Abstract
Eravacycline (TP-434 or 7-fluoro-9-pyrrolidinoacetamido-6-demethyl-6-deoxytetracycline) is a novel fluorocycline that was evaluated for antimicrobial activity against panels of recently isolated aerobic and anaerobic Gram-negative and Gram-positive bacteria. Eravacycline showed potent broad-spectrum activity against 90% of the isolates (MIC90) in each panel at concentrations ranging from ≤0.008 to 2 μg/ml for all species panels except those of Pseudomonas aeruginosa and Burkholderia cenocepacia (MIC90 values of 32 μg/ml for both organisms). The antibacterial activity of eravacycline was minimally affected by expression of tetracycline-specific efflux and ribosomal protection mechanisms in clinical isolates. Furthermore, eravacycline was active against multidrug-resistant bacteria, including those expressing extended-spectrum β-lactamases and mechanisms conferring resistance to other classes of antibiotics, including carbapenem resistance. Eravacycline has the potential to be a promising new intravenous (i.v.)/oral antibiotic for the empirical treatment of complicated hospital/health care infections and moderate-to-severe community-acquired infections.
INTRODUCTION
Multidrug-resistant bacteria pose a significant threat to public health. Antimicrobial resistance and its global spread threaten the continued effectiveness of many medicines used today, while at the same time, they jeopardize important medical procedures that require antimicrobial therapy to be successful (1). For example, the crude mortality rate was higher for adult patients with carbapenem-resistant Klebsiella pneumoniae infections than for those with carbapenem-susceptible K. pneumoniae infections (50.0% versus 25.7%) (2). Because carbapenem-resistant Enterobacteriaceae (CRE) are also resistant to most antibiotics, including cephalosporins, fluoroquinolones, and most aminoglycosides, few therapeutic options exist for the treatment of invasive infections caused by these pathogens (3–5). Of the 37 CRE that have been reported in the United States, the last 15 have been reported since July 2012 (6). In the United States, methicillin-resistant Staphylococcus aureus (MRSA) alone annually infects more than 94,000 people and kills nearly 19,000—more deaths than from homicides, HIV/AIDS, Parkinson's disease, or emphysema (5, 7). Additionally, resistant bacteria create an immense economic burden. The medical costs attributable to antimicrobial resistance ranged from $18,588 to $29,069 per patient in one sensitivity analysis of high-risk patients, with an excess duration of hospital stay of 6.4 to 12.7 days and with higher attributable mortality rates (8). Several studies have suggested that annual costs of antibiotic-resistant infections are a staggering $21 billion to $34 billion in the United States alone (9).
The need for new antibiotics to treat the increasing number of multidrug-resistant bacteria was recognized most recently in April 2011 by the World Health Organization's call for a six-point global policy package that includes joint planning, surveillance, drug regulation, rational use of medicines, infection prevention and control, and innovation and research (10). In some countries, there is little difference in the incidences of multidrug-resistant pathogens in the community and in the hospital; most notably, extended-spectrum β-lactamase (ESBL)-producing and/or carbapenem-resistant Enterobacteriaceae are being isolated in patients with no prior contact with the health care system, resulting in increased hospital stays for otherwise healthy adults with urinary tract infection or pyelonephritis (3, 11). In the United States, carbapenem-resistant health care-associated K. pneumoniae urinary tract infections are endemic in certain New York hospitals and carbapenem-resistant K. pneumoniae have spread to at least 33 U.S. states and have been described in many other countries (12, 13).
Eravacycline is a novel fluorocycline antibiotic designed to overcome resistance to common tetracycline-specific efflux and ribosomal protection mechanisms and is impervious to other antibiotic-specific resistance mechanisms (14–17). Similar to other members of the tetracycline antibiotic class, eravacycline has been shown to be a potent, mechanism-based inhibitor of the bacterial ribosome (16). It has modifications at both the C-7 (fluorine) and C-9 [2-(pyrrolidin-1-yl)ethanamido] positions on the tetracyclic core that were made possible by using a totally synthetic route (Fig. 1) (15, 18, 19). In this work, we show that eravacycline has broad-spectrum antimicrobial activity, with MIC90 values of ≤2 μg/ml against panels of all major bacterial species except for Pseudomonas aeruginosa and Burkholderia cenocepacia.
Fig 1.
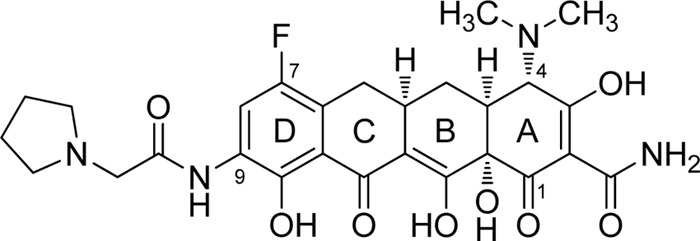
Chemical structure of eravacycline (TP-434).
MATERIALS AND METHODS
Bacterial strains.
Recently isolated, demographically diverse clinical isolates were obtained from or evaluated at Micromyx, LLC (Kalamazoo, MI); Eurofins Medinet (Chantilly, VA); International Health Management Associates, Inc. (IHMA; Schaumburg, IL); and Hershey Medical Center (Hershey, PA) and included over 200 baseline isolates from a phase 2 trial for treatment of complicated intra-abdominal infections conducted by Tetraphase Pharmaceuticals (20). Species-appropriate quality control (QC) strains were used to ensure laboratory standards, as guided by Clinical and Laboratory Standards Institute (CLSI) guidelines (21–23). The QC strains were obtained from the American Type Culture Collection (Manassas, VA). Staphylococcus aureus strains SA981 (original strain name, K28) and SA982 (original strain name, K40) are an isogenic pair, with SA982 overexpressing the NorA pump (24). S. aureus strain SA983 (original strain name, K181) is the parent of SA984 (original strain name, K2068), a strain that overexpresses mepA (25).
Genotypic detection of β-lactamases.
Detection of ESBL genes by PCR was done at the IHMA or by standard singleplex PCR methodology at Tetraphase Pharmaceuticals, using previously reported consensus primers for family or multiple-related families of genes, including blaOXA-1-like, blaSHV, blaCTX-M-1-3-15, blaCTX-M-2, blaCTX-M-9-14, blaCTX-M-8-25-26-39-41, and blaKPC (26). Plasmid-mediated ampC family-specific genes were distinguished by using primers described previously by Perez-Perez and Hanson (27) that targeted MOX-1, MOX-2, CMY-1, CMY-8 to CMY-11, LAT-1 to LAT-4, CMY-2 to CMY-7, BIL-1, DHA-1, DHA-2, ACC, MIR-1T, ACT-1, and FOX-1 to FOX-5b. Primers designed in-house were derived from reported GenBank sequences for blaNDM, blaTEM, blaSPM, blaGIM, blaIMP, blaVIM, blaSIM, blaKHM, blaAIM-1, blaPER, blaVEB, and blaADC. All in-house samples providing a PCR product were sequenced (Genewiz, South Plainfield, NJ) to confirm ESBL gene identity compared to reported GenBank sequences.
Source of antibiotics.
Commercial-grade antibiotics were obtained from the USP (Rockville, MD), ChemPacific Corp. (Baltimore, MD), or Sigma-Aldrich, (St. Louis, MO). Eravacycline was synthesized as described previously by Xiao et al. (15).
Antibiotic susceptibility.
MIC values were determined by using microtiter microdilution broth or agar dilution for aerobic and anaerobic organisms, respectively, according to CLSI standardized methodology (21–23). Antibiotic resistance or insensitivity was determined according to current CLSI guidelines (22).
RESULTS
Activity of eravacycline and comparators against Gram-negative pathogens.
The in vitro activity of eravacycline was evaluated against 2,644 Gram-negative aerobic isolates (Table 1). The collection of organisms contained clinically important species, and many of the isolates were resistant to one or more of the comparator compounds examined. In the vast majority of instances, the MIC90 value for eravacycline was equivalent to or lower than that of comparators for each organism/phenotypic grouping.
Table 1.
Susceptibilities of Gram-negative aerobic bacteria to eravacycline and comparatorsa
| Organism | MIC50/90 (μg/ml), MIC range (μg/ml), and no. of isolates |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ERV | TET | TGC | CARB | AG | 3rd GC | FQ | CST | PTZ | |
| Acinetobacter baumannii | 0.25/1 | 8/>32 | 0.5/4 | 2/32 | 8/>32 | >16/>32 | >2/>2 | 0.5/2 | >64/>128 |
| 0.016–8 | ≤0.25–>32 | ≤0.016–8 | 0.13–>32 | ≤0.25–>32 | 0.13–>64 | 0.016–>32 | 0.13–>4 | ≤0.5–>128 | |
| 188 | 159 | 188 | 188 | 188 | 128 | 188 | 155 | 128 | |
| Acinetobacter baumannii CARB-I/R,b FQ-R,d AG-R | 0.5/2 | >8/>32 | 2/8 | >8/>32 | >8/>32 | >32/>32 | >4/16 | 0.5/1 | >64/>128 |
| ≤0.016–4 | 2–>32 | 0.13–8 | >8–>32 | >8–>32 | >16–>32 | >2–>32 | 0.13–>4 | 64–>128 | |
| 52 | 43 | 52 | 52 | 52 | 37 | 52 | 43 | 44 | |
| Acinetobacter baumannii TET-R | 0.5/2 | >8/>32 | 2/4 | >8/>32 | >8/>32 | >32/>32 | >4/32 | 0.5/1 | >64/>128 |
| 0.06–2 | >8–>32 | 0.25–8 | ≤0.25–>32 | ≤0.25–>32 | 4–>64 | ≤0.25–>32 | 0.13–>4 | 4–>128 | |
| 69 | 69 | 69 | 69 | 69 | 39 | 69 | 68 | 56 | |
| Acinetobacter lwoffii | 0.13/0.25 | 1/2 | 0.13/0.5 | ≤1/4 | ≤0.25/1 | 1/16 | ≤0.25/≤0.25 | 0.25/>2 | ≤0.5/8 |
| 0.03–0.25 | ≤0.25–>8 | 0.06–0.5 | ≤0.25–>8 | ≤0.25–>8 | ≤0.5–>64 | ≤0.25–2 | ≤0.13–4 | ≤0.5–16 | |
| 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | |
| Burkholderia cenocepacia | 8/32 | >32/>32 | 8/32 | 32/>32 | >32/>32 | 16/32 | 4/8 | >32/>32 | 16/>128 |
| 0.13–32 | 16–>32 | 0.25–>32 | 1–>32 | >32–>32 | 2–>32 | 0.5–>32 | >32–>32 | 0.5–>128 | |
| 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Citrobacter freundii | 0.25/1 | 1/>8 | 0.5/2 | 0.5/2 | 0.5/>8 | 1/32 | <0.25/>2 | 0.5/1 | 4/>128 |
| 0.06–2 | 0.5–>8 | 0.13–8 | 0.004–>32 | ≤0.25–>32 | 0.06–>64 | 0.008–>4 | 0.25–>2 | 0.25–>128 | |
| 115 | 65 | 115 | 103 | 115 | 115 | 115 | 64 | 115 | |
| Citrobacter freundii 3rd-GC-I/Rc | 0.5/1 | 2/8 | 1/2 | 1/16 | 0.5/>32 | >16/>64 | 1/>4 | 0.25/1 | >64/>128 |
| 0.13–2 | 1–>8 | 0.25–8 | 0.25–>32 | ≤0.25–>32 | 4–>64 | 0.016–>4 | 0.25–>2 | 2–>128 | |
| 42 | 16 | 42 | 39 | 42 | 42 | 42 | 16 | 42 | |
| Enterobacter cloacae | 0.5/2 | 2/>8 | 0.5/2 | 0.5/2 | 0.5/8 | 2/>64 | ≤0.25/>4 | 0.5/>4 | 4/>64 |
| 0.03–4 | 0.5–>32 | 0.06–8 | 0.03–>32 | ≤0.25–>32 | 0.03–>64 | 0.008–>32 | ≤0.13–>32 | 0.5–>128 | |
| 270 | 218 | 270 | 270 | 270 | 246 | 270 | 178 | 220 | |
| Enterobacter cloacae 3rd-GC-I/R | 0.5/2 | 4/>8 | 1/4 | 0.5/4 | 0.5/16 | >32/>64 | 0.25/>4 | 0.25/>2 | >64/>128 |
| 0.03–4 | 1–>32 | 0.06–8 | 0.03–>32 | ≤0.25–>32 | 2–>64 | 0.008–>32 | ≤0.13–>32 | 2–>128 | |
| 122 | 93 | 122 | 122 | 122 | 122 | 122 | 81 | 107 | |
| Enterobacter cloacae CARB-I/R | 0.5/2 | 4/>32 | 0.5/4 | 2/16 | 1/>32 | >32/>64 | ≤0.25/>4 | 0.25/>4 | >64/>128 |
| 0.25–4 | 2–>32 | 0.13–4 | ≤0.016–>32 | ≤0.25–>32 | 0.13–>64 | 0.03–>32 | ≤0.13–>32 | 1–>128 | |
| 34 | 31 | 34 | 34 | 34 | 26 | 34 | 21 | 21 | |
| Enterobacter cloacae FQ-R | 2/4 | 8/>32 | 2/4 | 0.5/8 | 1/>32 | >32/>64 | >4/32 | 0.25/1 | >64/>128 |
| 0.25–4 | 2–>32 | 0.25–8 | ≤0.016–>32 | ≤0.25–>32 | 0.5–>64 | >2–>32 | ≤0.13–>4 | 2–>128 | |
| 36 | 29 | 36 | 36 | 36 | 35 | 36 | 21 | 27 | |
| Enterobacter cloacae AG-R | 0.5/2 | 8/>32 | 1/4 | 0.5/16 | 16/>32 | >32/>64 | >2/>4 | 0.25/1 | >64/>128 |
| 0.25–4 | 1–>32 | 0.25–8 | 0.13–>32 | >8–>32 | 0.25–>64 | ≤0.016–>32 | ≤0.13–1 | 2–>128 | |
| 26 | 20 | 26 | 26 | 26 | 26 | 26 | 15 | 22 | |
| Enterobacter cloacae TET-R | 2/4 | >8/>32 | 1/4 | 0.25/2 | 1/>32 | 32/>64 | 4/16 | 0.25/>4 | 16/>64 |
| 0.25–4 | >8–>32 | 0.25–8 | 0.03–>32 | ≤0.25–>32 | 0.13–>64 | 0.03–>32 | ≤0.13–>32 | 2–>128 | |
| 25 | 25 | 25 | 25 | 25 | 25 | 25 | 21 | 16 | |
| Enterobacter aerogenes | 0.25/1 | 2/8 | 0.5/2 | ≤1/1 | ≤0.25/0.5 | ≤0.5/>32 | ≤0.25/≤0.25 | 0.25/0.5 | 4/>64 |
| 0.13–2 | 0.5–>8 | 0.25–4 | ≤0.25–8 | ≤0.25–8 | ≤0.5–>64 | ≤0.25–>4 | ≤0.13–>4 | ≤0.5–>64 | |
| 77 | 77 | 77 | 77 | 77 | 77 | 77 | 77 | 77 | |
| Enterobacter aerogenes 3rd-GC-I/R | 0.25/1 | 2/8 | 0.5/2 | 0.5/1 | ≤0.25/1 | 32/>32 | ≤0.25/>4 | 0.25/1 | 64/>64 |
| 0.13–2 | 1–>8 | 0.25–4 | ≤0.25–8 | ≤0.25–2 | 4–>64 | ≤0.25–>4 | ≤0.13–1 | 8–>64 | |
| 27 | 27 | 27 | 27 | 27 | 27 | 27 | 27 | 27 | |
| Escherichia coli | 0.25/0.5 | 4/>32 | 0.25/0.5 | 0.25/0.5 | 1/>8 | ≤0.5/>32 | ≤0.25/>4 | 0.5/0.5 | 2/>64 |
| ≤0.016–4 | 0.25–>64 | 0.06–>8 | ≤0.002–>32 | ≤0.25–>32 | ≤0.016–>64 | ≤0.25–>32 | ≤0.13–4 | ≤0.5–>128 | |
| 445 | 390 | 445 | 445 | 445 | 445 | 445 | 216 | 359 | |
| Escherichia coli 3rd-GC-I/R | 0.25/0.5 | >8/>32 | 0.25/1 | 0.06/0.5 | 2/>32 | >32/>64 | >4/32 | 0.25/0.5 | 8/128 |
| ≤0.016–1 | 0.5–>32 | 0.03–>8 | ≤1–>32 | ≤0.25–>32 | 2–>64 | ≤0.25–>32 | ≤0.13–4 | ≤0.5–>128 | |
| 127 | 113 | 127 | 127 | 127 | 127 | 127 | 69 | 93 | |
| Escherichia coli FQ-R | 0.25/0.5 | >8/>32 | 0.25/0.5 | 0.13/≤0.5 | 4/>32 | >16/>64 | >4/32 | 0.25/0.5 | 8/>64 |
| ≤0.016–4 | 0.25–>32 | 0.06–>8 | ≤1–>32 | 0.25–>32 | 0.06–>64 | >2–>32 | ≤0.13–4 | 1–>128 | |
| 143 | 118 | 143 | 143 | 143 | 143 | 143 | 72 | 142 | |
| Escherichia coli AG-R | 0.25/0.5 | >8/>32 | 0.25/0.5 | 0.063/≤0.5 | >8/>32 | 32/>64 | >4/32 | 0.25/0.5 | 8/>64 |
| ≤0.016–1 | 0.25–>32 | 0.063–>8 | ≤1–>32 | >8–>32 | 0.06–>64 | ≤0.25–>32 | ≤0.13–0.5 | ≤0.5–>128 | |
| 79 | 69 | 79 | 79 | 79 | 79 | 79 | 44 | 78 | |
| Escherichia coli AG-R, FQ-R, 3rd-GC-I/R | 0.25/0.5 | >8/>32 | 0.25/0.5 | 0.063/0.5 | >32/>32 | >32/>64 | >4/32 | 0.25/0.5 | 8/>128 |
| ≤0.016–1 | 0.5–>32 | 0.063–>8 | ≤1–>32 | >8–>32 | 4–>64 | >2–>32 | ≤0.12–0.5 | 1–>128 | |
| 40 | 35 | 40 | 40 | 40 | 40 | 40 | 21 | 40 | |
| Escherichia coli TET-R | 0.25/0.5 | 16/>32 | 0.25/0.5 | 0.063/≤0.5 | 2/>8 | 4/>32 | 1/32 | 0.25/0.5 | 4/>64 |
| ≤0.016–2 | >8–>64 | 0.06–4 | ≤1–>32 | ≤0.25–>32 | 0.06–>64 | ≤0.25–>32 | ≤0.13–4 | ≤0.5–>128 | |
| 157 | 157 | 157 | 157 | 157 | 157 | 157 | 94 | 148 | |
| Haemophilus influenzae | 0.13/0.25 | 0.5/1 | 0.13/0.25 | 1/2 | ND | <0.03/0.13 | 0.016/0.03 | ND | ND |
| ≤0.016–0.5 | ≤0.06–16 | ≤0.016–1 | 0.06–8 | ND | ≤0.016–0.5 | 0.004–0.13 | ND | ND | |
| 114 | 114 | 114 | 101 | ND | 114 | 114 | ND | ND | |
| Klebsiella pneumoniae | 0.5/2 | 4/>32 | 0.5/2 | 0.25/>8 | 0.5/>8 | 8/>32 | 0.5/>32 | 0.5/1 | 8/>128 |
| 0.03–16 | ≤0.25–>64 | 0.13–16 | ≤0.002–>32 | ≤0.25–>32 | ≤0.016–>64 | ≤0.25–>64 | ≤0.13–>16 | ≤0.5–>128 | |
| 394 | 339 | 394 | 394 | 223 | 394 | 394 | 209 | 394 | |
| Klebsiella pneumoniae 3rd-GC-I/R | 0.5/2 | 8/>32 | 1/4 | 1/16 | 4/16 | >32/64 | >4/>32 | 0.5/4 | >64/>128 |
| 0.03–16 | 1–>64 | 0.13–16 | ≤1–>32 | 0.25–>32 | 4–>64 | ≤0.25–>64 | ≤0.13–>16 | 0.5–>128 | |
| 210 | 187 | 210 | 210 | 82 | 210 | 210 | 110 | 209 | |
| Klebsiella pneumoniae CARB-I/R | 0.5/2 | 8/>32 | 1/2 | >8/>32 | 4/>8 | >32/>32 | >4/>32 | 0.5/>4 | >64/>128 |
| 0.13–16 | 1–>32 | 0.25–16 | 2–>32 | 0.25–>32 | 1–>64 | 0.06–>64 | 0.13–>16 | 4–>128 | |
| 90 | 81 | 90 | 90 | 50 | 90 | 90 | 57 | 90 | |
| Klebsiella pneumoniae FQ-R | 0.5/2 | 8/>32 | 1/2 | 1/32 | 4/32 | >32/>32 | >4/>32 | 0.5/>4 | >64/>128 |
| 0.13–16 | 1–>32 | 0.13–16 | ≤1–>32 | ≤0.25–>32 | 0.25–>64 | >2–>64 | 0.13–>16 | 4–>128 | |
| 156 | 134 | 156 | 156 | 82 | 156 | 156 | 78 | 156 | |
| Klebsiella pneumoniae AG-R | 0.5/2 | 8/>32 | 1/4 | 0.5/32 | >8/>32 | >32/>64 | >4/>32 | 0.5/1 | >64/>128 |
| 0.06–16 | 1–>32 | 0.13–16 | ≤1–>32 | >8–>32 | 0.25–>64 | ≤0.25–>64 | ≤0.13–>16 | 2–>128 | |
| 119 | 106 | 119 | 119 | 59 | 119 | 119 | 61 | 118 | |
| Klebsiella pneumoniae AG-R, FQ-R, 3rd-GC-I/R | 0.5/2 | 8/>32 | 1/4 | 1/32 | >8/>32 | >32/>32 | 8/>32 | 0.5/1 | >64/>128 |
| 0.13–16 | 2–>32 | 0.13–16 | ≤1–>32 | >8–>32 | 8–>64 | >2–>64 | 0.13- >16 | 4–>128 | |
| 74 | 66 | 74 | 74 | 35 | 74 | 74 | 36 | 74 | |
| Klebsiella pneumoniae AG-R, FQ-R, CARB-I/R | 0.5/2 | 8/>32 | 1/2 | >8/>32 | >8/>32 | >32/>32 | >4/>32 | 0.5/>4 | >128/>128 |
| 0.13–16 | 4–>32 | 0.25–16 | 2–>32 | >8–>32 | >16–>64 | >2–>64 | 0.13–>16 | >64–>128 | |
| 37 | 33 | 37 | 37 | 21 | 37 | 37 | 21 | 37 | |
| Klebsiella oxytoca | 0.5/1 | 1/>32 | 0.5/2 | ≤1/≤1 | 0.5/>32 | ≤0.5/>32 | ≤0.25/4 | ≤0.13/0.13 | 2/16 |
| 0.03–2 | 0.5–>32 | 0.06–4 | 0.004–1 | ≤0.13–>32 | ≤0.016–>32 | 0.03–>32 | 0.03–>2 | ≤0.5–>64 | |
| 48 | 48 | 48 | 48 | 48 | 48 | 48 | 41 | 48 | |
| Klebsiella oxytoca 3rd-GC-I/R | 0.5/1 | >32/>32 | 0.25/0.5 | 0.06/0.25 | >32/>32 | >32/>32 | 0.5/>32 | 0.13/0.13 | 8/>32 |
| 0.03–1 | 0.5–>32 | 0.06–1 | 0.03–1 | 0.5–>32 | 4–>32 | 0.03–>32 | 0.03–0.13 | 0.5–>32 | |
| 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | |
| Legionella pneumophila | 1/2 | 4/8 | ND | ND | ND | ND | ND | ND | ND |
| 0.016–2 | 0.5–8 | ND | ND | ND | ND | ND | ND | ND | |
| 70 | 70 | ND | ND | ND | ND | ND | ND | ND | |
| Moraxella catarrhalis | 0.03/0.06 | ≤0.25/0.5 | 0.06/0.13 | ≤0.25/≤0.25 | ≤0.25/≤0.25 | ≤0.5/≤0.5 | ≤0.25/≤0.25 | 1/1 | ≤0.5/≤0.5 |
| ≤0.016–0.06 | ≤0.06–>32 | ≤0.016–0.13 | ≤0.25–≤0.25 | ≤0.06–0.25 | ≤0.5–2 | ≤0.25–≤0.25 | 0.5–2 | ≤0.5–2 | |
| 92 | 92 | 92 | 78 | 78 | 78 | 92 | 28 | 28 | |
| Morganella morganii | 1/2 | 2/>8 | 2/4 | ≤1/2 | 1/>8 | ≤0.5/8 | ≤0.25/4 | >2/>4 | ≤0.5/2 |
| 0.5–4 | 0.5–>8 | 0.25–8 | 0.008–4 | ≤0.25–>8 | ≤0.016–>16 | 0.03–>4 | >2–>4 | ≤0.5–>64 | |
| 43 | 43 | 43 | 43 | 43 | 43 | 43 | 39 | 43 | |
| Proteus mirabilis | 1/2 | >8/32 | 4/8 | 2/4 | 1/>8 | ≤0.5/1 | ≤0.25/>4 | >2/>4 | ≤0.5/2 |
| 0.25–16 | 2–>64 | 0.5–16 | 0.008–>32 | ≤0.25–>64 | ≤0.016–>64 | 0.016–>64 | >2–>4 | ≤0.016–64 | |
| 166 | 111 | 166 | 166 | 166 | 166 | 166 | 95 | 157 | |
| Proteus mirabilis 3rd-GC-I/R | 1/4 | >8/64 | 4/8 | 4/8 | 8/16 | 8/>32 | >2/8 | ND | 2/4 |
| 0.5–8 | >8–64 | 1–16 | 0.25–32 | 0.5–>64 | 4–>64 | ≤0.25–16 | >2–>4 | ≤0.5–64 | |
| 21 | 15 | 21 | 21 | 21 | 21 | 21 | 9 | 19 | |
| Proteus mirabilis CARB-R | 1/4 | >8/32 | 4/8 | 2/8 | 1/>8 | ≤0.5/2 | ≤0.25/>4 | >2/>4 | ≤0.5/2 |
| 0.25–16 | 2–>64 | 1–16 | 0.06–>32 | ≤0.25–>64 | ≤0.016–>64 | 0.016–>64 | >2–>4 | ≤0.06–>64 | |
| 136 | 81 | 136 | 136 | 136 | 136 | 136 | 67 | 127 | |
| Proteus mirabilis FQ-R | 2/4 | >8/64 | 4/8 | 4/8 | 2/16 | ≤0.5/16 | >4/16 | >2/>4 | 1/2 |
| 0.5–16 | >8–>64 | 1–16 | 0.25–32 | ≤0.25–>64 | ≤0.016–>64 | >2–>64 | >2–>4 | ≤0.13–64 | |
| 43 | 26 | 43 | 43 | 43 | 43 | 43 | 19 | 38 | |
| Proteus mirabilis AG-R | 2/4 | >8/>32 | 4/8 | 2/8 | >8/>64 | ≤0.5/32 | >2/>4 | >2/>4 | 0.5/4 |
| 0.5–8 | >8–64 | 2–8 | 0.25–32 | >8–>64 | ≤0.016–>64 | 0.016–32 | >2–>4 | ≤0.13–8 | |
| 24 | 16 | 24 | 24 | 24 | 24 | 24 | 12 | 23 | |
| Proteus mirabilis TET-R | 1/2 | >8/32 | 4/8 | 2/8 | 1/>8 | ≤0.5/1 | ≤0.25/>4 | >2/>4 | ≤0.5/2 |
| 0.25–16 | >8–>64 | 0.5–16 | 0.008–>32 | ≤0.25–>64 | ≤0.015–>64 | 0.03–>64 | >2–>4 | ≤0.5–>64 | |
| 109 | 109 | 109 | 109 | 109 | 109 | 109 | 93 | 100 | |
| Proteus vulgaris | 0.5/1 | 8/>8 | 2/4 | ≤1/2 | 1/4 | ≤0.5/32 | ≤0.25/0.5 | >2/>2 | ≤0.5/1 |
| 0.25–2 | 1–>8 | 0.5–8 | ≤0.5–4 | ≤0.25–>8 | ≤0.03–>64 | ≤0.25–4 | >2–>4 | ≤0.5–4 | |
| 55 | 55 | 55 | 55 | 55 | 55 | 55 | 55 | 55 | |
| Providencia stuartii | 1/2 | >8/>8 | 2/4 | 2/4 | 4/32 | <0.5/16 | >2/>4 | >2/>2 | 4/64 |
| 0.13–8 | <0.25–>8 | 0.06–16 | 0.25–16 | ≤0.25–>32 | ≤0.016–>64 | 0.016–>4 | >2–>4 | ≤0.13–>128 | |
| 101 | 51 | 101 | 101 | 101 | 101 | 101 | 51 | 101 | |
| Pseudomonas aeruginosa | 8/32 | >8/64 | 16/32 | 2/>8 | 2/>8 | >16/>32 | 1/>4 | 1/2 | 8/>128 |
| 1–>32 | 8–64 | 1–>32 | 0.13–>32 | 0.13–>32 | 1–>64 | 0.06–>32 | 0.25–4 | >64–>128 | |
| 145 | 93 | 145 | 145 | 145 | 145 | 145 | 85 | 145 | |
| Salmonella spp. | 0.25/0.25 | 1/>8 | 0.25/0.5 | ≤1/≤1 | 0.5/1 | ≤0.5/≤0.5 | ≤0.25/≤0.25 | ≤0.13/0.5 | 2/4 |
| 0.13–0.5 | 0.5–>8 | 0.13–1 | ≤1–8 | ≤0.25–>8 | ≤0.5–≤0.5 | ≤0.25–>4 | ≤0.13–2 | 1–64 | |
| 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | |
| Serratia marcescens | 1/1 | >8/>8 | 1/2 | 0.5/1 | 0.5/1 | ≤0.5/1 | ≤0.25/1 | >2/>4 | 2/4 |
| 0.25–8 | 2–>8 | 0.5–4 | ≤0.25–2 | ≤0.25–8 | ≤0.5–>64 | ≤0.25–>4 | 0.25–>4 | ≤0.5–>64 | |
| 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | 112 | |
| Shigella spp. | 0.13/0.5 | >8/>8 | 0.25/0.5 | ≤1/≤1 | 1/1 | ≤0.5/≤0.5 | ≤0.25/0.5 | ≤0.13/≤0.13 | 2/2 |
| 0.06–1 | ≤0.25–>8 | 0.13–1 | ≤1–≤1 | ≤0.25–>8 | ≤0.5–2 | ≤0.25–1 | ≤0.13–≤0.13 | ≤0.5–4 | |
| 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | |
| Stenotrophomonas maltophilia | 0.5/2 | >8/32 | 0.5/4 | >8/>32 | >8/>32 | >32/>32 | 1/>4 | >2/>32 | >64/>128 |
| ≤0.016–8 | 0.5–>32 | 0.03–8 | 2–>32 | ≤0.25–>32 | 1–>64 | 0.13–32 | ≤0.13–>32 | 8–>128 | |
| 105 | 105 | 105 | 105 | 105 | 105 | 105 | 104 | 105 | |
CARB, carbapenem (imipenem, meropenem, or ertapenem); AG, aminoglycoside (gentamicin or tobramycin); 3rd-GC, third-generation cephalosporin (ceftazidime, cefotaxime, or ceftriaxone); FQ, fluoroquinolone (levofloxacin or ciprofloxacin); ERV, eravacycline; TET, tetracycline; TGC, tigecycline; CST, colistin; PTZ, piperacillin-tazobactam; ND, not determined.
For Enterobacteriaceae, carbapenem-I/R isolates were defined as having an imipenem/meropenem MIC of ≥2 μg/ml or an ertapenem MIC of ≥1 μg/ml, and for Acinetobacter, carbapenem-I/R isolates were defined as having an imipenem/meropenem MIC of ≥16 μg/ml.
Third-generation cephalosporin-I/R isolates were defined as having a ceftazidime MIC of ≥8 μg/ml and a cefotaxime/ceftriaxone MIC of ≥2 μg/ml.
Fluoroquinolone-resistant (FQ-R) isolates were defined as having a levofloxacin MIC of ≥8 μg/ml or a ciprofloxacin MIC of ≥4 μg/ml.
Eravacycline exhibited MIC90 values of ≤0.5 μg/ml against Escherichia coli (including ESBL-producing isolates), Salmonella spp., Shigella spp., Haemophilus influenzae, Moraxella catarrhalis, and Acinetobacter lwoffii. Of the 445 E. coli isolates tested, 29% (n = 127) were intermediately resistant (I) or resistant (R) to third-generation cephalosporins, including isolates confirmed by PCR to contain one or more of the following ESBLs or carbapenemases: CTX-M (n = 53), TEM (n = 35), OXA (n = 16), SHV (n = 22), CMY (n = 13), NDM (n = 2), ACT-5 (n = 1), and DHA-1 (n = 1). In addition to eravacycline maintaining an MIC50/90 of 0.25/0.5 μg/ml against the subset of E. coli isolates with I/R phenotypes for third-generation cephalosporins, this antibiotic was also equally potent against the fluoroquinolone-resistant (n = 143), aminoglycoside-resistant (n = 79), and multidrug-resistant (resistant to all three antibiotic classes) (n = 40) subsets of isolates. The MIC50/90 value for eravacycline for a subset of 157 tetracycline-resistant E. coli isolates was also 0.25/0.5 μg/ml, consistent with previous work showing that eravacycline was minimally affected by major Gram-negative tetracycline-specific resistance mechanisms (16).
Eravacycline MIC90 values were 1 to 2 μg/ml against panels of clinical isolates of Acinetobacter baumannii, Citrobacter freundii, Enterobacter cloacae, Enterobacter aerogenes, K. pneumoniae, Klebsiella oxytoca, Legionella pneumophila, Morganella morganii, Proteus mirabilis, Proteus vulgaris, Providencia stuartii, Serratia marcescens, and Stenotrophomonas maltophilia (Table 1). Notably, eravacycline MIC90 values were unchanged (MIC50/90 = 0.5/2 μg/ml) for subsets of C. freundii, E. cloacae, E. aerogenes, K. pneumoniae, and K. oxytoca isolates displaying third-generation cephalosporin I or R phenotypes. Among the 210 and 90 K. pneumoniae isolates displaying I/R phenotypes for third-generation cephalosporins and carbapenems, respectively, were isolates confirmed by PCR to contain genes encoding one or more of the following: CTX-M (n = 29), TEM (n = 17), OXA (n = 6), SHV (n = 57), KPC (n = 20), NDM (n = 3), DHA (n = 1), and FOX (n = 1). Susceptibility to eravacycline was also unchanged (MIC50/90 = 0.5/2 μg/ml) against subsets of K. pneumoniae isolates displaying fluoroquinolone-resistant (n = 156), aminoglycoside-resistant (n = 119), and multidrug-resistant (aminoglycoside, fluoroquinolone, and either carbapenem I/R [n = 37] or third-generation cephalosporin I/R [n = 74]) phenotypes. For A. baumannii isolates (n = 52) displaying resistance to carbapenems, fluoroquinolones, and aminoglycosides, MIC50/90 values for eravacycline were 0.5/2 μg/ml, or 2-fold higher than those of the combined set of strains; eravacycline MIC50/90 values were also minimally affected by tetracycline resistance in a subset of A. baumannii isolates (n = 69; MIC50/90 = 0.5/2 μg/ml). Activity of eravacycline against P. mirabilis isolates expressing fluoroquinolone-resistant (n = 43; MIC50/90 = 2/4 μg/ml), aminoglycoside-resistant (n = 24; MIC50/90 = 2/4 μg/ml), third-generation cephalosporin-I/R (n = 21; MIC50/90 = 1/4 μg/ml), carbapenem I/R (n = 136; MIC50/90 = 1/4 μg/ml), and tetracycline-resistant (n = 109; MIC50/90 = 1/2 μg/ml) phenotypes was within 2-fold the MIC50/90 values for all P. mirabilis isolates combined (MIC50/90 = 1/2 μg/ml). Against carbapenem-I/R (n = 34), fluoroquinolone-resistant (n = 36), aminoglycoside-resistant (n = 26), and tetracycline-resistant (n = 25) E. cloacae isolates, eravacycline showed MIC50/90 values of 0.5/2, 2/4, 0.5/2, and 2/4 μg/ml, respectively. P. aeruginosa isolates (n = 145) and Burkholderia cenocepacia isolates (n = 10) were relatively less susceptible to eravacycline, with MIC50/90 values of 8/32 μg/ml for both organisms.
Activity of eravacycline against Gram-positive pathogens.
Eravacycline showed excellent in vitro potency, with MIC90 values ranging from 0.016 to 0.5 μg/ml against methicillin-susceptible Staphylococcus aureus (MSSA), methicillin-resistant S. aureus (MRSA), coagulase-negative staphylococci, vancomycin-susceptible Enterococcus faecium and Enterococcus faecalis (VSE), vancomycin-resistant Enterococcus faecium and Enterococcus faecalis (VRE), penicillin-susceptible and -resistant Streptococcus pneumoniae, and macrolide-resistant S. pneumoniae, Streptococcus pyogenes, and other important streptococcal species (Table 2). For S. aureus, the activity of eravacycline was independent of methicillin susceptibility or the expression of Panton-Valentine leukocidin, a pore-forming toxin contributing to the virulence of community-acquired MRSA (CA-MRSA) (Table 2) (28). Eravacycline also showed good potency against subsets of MRSA isolates expressing macrolide resistance (n = 132; MIC50/90 = 0.06/0.25 μg/ml), fluoroquinolone resistance (n = 178; MIC50/90 = 0.06/0.13 μg/ml), and resistance to both antibiotic classes (n = 83; MIC50/90 = 0.06/0.25 μg/ml). Eravacycline showed MIC values of ≤0.03 (n = 3) and 0.5 μg/ml (n = 2) against daptomycin-nonsusceptible MRSA isolates, while the daptomycin MIC values for these isolates ranged from 2 to 4 μg/ml. The MIC range of eravacycline against linezolid-resistant MRSA isolates (n = 9) was ≤0.03 to 0.25 μg/ml, while linezolid MIC values ranged from 8 to 64 μg/ml. Eravacycline was similarly highly active against both E. faecium and E. faecalis, independent of vancomycin resistance (MIC90 = 0.06 to 0.13 μg/ml) (Table 2). Eravacycline was also highly active against a subset of levofloxacin-resistant E. faecalis (n = 111; MIC50/90 = 0.06/0.13 μg/ml) and E. faecium (n = 127; MIC50/90 = 0.06/0.06 μg/ml) isolates. The activity of eravacycline was not impacted by linezolid-resistant isolates of E. faecalis (n = 2; MIC, ≤0.016 and 0.06 μg/ml) and E. faecium (n = 1; MIC, ≤0.016 μg/ml). Eravacycline also showed good potency against daptomycin-nonsusceptible isolates, with MIC50/90 values against E. faecium (n = 44) of 0.06/0.06 μg/ml and an MIC range against E. faecalis (n = 7) of ≤0.016 to 0.03 μg/ml.
Table 2.
Susceptibilities of Gram-positive aerobic bacteria to eravacycline and comparatorsa
| Organism | MIC50/90 (μg/ml), MIC range (μg/ml), and no. of isolates |
|||||||
|---|---|---|---|---|---|---|---|---|
| ERV | TET | TGC | DAP | LZD | VAN | LEV | MACRO | |
| Enterococcus faecalis | 0.06/0.13 | 32/>32 | 0.13/0.25 | 2/4 | 2/2 | 2/>64 | >8/>32 | >8/>8 |
| ≤0.016–0.13 | 0.13–>32 | ≤0.016–0.5 | 0.13–8 | ≤0.5–32 | 0.5–>64 | 0.25–>32 | ≤0.13–>8 | |
| 194 | 98 | 194 | 194 | 194 | 150 | 194 | 59 | |
| Enterococcus faecalis VSE | 0.06/0.13 | 32/>32 | 0.13/0.25 | 2/4 | 2/2 | 1/2 | 2/>32 | >4/>8 |
| ≤0.016–0.13 | 0.13–>32 | ≤0.016–0.5 | 0.13–8 | ≤0.5–32 | 0.5–4 | 0.25–>32 | ≤0.13–>8 | |
| 121 | 70 | 121 | 121 | 121 | 92 | 121 | 38 | |
| Enterococcus faecalis VRE | 0.06/0.13 | 32/>32 | 0.13/0.25 | 2/4 | 2/2 | >64/>64 | >32/>32 | >8/>8 |
| ≤0.016–0.13 | 1–>32 | 0.03–0.25 | 0.13–8 | 1–8 | >16–>64 | 0.25–>32 | 2–>8 | |
| 73 | 28 | 73 | 73 | 73 | 58 | 73 | 21 | |
| Enterococcus faecalis FQ-R | 0.06/0.13 | 32/>32 | 0.13/0.25 | 2/4 | 2/2 | >64/>64 | >32/>32 | >8/>8 |
| ≤0.016–0.13 | 0.13–>32 | ≤0.016–0.5 | ≤0.5–8 | 1–32 | 1–>64 | >4–>32 | ≤0.13–32 | |
| 111 | 48 | 111 | 111 | 111 | 87 | 111 | 34 | |
| Enterococcus faecium | 0.06/0.06 | ≤2/>32 | 0.06/0.13 | 4/8 | 2/4 | 2/>64 | >32/>32 | >8/>8 |
| ≤0.016–0.5 | 0.25–>32 | ≤0.016–0.5 | 1–16 | ≤0.5–32 | ≤0.5–>64 | 0.25–>32 | 0.25–>8 | |
| 153 | 59 | 153 | 153 | 153 | 108 | 153 | 56 | |
| Enterococcus faecium VSE | 0.06/0.13 | 1/>32 | 0.06/0.13 | 4/8 | 2/2 | 1/1 | >8/>32 | >8/>8 |
| 0.03–0.5 | 0.25–>32 | 0.03–0.25 | 1–8 | 1–4 | ≤0.5–4 | 0.25–>32 | 0.25–>8 | |
| 84 | 33 | 84 | 84 | 84 | 58 | 84 | 33 | |
| Enterococcus faecium VRE | 0.06/0.06 | 32/>32 | 0.06/0.13 | 4/8 | 2/4 | >64/>64 | >32/>32 | >8/>8 |
| ≤0.016–0.25 | 0.25–>32 | 0.03–0.5 | 1–16 | ≤0.5–32 | >16–>64 | 1–>32 | 8–>8 | |
| 69 | 26 | 69 | 69 | 69 | 49 | 69 | 24 | |
| Enterococcus faecium FQ-R | 0.06/0.06 | 2/>32 | 0.06/0.12 | 4/8 | 2/4 | 32/>64 | >32/>32 | >8/>8 |
| ≤0.016–0.5 | 0.25–>32 | ≤0.016–0.5 | 1–16 | ≤0.5–32 | ≤0.5–>64 | >4–>32 | >4–>8 | |
| 127 | 48 | 127 | 127 | 127 | 88 | 127 | 45 | |
| Enterococcus faecium DAP-NS | 0.06/0.06 | ND | 0.06/0.13 | 8/16 | 4/4 | >64/>64 | >32/>32 | ND |
| ≤0.016–0.5 | ND | 0.03–0.25 | 8–16 | 1–32 | 0.5–>64 | 2–>32 | ND | |
| 44 | ND | 44 | 44 | 44 | 44 | 44 | ND | |
| Enterococcus spp. | 0.03/0.06 | 0.5/32 | 0.13/0.13 | 0.5/2 | 2/2 | ND | 1/>8 | 0.5/>8 |
| ≤0.016–0.13 | ≤0.06–>32 | ≤0.016–0.25 | 0.25–4 | 1–2 | ND | ≤0.13–>8 | ≤0.13–>8 | |
| 29 | 29 | 29 | 29 | 29 | ND | 29 | 29 | |
| Staphylococcus aureus | 0.06/0.25 | 0.25/32 | 0.13/0.25 | 1/1 | 2/4 | 1/1 | 1/32 | >8/>32 |
| ≤0.016–4 | 0.06–>64 | ≤0.016–16 | 0.063–4 | 1–64 | 0.5–8 | 0.06–>64 | 0.13–>64 | |
| 408 | 245 | 408 | 407 | 407 | 258 | 399 | 237 | |
| MRSA | 0.06/0.13 | 0.25/32 | 0.13/0.25 | 1/1 | 2/4 | 1/1 | 8/>32 | >8/>32 |
| 0.016–4 | 0.063–>64 | ≤0.016–1 | 0.063–4 | 1–64 | 0.5–8 | 0.06–>64 | 0.13–>64 | |
| 284 | 177 | 284 | 283 | 283 | 202 | 275 | 169 | |
| MRSA PVL+ | 0.03/0.03 | 0.25/0.25 | 0.13/0.13 | 0.5/1 | 1/2 | 1/1 | 0.25/>2 | >4/>4 |
| ≤0.016–0.03 | 0.13–0.25 | 0.06–0.13 | 0.5–1 | 1–2 | 1–1 | 0.25–>2 | 1–>4 | |
| 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | |
| Staphylococcus aureus MACRO-Rb | 0.06/0.25 | 0.25/32 | 0.13/0.25 | 0.5/1 | 2/4 | 1/1 | 8/32 | >8/>32 |
| ≤0.016–4 | 0.06–>64 | ≤0.016–16 | 0.13–4 | 1–64 | 0.5–8 | 0.06–>64 | >4–>64 | |
| 132 | 132 | 132 | 131 | 131 | 70 | 132 | 132 | |
| Staphylococcus aureus FQ-R | 0.06/0.13 | 0.25/32 | 0.12/0.25 | 1/1 | 2/4 | 1/1 | >8/>32 | >8/>32 |
| ≤0.016–2 | 0.06–>64 | ≤0.016–16 | 0.125–2 | 1–64 | 0.5–8 | >2–>64 | 0.25–>64 | |
| 178 | 109 | 178 | 178 | 178 | 126 | 174 | 105 | |
| Staphylococcus aureus MACRO-R, FQ-R | 0.06/0.25 | 0.25/>32 | 0.13/0.25 | 0.5/1 | 2/4 | 1/2 | >8/32 | >8/>32 |
| ≤0.016–2 | 0.06–>64 | ≤0.016–16 | 0.13–2 | 1–64 | 0.5–8 | >2–>64 | >4–>64 | |
| 83 | 83 | 83 | 83 | 83 | 45 | 83 | 83 | |
| MSSA | 0.13/0.25 | 0.5/1 | 0.13/0.25 | 1/1 | 4/4 | 1/1 | 0.25/1 | 1/>8 |
| 0.03–0.25 | 0.25–32 | 0.06–0.25 | 0.25–1 | 2–4 | 0.5–2 | 0.13–>32 | 0.25–>8 | |
| 124 | 68 | 124 | 124 | 124 | 56 | 124 | 68 | |
| Coagulase-negative staphylococci | 0.06/0.5 | 0.25/32 | 0.25/1 | 1/1 | 2/2 | 1/2 | 0.5/>32 | 0.5/>8 |
| ≤0.016–2 | ≤0.06–>32 | 0.03–2 | 0.25–2 | ≤0.5–4 | 0.5–2 | 0.13–>32 | ≤0.13–>8 | |
| 165 | 59 | 165 | 165 | 165 | 111 | 165 | 59 | |
| Coagulase-negative staphylococci, methicillin sensitive | 0.06/0.5 | 0.25/32 | 0.25/1 | 1/1 | 2/2 | 1/2 | 0.25/>8 | 0.25/>8 |
| ≤0.016–1 | ≤0.06–>32 | 0.03–2 | 0.25–2 | ≤0.5–4 | 0.5–2 | 0.13–>32 | ≤0.13–>8 | |
| 89 | 37 | 89 | 89 | 89 | 54 | 89 | 37 | |
| Coagulase-negative staphylococci, methicillin resistant | 0.06/0.5 | 0.25/>32 | 0.13/0.5 | 1/1 | 1/2 | 1/2 | 8/>32 | >4/>8 |
| 0.03–2 | 0.25–>32 | 0.06–1 | 0.25–2 | ≤0.5–2 | 0.5–2 | 0.13–>32 | 0.25–>8 | |
| 76 | 22 | 76 | 76 | 76 | 57 | 76 | 22 | |
| Streptococcus pneumoniae | 0.016/0.016 | 0.25/>8 | 0.016/0.03 | ≤0.03/0.5 | 0.5/1 | 0.25/0.5 | 1/1 | 2/>2 |
| ≤0.008–0.03 | ≤0.03–>8 | ≤0.008–0.06 | ≤0.03–2 | ≤0.13–2 | 0.13–0.5 | ≤0.03–>8 | ≤0.03–>2 | |
| 182 | 100 | 182 | 182 | 182 | 82 | 182 | 100 | |
| Streptococcus pneumoniae penicillin resistantc | 0.016/0.016 | >8/>8 | 0.016/0.03 | ≤0.03/0.5 | 0.5/1 | 0.25/0.25 | 1/1 | >2/>2 |
| ≤0.008–0.03 | 0.13–>8 | ≤0.008–0.03 | ≤0.03–0.5 | 0.25–1 | 0.13–0.5 | 0.5–>8 | ≤0.03–>2 | |
| 60 | 33 | 60 | 60 | 60 | 27 | 60 | 33 | |
| Streptococcus pneumoniae MACRO-Rd | ≤0.008/0.016 | >8/>8 | 0.016/0.03 | ≤0.03/≤0.03 | 0.5/0.5 | ND | 1/1 | >2/>2 |
| ≤0.008–0.016 | ≤0.03–>8 | ≤0.008–0.03 | ≤0.03–0.13 | 0.25–1 | ND | 0.5–>8 | 2–>2 | |
| 53 | 53 | 53 | 53 | 53 | ND | 53 | 53 | |
| Streptococcus pneumoniae penicillin resistant, MACRO-R | ≤0.008/0.016 | >8/<8 | 0.016/0.03 | ≤0.03/≤0.03 | 0.5/0.5 | ND | 1/1 | >2/>2 |
| ≤0.008–0.016 | 0.13–>8 | ≤0.008–0.03 | ≤0.03–≤0.03 | 0.25–0.5 | ND | 0.5–>8 | 2–>2 | |
| 29 | 29 | 29 | 29 | 29 | ND | 29 | 29 | |
| Streptococcus pneumoniae TET-R | ≤0.008/0.016 | >8/>8 | 0.016/0.03 | ≤0.03/≤0.03 | 0.5/0.5 | ND | 1/1 | >2/>2 |
| ≤0.008–0.016 | >8–>8 | ≤0.008–0.03 | ≤0.03–0.06 | 0.25–0.5 | ND | 0.5–>2 | 2–>2 | |
| 34 | 34 | 34 | 34 | 34 | ND | 34 | 34 | |
| Streptococcus pyogenes | 0.03/0.03 | 0.25/0.25 | 0.03/0.06 | 0.13/0.13 | 1/2 | ND | 0.5/1 | 0.06/0.06 |
| 0.015–0.13 | 0.13–>8 | ≤0.016–0.13 | ≤0.03–0.25 | 0.5–2 | ND | 0.25–2 | ≤0.03–0.13 | |
| 74 | 20 | 74 | 74 | 74 | ND | 74 | 20 | |
| Streptococcus agalactiae | 0.03/0.06 | >8/>8 | 0.03/0.06 | 0.06/0.5 | 1/2 | 0.5/0.5 | 0.5/1 | 0.06/>4 |
| 0.016–0.06 | 0.25–>8 | 0.016–0.13 | ≤0.03–1 | 0.5–2 | 0.25–0.5 | 0.5–2 | ≤0.03–>4 | |
| 123 | 79 | 123 | 123 | 123 | 48 | 123 | 79 | |
| Streptococcus anginosus | 0.016/0.031 | 0.5/>16 | 0.016/0.06 | 0.25/0.5 | 1/2 | 0.5/1 | 0.5/1 | 0.03/>0.5 |
| ≤0.008–0.13 | ≤0.06–>16 | ≤0.008–0.25 | ≤0.03–0.5 | ≤0.25–2 | ≤0.008–1 | ≤0.25–2 | ≤0.016–>2 | |
| 47 | 47 | 47 | 25 | 47 | 46 | 25 | 25 | |
| Streptococcus intermedius | 0.016/0.06 | 0.25/>4 | 0.03/0.13 | 0.5/1 | 1/1 | 0.5/0.5 | 1/2 | 0.06/>0.5 |
| ≤0.008–0.06 | ≤0.06–>4 | ≤0.008–0.25 | ≤0.03–>1 | ≤0.25–1 | ≤0.06–0.5 | ≤0.25–>4 | ≤0.016–>0.5 | |
| 31 | 31 | 31 | 31 | 31 | 31 | 30 | 31 | |
| Streptococcus mitis | 0.016/0.06 | 0.5/>4 | 0.03/0.13 | 0.5/1 | 1/1 | 0.5/0.5 | 1/2 | >0.5/>0.5 |
| ≤0.008–0.06 | 0.13–>8 | ≤0.008–0.25 | 0.06–>1 | 0.5–1 | ≤0.06–1 | 0.5–>4 | ≤0.016–>2 | |
| 32 | 32 | 32 | 32 | 32 | 31 | 32 | 32 | |
| Streptococcus spp. | 0.016/0.13 | 1/>8 | 0.03/0.13 | 0.13/1 | 0.5/1 | 0.5/1 | 1/2 | 0.06/>2 |
| ≤0.008–0.25 | ≤0.06–>8 | ≤0.008–0.25 | ≤0.03–1 | ≤0.25–2 | ≤0.06–1 | ≤0.25–2 | ≤0.016–>2 | |
| 62 | 62 | 62 | 62 | 62 | 21 | 62 | 62 | |
MACRO, macrolide (erythromycin, azithromycin, or clarithromycin); ND, not determined; ERV, eravacycline; TET, tetracycline; TGC, tigecycline; DAP, daptomycin; LZD, linezolid; VAN, vancomycin; LEV, levofloxacin; DAP-NS, daptomycin nonsusceptible; PVL+, Panton-Valentine leukocidin positive.
Macrolide-resistant staphylococci were defined as having an erythromycin/azithromycin/clarithromycin MIC of ≥8 μg/ml.
Penicillin-resistant streptococcal isolates were defined as having an MIC of ≥2 μg/ml for the oral penicillin breakpoint.
Macrolide-resistant streptococcal isolates were defined as having an erythromycin/clarithromycin MIC of ≥1 μg/ml and an azithromycin MIC of ≥2 μg/ml.
Eravacycline was highly active against all streptococci, showing MIC90 values no higher than 0.13 μg/ml against all species, including S. pneumoniae, S. pyogenes, S. agalactiae, S. anginosus, S. intermedius, and S. mitis (Table 2). For S. pneumoniae, activity was unaffected by isolates expressing penicillin resistance, macrolide resistance (Table 2), or both phenotypes together (n = 29; MIC50/90, ≤0.008/0.016 μg/ml). Against tetracycline-resistant S. pneumoniae (n = 34), eravacycline displayed MIC50/90 values of ≤0.008/0.016 μg/ml.
Activity of eravacycline against anaerobic pathogens.
Eravacycline was tested against 292 clinical Gram-negative and Gram-positive anaerobic strains (Table 3). For Gram-negative species, eravacycline showed MIC50/90 values of 0.5/1 μg/ml against Bacteroides fragilis (n = 36), with similar potency against a subset of Cefinase-positive isolates (n = 20). Eravacycline was less active against Bacteroides ovatus and Bacteroides thetaiotaomicron (n = 11 for each species), with MIC50/90 values of 1/4 μg/ml, but showed MIC50/90 values of 0.25/0.25 μg/ml against Bacteroides vulgatus, 0.5/1 μg/ml against Parabacteroides distasonis (formerly of the Bacteroides genus), and 0.13/0.25 μg/ml against Fusobacterium spp., a group similar to Bacteroides. For other Gram-negative anaerobes (Porphyromonas asaccharolytica and Prevotella spp.), eravacycline MIC90 values ranged from 0.06 to 1 μg/ml.
Table 3.
Susceptibilities of anaerobic bacteria to eravacycline and comparatorsa
| Organism | MIC50/90 (μg/ml), MIC range (μg/ml), and no. of isolates |
||||
|---|---|---|---|---|---|
| ERV | TGC | CARB | MTZ | VAN | |
| Actinomyces spp. | ND | ND | ND | ND | ND |
| 0.25–0.25 | 0.25–0.5 | ND | 4–>16 | ND | |
| 5 | 5 | ND | 5 | ND | |
| Anaerococcus spp. | 0.13/0.13 | 0.13/0.25 | ND | 2/2 | ND |
| 0.03–0.25 | 0.06–0.25 | ND | 0.5–4 | ND | |
| 10 | 10 | ND | 10 | ND | |
| Bacteroides fragilis | 0.5/1 | 0.5/4 | 0.25/1 | 1/1 | >16/>16 |
| 0.06–2 | 0.13–8 | 0.13–4 | 0.25–>16 | 16–>16 | |
| 36 | 36 | 16 | 31 | 11 | |
| B. fragilis cefinase positive | 0.5/1 | 1/4 | ND | 1/1 | ND |
| 0.13–2 | 0.25–8 | ND | 0.25–1 | ND | |
| 20 | 20 | ND | 20 | ND | |
| Bacteroides ovatus | 1/4 | 0.5/16 | 0.25/0.25 | 1/2 | >16/>16 |
| 0.016–8 | 0.06–32 | 0.03–1 | 0.13–>16 | 8–>16 | |
| 11 | 11 | 11 | 10 | 10 | |
| Bacteroides thetaiotaomicron | 1/4 | 8/16 | 0.5/2 | 1/2 | >16/>16 |
| 0.13–4 | 0.25–16 | 0.13–4 | 0.5–>16 | 16–>16 | |
| 11 | 11 | 11 | 10 | 10 | |
| Bacteroides vulgatus | 0.25/0.25 | 0.5/0.5 | 0.25/1 | 0.5/1 | >16/>16 |
| 0.13–1 | 0.13–4 | 0.25–1 | 0.5–1 | 16–>16 | |
| 12 | 12 | 12 | 10 | 10 | |
| Bifidobacterium spp. | ND | ND | ND | ND | ND |
| 0.13–0.5 | 0.25–0.5 | ND | 2–>16 | ND | |
| 7 | 7 | ND | 6 | ND | |
| Clostridium difficile | 0.06/0.13 | 0.13/0.13 | 4/8 | 1/1 | 1/2 |
| 0.03–0.25 | 0.06–0.5 | 0.25–8 | 0.5–2 | 0.5–4 | |
| 11 | 11 | 11 | 11 | 11 | |
| Clostridium perfringens | 1/2 | 1/4 | 0.13/0.5 | 4/16 | 1/>16 |
| 0.06–4 | 0.13–8 | 0.06–1 | 2–>16 | 0.5–>16 | |
| 11 | 11 | 11 | 10 | 10 | |
| Eggerthella lenta | 0.25/0.25 | 0.5/0.5 | ND | 0.5/0.5 | ND |
| 0.25–0.25 | 0.25–0.5 | ND | 0.25–0.5 | ND | |
| 12 | 12 | ND | 12 | ND | |
| Finegoldia magna | 0.25/0.5 | 0.25/0.25 | ND | 0.5/1 | ND |
| 0.13–0.5 | 0.13–0.25 | ND | ≤0.13–1 | ND | |
| 10 | 10 | ND | 10 | ND | |
| Fusobacterium spp. | 0.13/0.25 | 0.13/0.5 | ND | ≤0.13/0.25 | ND |
| 0.03–0.25 | 0.06–0.5 | ND | ≤0.13–0.25 | ND | |
| 21 | 21 | ND | 21 | ND | |
| Lactobacillus spp. | 0.25/0.5 | 0.5/0.5 | ND | >16/>16 | ND |
| 0.25–1 | 0.25–1 | ND | >16–>16 | ND | |
| 7 | 7 | ND | 7 | ND | |
| Parabacteroides distasonis | 0.5/1 | 1/2 | ND | 1/1 | ND |
| 0.25–1 | 0.25–4 | ND | 0.5–1 | ND | |
| 10 | 10 | ND | 10 | ND | |
| Peptoniphilus asaccharolyticus | 0.06/0.13 | 0.13/0.25 | ND | 1/2 | ND |
| 0.03–0.13 | 0.06–0.25 | ND | 0.5–2 | ND | |
| 10 | 10 | ND | 10 | ND | |
| Peptostreptococcus anaerobius | 0.06/0.25 | 0.06/0.25 | 0.06/1 | 1/2 | 0.5/2 |
| 0.016–0.25 | 0.016–0.5 | 0.03–1 | 0.25–2 | 0.5–>16 | |
| 10 | 10 | 10 | 10 | 10 | |
| Peptostreptococcus micros | 0.016/0.25 | 0.03/0.25 | 0.016/0.03 | 0.25/>16 | 1/1 |
| 0.016–0.5 | 0.016–1 | ≤0.008–0.03 | ≤0.008–>16 | 0.5–2 | |
| 10 | 10 | 10 | 10 | 10 | |
| Porphyromonas asaccharolytica | 0.03/0.06 | 0.06/0.06 | 0.016/0.03 | 1/2 | 0.25/0.5 |
| 0.016–0.13 | 0.03–0.13 | ≤0.008–0.06 | 0.5–4 | 0.13–1 | |
| 10 | 10 | 10 | 10 | 10 | |
| Prevotella bivia | 1/1 | 1/2 | ND | 1/4 | ND |
| 0.13–1 | 0.03–2 | ND | 0.5–4 | ND | |
| 13 | 13 | ND | 13 | ND | |
| Prevotella buccae | 0.06/0.13 | 0.13/0.13 | ND | 0.5/1 | ND |
| 0.03–0.13 | 0.06–0.25 | ND | 0.25–1 | ND | |
| 10 | 10 | ND | 10 | ND | |
| Prevotella disiens | 0.13/0.25 | 0.25/0.5 | ND | 1/1 | ND |
| 0.06–0.25 | 0.13–0.5 | ND | 0.5–2 | ND | |
| 12 | 12 | ND | 12 | ND | |
| Prevotella intermedia | 0.06/0.13 | 0.25/0.25 | ND | 0.5/0.5 | ND |
| 0.03–0.13 | 0.13–0.25 | ND | 0.25–1 | ND | |
| 10 | 10 | ND | 10 | ND | |
| Prevotella melaninogenica | 0.13/1 | 0.5/1 | ND | 0.25/1 | ND |
| 0.06–1 | 0.06–4 | ND | ≤0.008–>16 | 1–>16 | |
| 13 | 13 | ND | 13 | 8 | |
| Prevotella spp. | ND | ND | ND | ND | ND |
| 0.03–1 | 0.06–0.5 | ND | 0.25–>16 | ND | |
| 7 | 7 | ND | 7 | ND | |
| Propionibacterium acnes | ND | ND | ND | ND | ND |
| 0.13–0.13 | 0.13–0.13 | ND | >16–>16 | ND | |
| 5 | 5 | ND | 5 | ND | |
ERV, eravacycline; TGC, tigecycline; MTZ, metronidazole; VAN, vancomycin; CARB, ertapenem or imipenem; ND, not determined.
Eravacycline showed MIC90 values of 0.13 to 0.5 μg/ml for Gram-positive anaerobes, including Clostridium difficile, Peptostreptococcus spp., Actinomyces spp., Anaerococcus spp., Bifidobacterium spp., Eggerthella spp., Finegoldia magna, Lactobacillus spp., Peptoniphilus asaccharolyticus, and Propionibacterium acnes. The MIC90 value was 2 μg/ml for 11 isolates of Clostridium perfringens. The anaerobic panels were biased to contain strains with therapeutically important antibiotic resistance phenotypes, and many of the Bacteroides species, Prevotella species, Peptostreptococcus species, Propionibacterium acnes, and Clostridium perfringens isolates were vancomycin resistant and/or metronidazole resistant; however, there was no impact on eravacycline activity in strains having the resistance phenotype(s). Eravacycline had the most consistent broad-spectrum activity against the anaerobic species compared to all comparators.
Eravacycline potency compared to that of tigecycline.
Tigecycline, a 9-t-butylglycylamido derivative of minocycline, is the most recent tetracycline to be approved for intravenous (i.v.) use in complicated intra-abdominal infections, complicated skin and skin structure infections, and complicated community-acquired bacterial pneumonia (29). For the vast majority of Gram-negative organisms tested in this study, the MIC90 values of eravacycline (Table 1) were found to be ≥2-fold lower than those of tigecycline; these organisms included A. baumannii, A. lwoffii, C. freundii, E. aerogenes, K. oxytoca, M. catarrhalis, M. morganii, P. mirabilis, P. vulgaris, P. stuartii, Salmonella spp., S. marcescens, and S. maltophilia, plus certain panels with I/R phenotypes for third-generation cephalosporins (C. freundii, E. aerogenes, E. cloacae, E. coli, K. pneumoniae, and P. mirabilis). Notably, eravacycline has MIC50/90 values of 1/2, 0.5/1, 1/1, and 1/2 μg/ml against P. mirabilis (n = 166), P. vulgaris (n = 55), P. stuartii (n = 101), and M. morganii (n = 43), respectively, compared to MIC50/90 values of 2/8, 2/4, 2/4, and 2/4 μg/ml for tigecycline against each species of the tribe Proteeae, respectively. For Gram-positive organisms, a ≥2-fold greater potency for eravacycline than for tigecycline by MIC90 value was noted for E. faecalis (VRE and VSE), E. faecium (VRE), Enterococcus spp., S. aureus (MRSA), coagulase-negative staphylococci (methicillin sensitive), S. pneumoniae, S. pyogenes, S. anginosus, S. intermedius, and S. mitis (Table 2), and similarly for anaerobes, eravacycline exhibited a ≥2-fold greater potency by MIC90 value than tigecycline for Anaerococcus spp., B. fragilis, B. ovatus, B. thetaiotaomicron, B. vulgatus, C. perfringens, Eggerthella lenta, Fusobacterium spp., P. distasonis, P. asaccharolyticus, Prevotella bivia, Prevotella disiens, and Prevotella intermedia (Table 3).
The relative MIC90 values of eravacycline and tigecycline were examined on a strain-by-strain basis for select Gram-negative pathogen panels (Table 4). This comparison revealed that eravacycline was ≥2-fold more active than tigecycline for 87% of A. baumannii isolates, 32% of E. coli isolates, 59% of E. cloacae isolates, 46% of K. pneumoniae isolates, 92% of P. mirabilis isolates, and 78% of B. fragilis isolates. For the majority of the remaining isolates in each panel, the activity of eravacycline was similar to that of tigecycline.
Table 4.
Distribution of tigecycline/eravacycline MIC ratiosa for individual isolates
| TGC/ERV MIC ratio | No. of isolates with TGC/ERV ratio |
|||||
|---|---|---|---|---|---|---|
| A. baumannii | E. coli | E. cloacae | K. pneumoniae | P. mirabilis | B. fragilis | |
| 32 | 2 | |||||
| 16 | 1 | 4 | 3 | |||
| 8 | 39 | 7 | 4 | 4 | 5 | 1 |
| 4 | 80 | 27 | 21 | 35 | 82 | 6 |
| 2 | 44 | 106 | 134 | 140 | 65 | 21 |
| 1 | 20 | 211 | 95 | 185 | 13 | 8 |
| 0.5 | 4 | 86 | 15 | 27 | 0 | |
| 0.25 | 2 | 1 | 1 | |||
| Total | 188 | 445 | 270 | 394 | 166 | 36 |
For each isolate within a given organism panel, the ratio of the tigecycline MIC to the eravacycline MIC (TGC/ERV MIC) was calculated.
Eravacycline was also evaluated against S. aureus isolates with upregulated expression of norA (24) or mepA (25), genes encoding pumps conferring antibiotic resistance to quinolones (NorA) and tigecycline (MepA), respectively (30, 31) (Table 5). Eravacycline retained activity in strains overexpressing either norA or mepA (MICs of ≤0.016 μg/ml), whereas tigecycline was 64-fold less active when mepA was overexpressed, and ciprofloxacin was 32-fold less active when norA was overexpressed.
Table 5.
Activity of eravacycline against S. aureus strains expressing NorA or MepA efflux pumps
| Compound | MIC (μg/ml) |
|||
|---|---|---|---|---|
| SA981 (parent) | SA982 (norA) | SA983 (parent) | SA984 (mepA) | |
| Eravacycline | 0.004 | 0.004 | 0.004 | 0.016 |
| Tigecycline | 0.063 | 0.13 | 0.016 | 1 |
| Tetracycline | 0.5 | 0.5 | 0.5 | 0.5 |
| Ciprofloxacin | 0.5 | 16 | 2 | 4 |
| Meropenem | 0.25 | 0.13 | 0.13 | 0.13 |
DISCUSSION
A recent survey of infectious disease specialists rated treatment for multidrug-resistant Gram-negative infections as the most important unmet clinical need in current practice, significantly outranking infections with MRSA and multidrug-resistant Mycobacterium tuberculosis (32). In the survey, 63% of physicians reported treating a patient in the past year whose infection was resistant to all available antibacterial agents. Multiple Gram-negative species are responsible for causing substantial increases in the rates of antibiotic-resistant infections and subsequent illness and death. For example, the rate of resistance to ceftazidime among K. pneumoniae strains isolated in the United States from 1998 to 2010 rose from 5.5 to 17.2% (33). Recent deaths at the Clinical Center of the U.S. National Institutes of Health due to K. pneumoniae, and the difficulty of eradication of this blaKPC clone, are illustrative of a Gram-negative problem with few to no treatment options (5). Infections due to antibiotic-resistant Gram-negative strains of Acinetobacter, Enterobacter, and Pseudomonas can be particularly life threatening, having mortality rates of 26%, 27%, and 21 to 54%, respectively, as well as causing increased hospital costs and length of stay (34–38).
Serious infections caused by Gram-negative bacteria such as A. baumannii and ESBL-producing Enterobacteriaceae are becoming increasingly more difficult to treat due to the evolution and spread of isolates expressing multiple antibiotic resistance mechanisms (39). ESBL-producing and carbapenem-resistant Enterobacteriaceae are frequently seen in complicated urinary tract infections (cUTIs) in patients from either the hospital or the community (3, 40, 41). Treatment with 1.5 mg/kg of body weight i.v. eravacycline every 24 h (q24h) provides urine concentrations within 8 h of the first dose that are 4- to 14-fold in excess of eravacycline's MIC90 values for common cUTI pathogens (MIC90 values of 0.5 to 2 μg/ml, except for P. aeruginosa) (42). This is in contrast to the reported urine levels of tigecycline of ∼0.3 μg/ml after a 100-mg i.v. dose (43).
Multidrug-resistant Gram-positive pathogens in hospital and community settings are of particular public health concern (44). Despite gains made in detecting and reducing MRSA infection during hospitalization, the risk of MRSA infection among critically and chronically ill carriers persists after discharge (45). The spread of resistance and the incidence of multidrug-resistant Streptococcus pneumoniae leave few alternatives to effectively treat severe respiratory infections empirically (46). The dissemination of multidrug-resistant Staphylococcus aureus, especially the now pandemic, highly virulent CA-MRSA, and vancomycin-resistant enterococci also leaves few empirical antibiotic options for treating serious infections caused by these organisms (28, 47). Eravacycline has the requisite potency in vitro against all species of Gram-positive bacteria and was found to cure 100% of patients who had a Gram-positive aerobe as one of their baseline pathogens in a phase 2 trial for treatment of complicated intra-abdominal infections (20).
Anaerobes are important pathogens, especially in patients with weakened immune systems, and are commonly recovered in complicated intra-abdominal infections and diabetic foot ulcers. The Bacteroides fragilis group constitutes the most important clinical group of these organisms, but infections with other anaerobes are increasingly being encountered.
Eravacycline possesses unique chemical modifications at C-9 and C-7 of the tetracycline core that confer potent, broad-spectrum antibacterial activity, especially against difficult-to-treat, multidrug-resistant pathogens. The activity of eravacycline was previously shown to be minimally affected by tetracycline-specific efflux and ribosome protection and inactivation (16). In the present study, eravacycline showed greater overall potency than other broad- and narrow-spectrum comparator antibiotics against large panels of isolates with significant representations of multidrug-resistant isolates. Eravacycline is differentiated from tigecycline, the most recently approved tetracycline-class antibiotic in clinical use, by its in vitro superior activity across multiple organisms, particularly multidrug-resistant Acinetobacter spp. and ESBL-producing Enterobacteriaceae, as well as by its promising pharmacokinetics, tolerability, and potential for oral dosing (48–51). The clinical efficacies in the microbiologically evaluable population were 92.9 and 100% for eravacycline intravenous doses of 1.5 mg/kg q24h and 1.0 mg/kg q12h, respectively, in a recent phase 2 trial for i.v. treatment of complicated intra-abdominal infections compared to the standard-of-care antibiotic ertapenem (92.3%). In this trial, 25% of the Gram-negative aerobic pathogens in the microbiological modified intent-to-treat population produced at least one ESBL, with 15.8% of the Enterobacteriaceae isolates being resistant to at least three antibiotics (20, 52). Eravacycline is a promising new therapy for empirical use for serious infections caused by new and emerging multidrug-resistant Gram-negative, Gram-positive, and anaerobic pathogens, and phase 3 clinical trials for treatment of complicated intra-abdominal infections and complicated urinary tract infections are planned (20, 48, 51).
ACKNOWLEDGMENT
This work was funded in part by the Biomedical Advanced Research and Development Authority (BARDA) through contract no. HHSO10020120002C.
Footnotes
Published ahead of print 26 August 2013
REFERENCES
- 1.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1–12 [DOI] [PubMed] [Google Scholar]
- 2.Correa L, Martino MD, Siqueira I, Pasternak J, Gales AC, Silva CV, Camargo TZ, Scherer PF, Marra AR. 2013. A hospital-based matched case-control study to identify clinical outcome and risk factors associated with carbapenem-resistant Klebsiella pneumoniae infection. BMC Infect. Dis. 13:80. 10.1186/1471-2334-13-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bratu S, Landman D, Haag R, Recco R, Eramo A, Alam M, Quale J. 2005. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch. Intern. Med. 165:1430–1435 [DOI] [PubMed] [Google Scholar]
- 4.van Duin D, Kaye KS, Neuner EA, Bonomo RA. 2013. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn. Microbiol. Infect. Dis. 75:115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snitkin ES, Zelazny AM, Thomas PJ, Stock F, Henderson DK, Palmore TN, Segre JA. 2012. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci. Transl. Med. 4:148ra116. 10.1126/scitranslmed.3004129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention 14 February 2013, posting date New carbapenem-resistant Enterobacteriaceae warrant additional action by healthcare providers. Centers for Disease Control and Prevention, Atlanta, GA: http://emergency.cdc.gov/HAN/han00341.asp [Google Scholar]
- 7.Liszewskik K. 2010. Resistant bugs necessitate tougher tactics. Genet. Eng. Biotechnol. News 30:1, 56–58 [Google Scholar]
- 8.Roberts RR, Hota B, Ahmad I, Scott RD, II, Foster SD, Abbasi F, Schabowski S, Kampe LM, Ciavarella GG, Supino M, Naples J, Cordell R, Levy SB, Weinstein RA. 2009. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin. Infect. Dis. 49:1175–1184 [DOI] [PubMed] [Google Scholar]
- 9.IDSA 2011. Combating antimicrobial resistance: policy recommendations to save lives. Clin. Infect. Dis. 52:S397–S428. 10.1093/cid/cir153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization 2011. World Health Day 2011: policy briefs. World Health Organization, Geneva, Switzerland: http://www.who.int/world-health-day/2011/policybriefs/en/index.html [Google Scholar]
- 11.Centers for Disease Control and Prevention 2013. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb. Mortal. Wkly. Rep. 62:165–170 [PMC free article] [PubMed] [Google Scholar]
- 12.Patel G, Bonomo RA. 2013. “Stormy waters ahead”: global emergence of carbapenemases. Front. Microbiol. 4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitchel B, Rasheed JK, Patel JB, Srinivasan A, Navon-Venezia S, Carmeli Y, Brolund A, Giske CG. 2009. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob. Agents Chemother. 53:3365–3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fyfe C, Grossman T, O'Brien W, Achorn C, Sutcliffe J. 2011. The novel broad-spectrum fluorocycline TP-434 is active against MDR gram-negative pathogens, abstr P-1149 Abstr. 21st Eur. Congr. Clin. Microbiol. Infect. Dis./27th Int. Congr. Chemother., Milan, Italy, 7 to 10 May 2011 [Google Scholar]
- 15.Xiao XY, Hunt DK, Zhou J, Clark RB, Dunwoody N, Fyfe C, Grossman TH, O'Brien WJ, Plamondon L, Ronn M, Sun C, Zhang WY, Sutcliffe JA. 2012. Fluorocyclines. 1. 7-Fluoro-9-pyrrolidinoacetamido-6-demethyl-6-deoxytetracycline: a potent, broad spectrum antibacterial agent. J. Med. Chem. 55:597–605 [DOI] [PubMed] [Google Scholar]
- 16.Grossman TH, Starosta AL, Fyfe C, O'Brien W, Rothstein DM, Mikolajka A, Wilson DN, Sutcliffe JA. 2012. Target- and resistance-based mechanistic studies with TP-434, a novel fluorocycline antibiotic. Antimicrob. Agents Chemother. 56:2559–2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutcliffe J, O'Brien W, Achorn C, Appelbaum P, Pillar C, Zurenko G. 2010. In vitro activity of fluorocycline TP-434 against panels of recent bacterial clinical isolates, abstr F1-2158 Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., 12 to 15 September 2010 [Google Scholar]
- 18.Clark RB, Hunt DK, He M, Achorn C, Chen CL, Deng Y, Fyfe C, Grossman TH, Hogan PC, O'Brien WJ, Plamondon L, Ronn M, Sutcliffe JA, Zhu Z, Xiao XY. 2012. Fluorocyclines. 2. Optimization of the C-9 side-chain for antibacterial activity and oral efficacy. J. Med. Chem. 55:606–622 [DOI] [PubMed] [Google Scholar]
- 19.Charest MG, Lerner CD, Brubaker JD, Siegel DR, Myers AG. 2005. A convergent enantioselective route to structurally diverse 6-deoxytetracycline antibiotics. Science 308:395–398 [DOI] [PubMed] [Google Scholar]
- 20.Solomkin JS, Cesnauskas G, Ramesh M, Walpole S, Sutcliffe J, Horn P. 2012. Efficacy and safety of TP-434 (eravacycline) versus ertapenem in complicated intra-abdominal infection (cIAI), abstr L1-1647a Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 9 to 12 September 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CLSI 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed. CLSI document M07-A9 CLSI, Wayne, PA [Google Scholar]
- 22.CLSI 2012. Performance standards for antimicrobial susceptibility testing: twenty-second informational supplement. CLSI document M100-S22 CLSI, Wayne, PA [Google Scholar]
- 23.CLSI 2012. Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standard, 8th ed. CLSI document M11-A8 CLSI, Wayne, PA [Google Scholar]
- 24.Kaatz GW, Seo SM. 1995. Inducible NorA-mediated multidrug resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 39:2650–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaatz GW, McAleese F, Seo SM. 2005. Multidrug resistance in Staphylococcus aureus due to overexpression of a novel multidrug and toxin extrusion (MATE) transport protein. Antimicrob. Agents Chemother. 49:1857–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dallenne C, Da Costa A, Decre D, Favier C, Arlet G. 2010. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 65:490–495 [DOI] [PubMed] [Google Scholar]
- 27.Perez-Perez FJ, Hanson ND. 2002. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40:2153–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otto M. 2010. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu. Rev. Microbiol. 64:143–162 [DOI] [PubMed] [Google Scholar]
- 29.Wyeth Pharmaceuticals, Inc November 2012. Tygacil (tigecycline) for injection for intravenous use, package insert, revised. Wyeth Pharmaceuticals, Inc, Philadelphia, PA [Google Scholar]
- 30.Yoshida H, Bogaki M, Nakamura S, Ubukata K, Konno M. 1990. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J. Bacteriol. 172:6942–6949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAleese F, Petersen P, Ruzin A, Dunman PM, Murphy E, Projan SJ, Bradford PA. 2005. A novel MATE family efflux pump contributes to the reduced susceptibility of laboratory-derived Staphylococcus aureus mutants to tigecycline. Antimicrob. Agents Chemother. 49:1865–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hersh AL, Newland JG, Beekmann SE, Polgreen PM, Gilbert DN. 2012. Unmet medical need in infectious diseases. Clin. Infect. Dis. 54:1677–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez GV, Master RN, Clark RB, Fyyaz M, Duvvuri P, Ekta G, Bordon J. 2013. Klebsiella pneumoniae antimicrobial drug resistance, United States, 1998-2010. Emerg. Infect. Dis. 19:133–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sunenshine RH, Wright M-O, Maragakis LL, Hariss AD, Song X, Hebden J, Cosgrove SE, Anderson A, Carnell J, Jernigan DB, Kleinbaum DG, Perl TM, Standiford HC, Srinivasan A. 2007. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg. Infect. Dis. 13:97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans HL, Lefrak SN, Lyman J, Smith RL, Chong TW, McElearney ST, Schulman AR, Hughes MG, Raymond DP, Pruett TL, Sawyer RG. 2007. Cost of Gram-negative resistance. Crit. Care Med. 35:89–95 [DOI] [PubMed] [Google Scholar]
- 36.Maragakis LL, Perencevich EN, Cosgrove SE. 2008. Clinical and economic burden of antimicrobial resistance. Expert Rev. Anti Infect. Ther. 6:751–763 [DOI] [PubMed] [Google Scholar]
- 37.Qureshi ZA, Paterson DL, Peleg AY, Adams-Haduch JM, Shutt KA, Pakstis DL, Sordillo E, Polsky B, Sandkovsky G, Bhussar MK, Doi Y. 2012. Clinical characteristics of bacteraemia caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae in the era of CTX-M-type and KPC-type beta-lactamases. Clin. Microbiol. Infect. 18:887–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirsch EB, Tam VH. 2010. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert Rev. Pharmacoecon. Outcomes Res. 10:441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peleg AY, Hooper DC. 2010. Hospital-acquired infections due to gram-negative bacteria. N. Engl. J. Med. 362:1804–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marchaim D, Chopra T, Perez F, Hayakawa K, Lephart PR, Bheemreddy S, Blunden C, Hujer AM, Rudin S, Shango M, Campbell M, Varkey J, Slim J, Ahmad F, Patel D, Chen TY, Pogue JM, Salimnia H, Dhar S, Bonomo RA, Kaye KS. 2011. Outcomes and genetic relatedness of carbapenem-resistant Enterobacteriaceae at Detroit medical center. Infect. Control Hosp. Epidemiol. 32:861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez F, Endimiani A, Ray AJ, Decker BK, Wallace CJ, Hujer KM, Ecker DJ, Adams MD, Toltzis P, Dul MJ, Windau A, Bajaksouzian S, Jacobs MR, Salata RA, Bonomo RA. 2010. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J. Antimicrob. Chemother. 65:1807–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutcliffe J, Grossman T, Ronn M, Xiao X, Leighton A. 2011. TP-434 has potential to treat complicated urinary tract infections (cUTI), abstr F1-1858 Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL, 17 to 20 September 2011 [Google Scholar]
- 43.Cunha BA. 2009. Pharmacokinetic considerations regarding tigecycline for multidrug-resistant (MDR) Klebsiella pneumoniae or MDR Acinetobacter baumannii urosepsis. J. Clin. Microbiol. 47:1613. 10.1128/JCM.00404-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rice LB. 2006. Antimicrobial resistance in gram-positive bacteria. Am. J. Med. 119:S11–S19; discussion S62–S70. 10.1016/j.amjmed.2006.03.012 [DOI] [PubMed] [Google Scholar]
- 45.Avery TR, Kleinman KP, Klompas M, Aschengrau A, Huang SS. 2012. Inclusion of 30-day postdischarge detection triples the incidence of hospital-onset methicillin-resistant Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 33:114–121 [DOI] [PubMed] [Google Scholar]
- 46.Van Bambeke F, Reinert RR, Appelbaum PC, Tulkens PM, Peetermans WE. 2007. Multidrug-resistant Streptococcus pneumoniae infections: current and future therapeutic options. Drugs 67:2355–2382 [DOI] [PubMed] [Google Scholar]
- 47.Calfee DP. 2012. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci, and other Gram-positives in healthcare. Curr. Opin. Infect. Dis. 25:385–394 [DOI] [PubMed] [Google Scholar]
- 48.Horn PT, Sutcliffe J, Walpole SM, Leighton A. 2011. Pharmacokinetics, safety and tolerability of a novel fluorocycline, TP-434, following multiple dose oral administration with and without food, abstr 603 Abstr. Infect. Dis. Soc. Am. 49th Annu. Meet., Boston, MA, 20 to 23 October 2011 [Google Scholar]
- 49.Seng Yue C, Sutcliffe JA, Colucci P, Sprenger CR, Ducharme MP. 2010. Population pharmacokinetic modeling of TP-434, a novel fluorocycline, following single and multiple dose administration, abstr A1-028 Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., 12 to 15 September 2010 [Google Scholar]
- 50.Leighton A, Zupanets I, Bezugla N, Plamondon L, Macdonald G, Sutcliffe J. 2011. Broad-spectrum fluorocycline TP-434 has oral bioavailability in humans, abstr P-1509 Abstr. 21st Eur. Congr. Clin. Microbiol. Infect. Dis./27th Int. Congr. Chemother., Milan, Italy, 7 to 10 May 2011 [Google Scholar]
- 51.Sutcliffe J, Ronn M, Leighton A, Sprenger C. 2010. Phase 1 single and multiple ascending dose studies of a broad-spectrum fluorocycline, TP-434, abstr A1-027 Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., 12 to 15 September 2010 [Google Scholar]
- 52.Fyfe C, Deane J, Grossman T, Horn P, Moore G, Sahm D, Studeny J, Walpole S, Sutcliffe J. 2013. Characterization of baseline pathogens and microbiological eradication in a phase 2 trial for treatment of complicated intra-abdominal infections comparing eravacycline (TP-434) to ertapenem, abstr O277 Abstr. 23rd Eur. Congr. Clin. Microbiol. Infect. Dis., Berlin, Germany, 27 to 30 April 2013 [Google Scholar]


