Abstract
In 2008, dihydroartemisinin (DHA)-piperaquine (PPQ) became the first-line treatment for uncomplicated Plasmodium falciparum malaria in western Cambodia. Recent reports of increased treatment failure rates after DHA-PPQ therapy in this region suggest that parasite resistance to DHA, PPQ, or both is now adversely affecting treatment. While artemisinin (ART) resistance is established in western Cambodia, there is no evidence of PPQ resistance. To monitor for resistance to PPQ and other antimalarials, we measured drug susceptibilities for parasites collected in 2011 and 2012 from Pursat, Preah Vihear, and Ratanakiri, in western, northern, and eastern Cambodia, respectively. Using a SYBR green I fluorescence assay, we calculated the ex vivo 50% inhibitory concentrations (IC50s) of 310 parasites to six antimalarials: chloroquine (CQ), mefloquine (MQ), quinine (QN), PPQ, artesunate (ATS), and DHA. Geometric mean IC50s (GMIC50s) for all drugs (except PPQ) were significantly higher in Pursat and Preah Vihear than in Ratanakiri (P ≤ 0.001). An increased copy number of P. falciparum mdr1 (pfmdr1), an MQ resistance marker, was more prevalent in Pursat and Preah Vihear than in Ratanakiri and was associated with higher GMIC50s for MQ, QN, ATS, and DHA. An increased copy number of a chromosome 5 region (X5r), a candidate PPQ resistance marker, was detected in Pursat but was not associated with reduced susceptibility to PPQ. The ex vivo IC50 and pfmdr1 copy number are important tools in the surveillance of multidrug-resistant (MDR) parasites in Cambodia. While MDR P. falciparum is prevalent in western and northern Cambodia, there is no evidence for PPQ resistance, suggesting that DHA-PPQ treatment failures result mainly from ART resistance.
INTRODUCTION
In 2000, artesunate (ATS) plus mefloquine (MQ) was introduced as first-line treatment for uncomplicated Plasmodium falciparum malaria in all regions of Cambodia. Since then, increasing proportions of patients have failed treatment with ATS-MQ in Pailin Province and other areas of western Cambodia (1, 2), prompting the National Malaria Control Program to recommend dihydroartemisinin (DHA)-piperaquine (PPQ) as the first-line treatment for most of western Cambodia in 2008. While DHA-PPQ has shown excellent efficacy in recent clinical trials in Africa (3–5) and Asia (6–13), a recent report from western Cambodia suggests that this fixed drug combination may be failing. Specifically, the efficacy of DHA-PPQ in Pailin decreased from 92% in 2009-2010 to 75% in 2010-2011; in Pursat Province, it was found to be 89% in 2010-2011 (14). Whether this decreased efficacy results from parasite resistance to artemisinin (ART) derivatives, PPQ, or both has not been determined. However, similar median 50% inhibitory concentrations (IC50s) for PPQ in Pailin, Pursat, Preah Vihear, and Ratanakiri Provinces in 2010 suggest that this decreased efficacy is unlikely due to PPQ resistance (14).
PPQ, a bisquinoline antimalarial drug, was widely used to treat chloroquine (CQ)-resistant P. falciparum malaria in southern China in the 1970s and 1980s. The use of PPQ monotherapy is believed to have selected PPQ-resistant parasites, which limited the use of PPQ by the late 1980s (15). The most recent data available for Cambodian P. falciparum isolates indicate that the geometric mean IC50s (GMIC50s) for CQ, MQ, quinine (QN), and ATS were significantly higher in western than eastern Cambodia from 2001 to 2011 (14, 16), confirming a remarkable disparity in parasite drug sensitivity between these two regions. These GMIC50s were also significantly higher in patients with late treatment failures after ATS-MQ therapy than in those who had adequate clinical and parasitological responses (16). Importantly, the GMIC50s for all four drugs increased significantly from 2006 to 2007 in northeastern Cambodia (16), suggesting that multidrug-resistant (MDR) parasites may be spreading to or emerging in areas other than Cambodia's western border with Thailand.
Here we present initial results from our longitudinal surveillance program to track the spread and emergence of MDR parasites in Cambodia. Specifically, we used an ex vivo drug susceptibility assay to establish baseline IC50s for six antimalarials (CQ, MQ, QN, PPQ, ATS, and DHA) in clinical P. falciparum isolates from western, northern, and eastern Cambodia. In addition, we tested the performance of a molecular marker (i.e., a P. falciparum mdr1 [pfmdr1] copy number of ≥2) in identifying MQ-resistant parasites in all three regions. Finally, we explored for the first time whether an increased copy number of a chromosome 5 region (X5r), a genetic marker associated with a high PPQ IC50 in the drug-selected P. falciparum PPQR4 line (17), correlates with reduced ex vivo susceptibility of clinical parasite isolates to PPQ.
MATERIALS AND METHODS
Parasite isolates.
In 2011-2012, we conducted studies to compare parasite clearance rates in response to ATS in three provinces: Pursat (western Cambodia), Preah Vihear (northern Cambodia), and Ratanakiri (eastern Cambodia) (Fig. 1). Patients who sought treatment for symptoms compatible with malaria were screened using a rapid diagnostic test (CareStart Malaria HRP-2/pLDH Pf/Pan; Access Bio Inc., Gyeongnam, South Korea). Patients with uncomplicated P. falciparum malaria were enrolled if they met the following inclusion criteria: age, 2 to 65 years; asexual parasite density, 10,000/μl to 200,000/μl whole blood; and hematocrit, ≥25%. Venous blood samples were collected into heparin Vacutainers (Becton, Dickinson, Franklin Lakes, NJ) prior to ATS treatment, transported on ice to Phnom Penh, and processed within 24 h. Adults or the parents/guardians of children gave written informed consent. The study protocol was approved by Cambodia's National Ethics Committee for Health Research, the National Institute of Allergy and Infectious Diseases Institutional Review Board, and the University of Oxford Tropical Research Ethics Committee. This study is registered with ClinicalTrials.gov (NCT01240603).
Fig 1.
Map of Cambodia showing the three study sites: Pursat (red), Preah Vihear (blue), and Ratanakiri (green) provinces.
Antimalarial drug preparation.
Stock solutions of chloroquine diphosphate (CQ), mefloquine hydrochloride (MQ), quinine sulfate (QN), ATS, and DHA (Sigma, Steinheim, Germany) were prepared in 70% ethanol. Stock solutions of piperaquine tetraphosphate (PPQ) were prepared in 0.5% lactic acid (Sigma) in culture water (Lonza, Walkersville, MD) to enhance its solubility (18). Twofold serial dilutions of stock solutions were prepared in culture water. Final drug concentrations ranged from 2.4 to 2,500 nM for CQ, 1.2 to 1,250 nM for MQ, 4.8 to 5,000 nM for QN, 0.97 to 1,000 nM for PPQ, 0.12 to 125 nM for ATS, and 0.09 to 100 nM for DHA. Fifty microliters of each diluted drug solution was added in duplicate to 96-well, flat-bottomed plates (Costar 3595; Corning, Lowell, MA), which were then air dried in a laminar flow hood and stored for up to 1 month at 4°C. Each plate included two drug-free wells as negative controls for each drug. The quality of each batch of plates was validated by measuring the antimalarial drug responses of the P. falciparum 3D7 line.
Ex vivo drug susceptibility assay.
Plasma and buffy coats were removed from venous blood samples, and red blood cells (RBCs) were washed three times with RPMI 1640 medium (Invitrogen, Carlsbad, CA) buffered with 25 mM HEPES. Parasitemias were counted from Giemsa-stained thin blood smears of RBCs. The ex vivo susceptibility of parasites to antimalarial drugs was assessed using a SYBR green I fluorescence assay (19). If parasitemia was 0.5% to 1%, assays were performed directly. If parasitemia was >1%, samples were diluted with uninfected RBCs to achieve 0.5% to 1% parasitemia. If parasitemia was <0.5%, parasites were cultured for no more than three cycles to 0.5 to 1% parasitemia. RBC suspensions (1% hematocrit) were prepared in RPMI 1640 medium supplemented with 0.5% AlbuMAX II lipid-rich bovine serum albumin (Invitrogen), 25 mM HEPES, 25 mM NaHCO3, and 0.005% (wt/vol) hypoxanthine and transferred (200 μl/well) in duplicate to drug-coated plates. Plates were incubated for 72 h at 37°C in an atmosphere of 5% CO2 and subsequently frozen and kept at −20°C. After the plates were thawed for 2 h at ambient temperature to lyse the RBCs and shaking them for 10 min, the contents of each well were carefully resuspended. A 100-μl cell suspension was removed from each well, and 100 μl of SYBR green I (Invitrogen) in lysis buffer (0.02%, vol/vol) was added. The lysis buffer contained Tris (20 mM, pH 7.5), EDTA (5 mM), saponin (0.008%, wt/vol), and Triton X-100 (0.08% vol/vol) (20). The plates were incubated at ambient temperature for 30 min, and SYBR green I fluorescence was quantified using a FLUOstar OPTIMA instrument (BMG Labtech, Cary, NC). The IC50, defined as the drug concentration at which the SYBR green I signal was 50% of that measured from drug-free control wells, was calculated from the inhibitory sigmoid maximum-effect (Emax) model (ICEstimator, version 1.2; http://www.antimalarial-icestimator.net/MethodIntro.htm) (21).
pfmdr1 copy number estimation.
Parasite DNA was extracted from frozen whole-blood aliquots (200 μl) using a QIAamp DNA minikit (Qiagen, Valencia, CA). The pfmdr1 copy number was estimated using a modified method as described previously (17, 22). The primer sequences used in the pfmdr1 copy number assay were 5′-CAA GTG AGT TCA GGA ATT GGT AC-3′ (forward) and 5′-GCC TCT TCT ATA ATG GAC ATG G-3′ (reverse) for pfmdr1 and 5′-AGG ACA ATA TGG ACA CTC CGA T-3′ (forward) and 5′-TTT CAG CTA TGG CTT CAT CAA A-3′ (reverse) for P. falciparum ldh (pfldh). Individual real-time PCRs were carried out in 20-μl volumes in a 96-well plate (Bio-Rad, Hercules, CA) with SensiMix SYBR No-Rox 2× PCR master mix containing SYBR green I (Bioline Inc., Taunton, MA), 300 nM each primer, and 2 μl genomic DNA template. Real-time PCR was performed in a CFX real-time PCR machine (Bio-Rad) as follows: 15 min at 95°C, followed by 40 cycles for 15 s at 95°C, 30 s at 58°C, and 20 s at 72°C. At the end of each reaction, the cycle threshold (CT) was manually set to the level reflecting the best kinetic PCR parameters, and melting curves were acquired and analyzed. Relative copy number was calculated on the basis of the ΔΔCT method (2ΔΔCT). ΔΔCT was calculated as (CTldh − CTpfmdr1)/(CTldh cal − CTpfmdr1 cal). DNAs from clinical parasite isolates and laboratory-adapted parasite lines (3D7 and Dd2) were analyzed on each plate in duplicate and triplicate, respectively. The 3D7 and Dd2 parasite lines possess 1 and 2 copies of pfmdr1, respectively.
Copy number of an X5r linked to PPQ resistance.
DNAs from Pursat and Ratanakiri parasite isolates were used to assess the copy number of a chromosome 5 region (X5r) amplified in an in vitro-selected PPQ-resistant parasite line (PPQR4) (17) using a SYBR green I fluorescence-based quantitative PCR (qPCR) method. Primer sequences are listed in Table S1 in the supplemental material. Specifically, copy number variations in 11 genes on X5r were interrogated: PF3D7_0519900, PF3D7_0520100, PF3D7_0520300, PF3D7_0520500, PF3D7_0520600, PF3D7_0520700, PF3D7_0520900, PF3D7_0521000, PF3D7_0521100, PF3D7_0521300, and PF3D7_0521500. PF3D7_1324900 (i.e., pfldh) was used as a control gene. Individual real-time PCRs were carried out in 20-μl volumes in a 96-well plate (Bio-Rad) with SensiMix SYBR No-Rox 2× PCR master mix (Bioline), 300 nM each primer, and 2 μl DNA genomic template. qPCR was performed in the CFX real-time PCR machine (Bio-Rad) as follows: 15 min at 95°C, followed by 40 cycles for 15 s at 95°C, 30 s at 57°C, and 20 s at 72°C. At the end of each reaction, the CT was manually set up at the level that reflected the best kinetic PCR parameters. Melting curve analysis was performed for each assay to verify that a single melting peak (from 55°C to 99°C) indicating a single specific PCR product for each reaction was produced. The assay was optimized to achieve approximately equal amplification efficiencies for the X5r genes and pfldh1 within the experimental range of DNA concentrations (from 1 ng to 100 ng template per reaction mixture). Relative copy number was calculated on the basis of the ΔΔCT method (2ΔΔCT). ΔΔCT was calculated as (CTldh − CTgene)/(CTldh cal − CTgene cal), where CTgene is the CT of the gene being tested. Control DNAs from P. falciparum lines Dd2-1pa and PPQR4 possess relative copy numbers of 1 and 2, respectively, for the X5r genes spanning PF3D7_0519900 to PF3D7_0521500. PPQR4 is a PPQ-resistant clone derived from the Dd2-1pa parasite lineage possessing a PPQ IC50 of 1,904 nM (R. T. Eastman and X.-Z. Su, unpublished data).
Statistical analysis.
All statistical analysis was performed using Stata/SE software, version 10 (Stata Corporation, College Station, TX). The ex vivo activity of each antimalarial was expressed as the GMIC50. Drug concentrations were log transformed. The Mann-Whitney test was used to determine whether the observed differences in IC50s, pfmdr1 copy number, or X5r copy number were significant. The chi-square test was used to compare categorical variables. Correlation analysis was used to assess the relationships between the IC50s of each drug. The level of significance was adjusted using the Bonferroni correction. For all statistical tests, P values of <0.05 were deemed significant.
RESULTS
A total of 360 P. falciparum isolates were collected from patients with uncomplicated malaria in Pursat (n = 120), Preah Vihear (n = 120), and Ratanakiri (n = 120) (Fig. 1). Parasitemias ranged from 0.02% to 13.80% (mean, 0.98%; 95% confidence interval [CI], 0.86 to 1.10) and did not differ significantly between sites. Of these, 310 (86.1%) parasites were tested for their ex vivo susceptibility to six antimalarials. Of these, 212 (68.4%) and 242 (78.1%) parasites yielded interpretable data for all six drugs and at least four drugs, respectively. The in vitro susceptibility of the P. falciparum 3D7 line to all six drugs was measured routinely for each batch of drug plates to control for assay consistency (see Fig. S1 in the supplemental material).
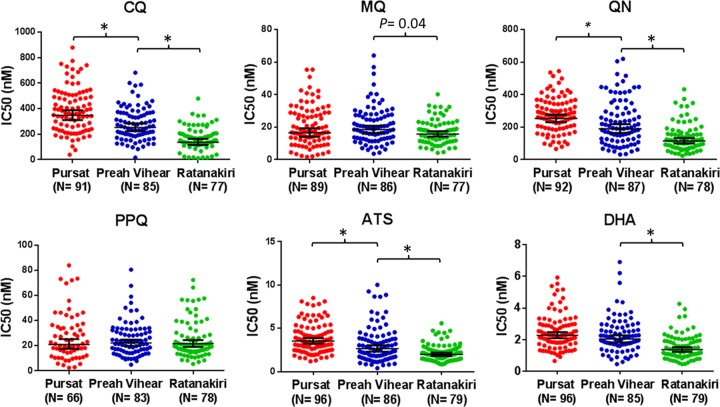
Table 1 shows the GMIC50s for all six drugs, stratified by Cambodian province. The GMIC50s for CQ, QN, and ATS were significantly higher in parasite isolates from Pursat than those from Preah Vihear (P ≤ 0.001) (Table 1; Fig. 2). The GMIC50s for CQ, MQ, QN, ATS, and DHA were significantly higher in parasites from Pursat and Preah Vihear than those from Ratanakiri (P ≤ 0.001; P = 0.04 for MQ) (Table 1; Fig. 2). In contrast, the GMIC50s for PPQ did not differ between provinces. Significant positive correlations between the IC50s of all pairwise drug combinations (P < 0.001) except for CQ versus MQ and all five drugs versus PPQ were observed (Table 2).
Table 1.
Ex vivo susceptibility of P. falciparum isolates to six antimalarial drugs in 2011-2012, stratified by Cambodian provincea
| Drug | Pursat |
Preah Vihear |
Ratanakiri |
|||
|---|---|---|---|---|---|---|
| n | GM (range) IC50 (nM) | n | GM (range) IC50 (nM) | n | GM (range) IC50 (nM) | |
| Chloroquine | 91 | 347.8 (38.4–880.2) | 85 | 251.8b (16.7–682.9) | 77 | 137.1c (11.8–479.5) |
| Mefloquine | 89 | 16.5 (1.7–55.4) | 86 | 18.5 (3.6–64.1) | 77 | 15.7c (4.2–40.2) |
| Quinine | 92 | 252.8 (65.5–544.9) | 87 | 189.3b (31.4–620.2) | 78 | 114.8c (23.2–433.0) |
| Piperaquine | 66 | 21.0 (2.5–83.8) | 83 | 22.2 (4.9–80.5) | 78 | 21.6 (5.5–72.3) |
| Artesunate | 96 | 3.5 (1.2–8.5) | 86 | 2.6b (0.4–10.0) | 79 | 1.9c (0.9–5.6) |
| DHA | 96 | 2.3 (0.6–5.9) | 85 | 2.0 (0.4–6.9) | 79 | 1.4c (0.4–4.3) |
n, number of isolates; GM, geometric mean.
P < 0.001, calculated using the Mann-Whitney test to compare IC50s between Pursat and Preah Vihear.
P < 0.001, calculated using the Mann-Whitney test to compare IC50s between Preah Vihear and Ratanakiri.
Fig 2.
Ex vivo susceptibility of P. falciparum isolates to six antimalarial drugs in 2011-2012, stratified by Cambodian province. The geometric mean IC50s (in nM) and 95% CIs are shown by the horizontal line and whiskers, respectively. *, P ≤ 0.001.
Table 2.
Pairwise correlations between IC50s for six antimalarial drugsa
| Drugs correlated | n | r | P value |
|---|---|---|---|
| ATS vs DHA | 260 | 0.701 | <0.001 |
| CQ vs QN | 252 | 0.600 | <0.001 |
| QN vs ATS | 252 | 0.589 | <0.001 |
| MQ vs QN | 251 | 0.545 | <0.001 |
| MQ vs ATS | 246 | 0.449 | <0.001 |
| QN vs DHA | 251 | 0.407 | <0.001 |
| CQ vs ATS | 247 | 0.337 | <0.001 |
| MQ vs DHA | 245 | 0.312 | <0.001 |
| CQ vs DHA | 246 | 0.281 | <0.001 |
| PPQ vs DHA | 221 | 0.183 | 0.096 |
| CQ vs PPQ | 222 | 0.172 | 0.150 |
| QN vs PPQ | 225 | 0.104 | 1.000 |
| CQ vs MQ | 248 | 0.108 | 1.000 |
| MQ vs PPQ | 221 | 0.087 | 1.000 |
| PPQ vs ATS | 222 | 0.042 | 1.000 |
Data for P. falciparum isolates from Pursat, Preah Vihear, and Ratanakiri were combined in this analysis. CQ, chloroquine; MQ, mefloquine; QN, quinine; PPQ, piperaquine; ATS, artesunate; DHA, dihydroartemisinin; n, number of isolates. Boldface indicates a statistically significant difference.
The pfmdr1 copy number was estimated successfully for all 360 P. falciparum isolates. Overall, the mean (range) pfmdr1 copy number was 1.8 (0.5 to 5.3) in Pursat, 1.5 (0.6 to 5.7) in Preah Vihear, and 1.1 (0.6 to 4.7) in Ratanakiri. The proportion of parasites with ≥2 pfmdr1 copies was significantly higher in Pursat (33%) than in Preah Vihear (15%, P < 0.001) and in Preah Vihear than in Ratanakiri (8%, P < 0.001) (Table 3). Table 4 shows the GMIC50s for all six drugs, stratified by pfmdr1 copy number. Compared to parasites with only one copy of pfmdr1, those with two or more copies of pfmdr1 had 28 to 50% higher GMIC50s for MQ, QN, ATS, and DHA (P ≤ 0.001) but not for CQ and PPQ. When data from Pursat were analyzed independently, parasites with only one copy of pfmdr1 had significantly higher GMIC50s for CQ (398.3 nM versus 270.8 nM, P < 0.001) and lower GMIC50s for MQ (13.3 nM versus 25.1 nM, P < 0.001).
Table 3.
pfmdr1 copy number in P. falciparum isolates in 2011-2012, stratified by Cambodian province
| pfmdr1 copy no. | Cutoff range | No. (%) of isolates |
||
|---|---|---|---|---|
| Pursat | Preah Vihear | Ratanakiri | ||
| 1 | 0.52–1.69 | 80 (67) | 102 (85a) | 111 (92b) |
| 2 | 1.70–2.49 | 12 (10) | 10 (8) | 8 (7) |
| 3 | 2.50–3.49 | 15 (12) | 2 (2) | 0 (0) |
| 4 | 3.50–4.49 | 8 (7) | 1 (1) | 0 (0) |
| 5 | 4.50–5.70 | 5 (4) | 5 (4) | 1 (1) |
P < 0.001, calculated using the Mann-Whitney test to compare the pfmdr1 copy number between Pursat and Preah Vihear.
P < 0.001, calculated using the Mann-Whitney test to compare the pfmdr1 copy number between Preah Vihear and Ratanakiri.
Table 4.
Ex vivo susceptibility of P. falciparum isolates to six antimalarial drugs in 2011-2012, stratified by pfmdr1 copy number
| Drug | One pfmdr1 copy |
Multiple pfmdr1 copies |
P valuea | ||
|---|---|---|---|---|---|
| nb | GMc (range) IC50 (nM) | n | GM (range) IC50 (nM) | ||
| Chloroquine | 205 | 231.6 (11.7–775.1) | 48 | 250.5 (38.3–880.2) | 0.536 |
| Mefloquine | 204 | 15.7 (1.7–55.4) | 48 | 22.9 (4.3–64.1) | <0.001 |
| Quinine | 208 | 166.9 (23.2–620.2) | 49 | 250.7 (61.8–605.6) | <0.001 |
| Piperaquine | 178 | 22.1 (4.9–83.8) | 49 | 20.2 (2.5–73.1) | 0.727 |
| Artesunate | 210 | 2.5 (0.4–8.8) | 51 | 3.7 (1.2–10.0) | <0.001 |
| DHA | 209 | 1.8 (0.5–6.2) | 51 | 2.3 (0.6–6.9) | 0.001 |
P values were calculated using the Mann-Whitney test. Boldface indicates a statistically significant difference.
n, number of isolates.
GM, geometric mean.
The X5r copy number was estimated successfully for all 66 Pursat parasite isolates for which a PPQ IC50 was obtained. Of the 11 X5r markers tested, only two showed copy number variation in Pursat: PF3D7_0520500 (1, 2, or 3 copies) and PF3D7_0520900 (1 or 2 copies). Parasites with multiple copies of PF3D7_0520500 (n = 16) had slightly higher PPQ IC50s than those with one copy of PF3D7_0520500 (n = 50) (GMIC50, 26.5 nM versus 19.5 nM; P = 0.082), but the difference was not significant. Only 4.5% (3/66) of parasites from Pursat had two copies of PF3D7_0520900. In a subset of 30 parasites from Ratanakiri, PF3D7_0520500 and PF3D7_0520900 showed no copy number variation.
DISCUSSION
The epicenter for emerging antimalarial drug resistance in P. falciparum has historically been western Cambodia, where clinical resistance to both ATS-MQ and ATS monotherapy has been reported over the past 10 years (1, 2, 23, 24). A recent report that parasite recrudescence rates after DHA-PPQ therapy in Pailin and Pursat were 25% and 11% (14), respectively, is particularly worrisome, suggesting that the first-line artemisinin-based combination therapy (ACT) for these provinces is already failing. While ART resistance is well documented in western Cambodia (23–26), PPQ resistance has not been reported from any area where malaria is endemic in recent years. To monitor P. falciparum resistance to first- and second-line antimalarial therapies, we used a SYBR green I fluorescence assay to measure the ex vivo susceptibilities of parasites to currently used drugs. This inexpensive method yields a high proportion of interpretable data and generates IC50s similar to those obtained using the “gold standard” isotopic method (20). Clinical parasite isolates were obtained from Pursat, western Cambodia (where MDR parasites have been identified); Preah Vihear, northern Cambodia (where MDR parasites are expected to emerge); and Ratanakiri, eastern Cambodia (where MDR parasites have not been documented). Our study, which defines baseline ex vivo IC50s for six antimalarials in three provinces for reference in ongoing surveillance efforts, indicates that MDR P. falciparum is now present in a major region of northern Cambodia where the efficacy of DHA-PPQ therapy was 100% as recently as 2009 (14).
Our study confirms the high prevalence in western Cambodia of MDR parasites showing reduced ex vivo susceptibility to CQ, MQ, QN, and ART derivatives, as well as an increased pfmdr1 copy number. Compared to parasites from Ratanakiri, those from Preah Vihear showed increased IC50s to CQ, MQ, and QN, as well as an increased pfmdr1 copy number, suggesting that MDR parasites have spread to or independently emerged in this northern Cambodian province. The finding that 8% of parasites from Ratanakiri have an increased pfmdr1 copy number suggests that MQ resistance may be spreading to or emerging independently in eastern Cambodia. The recent identification of multiple ART-resistant P. falciparum subpopulations (KH2, KH3, and KH4) predominating in Pursat and one ART-sensitive subpopulation (KH1) predominating in Ratanakiri (27) suggests that these parasite subpopulations may also differ in their ex vivo IC50s for CQ, MQ, and QN, all of which differ substantially between the two provinces. This possibility is supported by the presence of two parasite groups (PG1 and PG2) in Pursat which differ significantly in their ex vivo IC50s for MQ (23). Whether increases in antimalarial IC50s and pfmdr1 copy number in Preah Vihear reflect the spread of KH2, KH3, and KH4 parasites from western to northern Cambodia requires further investigation.
While nearly all Cambodian parasites showed reduced ex vivo susceptibility to CQ (IC50 > 100 nM) in this study, the GMIC50s for CQ differed significantly by province. Specifically, these GMIC50s decreased from Pursat to Preah Vihear to Ratanakiri, thus corresponding with the historical pattern of CQ resistance in Cambodia. Unlike previous studies (16, 28), the present study found no correlations between the ex vivo IC50s for CQ and MQ. Two previous studies have associated particular P. falciparum crt (pfcrt) genotypes with levels of ex vivo CQ susceptibility in Cambodian parasite isolates (29, 30). Understanding the relationships between ex vivo CQ IC50s and pfcrt haplotypes will likely benefit from more detailed analysis of whole-genome sequence data and an accounting of the P. falciparum population structure (27). How the variation in CQ IC50s and pfcrt haplotypes will be impacted by Cambodia's recent withdrawal of CQ as the first-line treatment for P. vivax malaria awaits future studies. To address these questions, all parasite isolates for which we have ex vivo IC50 data are presently being Illumina sequenced and genotyped at 420,000 worldwide single nucleotide polymorphisms (SNPs) in an effort to relate coding SNPs known or suspected to be associated with drug resistance to our ex vivo IC50 data.
In the present study, the GMIC50 for MQ was slightly lower in Pursat than in Preah Vihear, but this difference was not significant. Nevertheless, this pattern differs from that observed for MQ in a previous study (16) and for other quinolines (i.e., CQ and QN) in the present study, in which parasites showed significantly higher GMIC50s in Pursat than in Preah Vihear. One potential explanation for this is that MQ pressure was removed from Pursat in 2008 after ATS-MQ was replaced with DHA-PPQ as first-line therapy for P. falciparum malaria in western Cambodia. Our findings are consistent with those of Imwong et al., who found that the pfmdr1 copy number was significantly lower in parasite isolates obtained from western Cambodia in 2007 than in those obtained in 2005 (31). They are also consistent with experimental data showing that pfmdr1 amplification is associated with decreased in vitro survival fitness in the absence of MQ exposure (32). The recent alleviation of MQ pressure in Cambodia may thus lead to rapid outbreeding of parasite populations in the near future. On the other hand, the continued use of ART derivatives in Cambodia may also be maintaining or increasing MQ IC50s. In support of this possibility, we found significant correlations between the IC50s for MQ and those for ATS and DHA, suggesting cross-resistance between MQ and ART derivatives. Also, one study suggests that P. falciparum lines have the capacity to develop resistance to ART derivatives in vitro by amplifying and increasing expression of pfmdr1 (33), which is associated with MQ resistance. An improved understanding of the relationships between pfmdr1 copy number, MQ IC50s, and ATS-MQ failure rates requires coordinated studies of ex vivo drug susceptibility and in vivo drug efficacy.
Despite the more extensive use of PPQ in western Cambodia and the recent report of frequent DHA-PPQ treatment failures in Pailin and Pursat (14), we found that the ex vivo susceptibility of clinical parasite isolates to PPQ did not differ between Pursat, Preah Vihear, and Ratanakiri. This suggests that resistance to other quinoline drugs (e.g., CQ, MQ, and QN) does not induce significant cross-resistance to PPQ. In support of this, we found no correlations between the IC50s for PPQ and those for CQ, MQ, and QN. While presently there is no IC50 cutoff value for defining ex vivo PPQ resistance, we did find that PPQ IC50s do vary considerably, ranging from 2.5 to 83.8 nM in Pursat. To explore whether a candidate PPQ resistance marker (increased X5r copy number) (17) is associated with the natural variation in this phenotype, we genotyped Pursat parasites for copy number variation in 11 gene markers spanning X5r. While we did find copy number variation in PF3D7_0520500 (1, 2, or 3 copies) and PF3D7_0520900 (1 or 2 copies), an increased PF3D7_0520500 copy number did not significantly associate with higher ex vivo PPQ IC50s in Pursat, although the sample size was relatively small. As previously reported for Thai parasite isolates (34), we found no copy number variation in the other nine X5r gene markers tested. Whether ongoing use of PPQ-based ACTs in Cambodia and Thailand may eventually select for an increased X5r copy number awaits future studies. Analysis of ex vivo PPQ IC50s and whole-genome sequence data may reveal additional candidate molecular markers for PPQ resistance. The failure to demonstrate regional differences in parasite susceptibility to PPQ, while showing clear differences for the other drugs, does not exclude the possibility of the emergence of PPQ resistance in P. falciparum. However, it does suggest that the poor therapeutic responses to DHA-PPQ recently observed in western Cambodia are caused by parasite resistance to DHA and not to PPQ.
The ex vivo susceptibilities of parasite isolates to ATS and DHA were higher in Pursat and Preah Vihear than in Ratanakiri, but the clinical relevance of this finding is limited for two reasons. First, the mean IC50s for ATS and DHA are small relative to the variation in the range of IC50s at all three sites. Second, while parasite clearance rates in response to ATS are slower in western than in eastern Cambodia, parasite clearance half-lives do not correlate with DHA IC50s (23). Instead, several lines of evidence suggest that ART resistance reflects reduced ring-stage susceptibility to DHA, and so 48- to 72-h drug exposures in conventional in vitro tests may not capture the important differences between ART-resistant and ART-susceptible parasites. In a novel ring-stage survival assay (RSA) (35), Witkowski et al. found that in vitro-adapted parasites from Pailin survive high-dose pulses of DHA much better than those from Ratanakiri (35). The parasite survival rates measured in the RSA also do not correlate with IC50s for ART derivatives (35). Whether these survival rates correlate with parasite clearance half-lives is under investigation. While the SYBR green I fluorescence assay may be less useful than the RSA for studying the in vivo ART resistance phenotype of slow parasite clearance, ex vivo drug susceptibility data may inform basic scientific and clinical investigations of drug cross-resistance phenomena. Measuring the ex vivo susceptibility of P. falciparum isolates also has an important role in the surveillance of ACT partner drug resistance, the identification of molecular markers for drug-resistant parasites, and the confirmation of clinical ACT failures.
Supplementary Material
ACKNOWLEDGMENTS
This study was made possible by the gracious participation of the patients and their families and the dedication of provincial health department staff in Pursat, Preah Vihear, and Ratanakiri. We thank Mehul Dhorda, Robert Gwadz, Phansakunthea Hing, Lo Koung, Kean Hong Ky, Pengby Ngor, Vunsokserey Ou, Sonlay Sim, Khlem Sokun, Khoy Bun Thanny, and Thomas Wellems for their efforts in support of this work.
E.A.A., O.M., A.M.D., and N.J.W. are supported by the Wellcome Trust as part of the Wellcome Trust Mahidol University-Oxford Tropical Medicine Research Programme. This study was funded by the Intramural Research Program of the NIAID, NIH, and the Department for International Development, United Kingdom, as part of the Tracking Resistance to Artemisinins Collaboration (TRAC).
P.L., D.D., V.T., R.T.E., S.C., O.M., X.-Z.S., D.M., and R.M.F. performed laboratory studies to test P. falciparum isolates in drug susceptibility and molecular assays and analyzed the data. P.L., S. Sreng, S. Suon, S.M., C.S., B.S., E.A.A., A.M.D., N.J.W., M.C.C., J.M.A., C.A., and R.M.F. conducted and supervised the clinical protocol. P.L. and R.M.F. designed the study. P.L., D.M., and R.M.F. wrote the initial manuscript and incorporated edits from all authors.
None of the authors has a competing financial interest.
Footnotes
Published ahead of print 12 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00687-13.
REFERENCES
- 1.Denis MB, Tsuyuoka R, Poravuth Y, Narann TS, Seila S, Lim C, Incardona S, Lim P, Sem R, Socheat D, Christophel EM, Ringwald P. 2006. Surveillance of the efficacy of artesunate and mefloquine combination for the treatment of uncomplicated falciparum malaria in Cambodia. Trop. Med. Int. Health 11:1360–1366 [DOI] [PubMed] [Google Scholar]
- 2.Rogers WO, Sem R, Tero T, Chim P, Lim P, Muth S, Socheat D, Ariey F, Wongsrichanalai C. 2009. Failure of artesunate-mefloquine combination therapy for uncomplicated Plasmodium falciparum malaria in southern Cambodia. Malar. J. 8:10. 10.1186/1475-2875-8-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adam I, Salah MT, Eltahir HG, Elhassan AH, Elmardi KA, Malik EM. 2010. Dihydroartemisinin-piperaquine versus artemether-lumefantrine, in the treatment of uncomplicated Plasmodium falciparum malaria in central Sudan. Ann. Trop. Med. Parasitol. 104:319–326 [DOI] [PubMed] [Google Scholar]
- 4.Bassat Q, Mulenga M, Tinto H, Piola P, Borrmann S, Menendez C, Nambozi M, Valea I, Nabasumba C, Sasi P, Bacchieri A, Corsi M, Ubben D, Talisuna A, D'Alessandro U. 2009. Dihydroartemisinin-piperaquine and artemether-lumefantrine for treating uncomplicated malaria in African children: a randomised, non-inferiority trial. PLoS One 4:e7871. 10.1371/journal.pone.0007871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Four Artemisinin-Based Combinations (4ABC) Study Group 2011. A head-to-head comparison of four artemisinin-based combinations for treating uncomplicated malaria in African children: a randomized trial. PLoS Med. 8:e1001119. 10.1371/journal.pmed.1001119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denis MB, Davis TM, Hewitt S, Incardona S, Nimol K, Fandeur T, Poravuth Y, Lim C, Socheat D. 2002. Efficacy and safety of dihydroartemisinin-piperaquine (Artekin) in Cambodian children and adults with uncomplicated falciparum malaria. Clin. Infect. Dis. 35:1469–1476 [DOI] [PubMed] [Google Scholar]
- 7.Janssens B, van Herp M, Goubert L, Chan S, Uong S, Nong S, Socheat D, Brockman A, Ashley EA, Van Damme W. 2007. A randomized open study to assess the efficacy and tolerability of dihydroartemisinin-piperaquine for the treatment of uncomplicated falciparum malaria in Cambodia. Trop. Med. Int. Health 12:251–259 [DOI] [PubMed] [Google Scholar]
- 8.Myint HY, Ashley EA, Day NP, Nosten F, White NJ. 2007. Efficacy and safety of dihydroartemisinin-piperaquine. Trans. R. Soc. Trop. Med. Hyg. 101:858–866 [DOI] [PubMed] [Google Scholar]
- 9.Song J, Socheat D, Tan B, Seila S, Xu Y, Ou F, Sokunthea S, Sophorn L, Zhou C, Deng C, Wang Q, Li G. 2011. Randomized trials of artemisinin-piperaquine, dihydroartemisinin-piperaquine phosphate and artemether-lumefantrine for the treatment of multi-drug resistant falciparum malaria in Cambodia-Thailand border area. Malar. J. 10:231. 10.1186/1475-2875-10-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tjitra E, Hasugian AR, Siswantoro H, Prasetyorini B, Ekowatiningsih R, Yusnita EA, Purnamasari T, Driyah S, Salwati E, Yuwarni E, Januar L, Labora J, Wijayanto B, Amansyah F, Dedang TA, Purnama A. 2012. Efficacy and safety of artemisinin-naphthoquine versus dihydroartemisinin-piperaquine in adult patients with uncomplicated malaria: a multi-centre study in Indonesia. Malar. J. 11:153. 10.1186/1475-2875-11-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran TH, Nguyen TT, Nguyen HP, Boni MF, Ngo VT, Nguyen TN, Le HT, Cao QT, Pham VT, Phung DT, Le TL, Le TD, Merson L, Dolecek C, Stepniewska K, Ringwald P, White NJ, Farrar J, Wolbers M. 2012. In vivo susceptibility of Plasmodium falciparum to artesunate in Binh Phuoc Province, Vietnam. Malar. J. 11:355. 10.1186/1475-2875-11-355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valecha N, Phyo AP, Mayxay M, Newton PN, Krudsood S, Keomany S, Khanthavong M, Pongvongsa T, Ruangveerayuth R, Uthaisil C, Ubben D, Duparc S, Bacchieri A, Corsi M, Rao BH, Bhattacharya PC, Dubhashi N, Ghosh SK, Dev Kumar VA, Pukrittayakamee S. 2010. An open-label, randomised study of dihydroartemisinin-piperaquine versus artesunate-mefloquine for falciparum malaria in Asia. PLoS One 5:e11880. 10.1371/journal.pone.0011880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zwang J, Ashley EA, Karema C, D'Alessandro U, Smithuis F, Dorsey G, Janssens B, Mayxay M, Newton P, Singhasivanon P, Stepniewska K, White NJ, Nosten F. 2009. Safety and efficacy of dihydroartemisinin-piperaquine in falciparum malaria: a prospective multi-centre individual patient data analysis. PLoS One 4:e6358. 10.1371/journal.pone.0006358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leang R, Barrette A, Bouth DM, Menard D, Abdur R, Duong S, Ringwald P. 2013. Efficacy of dihydroartemisinin-piperaquine for treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax in Cambodia, 2008 to 2010. Antimicrob. Agents Chemother. 57:818–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis TM, Hung TY, Sim IK, Karunajeewa HA, Ilett KF. 2005. Piperaquine: a resurgent antimalarial drug. Drugs 65:75–87 [DOI] [PubMed] [Google Scholar]
- 16.Lim P, Wongsrichanalai C, Chim P, Khim N, Kim S, Chy S, Sem R, Nhem S, Yi P, Duong S, Bouth DM, Genton B, Beck HP, Gobert JG, Rogers WO, Coppee JY, Fandeur T, Mercereau-Puijalon O, Ringwald P, Le Bras J, Ariey F. 2010. Decreased in vitro susceptibility of Plasmodium falciparum isolates to artesunate, mefloquine, chloroquine, and quinine in Cambodia from 2001 to 2007. Antimicrob. Agents Chemother. 54:2135–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eastman RT, Dharia NV, Winzeler EA, Fidock DA. 2011. Piperaquine resistance is associated with a copy number variation on chromosome 5 in drug-pressured Plasmodium falciparum parasites. Antimicrob. Agents Chemother. 55:3908–3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basco LK, Ringwald P. 2003. In vitro activities of piperaquine and other 4-aminoquinolines against clinical isolates of Plasmodium falciparum in Cameroon. Antimicrob. Agents Chemother. 47:1391–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacon DJ, Latour C, Lucas C, Colina O, Ringwald P, Picot S. 2007. Comparison of a SYBR green I-based assay with a histidine-rich protein II enzyme-linked immunosorbent assay for in vitro antimalarial drug efficacy testing and application to clinical isolates. Antimicrob. Agents Chemother. 51:1172–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. 2004. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 48:1803–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Nagard H, Vincent C, Mentre F, Le Bras J. 2011. Online analysis of in vitro resistance to antimalarial drugs through nonlinear regression. Comput. Methods Programs Biomed. 104:10–18 [DOI] [PubMed] [Google Scholar]
- 22.Ferreira ID, Rosario VE, Cravo PV. 2006. Real-time quantitative PCR with SYBR green I detection for estimating copy numbers of nine drug resistance candidate genes in Plasmodium falciparum. Malar. J. 5:1. 10.1186/1475-2875-5-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amaratunga C, Sreng S, Suon S, Phelps ES, Stepniewska K, Lim P, Zhou C, Mao S, Anderson JM, Lindegardh N, Jiang H, Song J, Su XZ, White NJ, Dondorp AM, Anderson TJ, Fay MP, Mu J, Duong S, Fairhurst RM. 2012. Artemisinin-resistant Plasmodium falciparum in Pursat Province, western Cambodia: a parasite clearance rate study. Lancet Infect. Dis. 12:851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 359:2619–2620 [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization 2010. Global report on antimalarial drug efficacy and drug resistance: 2000–2010. World Health Organization, Geneva, Switzerland [Google Scholar]
- 27.Miotto O, Almagro-Garcia J, Manske M, Macinnis B, Campino S, Rockett KA, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Duong S, Nguon C, Chuor CM, Saunders D, Se Y, Lon C, Fukuda MM, Amenga-Etego L, Hodgson AV, Asoala V, Imwong M, Takala-Harrison S, Nosten F, Su XZ, Ringwald P, Ariey F, Dolecek C, Hien TT, Boni MF, Thai CQ, Amambua-Ngwa A, Conway DJ, Djimde AA, Doumbo OK, Zongo I, Ouedraogo JB, Alcock D, Drury E, Auburn S, Koch O, Sanders M, Hubbart C, Maslen G, Ruano-Rubio V, Jyothi D, Miles A, O'Brien J, Gamble C, Oyola SO, et al. 2013. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat. Genet. 45:648–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim P, Chim P, Sem R, Nemh S, Poravuth Y, Lim C, Seila S, Tsuyuoka R, Denis MB, Socheat D, Fandeur T. 2005. In vitro monitoring of Plasmodium falciparum susceptibility to artesunate, mefloquine, quinine and chloroquine in Cambodia: 2001-2002. Acta Trop. 93:31–40 [DOI] [PubMed] [Google Scholar]
- 29.Durrand V, Berry A, Sem R, Glaziou P, Beaudou J, Fandeur T. 2004. Variations in the sequence and expression of the Plasmodium falciparum chloroquine resistance transporter (Pfcrt) and their relationship to chloroquine resistance in vitro. Mol. Biochem. Parasitol. 136:273–285 [DOI] [PubMed] [Google Scholar]
- 30.Lim P, Chy S, Ariey F, Incardona S, Chim P, Sem R, Denis MB, Hewitt S, Hoyer S, Socheat D, Merecreau-Puijalon O, Fandeur T. 2003. pfcrt polymorphism and chloroquine resistance in Plasmodium falciparum strains isolated in Cambodia. Antimicrob. Agents Chemother. 47:87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imwong M, Dondorp AM, Nosten F, Yi P, Mungthin M, Hanchana S, Das D, Phyo AP, Lwin KM, Pukrittayakamee S, Lee SJ, Saisung S, Koecharoen K, Nguon C, Day NP, Socheat D, White NJ. 2010. Exploring the contribution of candidate genes to artemisinin resistance in Plasmodium falciparum. Antimicrob. Agents Chemother. 54:2886–2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preechapornkul P, Imwong M, Chotivanich K, Pongtavornpinyo W, Dondorp AM, Day NP, White NJ, Pukrittayakamee S. 2009. Plasmodium falciparum pfmdr1 amplification, mefloquine resistance, and parasite fitness. Antimicrob. Agents Chemother. 53:1509–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chavchich M, Gerena L, Peters J, Chen N, Cheng Q, Kyle DE. 2010. Role of pfmdr1 amplification and expression in induction of resistance to artemisinin derivatives in Plasmodium falciparum. Antimicrob. Agents Chemother. 54:2455–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veiga MI, Ferreira PE, Malmberg M, Jornhagen L, Bjorkman A, Nosten F, Gil JP. 2012. pfmdr1 amplification is related to increased Plasmodium falciparum in vitro sensitivity to the bisquinoline piperaquine. Antimicrob. Agents Chemother. 56:3615–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witkowski B, Khim N, Chim P, Kim S, Ke S, Kloeung N, Chy S, Duong S, Leang R, Ringwald P, Dondorp AM, Tripura R, Benoit-Vical F, Berry A, Gorgette O, Ariey F, Barale JC, Mercereau-Puijalon O, Menard D. 2013. Reduced artemisinin susceptibility of Plasmodium falciparum ring stages in western Cambodia. Antimicrob. Agents Chemother. 57:914–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.