Abstract
Development of anthrax countermeasures that may be used concomitantly in a postexposure setting requires an understanding of the interaction between these products. Anthrax immune globulin intravenous (AIGIV) is a candidate immunotherapeutic that contains neutralizing antibodies against protective antigen (PA), a component of anthrax toxins. We evaluated the interaction between AIGIV and BioThrax (anthrax vaccine adsorbed) in rabbits. While pharmacokinetics of AIGIV were not altered by vaccination, the vaccine-induced immune response was abrogated in AIGIV-treated animals.
TEXT
Bacillus anthracis, the etiologic agent of anthrax, causes human disease via the gastrointestinal, cutaneous, or inhalation route. Inhalational anthrax is the most lethal form of the disease and, if untreated, is nearly 100% fatal (1). Mortality may be prevented if treatment with antibiotics is initiated shortly after exposure to spores. However, a delay in initiating antimicrobial therapy may result in toxemia, which predominantly accounts for the morbidity and mortality associated with inhalational anthrax disease (2, 3).
Vaccines are being developed for postexposure prophylaxis of anthrax, to be used in combination with antibiotics (4). Additionally, polyclonal and monoclonal antibody-based antitoxins have been shown to improve survival when used to treat anthrax-induced toxemia in rabbits, guinea pigs, and cynomolgus macaques (5–7). Antibiotics do not appear to interfere with the concomitant active (vaccination) or passive (antitoxin) immunization against anthrax (4, 8). However, the effect of antitoxin on vaccine-induced immune response has not been elucidated.
Anthrivig (human anthrax immunoglobulin), also known as AIGIV, is purified from plasma of individuals vaccinated with the FDA-licensed anthrax vaccine BioThrax (anthrax vaccine adsorbed), also referred to as AVA (Emergent BioSolutions, Lansing, MI). AIGIV is produced using the same manufacturing process as that used for the manufacture of the FDA-licensed Gamunex-C [immune globulin injection (human), 10% caprylate/chromatography purified] (Grifols Therapeutics Inc., Clayton, NC). AIGIV contains antibodies against protective antigen (PA), a component of B. anthracis lethal toxin (LT) and edema toxin (ET), and neutralizes the effects of these toxins by binding to the PA component, as reported in the accompanying paper (9). We examined the interaction of AIGIV with AVA when they were coadministered in the New Zealand White (NZW) rabbit model.
Animal studies were conducted in compliance with the Animal Welfare Act and followed the principles outlined in the National Research Council Guide for the Care and Use of Laboratory Animals. All animal protocols were approved by the Institutional Animal Care and Use Committee. On study day 1, 4 groups of 8 rabbits (9.5 months of age, weighing between 3.2 and 4.7 kg) were administered AIGIV at a dose of 14.2 mg/kg of body weight or 21.3 mg/kg of anti-PA IgG (364 and 546 mg/kg of total IgG, respectively) via slow intravenous (IV) infusion. Control animals were infused with Gamunex (546 mg/kg of total IgG) (Table 1). Following infusion, animals in 4 out of 6 groups were immunized via intramuscular (IM) injection on study days 1 and 8 with 0.5 ml of AVA at a 1:16 dilution of the human dose. This dose of the vaccine was selected because it has been shown to elicit an immune response which lies within the linear portion of a vaccine dose-response curve in the NZW rabbit model (10). Serum AIGIV levels were assessed by measuring human anti-PA IgG by ELISA. The rabbit immune response to AVA was assessed by measuring rabbit anti-PA IgG by ELISA. Species-specific secondary antibodies were used to distinguish between human and rabbit anti-PA IgG. The ELISA values were used to perform PK analysis. The limit of quantitation (LOQ) of the human and rabbit anti-PA IgG ELISA was 9.27 and 5.0 μg/ml, respectively.
Table 1.
Study design
| Group | No. of animalsa | AVA dose (dilution of human dose), days 1 and 8 | AIGIV dose (mg/kg of anti-PA IgG), day 1 | Gamunex dose (mg/kg), day 1 |
|---|---|---|---|---|
| 1 | 8 | 1:16 | ||
| 2 | 8c | 14.2 | ||
| 3 | 8c | 21.3 | ||
| 4 | 8c | 1:16 | 14.2 | |
| 5 | 8 | 1:16 | 21.3 | |
| 6 | 8 | 1:16 | 546b |
Equal numbers of male and female NZW rabbits, weighing between 3.2 and 4.7 kg at the start of the study, were used.
Equivalent dose of total IgG as AIGIV at a dose of 21.3 mg/kg.
Three rabbits, one in each of groups 2, 3, and 4, did not receive a full dose of AIGIV and were therefore excluded from the PK analysis.
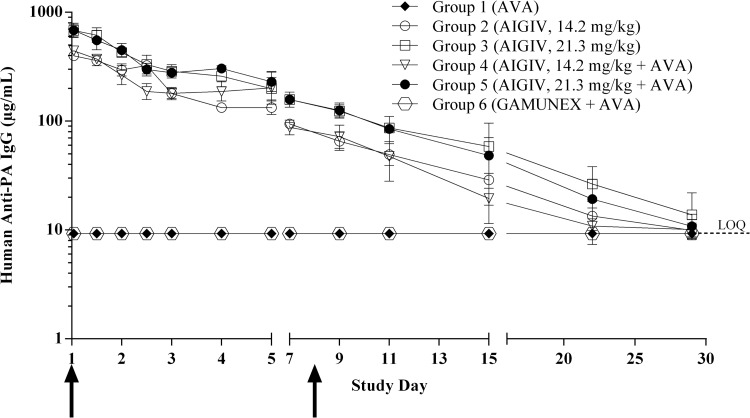
As expected, human anti-PA IgG levels in animals that received Gamunex remained below the LOQ for the duration of the experiment. In animals infused with AIGIV, the peak concentration of human anti-PA IgG was detected 1 h following AIGIV infusion. By study day 29, human anti-PA IgG levels dropped below the LOQ (Fig. 1). Group mean maximum concentration of AIGIV (Cmax) and area under the concentration-time curve (AUC) values increased in a dose-proportional manner (Table 2). Administration of AVA to animals that received AIGIV did not affect the AIGIV pharmacokinetic (PK) parameters (Fig. 1 and Table 2) (P > 0.18).
Fig 1.
Human anti-PA IgG levels in rabbit sera measured by ELISA. The data are presented as geometric mean human anti-PA IgG concentrations with 95% confidence intervals (CIs). Arrows represent the time points when vaccinations were administered, day 1 and day 8, respectively. Serum samples were collected 9 to 10 days prior to infusion (baseline), at 1, 12, 24, 36, 48, 72, and 96 h postinfusion, and on study days 7, 9, 11, 15, 22, and 29. Three rabbits, one in each of groups 2, 3, and 4, did not receive a full dose of AIGIV and were therefore excluded from the analysis. Values below the assay LOQ were replaced with the LOQ (9.27 μg/ml). The PK profile of AIGIV was not significantly different among groups 2 through 5 (P > 0.18).
Table 2.
PK parameters for human anti-PA IgGa
| Group (treatment)b | Cmax (μg/ml) | Tlast (days) | t1/2 (days) | AUClast (day × μg/ml) | AUC0-∞ (day × μg/ml) |
|---|---|---|---|---|---|
| 2 (AIGIV, 14.2 mg/kg of anti-PA IgG); n = 7c | 410.86 ± 37.38 | 26.00 ± 5.29 | 5.43 ± 2.48 | 1,818.57 ± 215.44 | 1,882.86 ± 254.93 |
| 3 (AIGIV, 21.3 mg/kg of anti-PA IgG); n = 7c | 705.14 ± 93.46 | 28.00 ± 0.00 | 6.91 ± 2.52 | 3,070.00 ± 192.61 | 3,251.43 ± 389.46 |
| 4 (AVA, 1:16; AIGIV, 14.2 mg/kg of anti-PA IgG); n = 7c | 445.00 ± 33.42 | 17.86 ± 7.84 | 4.04 ± 2.87 | 1,791.43 ± 386.11 | 1,881.43 ± 428.74 |
| 5 (AVA, 1:16; AIGIV, 21.3 mg/kg of anti-PA IgG); n = 8 | 714.38 ± 104.38 | 22.75 ± 9.85 | 4.17 ± 1.85 | 2,818.75 ± 537.41 | 2,946.25 ± 432.47 |
Cmax, maximum measured serum concentration during the period specified; Tmax, time to maximum measured serum concentration; Tlast, time of last measurable concentration; AUClast, area under the serum concentration-versus-time curve from time zero to the last quantifiable concentration; AUC0-∞, AUClast plus the additional area extrapolated to infinity, calculated using the terminal elimination rate constant; t1/2, elimination half-life. Values are means ± standard errors.
Anti-PA IgG ELISA was not performed on sera from animals in groups 1 (AVA alone) and 6 (AVA plus Gamunex), which were not infused with AIGIV.
Three rabbits, one in each of groups 2, 3, and 4, did not receive a full dose of AIGIV and were therefore excluded from the PK analysis.
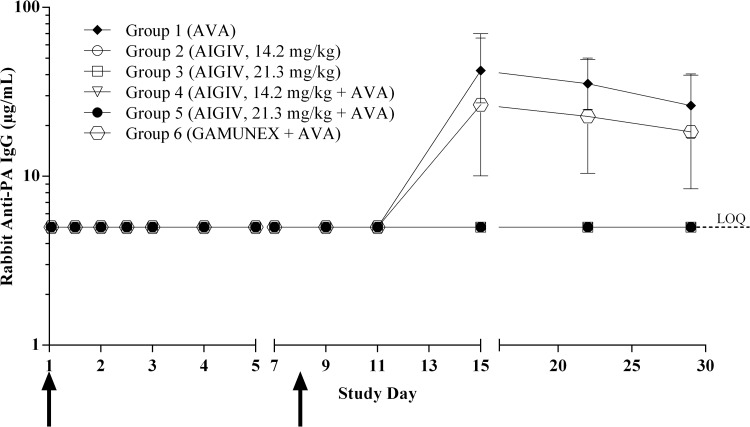
Following the second AVA vaccination, administered on study day 8, animals that received AVA alone or AVA and Gamunex developed a robust immune response, with the peak anti-PA IgG concentration occurring on day 15 (Fig. 2). In contrast, animals that received AIGIV at the dose of either 14.2 mg/kg or 21.3 mg/kg of anti-PA IgG had no detectable immune response to vaccination throughout the study (Fig. 2), indicating that under these experimental conditions, administration of AIGIV abrogated the immune response to AVA in the rabbit model (P < 0.003).
Fig 2.
Rabbit anti-PA IgG immune response to vaccination measured by ELISA. The data are presented as geometric mean rabbit anti-PA IgG concentrations with 95% CIs. Arrows represent the time points when vaccinations were administered, day 1 and day 8, respectively. Serum samples were collected 9 to 10 days prior to infusion (baseline), at 1, 12, 24, 36, 48, 72, and 96 h postinfusion, and on study days 7, 9, 11, 15, 22, and 29. Three rabbits, one in each of groups 2, 3, and 4, did not receive a full dose of AIGIV and were therefore excluded from the analysis. Values below the assay LOQ were replaced with the LOQ (5.0 μg/ml). The level of immune response in groups 1 and 6 was significantly higher than that in groups 2 through 5 (P < 0.003).
A possible explanation for the observed impairment of the serum antibody response to AVA in animals infused with AIGIV may be immunological interference. Interference between immune globulins and vaccines administered concomitantly has been observed previously in animal models and humans. Examples include concomitant active and passive immunization against plague (11) and hepatitis A (12). However, in other cases of coadministration of a vaccine and an immune globulin, including those against hepatitis B and poliovirus, inhibition of immune response to a vaccine does not occur (13, 14). In the case of concomitant administration of the tetanus-diphtheria vaccine and tetanus toxoid, a decrease in the vaccine-induced immune response has been observed in the short but not long term, and it appears to be dependent on the patients' age and prevaccination antibody titer (15, 16).
The mechanism of interference between active and passive immunization is not fully understood. One possible explanation for this finding is the formation of immune complexes between the passively acquired antibodies and the antigen supplied by the active immunization, resulting in inability of the antigen-specific B cells to recognize the antigen (17). For example, B-cell response to vaccination with a vector expressing a rabies virus glycoprotein was inhibited in mice passively immunized with rabies hyperimmune serum, resulting in vaccine failure upon challenge with a virulent strain of rabies virus (18).
It remains to be determined whether the observed suppression of the AVA-induced immune response under these experimental conditions would affect the memory response to AVA if the animals were subsequently reimmunized or exposed to anthrax spores at a later time point when the human anti-PA IgG levels have substantially decreased. Indeed, there have been reports of immune complexes eliciting a robust anamnestic response in the absence of a primary antibody response (19–21). In efficacy studies with other anti-PA antibody-based anthrax therapeutics, surviving animals were rechallenged with anthrax spores one or more months after the first challenge; i.e., after the passively administered antibody was expected to have been cleared from the animals (8, 22). All rechallenged animals survived the second challenge, suggesting that the passively administered antibodies did not interfere with the development of protective long-term immunological memory in response to B. anthracis infection.
Because AIGIV will likely be used in conjunction with antibiotics, we also examined the effect of AIGIV on serum levofloxacin levels in a separate experiment. NZW rabbits received 50 mg/kg of levofloxacin by oral gavage once daily for 6 days and a single IV infusion of AIGIV (14.2 or 21.3 mg/kg of anti-PA IgG; i.e., 364 or 546 mg/kg of total IgG, respectively) or Gamunex (546 mg/kg of total IgG) on day 4. Peak and trough concentrations of levofloxacin (as measured by high-pressure liquid chromatography [HPLC]) were comparable in all groups throughout the study period, indicating that concomitant administration of AIGIV did not affect serum levofloxacin levels (data not shown). The effect of a PA-based anthrax vaccine (4) and anti-PA-based antitoxin (8) coadministration on levofloxacin levels has also been examined previously in the rabbit model. The studies indicated that plasma antibiotic concentrations were not affected by the concomitant active or passive immunization.
It is not known whether the observed effect is common to any PA-based anthrax vaccine and anti-PA antibody-based therapeutic. Further studies in animal models and ultimately in humans should be conducted to elucidate the mechanism for the apparent decrease in vaccine-induced immune response when vaccination is coadministered with an immune globulin and to investigate potential strategies for overcoming this effect, such as delaying vaccination until levels of passively administered antibody have declined.
ACKNOWLEDGMENTS
We thank Monica Vegarra, Barbara Atkinson, and the technical staff at AVANZA Laboratories for conducting the animal studies; Frances Rabon and the technical staff at Battelle Biomedical Research Institute for performing serological analysis; Peter Hong, Gregory Stark, and Diane Sweeney for PK and statistical support; Mike Lacy for critical review of the manuscript; and Shannon Cureton, Christine Valencia, Bryan Fortson, and Tyler Laudenslager for programmatic and technical support.
This work was supported by a grant from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, Department of Health and Human Services (grant no. 5U01AI070486).
The authors are either current or former employees or contractors of Emergent BioSolutions, the developer of AIGIV and the manufacturer of BioThrax.
Footnotes
Published ahead of print 26 August 2013
REFERENCES
- 1.Grabenstein JD. 2008. Vaccines: countering anthrax: vaccines and immunoglobulins. Clin. Infect. Dis. 46:129–136 [DOI] [PubMed] [Google Scholar]
- 2.Abramova FA, Grinberg LM, Yampolskaya OV, Walker DH. 1993. Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc. Natl. Acad. Sci. U. S. A. 90:2291–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inglesby TV, O'Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Gerberding J, Hauer J, Hughes J, McDade J, Osterholm MT, Parker G, Perl TM, Russell PK, Tonat K. 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236–2252 [DOI] [PubMed] [Google Scholar]
- 4.Leffel EK, Bourdage JS, Williamson ED, Duchars M, Fuerst TR, Fusco PC. 2012. rPA anthrax vaccine improves survival when administered as a post-exposure prophylaxis countermeasure with antibiotic in the New Zealand White rabbit model of inhalation anthrax. Clin. Vaccine Immunol. 19:1158–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson JW, Comer JE, Noffsinger DM, Wenglikowski A, Walberg KG, Chatuev BM, Chopra AK, Stanberry LR, Kang AS, Scholz WW, Sircar J. 2006. Human monoclonal anti-protective antigen antibody completely protects rabbits and is synergistic with ciprofloxacin in protecting mice and guinea pigs against inhalation anthrax. Infect. Immun. 74:1016–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henning LN, Comer JE, Stark G, Ray BD, Tordoff KP, Knostman KA, Meister GT. 2012. Development of an inhalational Bacillus anthracis exposure therapeutic model in cynomolgus macaques. Clin. Vaccine Immunol. 19:1765–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobiler D, Gozes Y, Rosenberg H, Marcus D, Reuveny S, Altboum Z. 2002. Efficiency of protection of guinea pigs against infection with Bacillus anthracis spores by passive immunization. Infect. Immun. 70:544–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Migone TS, Subramanian GM, Zhong J, Healey LM, Corey A, Devalaraja M, Lo L, Ullrich S, Zimmerman J, Chen A, Lewis M, Meister G, Gillum K, Sanford D, Mott J, Bolmer SD. 2009. Raxibacumab for the treatment of inhalational anthrax. N. Engl. J. Med. 361:135–144 [DOI] [PubMed] [Google Scholar]
- 9.Mytle N, Hopkins RJ, Malkevich NV, Basu S, Meister GT, Sanford DC, Comer JE, Van Zandt KE, Al-Ibrahim M, Kramer WG, Howard C, Daczkowski N, Chakrabarti AC, Ionin B, Nabors GS, Skiadopoulos MH. 2013. Evaluation of intravenous anthrax immune globulin for treatment of inhalation anthrax. Antimicrob. Agents Chemother. 57:5684–5692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitt ML, Little SF, Ivins BE, Fellows P, Barth J, Hewetson J, Gibbs P, Dertzbaugh M, Friedlander AM. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19:4768–4773 [DOI] [PubMed] [Google Scholar]
- 11.Eyles JE, Butcher WA, Titball RW, Hill J. 2007. Concomitant administration of Yersinia pestis specific monoclonal antibodies with plague vaccine has a detrimental effect on vaccine mediated immunity. Vaccine 25:7301–7306 [DOI] [PubMed] [Google Scholar]
- 12.Zanetti A, Pregliasco F, Andreassi A, Pozzi A, Vigano P, Cargnel A, Briantais P, Vidor E. 1997. Does immunoglobulin interfere with the immunogenicity to Pasteur Merieux inactivated hepatitis A vaccine. J. Hepatol. 26:25–30 [DOI] [PubMed] [Google Scholar]
- 13.Szmuness W, Stevens CE, Olesko WR, Goodman A. 1981. Passive-active immunisation against hepatitis B: immunogenicity studies in adult Americans. Lancet i:575–577 [DOI] [PubMed] [Google Scholar]
- 14.Green MS, Melnick JL, Cohen D, Slepon R, Danon YL. 1990. Response to trivalent oral poliovirus vaccine with and without immune serum globulin in young adults in Israel in 1988. J. Infect. Dis. 162:971–974 [DOI] [PubMed] [Google Scholar]
- 15.Dal-Ré R, Gil A, González A, Lasheras L. 1995. Does tetanus immune globulin interfere with the immune response to simultaneous administration of tetanus-diphtheria vaccine? A comparative clinical trial in adults. J. Clin. Pharmacol. 35:420–425 [DOI] [PubMed] [Google Scholar]
- 16.Shin J, Kim J, Song K. 2012. Influences on formation of tetanus antibody after simultaneous injection of tetanus immunoglobulin with tetanus vaccine. J. Korean Med. Sci. 27:934–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowley DA, Fitch FW, Stuart FP, Kohler H, Cosenza H. 1973. Specific suppression of immune responses by antibody directed against either antigen or receptors for antigen can suppress immunity specifically. Science 181:1133–1141 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Xiang Z, Pasquini S, Ertl HC. 1998. Effect of passive immunization on maternally transferred immunity on the antibody response to a genetic vaccine to rabies virus. J. Virol. 72:1790–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nie X, Basu S, Cerny J. 1997. Immunization with immune complex alters the repertoire of antigen-reactive B cells in the germinal centers. Eur. J. Immunol. 27:3517–3525 [DOI] [PubMed] [Google Scholar]
- 20.Song H, Nie X, Basu S, Cerny J. 1998. Antibody feedback and somatic mutation in B cells: regulation of mutation by immune complexes with IgG antibody. Immunol. Rev. 162:211–218 [DOI] [PubMed] [Google Scholar]
- 21.Song H, Nie X, Basu S, Singh M, Cerny J. 1999. Regulation of Vh gene repertoire and somatic mutation in germinal centre B cells by passively administered antibody. Immunology 98:258–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson JW, Comer JE, Baze WB, Noffsinger DM, Wenglikowski A, Walberg KG, Hardcastle J, Pawlik J, Bush K, Taormina J, Moen S, Thomas J, Chatuev BM, Sower L, Chopra AK, Stanberry LR, Sawada R, Scholz WW, Sircar J. 2007. Human monoclonal antibody AVP-21D9 to protective antigen reduces dissemination of the Bacillus anthracis Ames strain from the lungs in a rabbit model. Infect. Immun. 75:3414–3424 [DOI] [PMC free article] [PubMed] [Google Scholar]