Abstract
Drug resistance has become a global threat that, if not addressed, may return us to the preantibiotic era. A way to overcome the problem of growing incidence of global antibiotic resistance is to introduce compounds belonging to classes that are new to the clinic. During a screening of the marine microbial extract library for new antibiotics, one of the extracts showed promising antibacterial activity against Gram-positive organisms. Bioactivity-guided isolation and characterization of active metabolites led to the discovery of a novel thiazolyl cyclic-peptide antibiotic, PM181104. It was isolated and characterized from a marine sponge-associated actinobacterium strain of the genus Kocuria (MTCC 5269). The compound exhibited a potent in vitro antibacterial activity against a broad range of Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE). The MIC values evaluated for the compound were found to be in the single-digit nanomolar range. In in vivo studies of PM181104 in a BALB/c murine septicemia model, the compound displayed 100% effective dose (ED100) values of 2.5 and 5.0 mg/kg of body weight against MRSA and 10.0 mg/kg against VRE. In this report, in vitro and in vivo studies of PM181104 are described.
INTRODUCTION
The past 2 decades have witnessed a dramatic rise in the prevalence of antibiotic resistance. This poses a significant clinical problem and has become a growing threat to public health worldwide. Infections caused by methicillin-resistant Staphylococcus aureus (MRSA), penicillin-resistant Streptococcus pneumoniae (PRSP), and vancomycin-resistant enterococci (VRE) are instances of such concern, as many of these organisms have developed resistance to several classes of established antibiotics (1). The most significant problem observed in clinical practice is the increase in incidences of MRSA infections. Numerous reports of emerging vancomycin resistance in some of the MRSA isolates have also been recorded (2, 3). The prospect for a major antibiotics emergency is well recapitulated by the Infectious Diseases Society of America (IDSA) (4, 5), which reports that there are only a few potential drugs in clinical development that provide significant benefits over existing drugs and that target hospital-based infections caused by Gram-negative organisms. Linezolid, a well-known antibiotic belonging to the oxazolidinone class of compounds, and daptomycin, a new cyclic lipopeptide, are currently considered the drugs of choice for the treatment of MRSA infections. However, due to rising incidences of resistance to even these products, there exists a need for the development of antibacterial agents with features aimed to act on novel targets (6, 7). Hence, the search for antibiotics with new scaffolds is of utmost importance at this juncture. While the search for new and novel classes of molecules continues, mining of marine microbial products for such molecules is considered an attractive proposition, and these products have been receiving immense interest from the majority of drug discovery organizations all over the globe (8).
In our continuing efforts for finding a new and novel class of antibiotics and other drug candidates from marine microorganisms, we have discovered a novel and potent thiazolyl cyclic-peptide antibiotic molecule, PM181104, from the sponge-associated actinobacterium strain of the genus Kocuria. It was isolated and characterized from the marine sponge Spirastrella inconstans var. digitata collected from Palk Bay in Tamil Nadu, India (9). A recent report from February 2013 (10) claims to have isolated a molecule called kocurin from a marine-derived strain of Kocuria palustris F-276,345. Kokurin was also reported to have been isolated from Kocuria sp. strain MI-67-EC3-038 (11). Interestingly, the former report (10) claims that kocurin and compound PM181104 are identical molecules, and the structure of kocurin (Fig. 1) is indeed the more acceptable structure of PM181104. Compound PM181104 has been studied by us extensively and has been discovered to possess potent in vitro anti-Gram-positive activity and in vivo efficacy in different murine models. In the current report, details of our in vitro and in vivo studies are presented.
Fig 1.
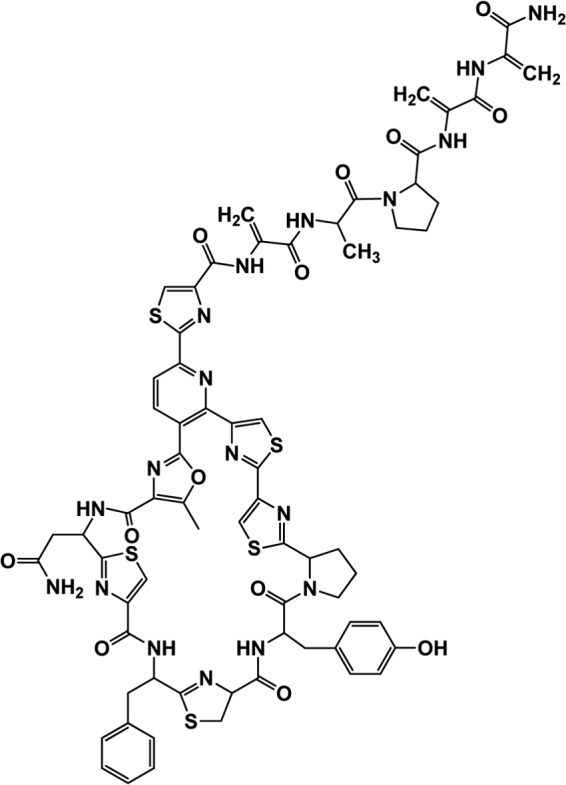
Structure of PM181104 (kocurin) (10).
MATERIALS AND METHODS
General experimental procedures.
All reagents and chemicals were obtained from Sigma and Merck unless otherwise specified. Linezolid was obtained from Glenmark Pharma Ltd. Amphotericin B, fluconazole, and vancomycin were purchased from Hi-Media. UV spectra were recorded on a Jasco spectrophotometer equipped with a Peltier temperature control unit. The sample was dissolved in dimethyl sulfoxide (DMSO). All relevant animal procurement and study protocols were reviewed, and requisite permissions were obtained from the Institutional Animal Safety Committee before initiating the in vivo efficacy studies.
In vitro antibacterial activity.
MIC values for the test and standard antibiotics were determined in Mueller-Hinton broth by the broth macrodilution method according to NCCLS guidelines, as per document no. M7-A5 (12). The medium with a 2-fold serial dilution of compound in Mueller-Hinton broth was inoculated with 105 CFU/ml of test culture and incubated at 37°C for 24 h. The MIC of PM181104 against 261 test organisms was determined, including clinical and standard strains of S. aureus, both MRSA and methicillin-sensitive S. aureus (MSSA), clinical and in-house strains of enterococci, both VRE and vancomycin-sensitive enterococci (VSE), Staphylococcus epidermidis strains, Bacillus spp., and 17 Gram-negative strains. American Type Culture Collection (ATCC) strains were also screened. Methanol was used as a solvent for making stock solutions of the test compound. Appropriate controls (positive, negative, solvent, and medium) were used in the assay, and the tubes were observed for growth of test cultures in terms of turbidity visible to the naked eye after 24 h of incubation. The MIC values of the compounds were recorded in μg/ml and expressed as MIC range, MIC50, and MIC90 for the test compound. MIC90 and MIC50 values were defined as the lowest concentrations of the antibiotic at which 90% and 50% of the isolates were inhibited, respectively. Linezolid and vancomycin were used as standard antibacterial agents.
In vivo activity of PM181104. (i) Preparation of bacterial culture for infection.
MRSA (strains E710 and ATCC 33591) and VRE (strain ATCC 47077) cultures were grown overnight in brain heart infusion broth. Log-phase cultures were prepared by subculturing the overnight growth into fresh broth and incubating for 2 h. The log-phase cultures were centrifuged, and the cell pellets were suspended in normal saline (0.85% sodium chloride solution). These culture suspensions were diluted further in normal saline to obtain the required CFU of bacteria for infection. To test the efficacy of PM181104 in animals, an injectable formulation comprising 6% (wt/wt) PEG 400 and 8% (wt/wt) Tween 80 was used. PM181104 was dissolved in this mixture and then diluted using sterile water for injection.
(ii) Efficacy of PM181104 against MRSA in a septicemia model.
Seven different groups of BALB/c mice (6 to 8 weeks of age, 19 to 22 g) were selected, each with 6 mice, which were infected intraperitoneally (i.p.) with 0.1 ml of MRSA strain E710 culture containing 108 to 109 CFU of bacteria. The PM181104 formulation was administered at intravenous (i.v.) doses of 1.25, 2.5, 5, and 10 mg/kg of body weight immediately postinfection to the assigned animals. One group received formulation excipients (vehicle control) by the i.v. route, and one group received 25 mg/kg standard antibiotic linezolid (batch number K2005028; Glenmark Pharma Ltd.) by the i.p. route. The dosing volume was 10 ml/kg body weight. One group of infected mice received no treatment (infection control). Mice were observed for mortality and morbidity for up to 10 days.
(iii) Efficacy of PM181104 against VRE in a septicemia model.
Six- to eight-week-old BALB/c mice were randomized into 6 groups (6 mice per group) and were infected intraperitoneally with 2.87 × 109 CFU of a VRE Enterococcus faecium strain ATCC 47077 culture. PM181104 was administered at i.v. doses of 2.5, 5, and 10 mg/kg immediately postinfection to 3 different groups. The vehicle control group received formulation excipients by the i.v. route, and the positive control (linezolid, 25 mg/kg) was by the i.p. route. The dosing volume was 10 ml/kg. One group of infected mice received no treatment (infection control). Mice were observed for mortality and morbidity for up to 10 days.
In vivo time-kill curve of VRE.
The neutropenic mice were infected in the thigh with 0.1 ml of VRE strain ATCC 47077 culture containing 4.16 × 106 CFU of bacteria. Three mice were sacrificed immediately postinfection, and their thighs were removed and processed for CFU determination. Two hours after infection, 30 mice were treated with a single i.v. dose of PM181104 (10 mg/kg) prepared as formulation PM-FO-52. The formulation contains 8% (wt/wt) PEG 400 and 8% (wt/wt) Tween 80. PM181104 was dissolved in this mixture, and the solution was then diluted using 0.9% saline. Another group of 30 mice were treated with blank formulation excipients (vehicle control) i.v., and the third group of 30 mice received 25 mg/kg linezolid intraperitoneally. The dosing volume was 10 ml/kg for all the groups. Six mice were sacrificed at the time of dosing, and their thighs were harvested for bacterial count determination. Six mice from each of the three treatment groups (PM181104, vehicle control, and standard antibiotic) were sacrificed at five sampling times over a time interval starting from 2 h postdosing up to 24 h postdosing. The thighs were removed at each time point and processed for CFU determination.
(iv) Efficacy of PM181104 against MRSA in a murine lung infection model.
Female BALB/c mice, weighing between 18 and 21 g, were rendered neutropenic by treatment with 200 mg/kg of cyclophosphamide (catalogue number C0768; Sigma) intraperitoneally at 4 and 2 days prior to infection. The mice were anesthetized through the i.p. route using a mixture of ketamine (50 mg/kg) and xylazine (10 mg/kg). Each animal was infected by placing 50 μl of bacterial inoculum containing approximately 2.7 × 106 CFU of MRSA strain E710 onto the tip of the nares. The animals were allowed to inhale the inoculum as small droplets and were then placed back into their respective cages for recovery and observation. Dosing of PM181104 was done 24 and 36 h postinfection. The dosing volume was 10 ml/kg for all the groups, and the group size for all experimental groups was 6 mice. PM181104 was administered at intravenous doses of 2.5, 5, and 10 mg/kg to the preassigned groups. Formulation excipients were administered by the same route and similar dosing volume to the control group; doses of 25 mg/kg standard antibiotic linezolid by the i.p. route and 110 mg/kg standard antibiotic vancomycin by the i.v. route were administered to the two positive-control groups. Mice were euthanized 48 h postinfection and their lungs collected for bacterial count determination.
(v) Efficacy of PM181104 against VRE in a murine kidney infection model.
The efficacy of PM181104 was tested in the λ-carrageenan-induced kidney infection model (13). Female BALB/c mice were pretreated with 0.2 ml of 2% λ-carrageenan, injected intravenously 7 days prior to bacterial challenge. VRE culture at a bacterial density of 8.29 × 109 CFU/ml was used to infect the mice. Mice were infected intravenously with 0.2 ml of the culture suspension containing 1.66 × 108 CFU. PM181104 was administered at 5, 10, and 15 mg/kg (i.v. dose) 4 h postinfection, which was continued once daily for 3 consecutive days. Two control groups, one treated with formulation excipients (group size, n = 4) and one without any treatment but only infection, formed part of the study. Two standard antibiotics were selected as positive controls: linezolid (25 mg/kg/day; i.p.) and vancomycin (150 mg/kg/day, i.v.) The group size was 4 to 6 mice. Mice were euthanized and their kidneys harvested 3 days postinfection.
RESULTS
In vitro activity.
Compound PM181104 exhibited potent growth-inhibitory activity against Gram-positive bacterial strains (Tables 1 and 2). It inhibited the growth of S. aureus (both MRSA and MSSA) with MICs in the range of 0.008 to 2.048 μg/ml and an MIC90 value of 0.064 μg/ml. The compound also acted on S. aureus carrying the cfr gene and on linezolid-resistant (LZDr) strains of S. aureus. It was effective against S. epidermidis with an MIC range of 0.008 to 1.024 μg/ml and an MIC90 value of 0.128 μg/ml. PM181104 showed better potency against VRE and VSE strains, exhibiting an MIC range of 0.004 to 1.024 μg/ml and an MIC90 value of 0.016 μg/ml. It also inhibited doxycycline-resistant strains of E. faecium and Enterococcus faecalis as well as linezolid- and gentamicin-resistant strains of E. faecalis. Clinical strains of enterococci were inhibited by the compound in the MIC range of 0.004 to 0.128 μg/ml with an MIC90 value of 0.064 μg/ml. Bacillus strains were also strongly inhibited by the compound, with an MIC range of 0.004 to 0.016 μg/ml. The compound is not active against either fungi or Gram-negative bacteria (data not shown). Thus, compound PM181104 showed very potent and specific in vitro activity against different strains of Gram-positive test organisms, including resistance strains. Moreover, our preliminary work indicated that antibiotic PM181104 inhibits bacterial growth by impeding its protein biosynthesis at the translation stage (14).
Table 1.
In vitro antibacterial activity of antibiotic PM181104
| Organism and resistance profilea (no. of test strains) | MIC range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) |
|---|---|---|---|
| S. aureus MRSA (67) | 0.008–2.048 | 0.032 | 0.064 |
| S. aureus MSSA (48) | 0.008–2.048 | 0.032 | 0.064 |
| S. aureus MRSA, inducible ERYr (1) | 1.024 | ||
| S. aureus carrying cfr (1) | 1.024 | ||
| S. aureus LZDr (6) | 0.250–0.500 | ||
| S. epidermidis (23) | 0.008–1.024 | 0.064 | 0.128 |
| E. faecium VSE (12) | 0.008–0.032 | 0.008 | 0.016 |
| E. faecalis VRE (12) | 0.064–1.024 | 0.064 | 0.128 |
| E. faecalis VSE (30) | 0.002–16 | 0.008 | 0.016 |
| E. faecium VRE (5) | 0.008–0.008 | ||
| E. faecium Dr (3) | 0.128–0.256 | ||
| E. faecalis LZDr (5) | 0.128–0.256 | ||
| E. faecalis GMr (6) | 0.128–0.512 | ||
| E. faecalis Dr (3) | 0.256–0.512 | ||
| Clinical VRE species (16) | 0.004–0.128 | 0.008 | 0.064 |
| Bacillus spp. (6) | 0.004–0.016 |
MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive S. aureus; ERYr, erythromycin resistant; LZDr, linezolid resistant; VRE, vancomycin-resistant enterococci; VSE, vancomycin-susceptible enterococci; Dr, doxycycline-resistant; GMr, gentamicin-resistant; cfr, gene that mediates the resistance phenotype (includes resistance to oxazolidinones, pleuromutilins-phenicols, lincosamides, streptogramin A antibiotics, etc.).
Table 2.
Comparison of in vitro antibacterial activities of PM181104 and standard antibiotic linezolida
| Organism (resistance profile)/no. of test strains | MIC (μg/ml) |
|
|---|---|---|
| PM181104 | Linezolid | |
| S. aureus ATCC 3066 (MRSA,b ERYr) | 0.032 | 0.512 |
| S. aureus E710 (MRSA, ERYr) | 0.016 | 2.560 |
| S. aureus ATCC 33591 (MRSAb) | 0.032 | 2.560 |
| E. faecium 02 D3 IP1 (VREd, Teichor) | 0.008 | 2.560 |
| E. faecium R-2 (VREd, vanA) | 0.008 | 2.560 |
| E. faecalis ATCC 29212 (VSEe) | 0.016 | 2.560 |
| E. faecium ATCC 19579 | 0.032 | 2.560 |
| E. faecalis ATCC 51299 (VREd, GMr, STRr, vanB) | 0.016 | 2.560 |
| E. faecalis ATCC 47077 | 0.032 | 1.280 |
| E. faecalis (LZDr)/5 | 0.128–0.256 | 8–32 |
| E. faecalis (GMr)/6c | 0.128–0.512 | 0.5–4 |
| E. faecalis (Dr)/3f | 0.256–0.512 | 1–2 |
MRSA, methicillin resistant Staphylococcus aureus; ERYr, erythromycin resistant; LZDr, linezolid resistant; VRE, vancomycin-resistant enterococci; VSE, vancomycin-susceptible enterococci; Dr, doxycycline resistant; GMr, gentamicin resistant; STRr, streptomycin resistant; Teichor, teichoplanin resistant.
MIC of oxacillin, >64 μg/ml.
MIC of gentamicin, >32 μg/ml.
MIC of vancomycin, >64 μg/ml.
MIC of vancomycin range, 0.5–2 μg/ml.
MIC of oxacillin range, 16–32 μg/ml.
To evaluate the cytotoxicity of PM181104 on mammalian cell lines, lung fibroblastic cells Wl-38 (a noncancerous cell line) were cultured and exposed to various concentrations of PM181104 (0.03 to 100 μg/ml) and checked for cell growth inhibition. The compound was found to be nontoxic up to 100 μg/ml (data not shown).
In vivo efficacy. (i) Efficacy of PM181104 against MRSA in the septicemia model.
Morbidity and mortality were observed in the vehicle control and infection control groups within 3 days of infection. The vehicle offered no protection from infection, as all the infected animals in this group died by day 3. At the 1.25-mg/kg dose, three mice survived up to day 10. All mice that received a 2.5- or a 5-mg/kg dose of PM181104 survived up to day 10 postinfection. Figure 2 shows a Kaplan-Meier survival plot of mice for up to 10 days.
Fig 2.
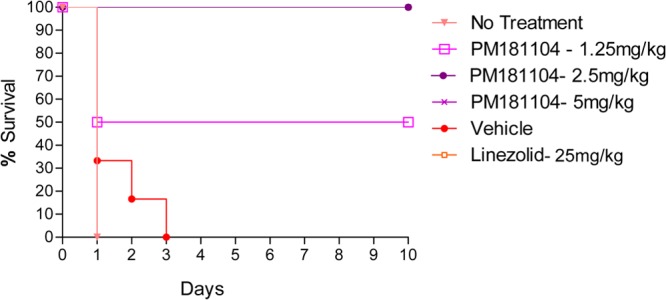
In vivo antibacterial activity of PM181104 against S. aureus E710 (MRSA) in the murine septicemia model when administered i.v. at 1.25 mg/kg, 2.5 mg/kg, and 5 mg/kg compared to the vehicle control, untreated control, and standard antibiotic, i.e., linezolid given i.p. at 25 mg/kg. Survival was monitored for 10 days.
(ii) Efficacy of PM181104 against VRE in the septicemia model.
A 100% mortality was observed in the vehicle control and infection control groups within 2 days of infection. At the 2.5-mg/kg dose, three mice survived up to day 10, and at the 5-mg/kg dose, 4 mice survived up to day 10. All mice that received the 10-mg/kg dose of PM181104 survived up to day 10 postinfection. Figure 3 shows the Kaplan-Meier survival plot of mice up to 10 days.
Fig 3.
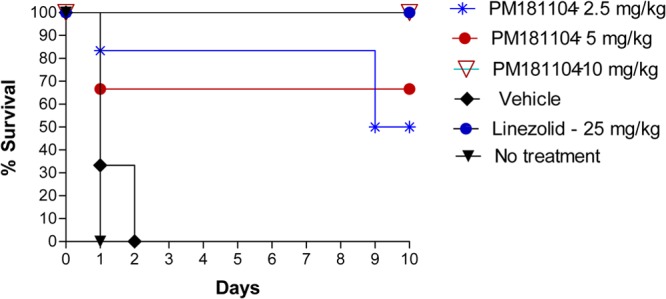
In vivo antibacterial activity of PM181104 against E. faecium ATCC 47077 (VRE) in the murine septicemia model when administered i.v. at 2.5 mg/kg, 5 mg/kg, and 10 mg/kg compared to the vehicle control, untreated control, and standard antibiotic, i.e., linezolid given i.p. at 25 mg/kg. Survival was monitored for 10 days.
(iii) In vivo time-kill curve of VRE.
The culture showed exponential growth phase up to 9 h in the control mice. In PM181104-treated mice, the bacterial growth up to 2 h was similar to that in the control group. However, between 4 and 9 h, there was an insignificant reduction in the bacterial growth in PM181104-treated mice compared to the control, with differences of 0.48 and 0.42 log CFU/g at 4 and 9 h, respectively, which was not comparable to that observed with linezolid. However, at 9 h the mean bacterial titer in PM181104-treated mice was on par with that of the linezolid standard. Figure 4 shows the mean log CFU/g for the three treatment groups at different time points up to 24 h.
Fig 4.
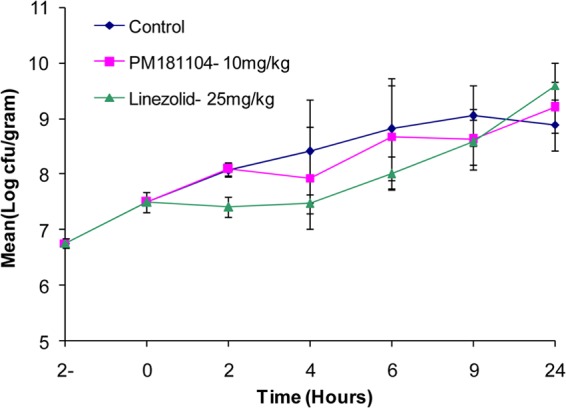
In vivo time-kill curve of E. faecium ATCC 47077 (VRE) in the neutropenic thigh model with i.v. administration of 10 mg/kg of PM181104 compared to the vehicle control and standard antibiotic, i.e., linezolid given i.p. at 25 mg/kg.
(iv) Efficacy of PM181104 against MRSA in the murine lung infection model.
The bacterial titer in the lungs of the treated mice was determined 48 h postinfection. PM181104 showed a decrease of 0.74 log CFU/g at the 2.5-mg/kg dose, 0.54 log CFU/g at the 5-mg/kg dose, and 0.68 log CFU/g at the 10-mg/kg dose compared to the control. The standard antibiotics linezolid and vancomycin showed bacterial count reductions of 0.90 log CFU/g and 1.56 log CFU/g, respectively, compared to the control. However, there was no dose response for PM181104 against MRSA in the murine lung infection model for reasons not fully understood. Further pharmacokinetic and tissue distribution studies may help us in a better understanding of PM181104 efficacy.
(v) Efficacy of PM181104 against VRE in the murine kidney infection model.
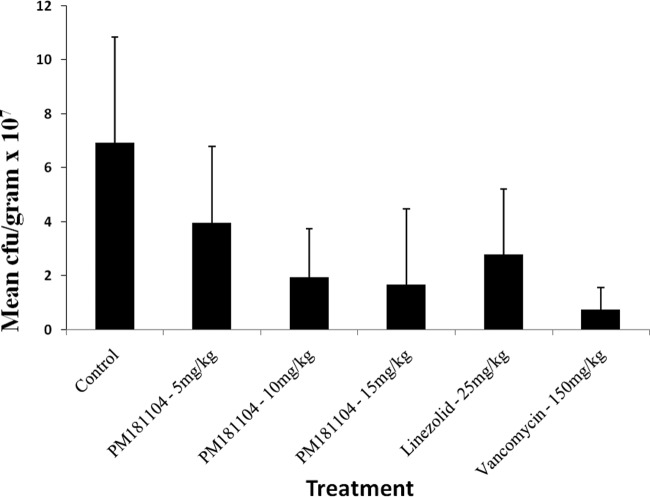
Untreated control mice showed no signs of systemic infection, and the infection was localized in the kidneys. PM181104 exhibited a decrease of 0.28 log CFU/g at the 5-mg/kg dose, 0.69 log CFU/g at the 10-mg/kg dose, and 0.73 log CFU/g at the 15-mg/kg dose compared to the infection control. PM181104 showed a dose-dependent reduction in bacterial titer in the kidney infection. The standard antibiotics linezolid and vancomycin showed bacterial count reductions of 0.53 log CFU/g and 1.31 log CFU/g, respectively, compared to the control. Figure 5 shows the mean bacterial titers ± standard deviations (SD) in the different treatment groups.
Fig 5.
In vivo antibacterial activity of PM181104 against E. faecium ATCC 47077 in the murine kidney infection model when administered intravenously once daily at 5 mg/kg, 10 mg/kg, and 15 mg/kg for 3 days, compared to the control and two standard antibiotics, i.e., linezolid given i.p. at 25 mg/kg and vancomycin given i.v. at 150 mg/kg.
DISCUSSION
Our continued efforts to discover novel antibiotic molecules from marine microbial sources have led to the discovery of PM181104, a potent thiazolyl cyclic-peptide molecule. In in vitro studies, the antibiotic PM181104 demonstrated potent anti-Gram-positive antibacterial activity, especially against drug-resistant bacteria such as MRSA and VRE. The in vitro activity translated well into the in vivo models. The molecule was able to protect the mice from systemic infection as well as from organ-specific infections. Antibiotic PM181104 or its variants reported elsewhere represent a promising scaffold for the treatment of Gram-positive bacterial infections, especially by S. aureus, as well as enterococcal infections caused by drug-resistant strains.
There are quite a few molecules reported from the class of PM181104 (10, 15). Most of them presented good in vitro activity against Gram-positive strains, especially MRSA. However, the development of this class of compounds as clinical agents has been hindered due to low aqueous solubility and unfavorable pharmacokinetics. For PM181104, after several attempts a suitable formulation was developed by us, which supported animal studies of PM181104.
In vivo efficacy of PM181104 was tested against MRSA in general-purpose models as well as tissue- or organ-specific infection models. In the general-purpose efficacy testing model of systemic disseminated septicemia, the mice were challenged with a lethal dose of MRSA strain E710 intraperitoneally and subsequently treated intravenously with different doses of PM181104. The compound showed 100% protection at a dose of 5 mg/kg.
Anti-infective activity of PM181104 for organ- or tissue-specific infection was evaluated in two mouse models: the skin abscess model (data not presented) and the lung infection model. The lung bacterial titer at 48 h postinfection in PM181104-treated mice showed a bacteriostatic effect with 2-log difference in the bacterial count compared to the control. In some of the models, the efficacy of PM181104 was comparable to that of the standard antibiotic linezolid administered intraperitoneally at a 25-mg/kg dose.
The in vivo antibacterial activity of PM181104 against VRE was tested in mice in a λ-carrageenan-induced kidney infection model. The bacterial count in the kidneys 72 h postinfection in the mice treated with the 5-mg/kg dose of PM181104 showed a reduction of 1 log CFU compared to the control. The efficacy of PM181104 in treating kidney infection was comparable to that of the standard antibiotics linezolid at 25 mg/kg and vancomycin at 150 mg/kg.
The in vivo activity of PM181104 has implications as an anti-infective therapeutic agent. The potent in vitro activity and successful in vivo results emphasize that the PM181104 class of compounds provides a great potential for the development of vitally needed antibiotics. However, further studies of pharmacokinetic parameters and toxicity and the development of a suitable formulation for human administration will decide the path of this compound.
ACKNOWLEDGMENTS
We thank Somesh Sharma and Arun Balakrishnan for their continuous help and encouragement during the course of this work. Also, we thank the Council of Scientific and Industrial Research (CSIR)-National Institute of Goa-India, the collaborative partner in this work. Special thanks to Rutuja Dikshit for her help in editing graphics in the manuscript.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 12 August 2013
This article is NIO contribution no. 5436.
REFERENCES
- 1.Menichetti F. 2005. Current and emerging serious Gram-positive infections. Clin. Microbiol. Infect. 11(Suppl 3):22–28 [DOI] [PubMed] [Google Scholar]
- 2.Aeschlimann JR, Rybak MJ. 1998. Pharmacodynamic analysis of the activity of quinupristin-dalfopristin against vancomycin-resistant Enterococcus faecium with differing MBCs via time-kill-curve and postantibiotic effect methods. Antimicrob. Agents Chemother. 42:2188–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tenover FC, Weigel LM, Appelbaum PC, McDougal LK, Chaitram J, McAllister S, Clark N, Killgore G, O'Hara CM, Jevitt L, Patel JB, Bozdogan B. 2004. vancomycin-resistant Staphylococcus aureus isolate from a patient in Pennsylvania. Antimicrob. Agents Chemother. 48:275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Infectious Diseases Society of America 2010. The 10 X '20 initiative: pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin. Infect. Dis. 15:1081–1083 [DOI] [PubMed] [Google Scholar]
- 5.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1–12 [DOI] [PubMed] [Google Scholar]
- 6.Mutnick AH, Enne V, Jones RN. 2003. Linezolid resistance since 2001: SENTRY Antimicrobial Surveillance Program. Ann. Pharmacother. 37:769–774 [DOI] [PubMed] [Google Scholar]
- 7.Skiest DJ. 2006. Treatment failure resulting from resistance of Staphylococcus aureus to daptomycin. J. Clin. Microbiol. 44:655–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayer AMS, Glaser KB, Cuevas C, Jacobs RS, Kem W, Little RD, McIntosh JM, Newman DJ, Potts BC, Shuster DE. 2010. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol. Sci. 31:255–265 [DOI] [PubMed] [Google Scholar]
- 9.Mahajan GB, George SD, Ranadive PV, Mishra PDS, Eyyammadichiyil SS, Panshikar RM, Sawant SN, Krishna S, Sivakumar M, Pari K, Prabha D, D'Souza L, Naik CG, Patel ZE, Sivakumar M, Thomas B, Vishwakarma R. 2007. PM181104 and related antibacterial compounds, production, pharmaceutical compositions, and therapeutic use. International Patent Cooperation Treaty Application WO/2007/119201 A3
- 10.Martín J, da Sousa ST, Crespo G, Palomo S, González I, Tormo JR, de la Cruz M, Anderson M, Hill RT, Vicente F, Genilloud O, Reyes F. 2013. Kocurin, the true structure of PM181104, an anti-methicillin-resistant Staphylococcus aureus (MRSA) thiazolyl peptide from the marine-derived bacterium Kocuria palustris. Mar. Drugs 11:387–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cañedo Hernández LM, Romero Millán F, Fernández Medarde A, Fernández Chimeno RI, Hidalgo Villar JC. 2012. Peptides as bioactive compounds. International Patent Cooperation Treaty Application WO/2012/062906
- 12.National Committee for Clinical Laboratory Standards 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A5, 5th ed. National Committee for Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 13.Alder J, Li T, Yu D, Morton L, Silverman J, Zhang XX, Critchley I, Thorne G. 2003. Analysis of daptomycin efficacy and breakpoint standards in a murine model of Enterococcus faecalis and Enterococcus faecium renal infection. Antimicrob. Agents Chemother. 47:3561–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahajan GB, Shanbhag P, Sivaramakrishnan H. 2011. Mode of action of antibiotic PM181104 on bacteria, abstr registration no. 5SN3KNFPHL 1st Global Forum on Bacterial Infections: Balancing Treatment Access and Antibiotic Resistance 2011. The Center for Disease Dynamics Economics & Policy, New Delhi, India [Google Scholar]
- 15.Zhang C, Herath K, Jayasuriya H, Ondeyka JG, Zink DL, Occi J, Birdsall G, Venugopal J, Ushio M, Burgess B, Masurekar P, Barrett JF, Singh SB. 2009. Thiazomycins, thiazolyl peptide antibiotics from Amycolatopsis fastidiosa. J. Nat. Prod. 72:841–847 [DOI] [PubMed] [Google Scholar]