Abstract
Dihydroxymethyl and monohydroxymethyl methylenecyclopropane nucleosides are effective inhibitors of both variants of human herpesvirus 6 (HHV-6). We investigated involvement of HHV-6 U69 protein kinase in their mechanism of action. Phosphorylation of the dihydroxymethyl analogue cyclopropavir and monohydroxymethyl nucleosides with either a 6-ether moiety (MBX 2168) or a 6-thioether moiety (MBX 1616) with purified U69 was examined. All three compounds were substrates of this viral kinase and had similar Michaelis-Menten kinetic parameters.
TEXT
Human herpesvirus6 (HHV-6) is widespread in the population, infecting over 90% of children by the age of 2 years (1, 2). Two closely related variants of HHV-6 exist, HHV-6A and HHV-6B. Primary infection with the HHV-6 variant B causes exanthem subitum (also known as roseola infantum), which manifests as mild skin rash and high fever, accompanied in a small percentage of children by febrile seizures (2, 3). After primary infection, the virus becomes latent and can reactivate in immunocompromised patients such as those undergoing bone marrow and solid-organ transplantations. This can lead to fever, skin rash, pneumonia, encephalitis, bone marrow suppression, and graft rejection (4). Drugs used in the clinic for treatment of HHV-6 infections are the same as those used for the treatment of human cytomegalovirus (HCMV), namely, ganciclovir (GCV), its prodrug valganciclovir, cidofovir, and foscarnet. However, no controlled clinical trials have been conducted to determine the efficacy of these drugs in HHV-6 infections (5–10).
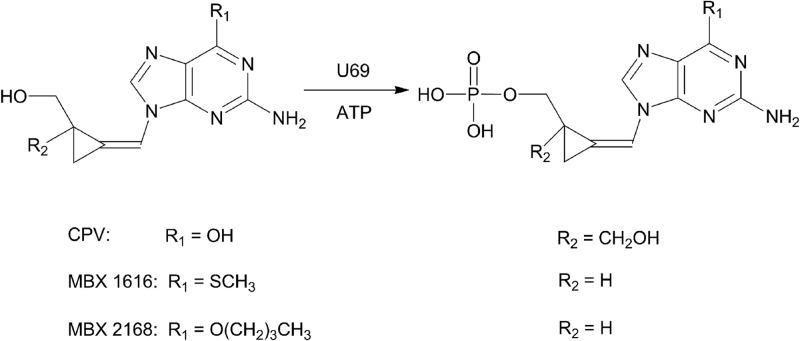
A number of methylenecyclopropane nucleosides (MCPNs) are effective inhibitors of both variants of human herpesvirus 6 in cell culture (11–13). The dihydroxymethyl MCPN analogue cyclopropavir (CPV) also inhibits human cytomegalovirus (HCMV), Epstein-Barr virus (EBV), and human herpesvirus 8 (HHV-8) (11–13). Cyclopropavir is currently in phase I clinical trials for the treatment of HCMV infections. A number of monohydroxymethyl MCPNs with ether or thioether substituents at the 6 position have increased broad-spectrum antiherpes activity, as they also inhibit herpes simplex viruses 1 and 2 and varicella-zoster virus in addition to HHV-6A, HHV-6B, HCMV, EBV, and HHV-8 (11–13). The proposed mechanism of action of these compounds involves sequential phosphorylation to a triphosphorylated drug, which inhibits viral DNA polymerase. The HHV-6 protein kinase U69 was shown previously to be involved in phosphorylation of the nucleoside analogue ganciclovir (14–17). In this study, we investigated the possibility that U69 phosphorylates MCPNs to their monophosphates (Fig. 1).
Fig 1.
Structures of CPV, MBX 1616, and MBX 2168 and representation of their phosphorylation by HHV-6 U69 kinase.
To determine if the dihydroxymethyl MCPN CPV and monohydroxymethyl analogues with either a 6-ether moiety (MBX 2168) or a 6-thioether moiety (MBX 1616) are substrates of the HHV-6A U69 protein kinase, we performed in vitro experiments with purified enzyme. Baculovirus expressing U69 with a glutathione S-transferase (GST) tag on the N terminus was constructed and used to express GST-U69 protein in SF21 insect cells. The protein was purified on a GSTtrap column followed by an anion exchange column (Fig. 2). Amino acid residue 219 in U69 from HHV-6 strain B is an invariant lysine required for U69 enzymatic activity (18). The corresponding residue in HHV-6 strain A is lysine 218. As a control, to exclude phosphorylation by a potential copurified host kinase, baculovirus expressing an inactive GST-U69 that has lysine 218 (K218) mutagenized to a methionine was constructed, and the protein was purified in the same manner as was the wild-type protein. The purified wild-type and K218M mutant proteins (10 ng/μl) were incubated in 125 μl of kinase buffer (50 mM Tris, pH 7.5, 50 mM KCl, 10 mM MgCl2, 1 mM dithiothreitol [DTT], 3 mM ATP) with either CPV, MBX 2168, or MBX 1616. At designated times, aliquots were removed and the reactions were stopped by addition of 2% trifluoroacetic acid (TFA). Samples were analyzed by reverse-phase high-pressure liquid chromatography (HPLC) using a Grace Alltima C18 column (Fig. 3). Liquid chromatography-mass spectrometry (LC-MS) (LCQ Fleet) was used to confirm the identity of drug monophosphate that was formed.
Fig 2.
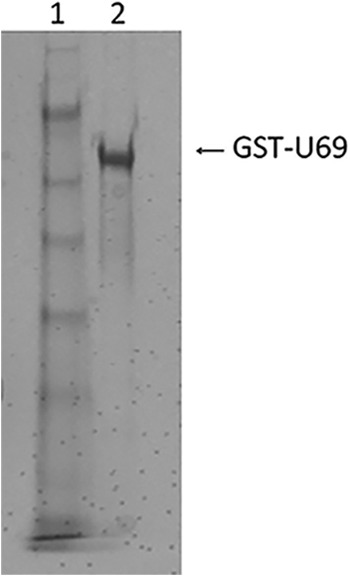
Coomassie blue-stained SDS-PAGE gel showing purified GST-U69. Baculovirus expressing U69 kinase as a GST fusion protein was constructed using the Bac-to-Bac baculovirus expression system from Invitrogen. SF21 insect cells were infected and harvested 65 h postinfection. GST-U69 was purified on a GST column followed by an anion exchange column. Lane 1, molecular weight markers (SeeBluePlus2; Invitrogen); lane 2, purified GST-U69.
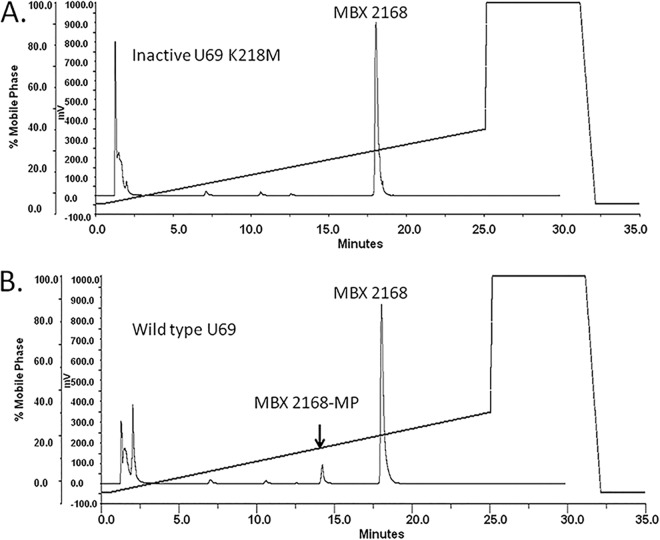
Fig 3.
HPLC analysis of MBX 2168 phosphorylation. MBX 2168 (2.5 mM) was incubated in a kinase buffer with either inactive GST-U69 (K218M) mutant (A) or wild-type GST-U69 kinase (B) at 37°C for 24 h. Samples were analyzed by reverse-phase HPLC using solvent A (0.1% TFA in H2O) and solvent B (0.1% TFA in 100% acetonitrile). Samples were resolved on a Grace Alltima 150-mm by 4.6-mm 5-μm C18 column with a gradient of 5% solvent B to 40% solvent B over 25 min. Mass spectrometry (via direct infusion of HPLC fractions) was used to confirm the identity of the monophosphorylated compound. The effluent was monitored as absorbance at 280 nm. Percent conversion of MBX 2168 to MBX 2168-MP in the presence of wild-type GST-U69 was 7.5%.
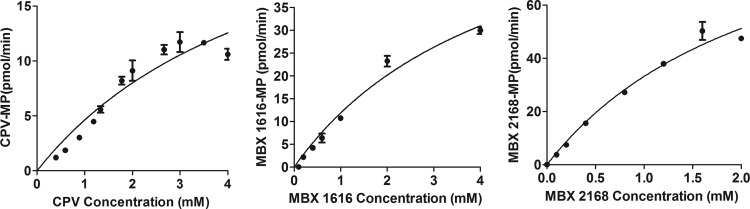
Time-dependent phosphorylation of CPV, MBX 1616, and MBX 2168 was observed in the presence of wild-type GST-U69, while the inactive GST-U69 K218M mutant was unable to phosphorylate these drugs, even after 24 h of incubation at 37°C (Fig. 3). Michaelis-Menten analysis of the phosphorylation of CPV by U69 gave an apparent Km of 2.9 ± 2.1 mM, Vmax of 29 ± 5 pmol/min, enzyme turnover number (kcat) of 0.054 s−1, and catalytic efficiency (kcat/Km) of 21 s−1 M−1. Phosphorylation of MBX 1616 by U69 gave an apparent Km value of 7.9 ± 2.8 mM, Vmax of 92 ± 24 pmol/min, enzyme turnover number (kcat) of 0.13 s−1, and catalytic efficiency (kcat/Km) of 16 s−1 M−1. Values for MBX 2168 were similar, with apparent Km of 5.1 ± 3.6 mM, Vmax of 170 ± 81 pmol/min, kcat of 0.23 s−1, and kcat/Km of 45 s−1 M−1 (Fig. 4).
Fig 4.
Michaelis-Menten curves for U69-mediated phosphorylation of CPV, MBX 1616, and MBX 2168. Enzyme velocity was measured during the linear phase of drug monophosphate formation (120 min). The amount of drug monophosphate formed was measured by UV absorbance on an HPLC. Mass spectrometry (via direct infusion of HPLC fractions) was used to confirm the identity of monophosphorylated compounds. The concentration of phosphorylated species was determined using standard curves of the corresponding unphosphorylated parent (molar absorptivity assumed to be equal). Data were analyzed using GraphPad Prism 5 software. The error bars show the standard errors of the means. Data are representative of three separate experiments, each done in duplicate.
Our data show that the nucleoside analogues CPV, MBX 1616, and MBX 2168 are substrates of HHV-6A U69 kinase. All three compounds displayed similar kinetic parameters even though they differ structurally, particularly at the 6 position of the purine, suggesting that modifications on this part of the molecule are tolerated by this viral kinase. Kinetic parameter values for their phosphorylation reactions were similar to those reported for a homologous kinase from HCMV (UL97) for phosphorylation of CPV (19). In the same study, it was reported that GCV phosphorylation by UL97 was about 45-fold less efficient (19), and GCV-monophosphate was detected only when tritium-labeled compound was used. We tested GCV but were unable to detect phosphorylation of GCV by U69 using our experimental design (UV detection). Another study has shown that HHV-6 U69 kinase is involved in the phosphorylation of GCV, but compared to UL97, the phosphorylation of GCV by U69 in human cells infected with U69-expressing recombinant vaccinia virus was approximately 10-fold lower (14). These observations, together with findings from the current study, lead us to hypothesize that CPV, MBX 2168, and MBX 1616 are better substrates of U69 than is GCV. This hypothesis could be tested by additional experiments using, for example, radiolabeled compounds to increase the sensitivity of detection of their phosphorylated forms. Published studies that have implicated U69 in GCV phosphorylation were based on experiments done in cell culture (14–17). Here, we show that purified U69 can phosphorylate a nucleoside analogue in a direct enzymatic assay. Our study shows that CPV, MBX 2168, and MBX 1616 are substrates of U69 kinase, but it does not exclude potential involvement of cellular kinases in their phosphorylation. We are currently investigating this possibility.
ACKNOWLEDGMENTS
We are thankful to Donald Moir for critical reading of the manuscript.
These studies were funded with federal funds from the National Institute of Allergy and Infectious Diseases under R44 AI 082799.
Footnotes
Published ahead of print 26 August 2013
REFERENCES
- 1.Okuno T, Takahashi K, Balachandra K, Shiraki K, Yamanishi K, Takahashi M, Baba K. 1989. Seroepidemiology of human herpesvirus 6 infection in normal children and adults. J. Clin. Microbiol. 27:651–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zerr DM, Meier AS, Selke SS, Frenkel LM, Huang ML, Wald A, Rhoads MP, Nguy L, Bornemann R, Morrow RA, Corey L. 2005. A population-based study of primary human herpesvirus 6 infection. N. Engl. J. Med. 352:768–776 [DOI] [PubMed] [Google Scholar]
- 3.Hall CB, Long CE, Schnabel KC, Caserta MT, McIntyre KM, Costanzo MA, Knott A, Dewhurst S, Insel RA, Epstein LG. 1994. Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. N. Engl. J. Med. 331:432–438 [DOI] [PubMed] [Google Scholar]
- 4.Yoshikawa T. 2003. Human herpesvirus-6 and -7 infections in transplantation. Pediatr. Transplant. 7:11–17 [DOI] [PubMed] [Google Scholar]
- 5.De Bolle L, Naesens L, De Clercq E. 2005. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin. Microbiol. Rev. 18:217–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reymen D, Naesens L, Balzarini J, Holy A, Dvorakova H, De Clercq E. 1995. Antiviral activity of selected acyclic nucleoside analogues against human herpesvirus 6. Antiviral Res. 28:343–357 [DOI] [PubMed] [Google Scholar]
- 7.De Clercq E, Naesens L, De Bolle L, Schols D, Zhang Y, Neyts J. 2001. Antiviral agents active against human herpesviruses HHV-6, HHV-7 and HHV-8. Rev. Med. Virol. 11:381–395 [DOI] [PubMed] [Google Scholar]
- 8.Williams-Aziz SL, Hartline CB, Harden EA, Daily SL, Prichard MN, Kushner NL, Beadle JR, Wan WB, Hostetler KY, Kern ER. 2005. Comparative activities of lipid esters of cidofovir and cyclic cidofovir against replication of herpesviruses in vitro. Antimicrob. Agents Chemother. 49:3724–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Clercq E, Naesens L. 2006. In search of effective anti-HHV-6 agents. J. Clin. Virol. 37(Suppl 1):S82–S86 [DOI] [PubMed] [Google Scholar]
- 10.Naesens L, Bonnafous P, Agut H, De Clercq E. 2006. Antiviral activity of diverse classes of broad-acting agents and natural compounds in HHV-6-infected lymphoblasts. J. Clin. Virol. 37(Suppl 1):S69–S75 [DOI] [PubMed] [Google Scholar]
- 11.Kushner NL, Williams SL, Hartline CB, Harden EA, Bidanset DJ, Chen X, Zemlicka J, Kern ER. 2003. Efficacy of methylenecyclopropane analogs of nucleosides against herpesvirus replication in vitro. Nucleosides Nucleotides Nucleic Acids 22:2105–2119 [DOI] [PubMed] [Google Scholar]
- 12.Zemlicka J. 2007. Methylenecyclopropane analogues of nucleosides as anti-herpes agents, p 113–165 In De ClercqE. (ed), Advances in antiviral drug design, vol 5 Elsevier Science, New York, NY [Google Scholar]
- 13.Prichard MN, Williams J, Komazin-Meredith G, Khan A, Price NB, Jefferson G, Harden EA, Hartline CB, Peet N, Bowlin TL. 2013. Synthesis and antiviral activity of methylenecyclopropane analogs with 6-alkoxy and 6-alkylthio substitutions that exhibit broad-spectrum antiviral activity against human herpesviruses. Antimicrob. Agents Chemother. 57:3518–3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Bolle L, Michel D, Mertens T, Manichanh C, Agut H, De Clercq E, Naesens L. 2002. Role of the human herpesvirus 6 U69-encoded kinase in the phosphorylation of ganciclovir. Mol. Pharmacol. 62:714–721 [DOI] [PubMed] [Google Scholar]
- 15.Ansari A, Emery VC. 1999. The U69 gene of human herpesvirus 6 encodes a protein kinase which can confer ganciclovir sensitivity to baculoviruses. J. Virol. 73:3284–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safronetz D, Petric M, Tellier R, Parvez B, Tipples GA. 2003. Mapping ganciclovir resistance in the human herpesvirus-6 U69 protein kinase. J. Med. Virol. 71:434–439 [DOI] [PubMed] [Google Scholar]
- 17.Manichanh C, Olivier-Aubron C, Lagarde JP, Aubin JT, Bossi P, Gautheret-Dejean A, Huraux JM, Agut H. 2001. Selection of the same mutation in the U69 protein kinase gene of human herpesvirus-6 after prolonged exposure to ganciclovir in vitro and in vivo. J. Gen. Virol. 82:2767–2776 [DOI] [PubMed] [Google Scholar]
- 18.Prichard MN, Frederick SL, Daily S, Borysko KZ, Townsend LB, Drach JC, Kern ER. 2011. Benzimidazole analogs inhibit human herpesvirus 6. Antimicrob. Agents Chemother. 55:2442–2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gentry BG, Kamil JP, Coen DM, Zemlicka J, Drach JC. 2010. Stereoselective phosphorylation of cyclopropavir by pUL97 and competitive inhibition by maribavir. Antimicrob. Agents Chemother. 54:3093–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]