | Healthy children | 73 | |
| 2008 | Madagascar | 244 | 22.1 (54) | NP | Ecol (24/54), Kp (24/54), Eclo, other EB | NP | NP | NP | Hospitalization during the last 30 days | Pediatric admissions | 76 | |
| 2009 | Madagascar | 484 | 10.1 (49) | 94.2 (49/52) | Ecol (31/49), Kp, Eclo, Cf, Klu, Pan | 15 (46/49), 3, 1 | 8/53 in 3 Ecol clones (rep, ERIC) | NP | Unemployment | Healthy individuals | 75 | |
| 2009 | Cameroon | 150 | 6.7 (10) | 100 (10/10) | Ecol (9/10), Kp | 15 | NP | NP | None | Healthy students | 149 | |
| Southeast Asia | 2001–2002 | Indonesia | 1,998 | 0 (0/1,998) | NA | NA | NA | NA | NP | NP | Healthy individuals, only the dominant flora was studied | 77 |
| 2008 | Thailand | 160 | 61.7 (87) | 94.3 (82/87) | Ecol (73/82), Cit spp., Kp, Ent spp., Salm spp., other EB | Group 9 (71/82), group 1 | NP | NP | NP | Healthy individuals | 22 | |
| 2009 | Thailand | 445 | 40 (177) | 92.7 (164/177) | Ecol (149/164), Cit spp., other | Group 9 (127/164), group 1, group 8 | NP | NP | Antibiotic exposure (?) | Healthy individuals | 79 | |
| 2010 | Thailand | 417 | 69.3 (289) | 94.8 (274/289) | Ecol (234/274), Kp, Cit spp., Ent spp. | Group 9 (166/274), group 1, group 8 | NP | NP | Formal education, hospitalization over past year, antibiotic use during last 3 months | Healthy individuals | 78 | |
| Western Pacific region | 2002 | China | 53 | 11.3 (6) | 100 (6/6) | Kp (4/6), Ecol | 24 (2/6), 38 (2/6), 9, 14 | 4/6 in 2 clones (PFGE) | NP | NP | Healthy students | 150 |
| 2007* | China | 270 | 7.0 (19) | 100 (19/19) | Ecol | 14 (11/19), 22, 79, 24 | None (PFGE) | NP | Antibiotic use in past <3 months | Elderly people | 81 | |
| 2007–2008 | China | 225 | 45.8 (103) | 95.4 (104/109) | Ecol (88/109), Kp | 14 (65/104), 15, 3, 24, 27, 55, 57 | 15/109 in 6 clones (PFGE) | NP | Amount of living space per person | Pediatric admissions and household contacts | 98 | |
| 2009 | China | 109 | 50.6 (55) | 100 (55/55) | Ecol | 14 (39/55), 15, 55, 79, 3, 24, 27 | None (PFGE) | NP | NP | Healthy individuals | 80 | |
| 2009–2010 | Japan | 218 | 6.4 (14) | 92.9 (13/14) | Ecol (11/13), Kp | 14 (5/13), 2, 8, 3, 15 | NP | NP | None | Healthy individuals | 151 | |
| 2011 | Korea | 290 | 20.3 (59) | NP | Ecol (57/59), Kp | NP | NP | NP | NP | Healthy individuals | 152 | |
| The Americas | 2002 | Bolivia and Peru | 3,208 | 0.1 (4) | 100 (4/4) | Ecol | 2 (3/4), 15 | NP | NP | NP | Healthy children, only Ecol was selected | 83 |
| 2005 | Bolivia and Peru | 3,193 | 1.7 (50) | 88.0 (44/50) | Ecol | 2 (16/44), 15, 14, 24, 56 | 19/44 in 6 clones (RAPD) | NP | NP | Healthy children, only Ecol was selected | 153 | |
| 2006 | French Guiana | 163 | 8.0 (13) | 86.0 (12/14) | Ecol | 2 (11/15), 8 | 9/14 in 2 clones (rep) | NP | Overall antibiotic pressure (?) | Healthy individuals | 85 | |
| 2011 | Bolivia and Peru | 482 | 12.4 (60) | 96.7 (58/60) | Ecol | 15 (23/58), 65, 8, 14, 2, 3 | 25/58 in 9 clones (RAPD) | NP | Antibiotic use | Healthy children, only Ecol was selected | 84 | |
| Travelers | 2006–2008 | UK | 1,031 | 17.7# (182) | 100 (182/182) | Ecol | 15 (174/182), group 9, group 2 | 1 clone of 21 ST131 clones identified (MLST, PFGE) | Only ST131 strains were detected | NP | Travelers with diarrhea | 89 |
| 2007–2008 | Sweden | 101 (before) | 1.0 (1) | 100 (24/24) | Ecol | 15 (13/24), 14, 9, 27, 1 | NP | NP | Visiting India, gastroenteritis | Travelers (before versus after travel) | 91 | |
| 100 (after) | 24.0# (24) | |||||||||||
| 2008 | Sweden | 242 | 24.0# (58) | 90 (52/58) | Ecol | Group 1 (35/52), group 9 | None (rep) | NP | Travel, excluding Europe (Egypt, Thailand, India, Middle East, Southeast Asia) | Travelers with diarrhea | 90 | |
| 2009–2010 | USA | 60 (before) | 1.6 (1) | NP | NP | NP | NP | NA | NP | Travelers (before versus after travel) | 86 | |
| 28 (after) | 25.0 (7) | 71.4 (5/7) | Ecol | 14 (3/5), 15 | None (MLST) | 39, 8, 37, 399, 8, 437,83 | NP |
Studies were grouped together according to WHO geographical areas (http://www.who.int/healthinfo/global_burden_disease/definition_regions/en/index.html; accessed 18 December 2012).
*, studies in which the year of sampling was not specified. In those cases, the year indicated is the year before the year of publication.
#, the carriage rate value was not included in the statistical analysis.
NA, not applicable; NP, not performed.
Ecol, Escherichia coli; Salm, Salmonella; Pmir, Proteus mirabilis; Eclo, Enterobacter cloacae; Kp, Klebsiella pneumoniae; Ckos, Citrobacter koseri; Ent, Enterobacter; Cit, Citrobacter; Ko, Klebsiella oxytoca; EB, Enterobacteriaceae; Cf, Citrobacter freundii; Klu, Kluyvera; Pan, Pantoea; Easb, Enterobacter asburiae.
The dominant enterobacterial species is shown in bold. The dominant species is either the dominant species among CTX-M strains (when such data are known) or the dominant species among ESBL strains.
The dominant allele or allelic group is shown in bold.
RAPD, random amplification of polymorphic DNA; PFGE, pulsed-field gel electrophoresis; rep, repetitive element palindromic PCR; MLST, multilocus sequence typing; ERIC, enterobacterial repetitive intragenic consensus PCR.
Eastern Mediterranean
The first data from the Eastern Mediterranean region were published in 2005. They reported an ESBL carriage rate of 2.4% in young healthy students (70), pointing out the community nature of the pandemic early. Since then, carriage rates have ranged from 7.3% in Tunisia in 2010 (71) to 63.3% in Egypt in 2011 (72), reflecting the sharp increase and wide variations in carriage rates in this area. The very scarce CTX-M enzymes identified were of alleles 1 and 15 (Table 1).
Africa
Community carriage in Africa has been studied very poorly. Reported rates appear to be quite high, from 10.0% in Senegal (73) to 30.9% in Niger (74). Poor populations were found to be particularly affected in Madagascar in 2009 (75). Both there (76) and in Niger (74), it was shown that children were often carrying ESBL-E upon admission to hospital. Moreover, antibiotic use and hygiene failures in hospitals further dramatically increased transmission and dissemination among patients. In cases where they were identified, CTX-M enzymes were practically exclusively of allele 15 (Table 1).
Southeast Asia
The first report from Southeast Asia—from Java in 2001 to 2002—detected no community carriage (77). However, this study had technical limitations. Only predominant fecal enterobacteria were studied, and the subjects included were from a single area. Since then, data from rural Thailand have indicated very high rates, reaching 69.3% in 2010 (78). Interestingly, in another study from Thailand, there were significant variations in carriage rates between subjects from three separate provinces. These differences seemed to be linked to variations in overall antibiotic use between these populations but not to individual risk factors (79). The great majority of CTX-M alleles identified in Thailand belonged to group 9. However, data from Indonesia reported the presence of alleles 14 and 15 as early as 2001 to 2002 (Table 1). Data from other countries in the region, including India, are lacking.
Western Pacific
The number of studies available from the Western Pacific region is strikingly low considering the diversity and size of the populations living in this region. Wide differences are reported in two studies available from China, ranging from 7% in Shenyang in 2007 to 50% in Fujian in 2009 (80, 81). This underscores the magnitude of the variations that can be observed between areas and populations in such a vast country. Available data clearly indicate the predominance of the CTX-M-14 allele. Interestingly, CTX-M-15 ranks second in two recent studies from China, from 2007 and 2009, suggesting that this allele has now emerged there (Table 1).
The Americas
Carriage rates have been assessed repeatedly in poor children in urban areas in Latin America, which is of great interest. The carriage rate was as low as 0.1% in 2002 but jumped to 1.7% in 2005 and reached 12.4% in 2011 (82–84). Just as in the Thai study described above (79), no individual risk factor (including antibiotic intake) was associated with carriage (84). In contrast, this seemed to correlate with the overall exposure of the population to antibiotics, as observed in a remote community of Amerindians from French Guiana (85). Although the CTX-M-2 allele was predominant in early studies from South America, longitudinal data from Peru and Bolivia tend to demonstrate that CTX-M-15 is also emerging there, as in other regions (Table 1). Data from North America are virtually nonexistent, and to our knowledge, only the 24 U.S. cases explored in a study of travelers (86) could be considered community related, suggesting a 1.6% carriage rate (Table 1). This rate seems to be corroborated by the rather low hospital rates of ESBL carriage observed in neighboring Canada (87).
Travelers
Carriage rates in Europe are lower overall than those in other parts of the world, and carriage of resistant bacteria has been associated in the past with international travel (88). The suspicion thus emerged that travel of subjects from countries with low ESBL-E carriage rates to places with high ESBL-E carriage rates might be a source of colonization. Indeed, ESBL-E carriage rates in European subjects with traveler's diarrhea, upon return from diverse overseas areas, were 24% and 18% in 2006 and 2008, respectively (89, 90). These rates increased significantly after travel to Egypt, India, Southeast Asia, Thailand, and the Middle East (90). A prospective study of healthy subjects confirmed the high acquisition rates associated with travel to India, Asia, and the Middle East (91). Finally, in a prospective case-control study performed in 2008, European travelers had a 23% ESBL-E carriage rate, which was significantly more than the 4% found in nontravelers. Upon return from India, Africa, or Asia, the ESBL-E carriage rate reached 46% (92).
The duration of carriage after travel seems to be relatively short, lasting only a few weeks (93). This contrasts with the mean carriage duration of 6.6 months in patients who were colonized during hospitalization (94). However, carriage can be far more prolonged in travelers with diarrhea or exposed to antibiotics while abroad (93, 95) and in native African children arriving in Europe after adoption (96).
DISSEMINATION ROUTES, STRAIN DIVERSITY, AND TRANSMISSION
As mentioned above, dissemination within households was first demonstrated in a Spanish study, where carriage rates were found to be significantly higher among relatives of patients with ESBL-producing E. coli UTI than in nonrelative controls (23.8% versus 7.4%; P < 0.01) and possibly higher in household relatives than in nonhousehold ones (27.4% versus 15.6%; P = 0.1) (57). However, molecular characterization of the isolates showed that although there was a particular strain with a given ESBL allele disseminated between subjects in nearly half of the families, strains producing the same ESBL allele but with different genetic backgrounds were also found. This suggested to the authors that diverse modes of transmission of resistance were involved, with a possible major role of plasmids, as earlier suspected in hospital settings (97). Similar results were reported in another study, again from Spain, in which up to 66% of the isolates from patients with community-acquired ESBL-producing E. coli infections were indistinguishable from those isolated from fecal samples from their household members. Again, this suggested that patients with community infections and members of their households were a reservoir for ESBL-producing strains (58). In China, the carriage rates were higher in families with at least one individual with a history of out-of-town residence and were inversely correlated with living space (98). Finally, transmission of ESBL-E within the family does not seem to be limited to E. coli but may also occur for other enterobacteria, such as Salmonella (96).
Studies describing the genetic relatedness of ESBL-E isolates are limited, and different methods, including repetitive element palindromic PCR (rep-PCR), random amplification of polymorphic DNA (RAPD), and pulsed-field gel electrophoresis (PFGE), were used. It is clear, however, that diverse ESBL-producing E. coli clones are present in the community. No widely distributed clone appears to have emerged, in contrast with what has been described for infectious strains, where a pandemic clone, ST131, was pinpointed (66). The ST131 clone was, however, also sometimes found in carriers from Madagascar, India, and Pakistan (75, 89), and also in France (69) and Niger (74). Altogether, the general picture seems to be that each carrier has his/her own strain that disseminates exclusively in its immediate surroundings. This is in sharp contrast to what was observed in the 1980s and -90s, when TEM and SHV types of ESBL were prevalent and frequently caused clonal outbreaks in hospitalized patients, in whom the forces underpinning the dynamics of dissemination are different (6).
ENZYMES
In cases where they were analyzed, CTX-M alleles generally accounted for more than 90% of the ESBL-producing strains from community individuals (Table 1). Although there were some early studies which suggested that there was some degree of CTX-M allele specificity between regions, a trend toward the dominance of the CTX-M-15 allele appeared afterwards, except in the Western Pacific region, where CTX-M-14 continues to be the predominant allele (Table 1). It may be that specific biological properties are associated with this allele that could explain its propensity to disseminate, but this still remains to be elucidated fully.
HOW TO DEAL WITH CARRIERS UPON ADMISSION TO HOSPITAL
Despite the global extent of the pandemic, there are currently no precise guidelines about how to screen for and deal with ESBL-E carriers in hospitals (99). This is partially due to the paucity of studies on the efficacy of ESBL-E screening to ascertain the spread of hospital-acquired infections in nonoutbreak situations. Although this may be changing rapidly (100), the current situation is that recommendations regarding this issue are not homogeneous, even within a single geographic area (101). In hospitals, clonal outbreaks of CTX-M-producing E. coli have been described (102, 103) but are not frequent (104). This may be because the mode of dissemination of the current CTX-M-type ESBL genes is due more often to plasmids than to strain transmission and may go unnoticed and therefore underestimated (74). Currently, the type of unit to which the patient is admitted, the presence of risk factors, and/or the risk of environmental contamination is taken into account to define the best screening strategies.
In many hospitals, screening for ESBL-E carriage is systematic for ICU patients, and carriers are often administered carbapenems as empirical therapy for hospital-acquired infections. Another reason for systematic screening of ICU patients is that the likelihood of outbreaks is higher there than in other wards because of the very large number of medical procedures performed which promote indirect transmission of strains between patients (105). Although it has clearly been demonstrated that recent hospitalization or transfer, comorbidities, previous antibiotic treatment, urinary catheterization, and age are independently associated with ESBL-E carriage, the absence of any of these items does not guarantee the absence of ESBL-E carriage (106). Finally, patients likely to disseminate high loads of ESBL-producing strains in the environment (because of wounds, diarrhea, or secretions) should always be screened (107).
Once identified, ESBL-E carriers in most cases are isolated in single rooms, where contact precautions are recommended to prevent cross-transmission. However, this is applied unevenly (101), and its effectiveness is debated (108). Apart from when the clone is particularly virulent or the surrounding patients are at particular risk (109), cohorting does not appear to be applied anymore.
At this time, systematic screening for ESBL-E carriage upon admission to hospital is not recommended. Systematic screening is costly, and its effectiveness and that of associated policies of isolation have not been demonstrated (110). In addition, the spread of ESBL-producing strains seems to be species dependent. For example, Klebsiella pneumoniae tends to be cross-transmitted more frequently than E. coli (111). In the context of a likely future increase in ESBL-E carriage, it is a commonly accepted view that strict adherence to standard contact precautions (107) by all medical and nonmedical staff members will be the cornerstone of the control of ESBL dissemination in hospitals. However, the topic is the subject of intense debate (112) and research. Things might change when rapid carriage detection methods that can be used on a large scale become available.
HOW TO PREVENT AND REDUCE ESBL CARRIAGE IN COMMUNITY POPULATIONS
We badly lack adequate recommendations to prevent the emergence and spread of ESBL-E through fecal carriage in the community. Interventions could be targeted at several levels. First, one could try to reduce the circulation of resistant bacteria in the environment where they circulate. This would join with general efforts for better water sanitation, which is far beyond the scope of this review. It is notable that transmission of antimicrobial resistance is not currently listed in the Water Quality and Health Strategy 2013–2020 report from WHO (113). However, it has been shown that urban wastewater treatment plants are hot spots for antibiotic-resistant bacteria and genes spread into the environment (114). Hospital effluents may be a vehicle for ESBL-E (115). Methods to reduce resistant bacterial loads in wastewater and the amounts of antimicrobial agents, in most cases originated from hospitals and farms, include optimization of disinfection procedures and management of wastewater and manure. A policy for preventing mixing of human-originated and animal-originated bacteria with environmental organisms would certainly be advisable (116). However, no recommended method has yet proven sufficiently efficient, safe, and cheap to use on a large scale, particularly in developing countries. Note that antibacterials are not among the products currently listed by the EU Council directive on environmental quality standards in the field of water policy (117).
A second suggestion is to implement measures targeting patients. Since one of the major drivers of bacterial resistance is the accumulation of antibiotic residues in the colonic microbiota (118), the development of colon-targeted companion treatments has been proposed to destroy (119) or inhibit (120) these residues without exerting an impact on the systemic efficacy of antibiotic therapies. However, as promising as these approaches might appear, they have not been tested to prevent the emergence of ESBL-E and still have to prove their efficacy in large-scale clinical trials.
Lastly, the question of decontamination of colonized patients has been raised, mimicking what is done to prevent the dissemination of resistant bacteria and infection in intensive care patients by selective digestive decontamination (121). Although the initial results for carbapenemase carriers appeared promising (122), those obtained subsequently with ESBL-E carriers were disappointing, with short-lived ESBL-E elimination after the end of the procedure (123). Others have tried to use probiotics (124), but this was also a failure. Overall, it seems that far more research is warranted in the field before any practical solution can be proposed. Indeed, there will be a need to clarify the regulatory procedure before envisaging significant developments (125).
However, besides these promising current developments, the simplest hygienic behaviors should not be forgotten. Indeed, in many parts of the world, hand washing remains inadequate (126). Although the effects of promoting hand washing on ESBL-E spread have not been evaluated specifically, its benefits regarding the control of fecally-orally transmitted diseases are unequivocal (127–129). This is why hand washing appears to be a necessary step for the control of ESBL-E in the community. Moreover, since the costs associated with soap supplies are low, hand-washing education should be among public health priorities.
CONCLUSIONS
The main information gained by this review is that the ESBL enzyme pandemic emerged in the community in the early 2000s and has since increased regularly in all regions in a significant manner, sometimes dramatically, with carriage rates exceeding 50% after 2008 in part of Southeast Asia. Differences in the increases in carriage rates were highly significant between Europe, where they are currently around 10%, and less developed regions, where they are higher, explaining why travelers are at risk of becoming colonized while visiting countries abroad. From the beginning, CTX-M alleles accounted for the majority of cases, very often exceeding 90% (Table 1).
The biological characteristics of the colonizing strains may bring some light to bear on why and how resistance has disseminated so well. Although specific clones such as ST131 may play a role in the pandemic (66), the usual lack of clonal relatedness between strains from different carriers suggests that the predominant CTX-M genes are carried by genetic elements that are highly mobile between strains. In addition, CTX-M alleles, which were first found to be different between regions, tend to homogenize. Today, CTX-M-15 ranks first in most regions and is challenging CTX-M-14 in Southeast Asia and CTX-M-2 in South America. This review supports the contention that mobile genetic elements are the cornerstone of the current CTX-M pandemic, as recently reviewed (11). Plasmids have been involved in the intercontinental spread of CTX-M-15 (130), and community outbreaks may also result from strain-to-strain transmission of plasmids. The plausibility of this scenario is also supported by the reported association between CTX-M enzymes and E. coli IncF resident plasmids (12, 131), which have the ability to interchange between E. coli strains, to which they are particularly well adapted (132). Full sequencing of plasmids should shed further light on this matter in the near future.
This history of CTX-M enzyme dissemination shows parallels with the emergence of TEM-1, a well-known wild-type narrow-spectrum penicillinase which was first isolated in 1965 in Greece (133) and later spread through healthy populations worldwide (134–137). The rapid and wide dissemination of this gene in cattle as well as in food, pets, and environments (138–142) is also paralleled by recent observations made for CTX-M (143). The community certainly appears to be the major reservoir of CTX-M-type ESBL (Fig. 3). The presence of this type of enzyme in hospitalized patients is probably only a secondary consequence (144), but it can, however, give rise to subsequent nosocomial outbreaks (103).
A limitation of this analysis is the fact that there was no study for many areas, such as Eastern Europe, Australia, and North America. Also, the question may be posed as to whether the data analyzed are representative of the current trends in ESBL-E epidemiology. Indeed, only studies focusing on community settings were selected; therefore, some important information may have been excluded. In addition, our data analysis may be biased as a result of the diverse methods used by the authors who collected them. Overall, the definition of the healthy community population is not univocal, and medication, chronic diseases, and antibiotic exposure were sometimes considered exclusion criteria. Heterogeneous screening methods (antibiotic agent and concentration used in the selective media) and epidemiological designs (mainly cross-sectional studies, but also case-control studies or cohorts) were used as well. Nonetheless, our analysis probably provides the most relevant conclusions that can be drawn from current literature. They also show that apart from a reduction in antibiotic usage and promotion of hand washing, our means of action are very limited.
Altogether, our results show not only that CTX-M-type ESBL have spread to communities but also that carriage rates are on the rise. This is obviously a major public health concern, particularly in the regions where the rates are very high. The drivers of this catastrophic epidemiology are not fully understood. They may include (i) the genetic material bearing these enzymes, which appears to be extremely well adapted to their bacterial hosts; (ii) the predominance of E. coli, an intestinal commensal species widely distributed in both humans and animals, as a bacterial host; (iii) the vast dissemination of CTX-M-type ESBL strains in all kinds of environments; and (iv) the increase in selective pressure due to the multiple uses of extended-spectrum cephalosporins, which are now cheap and widely available as generics. In addition to strong policies to reduce antibiotic misuse in all parts of the world, detailed studies and applied research are urgently needed to determine the best countermeasures which can be implemented.
ACKNOWLEDGMENTS
We thank France Mentré for helpful discussions and Lorna Saint Ange for editing.
There are no reported conflicts of interest.
This work was supported in part by EU-FP7 R-Gnosis and EvoTAR programs and by OSEO-Nosobio.
Biographies

Paul-Louis Woerther, M.D., Ph.D., was a fellow at Bichat-Claude Bernard Hospital in Paris until 2011 and has worked on the in vivo emergence of antibiotic resistance. He is now a Microbiologist at the Gustave Roussy Institute, a comprehensive anticancer center located at the doorstep of Paris, France, where he is working in collaboration with clinicians on the diagnosis and treatment of multidrug-resistant infections. His main research interests include the molecular epidemiology of resistant enterobacterial carriage in both community and hospital settings. He is also involved in projects aimed at reducing antibiotic selective pressure in immunocompromised patients.

Charles Burdet, M.D., M.P.H., was trained in public health at the Paris 5-René Descartes University, France. He currently specializes in infectious diseases. Since 2011, he has been working at the Bichat-Claude Bernard Hospital (AP-HP, Paris, France), in collaboration with clinicians and microbiologists, with a particular focus on statistical modeling in infectious diseases. His main areas of interest include the pharmacokinetics of antimicrobial agents and the emergence of bacterial resistance. In 2014, he will join the Department of Infectious Diseases at the Bicêtre Hospital, Le Kremlin Bicêtre, where he will work as a fellow.

Elisabeth Chachaty obtained her Pharm.D. at the University of Nancy in 1991 and her Ph.D. at the University of Paris-South in 1993, as a microbiology specialist. Her main fields of research focus on resistance to antibiotics and the deleterious effects of antibiotic treatments on the human intestinal microflora. She is currently Head of the Laboratory of Microbiology at the Institut de Cancérologie Gustave Roussy, Villejuif, France, and specializes in the diagnosis and epidemiology of infections in immunocompromised patients.

Antoine Andremont obtained his M.D. at the University of Tours in 1976 and his Ph.D. at the University of Paris-South in 1986. Initially trained in pediatrics, microbiology, and tropical medicine in France, the United States, and Madagascar, he is currently a Full Professor of Microbiology at the University of Paris 7 Medical School and Head of the Bacteriology Laboratory of Bichat-Claude Bernard University Hospital in Paris (France). He specializes on the impact of antibiotics on the dynamics of bacterial resistance in the intestinal microbiota and has conducted numerous studies in this field in various parts of the world.
REFERENCES
- 1.Knothe H, Shah P, Krcmery V, Antal M, Mitsuhashi S. 1983. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 11:315–317 [DOI] [PubMed] [Google Scholar]
- 2.Jacoby GA, Medeiros AA. 1991. More extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 35:1697–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kliebe C, Nies BA, Meyer JF, Tolxdorff-Neutzling RM, Wiedemann B. 1985. Evolution of plasmid-coded resistance to broad-spectrum cephalosporins. Antimicrob. Agents Chemother. 28:302–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petit A, Gerbaud G, Sirot D, Courvalin P, Sirot J. 1990. Molecular epidemiology of TEM-3 (CTX-1) beta-lactamase. Antimicrob. Agents Chemother. 34:219–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Philippon A, Ben Redjeb S, Fournier G, Ben Hassen A. 1989. Epidemiology of extended spectrum beta-lactamases. Infection 17:347–354 [DOI] [PubMed] [Google Scholar]
- 6.de Champs C, Sirot D, Chanal C, Poupart MC, Dumas MP, Sirot J. 1991. Concomitant dissemination of three extended-spectrum beta-lactamases among different Enterobacteriaceae isolated in a French hospital. J. Antimicrob. Chemother. 27:441–457 [DOI] [PubMed] [Google Scholar]
- 7.Pitout JD, Nordmann P, Laupland KB, Poirel L. 2005. Emergence of Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs) in the community. J. Antimicrob. Chemother. 56:52–59 [DOI] [PubMed] [Google Scholar]
- 8.Bush K, Jacoby GA. 2010. Updated functional classification of beta-lactamases. Antimicrob. Agents Chemother. 54:969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tenaillon O, Skurnik D, Picard B, Denamur E. 2010. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 8:207–217 [DOI] [PubMed] [Google Scholar]
- 10.Bauernfeind A, Stemplinger I, Jungwirth R, Ernst S, Casellas JM. 1996. Sequences of beta-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequences with those of other beta-lactamases. Antimicrob. Agents Chemother. 40:509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canton R, Gonzalez-Alba JM, Galan JC. 2012. CTX-M enzymes: origin and diffusion. Front. Microbiol. 3:110. 10.3389/fmicb.2012.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcade G, Deschamps C, Boyd A, Gautier V, Picard B, Branger C, Denamur E, Arlet G. 2009. Replicon typing of plasmids in Escherichia coli producing extended-spectrum beta-lactamases. J. Antimicrob. Chemother. 63:67–71 [DOI] [PubMed] [Google Scholar]
- 13.Coque TM, Baquero F, Canton R. 2008. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill. 13:1–11 [PubMed] [Google Scholar]
- 14.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 52:e103–e120 [DOI] [PubMed] [Google Scholar]
- 15.Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. 2012. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum beta-lactamases: a systematic review and meta-analysis. J. Antimicrob. Chemother. 67:2793–2803 [DOI] [PubMed] [Google Scholar]
- 16.Randrianirina F, Vaillant L, Ramarokoto CE, Rakotoarijaona A, Andriamanarivo ML, Razafimahandry HC, Randrianomenjanahary J, Raveloson JR, Hariniana ER, Carod JF, Talarmin A, Richard V. 2010. Antimicrobial resistance in pathogens causing nosocomial infections in surgery and intensive care units of two hospitals in Antananarivo, Madagascar. J. Infect. Dev. Ctries. 4:74–82 [DOI] [PubMed] [Google Scholar]
- 17.Carlet J. 2012. The gut is the epicentre of antibiotic resistance. Antimicrob. Resist. Infect. Control 1:39. 10.1186/2047-2994-1-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donskey CJ. 2004. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin. Infect. Dis. 39:219–226 [DOI] [PubMed] [Google Scholar]
- 19.Duval-Iflah Y, Raibaud P, Tancrede C, Rousseau M. 1980. R-plasmic transfer from Serratia liquefaciens to Escherichia coli in vitro and in vivo in the digestive tract of gnotobiotic mice associated with human fecal flora. Infect. Immun. 28:981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franiczek R, Sobieszczanska B, Grabowski M, Mowszet K, Pytrus T. 2003. Occurrence of extended-spectrum beta-lactamases among Escherichia coli isolates from hospitalized and healthy children. Folia Microbiol. (Praha) 48:243–247 [DOI] [PubMed] [Google Scholar]
- 21.Mirelis B, Navarro F, Miro E, Mesa RJ, Coll P, Prats G. 2003. Community transmission of extended-spectrum beta-lactamase. Emerg. Infect. Dis. 9:1024–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasaki T, Hirai I, Niki M, Nakamura T, Komalamisra C, Maipanich W, Kusolsuk T, Sa-Nguankiat S, Pubampen S, Yamamoto Y. 2010. High prevalence of CTX-M beta-lactamase-producing Enterobacteriaceae in stool specimens obtained from healthy individuals in Thailand. J. Antimicrob. Chemother. 65:666–668 [DOI] [PubMed] [Google Scholar]
- 23.Schoevaerdts D, Verroken A, Huang TD, Frennet M, Berhin C, Jamart J, Bogaerts P, Swine C, Glupczynski Y. 2012. Multidrug-resistant bacteria colonization amongst patients newly admitted to a geriatric unit: a prospective cohort study. J. Infect. 65:109–118 [DOI] [PubMed] [Google Scholar]
- 24.Lu SY, Zhang YL, Geng SN, Li TY, Ye ZM, Zhang DS, Zou F, Zhou HW. 2010. High diversity of extended-spectrum beta-lactamase-producing bacteria in an urban river sediment habitat. Appl. Environ. Microbiol. 76:5972–5976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolejska M, Frolkova P, Florek M, Jamborova I, Purgertova M, Kutilova I, Cizek A, Guenther S, Literak I. 2011. CTX-M-15-producing Escherichia coli clone B2-O25b-ST131 and Klebsiella spp. isolates in municipal wastewater treatment plant effluents. J. Antimicrob. Chemother. 66:2784–2790 [DOI] [PubMed] [Google Scholar]
- 26.Reinthaler FF, Feierl G, Galler H, Haas D, Leitner E, Mascher F, Melkes A, Posch J, Winter I, Zarfel G, Marth E. 2010. ESBL-producing E. coli in Austrian sewage sludge. Water Res. 44:1981–1985 [DOI] [PubMed] [Google Scholar]
- 27.Diwan V, Chandran SP, Tamhankar AJ, Stalsby Lundborg C, Macaden R. 2012. Identification of extended-spectrum beta-lactamase and quinolone resistance genes in Escherichia coli isolated from hospital wastewater from central India. J. Antimicrob. Chemother. 67:857–859 [DOI] [PubMed] [Google Scholar]
- 28.Chagas TP, Seki LM, Cury JC, Oliveira JA, Davila AM, Silva DM, Asensi MD. 2011. Multiresistance, beta-lactamase-encoding genes and bacterial diversity in hospital wastewater in Rio de Janeiro, Brazil. J. Appl. Microbiol. 111:572–581 [DOI] [PubMed] [Google Scholar]
- 29.De Boeck H, Lunguya O, Muyembe JJ, Glupczynski Y, Jacobs J. 2012. Presence of extended-spectrum beta-lactamase-producing Enterobacteriaceae in waste waters, Kinshasa, the Democratic Republic of the Congo. Eur. J. Clin. Microbiol. Infect. Dis. 31:3085–3088 [DOI] [PubMed] [Google Scholar]
- 30.Amaya E, Reyes D, Paniagua M, Calderon S, Rashid MU, Colque P, Kuhn I, Mollby R, Weintraub A, Nord CE. 2012. Antibiotic resistance patterns of Escherichia coli isolates from different aquatic environmental sources in Leon, Nicaragua. Clin. Microbiol. Infect. 18:E347–E354 [DOI] [PubMed] [Google Scholar]
- 31.Zurfluh K, Hachler H, Nuesch-Inderbinen M, Stephan R. 2013. Characteristics of extended-spectrum beta-lactamase- and carbapenemase-producing Enterobacteriaceae isolates from rivers and lakes in Switzerland. Appl. Environ. Microbiol. 79:3021–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhanji H, Murphy NM, Akhigbe C, Doumith M, Hope R, Livermore DM, Woodford N. 2011. Isolation of fluoroquinolone-resistant O25b:H4-ST131 Escherichia coli with CTX-M-14 extended-spectrum beta-lactamase from UK river water. J. Antimicrob. Chemother. 66:512–516 [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Shu W, Chang X, Chen JA, Guo Y, Tan Y. 2010. The profile of antibiotics resistance and integrons of extended-spectrum beta-lactamase producing thermotolerant coliforms isolated from the Yangtze River basin in Chongqing. Environ. Pollut. 158:2459–2464 [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Kang HY, Lee Y. 2008. The identification of CTX-M-14, TEM-52, and CMY-1 enzymes in Escherichia coli isolated from the Han River in Korea. J. Microbiol. 46:478–481 [DOI] [PubMed] [Google Scholar]
- 35.Tacao M, Correia A, Henriques I. 2012. Resistance to broad-spectrum antibiotics in aquatic systems: anthropogenic activities modulate the dissemination of blaCTX-M-like genes. Appl. Environ. Microbiol. 78:4134–4140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chouchani C, Marrakchi R, Henriques I, Correia A. 2013. Occurrence of IMP-8, IMP-10, and IMP-13 metallo-beta-lactamases located on class 1 integrons and other extended-spectrum beta-lactamases in bacterial isolates from Tunisian rivers. Scand. J. Infect. Dis. 45:95–103 [DOI] [PubMed] [Google Scholar]
- 37.Alouache S, Kada M, Messai Y, Estepa V, Torres C, Bakour R. 2012. Antibiotic resistance and extended-spectrum beta-lactamases in isolated bacteria from seawater of Algiers beaches (Algeria). Microbes Environ. 27:80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez J, Stedt J, Bonnedahl J, Molin Y, Drobni M, Calisto-Ulloa N, Gomez-Fuentes C, Astorga-Espana MS, Gonzalez-Acuna D, Waldenstrom J, Blomqvist M, Olsen B. 2012. Human-associated extended-spectrum beta-lactamase in the Antarctic. Appl. Environ. Microbiol. 78:2056–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall BM, Levy SB. 2011. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 24:718–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geser N, Stephan R, Hachler H. 2012. Occurrence and characteristics of extended-spectrum beta-lactamase (ESBL) producing Enterobacteriaceae in food producing animals, minced meat and raw milk. BMC Vet. Res. 8:21. 10.1186/1746-6148-8-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filippini M, Masiero G, Moschetti K. 2006. Socioeconomic determinants of regional differences in outpatient antibiotic consumption: evidence from Switzerland. Health Policy 78:77–92 [DOI] [PubMed] [Google Scholar]
- 42.Kluytmans JA, Overdevest IT, Willemsen I, Kluytmans-van den Bergh MF, van der Zwaluw K, Heck M, Rijnsburger M, Vandenbroucke-Grauls CM, Savelkoul PH, Johnston BD, Gordon D, Johnson JR. 2013. Extended-spectrum beta-lactamase-producing Escherichia coli from retail chicken meat and humans: comparison of strains, plasmids, resistance genes, and virulence factors. Clin. Infect. Dis. 56:478–487 [DOI] [PubMed] [Google Scholar]
- 43.Leverstein-van Hall MA, Dierikx CM, Cohen Stuart J, Voets GM, van den Munckhof MP, van Essen-Zandbergen A, Platteel T, Fluit AC, van de Sande-Bruinsma N, Scharinga J, Bonten MJ, Mevius DJ. 2011. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin. Microbiol. Infect. 17:873–880 [DOI] [PubMed] [Google Scholar]
- 44.Costa D, Poeta P, Brinas L, Saenz Y, Rodrigues J, Torres C. 2004. Detection of CTX-M-1 and TEM-52 beta-lactamases in Escherichia coli strains from healthy pets in Portugal. J. Antimicrob. Chemother. 54:960–961 [DOI] [PubMed] [Google Scholar]
- 45.O'Keefe A, Hutton TA, Schifferli DM, Rankin SC. 2010. First detection of CTX-M and SHV extended-spectrum beta-lactamases in Escherichia coli urinary tract isolates from dogs and cats in the United States. Antimicrob. Agents Chemother. 54:3489–3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Y, Zeng Z, Chen S, Ma J, He L, Liu Y, Deng Y, Lei T, Zhao J, Liu JH. 2010. High prevalence of blaCTX-M extended-spectrum beta-lactamase genes in Escherichia coli isolates from pets and emergence of CTX-M-64 in China. Clin. Microbiol. Infect. 16:1475–1481 [DOI] [PubMed] [Google Scholar]
- 47.Huber H, Zweifel C, Wittenbrink MM, Stephan R. 2013. ESBL-producing uropathogenic Escherichia coli isolated from dogs and cats in Switzerland. Vet. Microbiol. 162:992–996 [DOI] [PubMed] [Google Scholar]
- 48.Ewers C, Grobbel M, Stamm I, Kopp PA, Diehl I, Semmler T, Fruth A, Beutlich J, Guerra B, Wieler LH, Guenther S. 2010. Emergence of human pandemic O25:H4-ST131 CTX-M-15 extended-spectrum-beta-lactamase-producing Escherichia coli among companion animals. J. Antimicrob. Chemother. 65:651–660 [DOI] [PubMed] [Google Scholar]
- 49.Platell JL, Cobbold RN, Johnson JR, Heisig A, Heisig P, Clabots C, Kuskowski MA, Trott DJ. 2011. Commonality among fluoroquinolone-resistant sequence type ST131 extraintestinal Escherichia coli isolates from humans and companion animals in Australia. Antimicrob. Agents Chemother. 55:3782–3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson JR, Miller S, Johnston B, Clabots C, Debroy C. 2009. Sharing of Escherichia coli sequence type ST131 and other multidrug-resistant and urovirulent E. coli strains among dogs and cats within a household. J. Clin. Microbiol. 47:3721–3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poirel L, Nordmann P, Ducroz S, Boulouis HJ, Arne P, Millemann Y. 2013. Extended-spectrum beta-lactamase CTX-M-15-producing Klebsiella pneumoniae of sequence type ST274 in companion animals. Antimicrob. Agents Chemother. 57:2372–2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tzouvelekis LS, Tzelepi E, Tassios PT, Legakis NJ. 2000. CTX-M-type beta-lactamases: an emerging group of extended-spectrum enzymes. Int. J. Antimicrob. Agents 14:137–142 [DOI] [PubMed] [Google Scholar]
- 53.Castillo Garcia FJ, Seral Garcia C, Pardos De la Gandara M, Millan Lou MI, Pitart Ferre C. 2007. Prevalence of fecal carriage of ESBL-producing Enterobacteriaceae in hospitalized and ambulatory patients during two non-outbreak periods. Eur. J. Clin. Microbiol. Infect. Dis. 26:77–78 [DOI] [PubMed] [Google Scholar]
- 54.Valverde A, Coque TM, Sanchez-Moreno MP, Rollan A, Baquero F, Canton R. 2004. Dramatic increase in prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae during nonoutbreak situations in Spain. J. Clin. Microbiol. 42:4769–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mesa RJ, Blanc V, Blanch AR, Cortes P, Gonzalez JJ, Lavilla S, Miro E, Muniesa M, Saco M, Tortola MT, Mirelis B, Coll P, Llagostera M, Prats G, Navarro F. 2006. Extended-spectrum beta-lactamase-producing Enterobacteriaceae in different environments (humans, food, animal farms and sewage). J. Antimicrob. Chemother. 58:211–215 [DOI] [PubMed] [Google Scholar]
- 56.Miro E, Mirelis B, Navarro F, Rivera A, Mesa RJ, Roig MC, Gomez L, Coll P. 2005. Surveillance of extended-spectrum beta-lactamases from clinical samples and faecal carriers in Barcelona, Spain. J. Antimicrob. Chemother. 56:1152–1155 [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez-Bano J, Lopez-Cerero L, Navarro MD, Diaz de Alba P, Pascual A. 2008. Faecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli: prevalence, risk factors and molecular epidemiology. J. Antimicrob. Chemother. 62:1142–1149 [DOI] [PubMed] [Google Scholar]
- 58.Valverde A, Grill F, Coque TM, Pintado V, Baquero F, Canton R, Cobo J. 2008. High rate of intestinal colonization with extended-spectrum-beta-lactamase-producing organisms in household contacts of infected community patients. J. Clin. Microbiol. 46:2796–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guimaraes B, Barreto A, Radhouani H, Figueiredo N, Gaspar E, Rodrigues J, Torres C, Igrejas G, Poeta P. 2009. Genetic detection of extended-spectrum beta-lactamase-containing Escherichia coli isolates and vancomycin-resistant enterococci in fecal samples of healthy children. Microb. Drug Resist. 15:211–216 [DOI] [PubMed] [Google Scholar]
- 60.Hammerum AM, Lester CH, Jakobsen L, Porsbo LJ. 2011. Faecal carriage of extended-spectrum beta-lactamase-producing and AmpC beta-lactamase-producing bacteria among Danish army recruits. Clin. Microbiol. Infect. 17:566–568 [DOI] [PubMed] [Google Scholar]
- 61.Janvier F, Merens A, Delaune D, Soler C, Cavallo JD. 2011. Fecal carriage of third-generation cephalosporins-resistant Enterobacteriaceae in asymptomatic young adults: evolution between 1999 and 2009. Pathol. Biol. (Paris) 59:97–101 [DOI] [PubMed] [Google Scholar]
- 62.Leflon-Guibout V, Blanco J, Amaqdouf K, Mora A, Guize L, Nicolas-Chanoine MH. 2008. Absence of CTX-M enzymes but high prevalence of clones, including clone ST131, among fecal Escherichia coli isolates from healthy subjects living in the area of Paris, France. J. Clin. Microbiol. 46:3900–3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Munday CJ, Whitehead GM, Todd NJ, Campbell M, Hawkey PM. 2004. Predominance and genetic diversity of community- and hospital-acquired CTX-M extended-spectrum beta-lactamases in York, UK. J. Antimicrob. Chemother. 54:628–633 [DOI] [PubMed] [Google Scholar]
- 64.Stromdahl H, Tham J, Melander E, Walder M, Edquist PJ, Odenholt I. 2011. Prevalence of faecal ESBL carriage in the community and in a hospital setting in a county of Southern Sweden. Eur. J. Clin. Microbiol. Infect. Dis. 30:1159–1162 [DOI] [PubMed] [Google Scholar]
- 65.Geser N, Stephan R, Korczak BM, Beutin L, Hachler H. 2012. Molecular identification of extended-spectrum-beta-lactamase genes from Enterobacteriaceae isolated from healthy human carriers in Switzerland. Antimicrob. Agents Chemother. 56:1609–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicolas-Chanoine MH, Blanco J, Leflon-Guibout V, Demarty R, Alonso MP, Canica MM, Park YJ, Lavigne JP, Pitout J, Johnson JR. 2008. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 61:273–281 [DOI] [PubMed] [Google Scholar]
- 67.Ruppe E, Pitsch A, Tubach F, de Lastours V, Chau F, Pasquet B, Lucet JC, Andremont A, Fantin B. 2012. Clinical predictive values of extended-spectrum beta-lactamase carriage in patients admitted to medical wards. Eur. J. Clin. Microbiol. Infect. Dis. 31:319–325 [DOI] [PubMed] [Google Scholar]
- 68.Wickramasinghe NH, Xu L, Eustace A, Shabir S, Saluja T, Hawkey PM. 2012. High community faecal carriage rates of CTX-M ESBL-producing Escherichia coli in a specific population group in Birmingham, UK. J. Antimicrob. Chemother. 67:1108–1113 [DOI] [PubMed] [Google Scholar]
- 69.Nicolas-Chanoine MH, Gruson C, Bialek-Davenet S, Bertrand X, Thomas-Jean F, Bert F, Moyat M, Meiller E, Marcon E, Danchin N, Noussair L, Moreau R, Leflon-Guibout V. 2013. 10-fold increase (2006–11) in the rate of healthy subjects with extended-spectrum beta-lactamase-producing Escherichia coli faecal carriage in a Parisian check-up centre. J. Antimicrob. Chemother. 68:562–568 [DOI] [PubMed] [Google Scholar]
- 70.Moubareck C, Daoud Z, Hakime NI, Hamze M, Mangeney N, Matta H, Mokhbat JE, Rohban R, Sarkis DK, Doucet-Populaire F. 2005. Countrywide spread of community- and hospital-acquired extended-spectrum beta-lactamase (CTX-M-15)-producing Enterobacteriaceae in Lebanon. J. Clin. Microbiol. 43:3309–3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ben Sallem R, Ben Slama K, Estepa V, Jouini A, Gharsa H, Klibi N, Saenz Y, Ruiz-Larrea F, Boudabous A, Torres C. 2012. Prevalence and characterisation of extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli isolates in healthy volunteers in Tunisia. Eur. J. Clin. Microbiol. Infect. Dis. 31:1511–1516 [DOI] [PubMed] [Google Scholar]
- 72.Abdul Rahman EM, El-Sherif RH. 2011. High rates of intestinal colonization with extended-spectrum lactamase-producing Enterobacteriaceae among healthy individuals. J. Invest. Med. 59:1284–1286 [DOI] [PubMed] [Google Scholar]
- 73.Ruppe E, Woerther PL, Diop A, Sene AM, Da Costa A, Arlet G, Andremont A, Rouveix B. 2009. Carriage of CTX-M-15-producing Escherichia coli isolates among children living in a remote village in Senegal. Antimicrob. Agents Chemother. 53:3135–3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woerther PL, Angebault C, Jacquier H, Hugede HC, Janssens AC, Sayadi S, El Mniai A, Armand-Lefevre L, Ruppe E, Barbier F, Raskine L, Page AL, de Rekeneire N, Andremont A. 2011. Massive increase, spread, and exchange of extended spectrum beta-lactamase-encoding genes among intestinal Enterobacteriaceae in hospitalized children with severe acute malnutrition in Niger. Clin. Infect. Dis. 53:677–685 [DOI] [PubMed] [Google Scholar]
- 75.Herindrainy P, Randrianirina F, Ratovoson R, Ratsima Hariniana E, Buisson Y, Genel N, Decre D, Arlet G, Talarmin A, Richard V. 2011. Rectal carriage of extended-spectrum beta-lactamase-producing gram-negative bacilli in community settings in Madagascar. PLoS One 6:e22738. 10.1371/journal.pone.0022738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andriatahina T, Randrianirina F, Hariniana ER, Talarmin A, Raobijaona H, Buisson Y, Richard V. 2010. High prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a pediatric unit in Madagascar. BMC Infect. Dis. 10:204. 10.1186/1471-2334-10-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Severin JA, Lestari ES, Kloezen W, Lemmens-den Toom N, Mertaniasih NM, Kuntaman K, Purwanta M, Offra Duerink D, Hadi U, van Belkum A, Verbrugh HA, Goessens WH. 2012. Faecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae among humans in Java, Indonesia, in 2001–2002. Trop. Med. Int. Health 17:455–461 [DOI] [PubMed] [Google Scholar]
- 78.Luvsansharav UO, Hirai I, Nakata A, Imura K, Yamauchi K, Niki M, Komalamisra C, Kusolsuk T, Yamamoto Y. 2012. Prevalence of and risk factors associated with faecal carriage of CTX-M beta-lactamase-producing Enterobacteriaceae in rural Thai communities. J. Antimicrob. Chemother. 67:1769–1774 [DOI] [PubMed] [Google Scholar]
- 79.Luvsansharav UO, Hirai I, Niki M, Sasaki T, Makimoto K, Komalamisra C, Maipanich W, Kusolsuk T, Sa-Nguankiat S, Pubampen S, Yamamoto Y. 2011. Analysis of risk factors for a high prevalence of extended-spectrum beta-lactamase-producing Enterobacteriaceae in asymptomatic individuals in rural Thailand. J. Med. Microbiol. 60:619–624 [DOI] [PubMed] [Google Scholar]
- 80.Li B, Sun JY, Liu QZ, Han LZ, Huang XH, Ni YX. 2011. High prevalence of CTX-M beta-lactamases in faecal Escherichia coli strains from healthy humans in Fuzhou, China. Scand. J. Infect. Dis. 43:170–174 [DOI] [PubMed] [Google Scholar]
- 81.Tian SF, Chen BY, Chu YZ, Wang S. 2008. Prevalence of rectal carriage of extended-spectrum beta-lactamase-producing Escherichia coli among elderly people in community settings in China. Can. J. Microbiol. 54:781–785 [DOI] [PubMed] [Google Scholar]
- 82.Pallecchi L, Bartoloni A, Fiorelli C, Mantella A, Di Maggio T, Gamboa H, Gotuzzo E, Kronvall G, Paradisi F, Rossolini GM. 2007. Rapid dissemination and diversity of CTX-M extended-spectrum beta-lactamase genes in commensal Escherichia coli isolates from healthy children from low-resource settings in Latin America. Antimicrob. Agents Chemother. 51:2720–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pallecchi L, Malossi M, Mantella A, Gotuzzo E, Trigoso C, Bartoloni A, Paradisi F, Kronvall G, Rossolini GM. 2004. Detection of CTX-M-type beta-lactamase genes in fecal Escherichia coli isolates from healthy children in Bolivia and Peru. Antimicrob. Agents Chemother. 48:4556–4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bartoloni A, Pallecchi L, Riccobono E, Mantella A, Magnelli D, Di Maggio T, Villagran AL, Lara Y, Saavedra C, Strohmeyer M, Bartalesi F, Trigoso C, Rossolini GM. 2013. Relentless increase of resistance to fluoroquinolones and expanded-spectrum cephalosporins in Escherichia coli: 20 years of surveillance in resource-limited settings from Latin America. Clin. Microbiol. Infect. 19:356–361 [DOI] [PubMed] [Google Scholar]
- 85.Woerther PL, Angebault C, Lescat M, Ruppe E, Skurnik D, Mniai AE, Clermont O, Jacquier H, Costa AD, Renard M, Bettinger RM, Epelboin L, Dupont C, Guillemot D, Rousset F, Arlet G, Denamur E, Djossou F, Andremont A. 2010. Emergence and dissemination of extended-spectrum beta-lactamase-producing Escherichia coli in the community: lessons from the study of a remote and controlled population. J. Infect. Dis. 202:515–523 [DOI] [PubMed] [Google Scholar]
- 86.Weisenberg SA, Mediavilla JR, Chen L, Alexander EL, Rhee KY, Kreiswirth BN, Jenkins SG. 2012. Extended spectrum beta-lactamase-producing Enterobacteriaceae in international travelers and non-travelers in New York City. PLoS One 7:e45141. 10.1371/journal.pone.0045141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simner PJ, Zhanel GG, Pitout J, Tailor F, McCracken M, Mulvey MR, Lagace-Wiens PR, Adam HJ, Hoban DJ. 2011. Prevalence and characterization of extended-spectrum beta-lactamase- and AmpC beta-lactamase-producing Escherichia coli: results of the CANWARD 2007–2009 study. Diagn. Microbiol. Infect. Dis. 69:326–334 [DOI] [PubMed] [Google Scholar]
- 88.Murray BE, Mathewson JJ, DuPont HL, Ericsson CD, Reves RR. 1990. Emergence of resistant fecal Escherichia coli in travelers not taking prophylactic antimicrobial agents. Antimicrob. Agents Chemother. 34:515–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dhanji H, Patel R, Wall R, Doumith M, Patel B, Hope R, Livermore DM, Woodford N. 2011. Variation in the genetic environments of blaCTX-M-15 in Escherichia coli from the faeces of travellers returning to the United Kingdom. J. Antimicrob. Chemother. 66:1005–1012 [DOI] [PubMed] [Google Scholar]
- 90.Tham J, Odenholt I, Walder M, Brolund A, Ahl J, Melander E. 2010. Extended-spectrum beta-lactamase-producing Escherichia coli in patients with travellers' diarrhoea. Scand. J. Infect. Dis. 42:275–280 [DOI] [PubMed] [Google Scholar]
- 91.Tangden T, Cars O, Melhus A, Lowdin E. 2010. Foreign travel is a major risk factor for colonization with Escherichia coli producing CTX-M-type extended-spectrum beta-lactamases: a prospective study with Swedish volunteers. Antimicrob. Agents Chemother. 54:3564–3568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peirano G, Laupland KB, Gregson DB, Pitout JD. 2011. Colonization of returning travelers with CTX-M-producing Escherichia coli. J. Travel Med. 18:299–303 [DOI] [PubMed] [Google Scholar]
- 93.Rogers BA, Kennedy KJ, Sidjabat HE, Jones M, Collignon P, Paterson DL. 2012. Prolonged carriage of resistant E. coli by returned travellers: clonality, risk factors and bacterial characteristics. Eur. J. Clin. Microbiol. Infect. Dis. 31:2413–2420 [DOI] [PubMed] [Google Scholar]
- 94.Birgand G, Armand-Lefevre L, Lolom I, Ruppe E, Andremont A, Lucet JC. 2013. Duration of colonization by extended-spectrum beta-lactamase-producing Enterobacteriaceae after hospital discharge. Am. J. Infect. Control 41:443–447 [DOI] [PubMed] [Google Scholar]
- 95.Tham J, Walder M, Melander E, Odenholt I. 2012. Duration of colonization with extended-spectrum beta-lactamase-producing Escherichia coli in patients with travellers' diarrhoea. Scand. J. Infect. Dis. 44:573–577 [DOI] [PubMed] [Google Scholar]
- 96.Tande D, Boisrame-Gastrin S, Munck MR, Hery-Arnaud G, Gouriou S, Jallot N, Nordmann P, Naas T. 2010. Intrafamilial transmission of extended-spectrum-beta-lactamase-producing Escherichia coli and Salmonella enterica Babelsberg among the families of internationally adopted children. J. Antimicrob. Chemother. 65:859–865 [DOI] [PubMed] [Google Scholar]
- 97.Baraniak A, Fiett J, Sulikowska A, Hryniewicz W, Gniadkowski M. 2002. Countrywide spread of CTX-M-3 extended-spectrum beta-lactamase-producing microorganisms of the family Enterobacteriaceae in Poland. Antimicrob. Agents Chemother. 46:151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lo WU, Ho PL, Chow KH, Lai EL, Yeung F, Chiu SS. 2010. Fecal carriage of CTX-M type extended-spectrum beta-lactamase-producing organisms by children and their household contacts. J. Infect. 60:286–292 [DOI] [PubMed] [Google Scholar]
- 99.Goddard S, Muller MP. 2011. The efficacy of infection control interventions in reducing the incidence of extended-spectrum beta-lactamase-producing Enterobacteriaceae in the nonoutbreak setting: a systematic review. Am. J. Infect. Control 39:599–601 [DOI] [PubMed] [Google Scholar]
- 100.Lowe CF, Katz K, McGeer AJ, Muller MP. 2013. Efficacy of admission screening for extended-spectrum beta-lactamase producing Enterobacteriaceae. PLoS One 8:e62678. 10.1371/journal.pone.0062678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lowe C, Katz K, McGeer A, Muller MP. 2012. Disparity in infection control practices for multidrug-resistant Enterobacteriaceae. Am. J. Infect. Control 40:836–839 [DOI] [PubMed] [Google Scholar]
- 102.Naseer U, Natas OB, Haldorsen BC, Bue B, Grundt H, Walsh TR, Sundsfjord A. 2007. Nosocomial outbreak of CTX-M-15-producing E. coli in Norway. APMIS 115:120–126 [DOI] [PubMed] [Google Scholar]
- 103.Oteo J, Cercenado E, Fernandez-Romero S, Saez D, Padilla B, Zamora E, Cuevas O, Bautista V, Campos J. 2012. Extended-spectrum-beta-lactamase-producing Escherichia coli as a cause of pediatric infections: report of a neonatal intensive care unit outbreak due to a CTX-M-14-producing strain. Antimicrob. Agents Chemother. 56:54–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harris AD, Kotetishvili M, Shurland S, Johnson JA, Morris JG, Nemoy LL, Johnson JK. 2007. How important is patient-to-patient transmission in extended-spectrum beta-lactamase Escherichia coli acquisition. Am. J. Infect. Control 35:97–101 [DOI] [PubMed] [Google Scholar]
- 105.Bou R, Aguilar A, Perpinan J, Ramos P, Peris M, Lorente L, Zuniga A. 2006. Nosocomial outbreak of Pseudomonas aeruginosa infections related to a flexible bronchoscope. J. Hosp. Infect. 64:129–135 [DOI] [PubMed] [Google Scholar]
- 106.Tumbarello M, Trecarichi EM, Bassetti M, De Rosa FG, Spanu T, Di Meco E, Losito AR, Parisini A, Pagani N, Cauda R. 2011. Identifying patients harboring extended-spectrum-beta-lactamase-producing Enterobacteriaceae on hospital admission: derivation and validation of a scoring system. Antimicrob. Agents Chemother. 55:3485–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007. 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am. J. Infect. Control 35:S65–S164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kirkland KB. 2009. Taking off the gloves: toward a less dogmatic approach to the use of contact isolation. Clin. Infect. Dis. 48:766–771 [DOI] [PubMed] [Google Scholar]
- 109.Cantey JB, Sreeramoju P, Jaleel M, Trevino S, Gander R, Hynan LS, Hill J, Brown C, Chung W, Siegel JD, Sanchez PJ. Prompt control of an outbreak caused by extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit. J. Pediatr., in press [DOI] [PubMed] [Google Scholar]
- 110.Conterno LO, Shymanski J, Ramotar K, Toye B, Zvonar R, Roth V. 2007. Impact and cost of infection control measures to reduce nosocomial transmission of extended-spectrum beta-lactamase-producing organisms in a non-outbreak setting. J. Hosp. Infect. 65:354–360 [DOI] [PubMed] [Google Scholar]
- 111.Shu JC, Chia JH, Kuo AJ, Su LH, Wu TL. 2010. A 7-year surveillance for ESBL-producing Escherichia coli and Klebsiella pneumoniae at a university hospital in Taiwan: the increase of CTX-M-15 in the ICU. Epidemiol. Infect. 138:253–263 [DOI] [PubMed] [Google Scholar]
- 112.Tschudin-Sutter S, Frei R, Dangel M, Stranden A, Widmer AF. 2012. Rate of transmission of extended-spectrum beta-lactamase-producing Enterobacteriaceae without contact isolation. Clin. Infect. Dis. 55:1505–1511 [DOI] [PubMed] [Google Scholar]
- 113.WHO 2013. Water quality and health strategy 2013–2020. WHO, Geneva, Switzerland: http://www.who.int/water_sanitation_health/publications/2013/water_quality_strategy.pdf Accessed 3 June 2013 [Google Scholar]
- 114.Rizzo L, Manaia C, Merlin C, Schwartz T, Dagot C, Ploy MC, Michael I, Fatta-Kassinos D. 2013. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: a review. Sci. Total Environ. 447:345–360 [DOI] [PubMed] [Google Scholar]
- 115.Prado T, Pereira WC, Silva DM, Seki LM, Carvalho AP, Asensi MD. 2008. Detection of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in effluents and sludge of a hospital sewage treatment plant. Lett. Appl. Microbiol. 46:136–141 [DOI] [PubMed] [Google Scholar]
- 116.Baquero F, Martinez JL, Canton R. 2008. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 19:260–265 [DOI] [PubMed] [Google Scholar]
- 117.European Parliament and the Council of the European Union 2008. Directive 2008/105/EC of the European Parliament and of the Council. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:348:0084:0097:EN:PDF Accessed 3 June 2013
- 118.Fantin B, Duval X, Massias L, Alavoine L, Chau F, Retout S, Andremont A, Mentre F. 2009. Ciprofloxacin dosage and emergence of resistance in human commensal bacteria. J. Infect. Dis. 200:390–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pitout JD. 2009. IPSAT P1A, a class A beta-lactamase therapy for the prevention of penicillin-induced disruption to the intestinal microflora. Curr. Opin. Invest. Drugs 10:838–844 [PubMed] [Google Scholar]
- 120.Khoder M, Tsapis N, Domergue-Dupont V, Gueutin C, Fattal E. 2010. Removal of residual colonic ciprofloxacin in the rat by activated charcoal entrapped within zinc-pectinate beads. Eur. J. Pharm. Sci. 41:281–288 [DOI] [PubMed] [Google Scholar]
- 121.Daneman N, Sarwar S, Fowler RA, Cuthbertson BH. 2013. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect. Dis. 13:328–341 [DOI] [PubMed] [Google Scholar]
- 122.Saidel-Odes L, Polachek H, Peled N, Riesenberg K, Schlaeffer F, Trabelsi Y, Eskira S, Yousef B, Smolykov R, Codish S, Borer A. 2012. A randomized, double-blind, placebo-controlled trial of selective digestive decontamination using oral gentamicin and oral polymyxin E for eradication of carbapenem-resistant Klebsiella pneumoniae carriage. Infect. Control Hosp. Epidemiol. 33:14–19 [DOI] [PubMed] [Google Scholar]
- 123.Huttner B, Haustein T, Uçkay I, Renzi G, Stewardson A, Schaerrer D, Agostinho A, Andremont A, Schrenzel J, Pittet D, Harbarth S. Decolonization of intestinal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae with oral colistin and neomycin: a randomized, double-blind, placebo-controlled trial. J. Antimicrob. Chemother., in press [DOI] [PubMed] [Google Scholar]
- 124.Tannock GW, Tiong IS, Priest P, Munro K, Taylor C, Richardson A, Schultz M. 2011. Testing probiotic strain Escherichia coli Nissle 1917 (Mutaflor) for its ability to reduce carriage of multidrug-resistant E. coli by elderly residents in long-term care facilities. J. Med. Microbiol. 60:366–370 [DOI] [PubMed] [Google Scholar]
- 125.Andremont A, Bonten M, Kluytmans J, Carmeli Y, Cars O, Harbarth S. 2011. Fighting bacterial resistance at the root: need for adapted EMEA guidelines. Lancet Infect. Dis. 11:6–8 [DOI] [PubMed] [Google Scholar]
- 126.Halder AK, Tronchet C, Akhter S, Bhuiya A, Johnston R, Luby SP. 2010. Observed hand cleanliness and other measures of handwashing behavior in rural Bangladesh. BMC Public Health 10:545. 10.1186/1471-2458-10-545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Luby SP, Agboatwalla M, Feikin DR, Painter J, Billhimer W, Altaf A, Hoekstra RM. 2005. Effect of handwashing on child health: a randomised controlled trial. Lancet 366:225–233 [DOI] [PubMed] [Google Scholar]
- 128.Luby SP, Agboatwalla M, Painter J, Altaf A, Billhimer WL, Hoekstra RM. 2004. Effect of intensive handwashing promotion on childhood diarrhea in high-risk communities in Pakistan: a randomized controlled trial. JAMA 291:2547–2554 [DOI] [PubMed] [Google Scholar]
- 129.Luby SP, Halder AK, Huda T, Unicomb L, Johnston RB. 2011. The effect of handwashing at recommended times with water alone and with soap on child diarrhea in rural Bangladesh: an observational study. PLoS Med. 8:e1001052. 10.1371/journal.pmed.1001052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boyd DA, Tyler S, Christianson S, McGeer A, Muller MP, Willey BM, Bryce E, Gardam M, Nordmann P, Mulvey MR. 2004. Complete nucleotide sequence of a 92-kilobase plasmid harboring the CTX-M-15 extended-spectrum beta-lactamase involved in an outbreak in long-term-care facilities in Toronto, Canada. Antimicrob. Agents Chemother. 48:3758–3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carattoli A, Garcia-Fernandez A, Varesi P, Fortini D, Gerardi S, Penni A, Mancini C, Giordano A. 2008. Molecular epidemiology of Escherichia coli producing extended-spectrum beta-lactamases isolated in Rome, Italy. J. Clin. Microbiol. 46:103–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Datta N, Dacey S, Hughes V, Knight S, Richards H, Williams G, Casewell M, Shannon KP. 1980. Distribution of genes for trimethoprim and gentamicin resistance in bacteria and their plasmids in a general hospital. J. Gen. Microbiol. 118:495–508 [DOI] [PubMed] [Google Scholar]
- 133.Datta N, Kontomichalou P. 1965. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature 208:239–241 [DOI] [PubMed] [Google Scholar]
- 134.Shanahan PM, Thomson CJ, Amyes SG. 1994. Beta-lactam resistance in aerobic faecal flora from general practice patients in the UK. Eur. J. Clin. Microbiol. Infect. Dis. 13:760–763 [DOI] [PubMed] [Google Scholar]
- 135.Shanahan PM, Thomson CJ, Amyes SG. 1994. Beta-lactam resistance in aerobic commensal faecal flora. Int. J. Antimicrob. Agents 3:259–266 [DOI] [PubMed] [Google Scholar]
- 136.Shanahan PM, Thomson CJ, Amyes SG. 1995. Beta-lactam resistance in normal faecal flora from South Africa. Epidemiol. Infect. 115:243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shanahan PM, Wylie BA, Adrian PV, Koornhof HJ, Thomson CJ, Amyes SG. 1993. The prevalence of antimicrobial resistance in human faecal flora in South Africa. Epidemiol. Infect. 111:221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Babcock GF, Berryhill DL, Marsh DH. 1973. R-factors of Escherichia coli from dressed beef and humans. Appl. Microbiol. 25:21–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fein D, Burton G, Tsutakawa R, Blenden D. 1974. Matching of antibiotic resistance patterns of Escherichia coli of farm families and their animals. J. Infect. Dis. 130:274–279 [DOI] [PubMed] [Google Scholar]
- 140.Grabow WO, Prozesky OW. 1973. Drug resistance of coliform bacteria in hospital and city sewage. Antimicrob. Agents Chemother. 3:175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Niemi M, Sibakov M, Niemela S. 1983. Antibiotic resistance among different species of fecal coliforms isolated from water samples. Appl. Environ. Microbiol. 45:79–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Siegel D, Huber WG, Enloe F. 1974. Continuous non-therapeutic use of antibacterial drugs in feed and drug resistance of the gram-negative enteric florae of food-producing animals. Antimicrob. Agents Chemother. 6:697–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hartmann A, Locatelli A, Amoureux L, Depret G, Jolivet C, Gueneau E, Neuwirth C. 2012. Occurrence of CTX-M producing Escherichia coli in soils, cattle, and farm environment in France (Burgundy region). Front. Microbiol. 3:83. 10.3389/fmicb.2012.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ben-Ami R, Schwaber MJ, Navon-Venezia S, Schwartz D, Giladi M, Chmelnitsky I, Leavitt A, Carmeli Y. 2006. Influx of extended-spectrum beta-lactamase-producing Enterobacteriaceae into the hospital. Clin. Infect. Dis. 42:925–934 [DOI] [PubMed] [Google Scholar]
- 145.Vinue L, Saenz Y, Martinez S, Somalo S, Moreno MA, Torres C, Zarazaga M. 2009. Prevalence and diversity of extended-spectrum beta-lactamases in faecal Escherichia coli isolates from healthy humans in Spain. Clin. Microbiol. Infect. 15:954–957 [DOI] [PubMed] [Google Scholar]
- 146.Meyer E, Gastmeier P, Kola A, Schwab F. 2012. Pet animals and foreign travel are risk factors for colonisation with extended-spectrum beta-lactamase-producing Escherichia coli. Infection 40:685–687 [DOI] [PubMed] [Google Scholar]
- 147.Kader AA, Kumar A, Kamath KA. 2007. Fecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in patients and asymptomatic healthy individuals. Infect. Control Hosp. Epidemiol. 28:1114–1116 [DOI] [PubMed] [Google Scholar]
- 148.Kader AA, Kamath KA. 2009. Faecal carriage of extended-spectrum beta-lactamase-producing bacteria in the community. East Mediterr. Health J. 15:1365–1370 [PubMed] [Google Scholar]
- 149.Lonchel CM, Meex C, Gangoue-Pieboji J, Boreux R, Assoumou MC, Melin P, De Mol P. 2012. Proportion of extended-spectrum β-lactamase-producing Enterobacteriaceae in community setting in Ngaoundere, Cameroon. BMC Infect. Dis. 12:53. 10.1186/1471-2334-12-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ho PL, Wong RC, Chow KH, Yip K, Wong SS, Que TL. 2008. CTX-M type beta-lactamases among fecal Escherichia coli and Klebsiella pneumoniae isolates in non-hospitalized children and adults. J. Microbiol. Immunol. Infect. 41:428–432 [PubMed] [Google Scholar]
- 151.Luvsansharav UO, Hirai I, Niki M, Nakata A, Yoshinaga A, Moriyama T, Yamamoto Y. 2011. Prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae among healthy adult people in Japan. J. Infect. Chemother. 17:722–725 [DOI] [PubMed] [Google Scholar]
- 152.Ko YJ, Moon HW, Hur M, Park CM, Cho SE, Yun YM. 2013. Fecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Korean community and hospital settings. Infection 41:9–13 [DOI] [PubMed] [Google Scholar]
- 153.Pallecchi L, Lucchetti C, Bartoloni A, Bartalesi F, Mantella A, Gamboa H, Carattoli A, Paradisi F, Rossolini GM. 2007. Population structure and resistance genes in antibiotic-resistant bacteria from a remote community with minimal antibiotic exposure. Antimicrob. Agents Chemother. 51:1179–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]