Abstract
Bacillus anthracis toxins can be neutralized by antibodies against protective antigen (PA), a component of anthrax toxins. Anthrivig (human anthrax immunoglobulin), also known as AIGIV, derived from plasma of humans immunized with BioThrax (anthrax vaccine adsorbed), is under development for the treatment of toxemia following exposure to anthrax spores. The pharmacokinetics (PK) of AIGIV was assessed in naive animals and healthy human volunteers, and the efficacy of AIGIV was assessed in animals exposed via inhalation to aerosolized B. anthracis spores. In the clinical study, safety, tolerability, and PK were evaluated in three dose cohorts (3.5, 7.1, and 14.2 mg/kg of body weight of anti-PA IgG) with 30 volunteers per cohort. The elimination half-life of AIGIV in rabbits, nonhuman primates (NHPs), and humans following intravenous infusion was estimated to be approximately 4, 12, and 24 days, respectively, and dose proportionality was observed. In a time-based treatment study, AIGIV protected 89 to 100% of animals when administered 12 h postexposure; however, a lower survival rate of 39% was observed when animals were treated 24 h postexposure, underscoring the need for early intervention. In a separate set of studies, animals were treated on an individual basis upon detection of a clinical sign or biomarker of disease, namely, a significant increase in body temperature (SIBT) in rabbits and presence of PA in the serum of NHPs. In these trigger-based intervention studies, AIGIV induced up to 75% survival in rabbits depending on the dose and severity of toxemia at the time of treatment. In NHPs, up to 33% survival was observed in AIGIV-treated animals. (The clinical study has been registered at ClinicalTrials.gov under registration no. NCT00845650.)
INTRODUCTION
Anthrax is an acute infectious disease caused by Bacillus anthracis. The spore form is the predominant phase of the bacterium in the environment, and it is largely as a result of exposure to spores that anthrax is contracted. The high lethality of anthrax, the stability of anthrax spores in the environment, and the relative ease of production and dispersal make B. anthracis spores an ideal biological weapon.
The fatality rate for inhalation anthrax is high, approaching 100% in the absence of treatment (1, 2). The onset of disease is reported to occur anywhere from 2 to 43 days after exposure to aerosolized spores (3). In most cases, however, initial symptoms develop within 5 days (1). After a brief period of improvement, the continued production of anthrax toxins may cause an abrupt deterioration in the health of the infected individual, with the sudden onset of symptoms, including fever, shock, and respiratory failure due to pulmonary edema. Hemorrhagic meningitis is also common (3). This later stage of disease is termed the fulminant phase. Death can occur within 24 h after the onset of advanced respiratory complications (1, 2, 4).
Anthrax virulence factors include an antiphagocytic polypeptide capsule and three proteins known as protective antigen (PA), lethal factor (LF), and edema factor (EF). Individually, these proteins are not cytotoxic, but the combination of PA with LF or EF results in the formation of the cytotoxic lethal toxin (LT) and edema toxin (ET), respectively. ET impairs neutrophil function in vivo and affects water homeostasis, leading to edema, while LT causes release of tumor necrosis factor alpha and interleukin-1β, which is believed to be linked to the development of shock and subsequent death in severe anthrax infection (5). Neutralizing antibodies generated against the PA component of anthrax toxins have been shown to confer protection against anthrax (6–10).
Although antimicrobials reduce the incidence or progression of disease following exposure to aerosolized B. anthracis spores, delayed initiation of treatment may result in increased mortality, as the disease progresses rapidly to the toxemic phase. Among the 11 cases of inhalation anthrax linked to the 2001 anthrax letter attacks in the United States, five were fatal despite aggressive medical care, including antibiotic therapy (11, 12). Antibody-based antitoxin therapy, which has a long history of successful use as a medical countermeasure for bacterial toxins, such as tetanus (13, 14) and botulinum (15), could serve as adjunct therapy for toxemic patients.
Anthrivig (human anthrax immunoglobulin), also known as AIGIV, is derived from plasma of humans immunized with BioThrax (adsorbed anthrax vaccine). AIGIV is produced using the same process that is used for the manufacture of the FDA-licensed Gamunex-C (immune globulin injection [human], 10% caprylate/chromatography purified) (Grifols Therapeutics Inc., Clayton, NC) and contains the same excipients as Gamunex. AIGIV contains polyclonal toxin-neutralizing antibodies against PA and is being developed as a countermeasure for treatment of B. anthracis-induced toxemia in combination with antimicrobial treatment.
Clinical studies aimed at demonstrating efficacy of anthrax countermeasures are not feasible because the incidence of naturally occurring anthrax is too low in the United States (16) and because it is unethical to intentionally expose humans to anthrax. An alternative approach is to perform animal efficacy studies and use pharmacokinetic (PK) assessment to correlate the efficacious dose in animals to a comparable dose in humans (17, 18). Rabbits and nonhuman primates (NHPs) are considered the preferred models of inhalation anthrax. In the studies described here, PK and tolerability of AIGIV were evaluated in healthy rabbits and NHPs, and PK and safety were evaluated in healthy human volunteers. Efficacy was assessed in rabbits and NHPs exposed via inhalation to aerosolized B. anthracis spores.
MATERIALS AND METHODS
Test and control articles.
AIGIV was derived from plasma collected from healthy volunteers who had been immunized with at least four doses of BioThrax. Gamunex was used as a placebo control. In the animal PK studies, saline was also used as a placebo control.
Experimental animals.
Animal studies were conducted at Battelle (Columbus, OH). The work was performed in compliance with the Animal Welfare Act and followed the principles outlined in the National Research Council Guide for the Care and Use of Laboratory Animals. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC).
Specific-pathogen-free New Zealand White (NZW) rabbits (Oryctolagus cuniculus), weighing between 2.8 and 4.0 kg, and cynomolgus macaques (Macaca fascicularis) of Asian origin, weighing between 1.5 and 3.5 kg (1.5 to 4.0 years old), were procured from Covance Laboratories (Denver, PA). Only healthy animals of the specified weight range and free of obvious clinical signs of disease or malformations were placed in each study, and equal numbers of male and female animals were assigned to each treatment group. Monkeys were tested and verified as being negative for tuberculosis, simian immunodeficiency virus, simian T-lymphotropic virus 1, herpesvirus B, and simian retrovirus 1 and 2. The monkeys were also confirmed to be seronegative for anti-PA immunoglobulin G (IgG) by enzyme-linked immunosorbent assay (ELISA).
Clinical study.
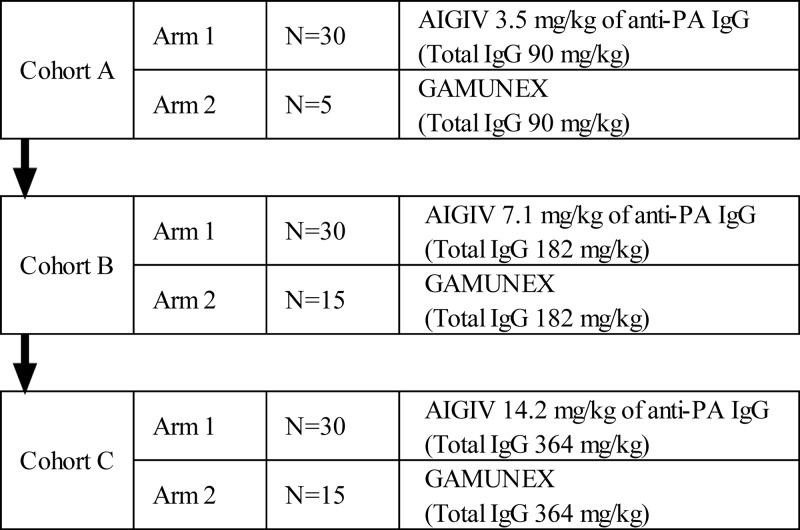
A randomized, double-blind, dose escalation, single-center PK and safety trial was conducted at SNBL Clinical Pharmacology Center (Baltimore, MD). The clinical study was conducted in accordance with the principles of the International Conference on Harmonization (ICH) E6 Guideline on Good Clinical Practices (GCP) and the principles of the Declaration of Helsinki and was approved by the Chesapeake Institutional Review Board (Columbia, MD). A total of 125 subjects were enrolled in one of three dose-escalating cohorts and within cohorts were randomized to receive either AIGIV or Gamunex (Fig. 1). Safety was assessed by adverse-event reporting and concomitant medication collection at each visit from screening through day 90 as well as at the early-withdrawal visit, if applicable. Additionally, safety laboratory assessments and physical examinations were conducted. (This study has been registered at ClinicalTrials.gov under registration no. NCT00845650.)
Fig 1.
Design of the human safety and PK trial.
Laboratory assays.
PK assessments were performed on human and animal serum samples using the toxin-neutralizing antibody (TNA) assay as described by Quinn et al. (19). The limit of detection (LOD) of the assay was equal to a 50% neutralization factor (NF50) of 0.054 and 0.074 for rabbit and NHP sera, respectively. Where applicable, human anti-PA immunoglobulin G (IgG) concentrations in animal sera were assessed by ELISA as described previously by Quinn et al. (20). Complete blood count (CBC), C-reactive protein (CRP) levels, and bacteremia were assessed in animal studies as described by Comer et al. (21), and the presence of PA in animal sera was determined by an electrochemiluminescence (ECL)-based method as described by Henning et al. (22). The limits of detection (LOD) of the assay were 2.0 and 0.5 ng/ml for rabbits and NHPs, respectively.
Intravenous infusion.
Animals were administered intravenous (IV) infusions at the rate of 0.08 ml/kg/min via PortHold (Instech Laboratories, Plymouth Meeting, PA) or plastic SoloPort MID configuration (Instech Labs Plymouth Meeting, PA) vascular access ports (VAPs) surgically implanted into the jugular vein. Animals were not anesthetized during infusions. Patency of VAPs was maintained by flushing with a 5% dextrose solution and locking with 0.9 ml of heparinized saline. Ports were flushed with 5% dextrose solution prior to needle placement for infusion and immediately after the infusion. To reduce stress, infusions were administered using an ambulatory infusion system with an in-pump jacket (Strategic Applications Inc., Libertyville, IL), and animals were acclimated to the jackets prior to infusion. Humans were administered IV infusions at an initial rate of 0.01 ml/kg/min, which was gradually increased after 30 min to 0.08 ml/kg/min based on tolerability.
Challenge of animals with aerosolized B. anthracis spores.
B. anthracis (Ames strain) spores were aerosolized by a Collison nebulizer (BGI, Waltham, MA). Animals were placed individually in a plethysmography chamber inside a class III biosafety cabinet and challenged via the inhalation route with a target dose of 200 times the median lethal dose (LD50) of the aerosolized spores, which is equivalent to 2.1 × 107 spores/rabbit and 1.2 × 107 spores/NHP based on published LD50 data (23, 24). NHPs were anesthetized with Telazol (1 to 6 mg/kg, intramuscularly) before the challenge; rabbits were not anesthetized prior to challenge.
To estimate inhaled aerosol concentrations of B. anthracis, effluent aerosol streams were collected directly from an animal exposure port via an inline impinger (model 7541; Ace Glass Incorporated, Vineland, NJ). Serial dilutions of impinger samples were plated on Trypticase soy agar plates, and CFU were enumerated.
Experimental design. (i) Pharmacokinetic studies.
PK of AIGIV was assessed in human subjects and animals by measuring serum TNA levels at selected time points following intravenous infusion of AIGIV. The 50% effective dilution (ED50; the dilution of serum that inhibits 50% of the cytotoxic effect of lethal toxin) was calculated for each test sample, and the TNA level was reported as the 50% neutralization factor (NF50; the ED50 of the test sample divided by the ED50 of the reference standard). A human serum reference standard AVR801 (BEI Resources, Manassas, VA) was used for all human and animal TNA analysis, and the data analysis was conducted using a SAS platform and the analysis algorithm developed by the CDC (25). The mean ED50 of the AVR801 reference standard was 656 (26). The tolerability assessment in animals included clinical observations, hematology and clinical chemistry analyses, and gross necropsy on all animals at study termination.
(ii) Efficacy studies.
A dose-ranging efficacy study in NZW rabbits was conducted with AIGIV or Gamunex administered at 12 or 24 h postexposure (time-based intervention). AIGIV was administered by slow IV infusion via surgically implanted VAPs at a dose of 14.2 or 21.3 mg/kg of anti-PA IgG (364 or 546 mg/kg of total IgG) either 12 or 24 (±2) hours postchallenge. Gamunex (546 mg/kg of total IgG) was administered by slow IV infusion 12 h postchallenge.
Two additional dose-ranging efficacy studies were performed in NZW rabbits and cynomolgus macaques, in which AIGIV or Gamunex was administered on an individual-animal basis using a clinical sign of the disease or a biological marker of disease as a trigger for treatment (trigger-based intervention). Rabbits and NHPs were monitored for mortality for 28 and 60 days postexposure, respectively. All animals that died or were euthanized due to moribund condition prior to scheduled termination were subjected to complete gross necropsy and histopathology in order to confirm death from inhalation anthrax.
In a trigger-based intervention study in rabbits, AIGIV at a dose of 7.1 mg/kg, 14.2 mg/kg, or 21.3 mg/kg of anti-PA IgG or Gamunex at a dose of 546 mg/kg of total IgG was administered postexposure by slow IV infusion via surgically implanted VAPs upon detection of a significant increase in body temperature (SIBT). SIBT was defined as an increase equal to or greater than two standard deviations above each individual rabbit's average baseline body temperature, which had been shown to correlate with disease progression, bacteremia, toxemia, and mortality (21). Body temperatures were recorded via an implantable programmable temperature transponder (IPTT-300; BMDS, Seaford, DE). Temperatures were recorded twice daily, starting at 7 days prior to challenge, and hourly from approximately 9 to 72 h postchallenge. Therapeutic intervention was initiated when animals exhibited either three consecutive SIBT readings or two consecutive SIBT readings twice (21).
In a trigger-based intervention efficacy study in NHPs, AIGIV at a dose of 7.1 mg/kg, 14.2 mg/kg, or 21.3 mg/kg of anti-PA IgG or Gamunex at a dose of 546 mg/kg of total IgG was administered by slow IV infusion via surgically implanted VAPs upon detection of antigenemia. Antigenemia was defined as the presence of anthrax PA in the serum as determined by ECL assay (21, 22). The assay limit of quantitation was 2.0 ng of PA per ml.
Statistical analysis.
PK parameters in human subjects and animals were estimated based on TNA titers using noncompartmental analysis. SAS (SAS Institute, Cary, NC) and WinNonlin v.5.0.1 (Pharsight Corp., Mountain View, CA) software was used to determine PK parameters in humans and animals, respectively. Analysis of variance (ANOVA) models were fitted to the PK parameters to compare groups, and Tukey's multiple comparison tests were used to test for significant differences between groups. Fisher's exact test was used to test for significant differences in the survival rates between each treated group and the control group in the nonclinical efficacy studies. For animal efficacy studies, time-to-death data were depicted using Kaplan-Meier curves, and the log-rank test was used to test for significant differences in survival between the treated groups and the control group. The Bonferroni-Holm adjustment for multiple comparisons was used to assess significance in the differences between the groups.
RESULTS
PK and tolerability of AIGIV in rabbits and NHPs.
The efficacious human dose of AIGIV may be determined by identifying the human dose that results in PK levels that are associated with protection in the animal efficacy studies (17). Therefore, the PK of AIGIV was evaluated in the relevant animal models at the doses intended to be used in the efficacy studies. Additionally, tolerability of these doses of AIGIV in the animal models was assessed.
The PK analysis in rabbits and NHPs was performed by measuring TNA levels at selected time points following AIGIV infusion, as shown in Tables 1 and 2, respectively. The AIGIV doses of 7.1, 14.2, and 21.3 mg/kg of anti-PA IgG used in animal PK and efficacy studies represented one-, two-, and threefold multiples of 7.1 mg/kg, the putative target dose based on a dose of 500 mg of anti-PA IgG, which had been proposed by a CDC panel of experts for therapy of anthrax for an adult and corresponds to 7.1 mg/kg for a 70-kg person (C. Dykewicz, presented at an external review committee of anthrax and immune globulin experts of CDC, NIH, and USAMRID, 2003).
Table 1.
Design of PK study in rabbitsa
| Treatment groupb | Product | Anti-PA IgG (mg/kg) | Total IgG (mg/kg) |
|---|---|---|---|
| 1 | AIGIV | 7.1 | 182 |
| 2 | AIGIV | 14.2 | 364 |
| 3 | AIGIV | 21.3 | 546 |
| 4 | Gamunex | 0 | 546 |
| 5 | Saline | 0 | 0 |
Blood was collected at −3 days, 1, 4, 12, 24, 48, 72, and 96 h, and 10, 20, and 30 days postinfusion.
Each group contained 5 males and 5 females.
Table 2.
Design of PK study in NHPsa
| Treatment group | Product | No. of animals per groupb | Anti-PA IgG (mg/kg) | Total IgG (mg/kg) |
|---|---|---|---|---|
| 1 | AIGIV | 8 | 7.1 | 182 |
| 2 | AIGIV | 8 | 14.2 | 364 |
| 3 | AIGIV | 8 | 21.3 | 546 |
| 4 | Gamunex | 6 | 0 | 546 |
| 5 | Saline | 4 | 0 | 0 |
Blood was collected at −3 days, 1, 4, 12, 24, 48, 72, and 96 h, and 10, 20, 30, and 45 days postinfusion.
Equal numbers of males and females were used.
The infusion times in rabbits ranged between 20 and 25 min, 40 and 90 min, and 59 and 72 min for the doses of 7.1, 14.2, and 21.3 mg/kg anti-PA IgG, respectively. In NHPs, the infusion times ranged between 22 and 25 min, 45 and 46 min, and 69 and 70 min for 7.1, 14.2, and 21.3 mg/kg anti-PA IgG, respectively. Minimum infusion times for humans were 38 min for 3.5 mg/kg of anti-PA IgG and 90 mg/kg of Gamunex total IgG, 49 min for 7.1 mg/kg of anti-PA IgG and 180 mg/kg of Gamunex total IgG, and 71 min for 14.2 mg/kg of anti-PA IgG and 360 mg/kg of Gamunex total IgG. All AIGIV-infused groups of animals exhibited dose proportionality based on the peak TNA level (Cmax) and area under the curve through infinity (AUC0-∞) (Table 3). The group means of the time to peak TNA level (Tmax), time to last measurable TNA level (Tlast), and elimination half-life (t1/2) were comparable across the doses (Table 3). There were no overt gender-related differences in PK parameters of AIGIV for a given dose. There was a significant dose effect for Cmax, AUC through Tlast (AUClast), and AUC0-∞.
Table 3.
PK of AIGIV in rabbits, NHPs, and humansa
| Subject | Dose (mg/kg) | Cmax (NF50) | Tmax (h) | t1/2 (days) | AUClast (days × NF50) | AUC0-∞ (days × NF50) |
|---|---|---|---|---|---|---|
| Rabbits | 7.1 | 2.11 ± 0.18 | 9.12 ± 7.06 | 3.82 ± 0.63 | 8.21 ± 1.01 | 9.29 ± 1.21 |
| 14.2 | 4.07 ± 0.48 | 3.60 ± 2.28 | 4.23 ± 0.58 | 17.20 ± 2.10 | 21.10 ± 1.90 | |
| 21.3 | 7.29 ± 0.32 | 1.18 ± 0.31 | 4.47 ± 0.59 | 29.00 ± 2.50 | 30.80 ± 2.60 | |
| NHPs | 7.1 | 1.63 ± 0.14 | 4.94 ± 2.71 | 14.50 ± 1.90 | 10.40 ± 0.60 | 11.60 ± 0.90 |
| 14.2 | 3.14 ± 0.18 | 1.40 ± 0.37 | 11.40 ± 1.70 | 19.70 ± 1.50 | 21.30 ± 2.00 | |
| 21.3 | 4.72 ± 0.51 | 8.21 ± 5.86 | 11.00 ± 0.60 | 31.70 ± 2.40 | 33.60 ± 2.70 | |
| Humans | 3.5 | 1.07 ± 0.26 | 4.49 ± 2.63 | 22.29 ± 6.20 | 13.45 ± 13.04 | 13.70 ± 2.12 |
| 7.0 | 2.21 ± 0.48 | 7.81 ± 4.36 | 23.25 ± 6.33 | 26.50 ± 4.79 | 29.08 ± 4.91 | |
| 14.0 | 4.30 ± 0.98 | 7.35 ± 4.26 | 25.3 ± 7.50 | 53.20 ± 7.75 | 58.37 ± 9.04 |
Cmax, maximum measured serum concentration during the time period specified; Tmax, time of maximum measured serum concentration; t1/2, elimination half-life; AUClast, area under the serum concentration-versus-time curve from time zero to the last quantifiable concentration; AUC0-∞, AUClast plus the additional area extrapolated to infinity, calculated using the terminal elimination rate constant. Values are means ± standard errors of the means.
A single infusion of AIGIV at doses of 7.1, 14.2, and 21.3 mg/kg of anti-PA IgG was well tolerated by rabbits and NHPs. No abnormalities were noted in any of the evaluated parameters, including body weights, clinical chemistry, hematology, and gross necropsy, and no abnormal clinical observations were reported in any of the treatment groups.
Postexposure efficacy (time-based intervention) in rabbits.
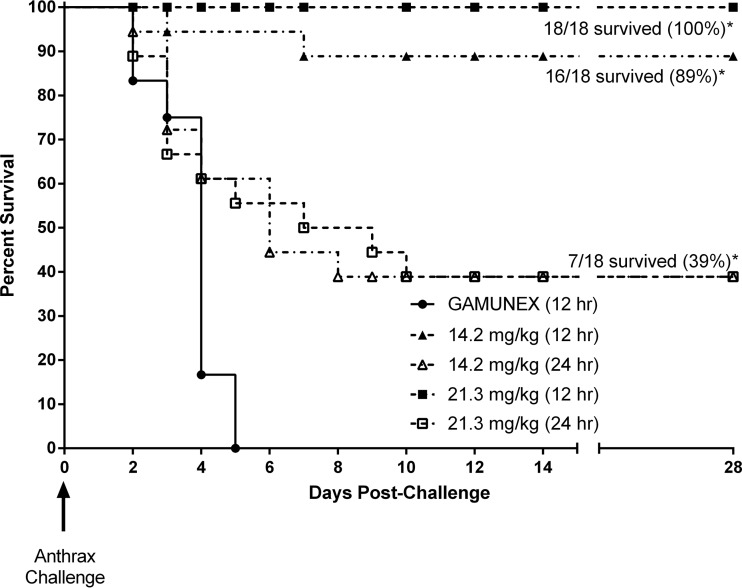
To evaluate postexposure efficacy of AIGIV, a time-based intervention study was conducted where rabbits were treated 12 or 24 h postchallenge (Table 4). The average actual inhaled aerosol dose was 204 ± 47 LD50 (mean ± standard deviation), which corresponds to 2.2 × 107 ± 5.4 × 106 CFU/animal based on the published LD50 (24). Treatment with AIGIV at doses of 14.2 and 21.3 mg/kg of anti-PA IgG administered at 12 h postchallenge afforded 89% and 100% protection, respectively. When the treatment was delayed to 24 h postchallenge, 39% of animals survived regardless of the dose administered. Survival was slightly lower, 27%, among animals that were bacteremic prior to AIGIV treatment initiation and therefore received treatment after the onset of systemic anthrax (i.e., a therapeutic rather than postexposure prophylactic scenario). All control animals, treated with Gamunex 12 h postchallenge, succumbed to inhalation anthrax within 5 days following challenge (Fig. 2). Gross necropsy and/or histopathology analysis confirmed anthrax as the cause of death for all rabbits that died prior to the end of the 28-day observation period.
Table 4.
Design of postexposure efficacy study in rabbits (time-based intervention)
| Treatment group | Product | No. of animals/groupa | IgG (mg/kg) |
Time of treatment postchallenge (h) | Inhaled dose (LD50) |
Blood collection time pointsb |
|||
|---|---|---|---|---|---|---|---|---|---|
| Anti-PA | Total | Target | Actual [mean ± SD (range)] | PA (by ELISA) and bacteremia | Anti-PA and TNA levels | ||||
| 1 | AIGIV | 18 | 14.2 | 364 | 12 | 200 | 194 ± 40 (86–256) | Prechallenge day −7 | Prechallenge day −7 |
| 2 | AIGIV | 18 | 21.3 | 546 | 12 | 200 | 220 ± 43 (140–389) | Postchallenge, PTI | Postchallenge, PTI |
| 3 | AIGIV | 18 | 14.2 | 364 | 24 | 200 | 222 ± 58 (116–397) | Postinfusion days 2, 6, 10, and 28 | Postinfusion hours 1, 4, 12, 24, 48, 72, and 96 |
| 4 | AIGIV | 18 | 21.3 | 546 | 24 | 200 | 194 ± 35 (120–253) | Postinfusion days 6, 10, 21 and 28 | |
| 5 | Gamunex | 12 | 0 | 546 | 12 | 200 | 182 ± 51 (124–281) | ||
Equal number of males and females were used.
PTI, prior to infusion.
Fig 2.
Survival results (Kaplan-Meier plot) of a postexposure efficacy study in rabbits (time-based intervention). Animals were challenged with a target dose of 200 LD50 of B. anthracis Ames strain spores. *, survival in AIGIV-treated groups was significantly greater than that in the Gamunex control group (P < 0.02).
Survival among AIGIV-treated animals was significantly greater than that in the Gamunex control group (P < 0.02). Furthermore, a correlation between the time of treatment and the likelihood of survival was observed, with earlier intervention affording significantly greater protection (P < 0.0001).
Therapeutic efficacy in NZW rabbits (trigger-based intervention).
The efficacy of AIGIV in symptomatic animals was also evaluated in the rabbit model, with treatment being administered on an individual-animal basis using SIBT as a trigger for treatment (Table 5). The average actual inhaled aerosol dose was 188 ± 43 LD50 (mean ± standard deviation), which corresponds to 2.0 × 107 ± 4.6 × 106 CFU/animal based on the published LD50 (24). SIBT has been shown to correlate with disease progression, bacteremia, toxemia, and mortality (21). All animals exhibited SIBT at 28 ± 6 h postchallenge, which is consistent with the 27 ± 7 h postchallenge reported previously (21). All animals became bacteremic, as determined retrospectively by culture and quantitative PCR at 26 ± 6 h, and antigenemic, as determined retrospectively by ECL and ELISA, at 28 ± 15 h postchallenge. Necropsy and histopathology analysis confirmed that all animals that died prior to the scheduled study termination exhibited typical anthrax lesions.
Table 5.
Design of therapeutic efficacy study in NZW rabbits (trigger-based intervention)
| Treatment group | Product | No. of animals/group | IgG (mg/kg) |
Inhaled dose (LD50) |
Blood collection time pointsa |
|||
|---|---|---|---|---|---|---|---|---|
| Anti-PA | Total | Target | Actual [mean ± SD (range)] | PA (by ECL) and bacteremia | PA (by ELISA) and TNA levels | |||
| 1 | AIGIV | 8 | 7.1 | 182 | 200 | 190 ± 39 (137–257) | Prechallenge day −7 | Prechallenge day −7 |
| 2 | AIGIV | 8 | 14.2 | 364 | 200 | 209 ± 50 (150–307) | Postchallenge hours 12, 18, 24, 30, 36, 42, and 72 and PTI | Postchallenge, PTI |
| 3 | AIGIV | 8 | 21.3 | 546 | 200 | 181 ± 37 (127–222) | Postinfusion hours 48 and 96 | Postinfusion hours 1, 4, 12, 24, 48, 72, and 96 |
| 4 | Gamunex | 8 | 0 | 546 | 200 | 170 ± 43 (124–265) | Postinfusion days 6, 10, 20, and 28 | Postinfusion days 6, 10, 20, and 28 |
PTI, prior to infusion.
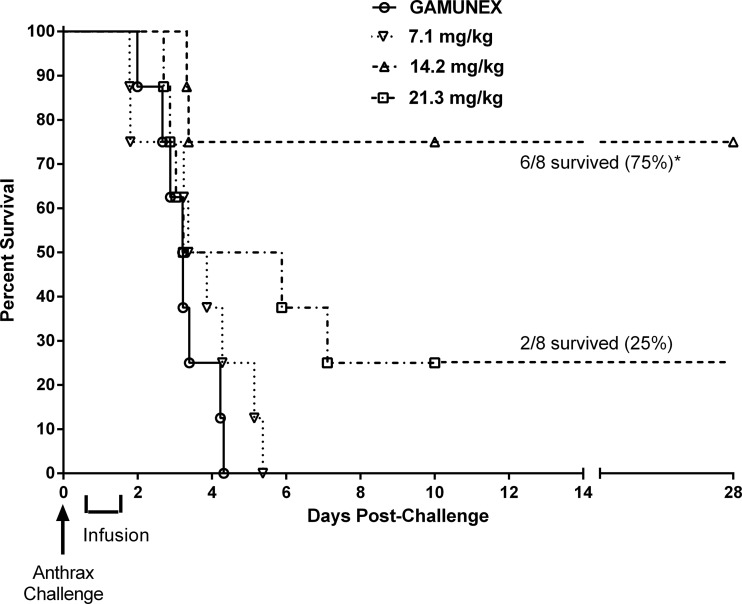
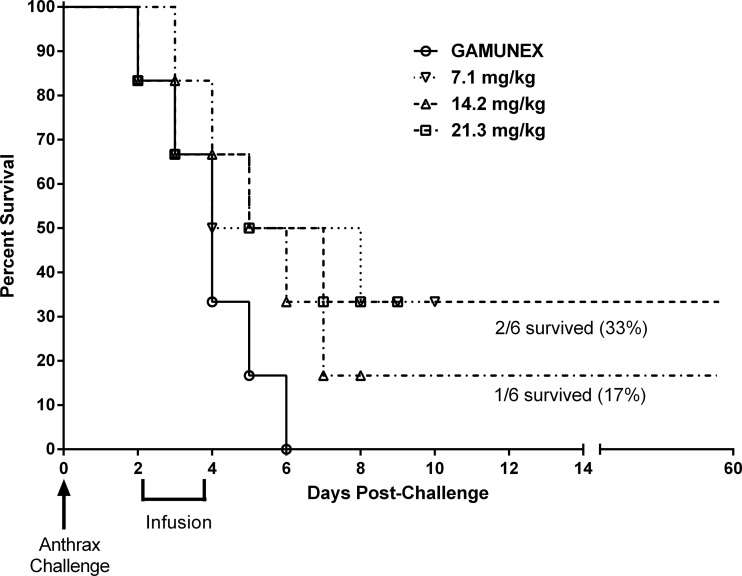
At the doses of 14.2 mg/kg and 21.3 mg/kg of anti-PA IgG, survival rates of 75% and 25%, respectively, were observed. There were no survivors in groups dosed with 7.1 mg/kg of AIGIV or Gamunex (Fig. 3). Survival in the group treated with 14.2 mg/kg of anti-PA IgG was significantly (P < 0.05) higher than in the Gamunex control group and in the group treated with 7.1 mg/kg of anti-PA IgG. Additionally, time to death increased significantly (P < 0.05) in the groups administered 14.2 and 21.3 mg/kg of anti-PA IgG compared to the control group. However, the differences in survival between these two groups were not significant, as the small sample size in this pilot study did not provide sufficient power to differentiate between two AIGIV-treated groups with respect to survival outcome.
Fig 3.
Survival results (Kaplan-Meier plot) of a therapeutic efficacy study in rabbits (trigger-based intervention). Animals were challenged with a target dose of 200 LD50 of B. anthracis Ames strain spores. *, survival in the group treated with 14.2 mg/kg of anti-PA IgG was significantly greater than that in the Gamunex control group (P < 0.05).
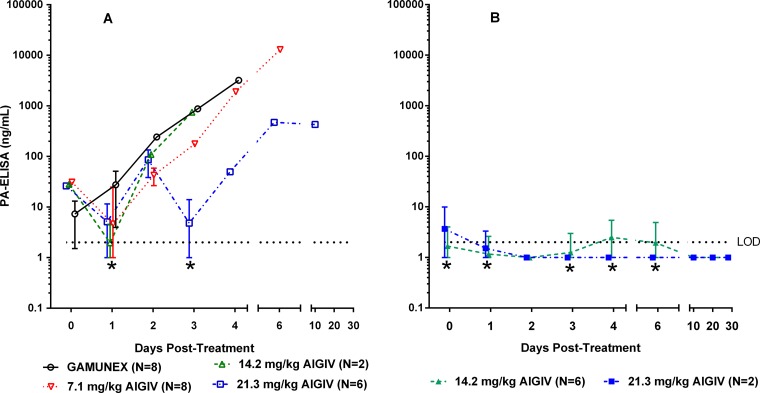
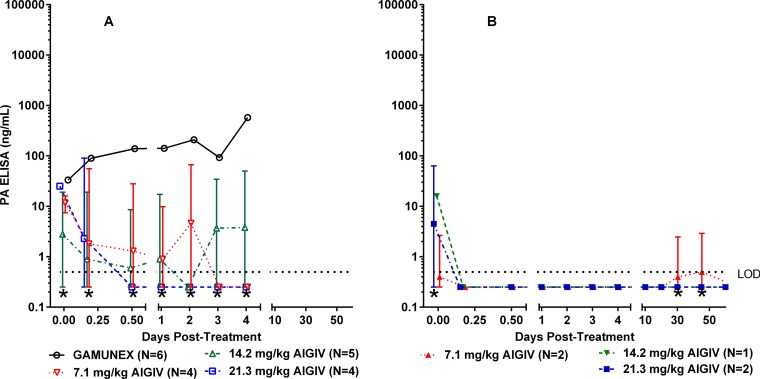
All rabbits that succumbed to the disease, in groups dosed with 7.1 mg/kg anti-PA IgG of AIGIV or Gamunex, exhibited high levels of PA in the serum prior to treatment, and their PA levels continued to rise throughout the observation period (Fig. 4A). In contrast, animals that were treated with 14.2 and 21.3 mg/kg of anti-PA IgG and survived exhibited lower PA levels prior to treatment, and the low levels of PA were maintained in these animals until the end of the study (Fig. 4B). Importantly, the increase in the AIGIV dose appeared to correlate with a decrease in postinfusion levels of PA in the surviving animals, as quantified by ELISA (Fig. 4B). For example, on day 3 postinfusion, the geometric mean PA levels in animals treated with AIGIV at the dose of 14.2 mg/kg and 21.3 mg/kg of anti-PA IgG were, respectively, 280 and 400 times lower (P < 0.05) than those in the control group. The inverse correlation between the dose of AIGIV and serum PA concentration postinfusion was further supported when areas under the PA concentration-time curve (AUCPA) were examined from the time point immediately prior to treatment until day 4, the time point by which all Gamunex-treated control animals succumbed to the disease. The AUCPA for animals treated with Gamunex and with 7.1, 14.2, and 21.3 mg/kg of anti-PA IgG, were 2,753, 1,194, 11, and 46 ng · day/ml, respectively. These values represent 250- and 60-fold decreases in AUCPA in animals infused with 14.2 and 21.3 mg/kg of anti-PA IgG, respectively, compared to the Gamunex control group.
Fig 4.
Circulating PA levels in a therapeutic efficacy-based study in rabbits (trigger-based intervention). Geometric mean PA concentrations with standard deviations, as measured by ELISA, in rabbits (n = 32) exposed to 188 ± 43 (mean ± standard deviation) LD50 of B. anthracis Ames strain spores via aerosol inhalation are shown. (A) Nonsurvivors; (B) survivors. Limit of detection (LOD) = 2.0 ng/ml. Values below the LOD were replaced with one-half the LOD (1.0 ng/ml). *, standard deviations below the limit of detection were truncated for plotting purposes.
Therapeutic efficacy in cynomolgus macaques (trigger-based intervention).
Therapeutic efficacy of AIGIV was also evaluated in the NHP model (Table 6). In this study, cynomolgus macaques were administered AIGIV or Gamunex on an individual-animal basis upon detection of the presence of PA in serum by ECL-based immunoassay (22). While fever is not a reliable predictor of disease progression in NHPs, detection of anthrax PA in the serum by ECL can be used as a trigger for treatment because the longer time frame for the progression of the disease in this animal model allows sufficient time for detection of this biomarker and initiation of treatment (23, 27).
Table 6.
Design of therapeutic efficacy study in NHPs (trigger-based intervention)
| Treatment group | Product | No. of animals/groupa | IgG (mg/kg) |
Inhaled dose (LD50) |
Blood collection time pointsb |
|||
|---|---|---|---|---|---|---|---|---|
| Anti-PA | Total | Target | Actual [mean ± SD (range)] | PA (by ECL) and bacteremia | PA (by ELISA) and TNA levels | |||
| 1 | AIGIV | 6 | 21.3 | 546 | 200 | 278 ± 58 (214–338) | Prechallenge day −7 | Prechallenge day −7 |
| 2 | AIGIV | 6 | 14.2 | 364 | 200 | 314 ± 57 (228–365) | Postchallenge hours 24, 30, 36, 42, 48, 54, 60, and 66 and PTI | Postchallenge, PTI |
| 3 | AIGIV | 6 | 7.1 | 182 | 200 | 254 ± 34 (207–308) | Postinfusion hours 12, 48, 72, and 96 | Postinfusion hours 1, 4, 12, 24, 48, 72, and 96 |
| 4 | Gamunex | 6 | 0 | 546 | 200 | 279 ± 55 (197–347) | Postinfusion days 10, 20, 30, 45, and 60 | Postinfusion days 10, 20, 30, 45, and 60 |
Equal number of males and females were used.
Time points represent all of the possible blood collection time points. Once an NHP was positive for PA ECL and the PTI sample was taken, the subsequent blood draw schedule adhered to the postinfusion time points. PTI, prior to infusion.
The average actual inhaled aerosol exposure dose was 281 ± 51 LD50 (mean ± standard deviation), which is equivalent to 1.6 × 107 ± 3.3 × 106 CFU/animal based on the published LD50 (23).
The onset of antigenemia, as evidenced by detection of PA in circulation by ELISA, occurred, on average, 48 h postchallenge, which was significantly later (P < 0.05) than the onset of bacteremia, which occurred, on average, 33 h postchallenge. All animals treated with AIGIV, regardless of survival outcome, showed a decrease in circulating PA levels following infusion (Fig. 5A and B). In contrast, PA levels in Gamunex-treated animals remained high at all time points postinfusion (Fig. 5A).
Fig 5.
Circulating PA levels in a therapeutic efficacy-based study in NHPs (trigger-based intervention). Geometric mean PA concentrations with standard deviations, as measured by ELISA, in cynomolgus macaques (n = 24) challenged with a dose of 281 ± 51 (mean ± standard deviation) of B. anthracis (Ames strain) spores via aerosol inhalation are shown. (A) Nonsurvivors; (B) survivors. Limit of detection (LOD) = 0.5 ng/ml. Values below the LOD were replaced with one-half the LOD (0.25 ng/ml). *, standard deviations below the limit of detection were truncated for plotting purposes.
All Gamunex-treated control animals succumbed to the disease, while up to 33% of AIGIV-treated animals survived (Fig. 6). However, the observed differences in survival were not statistically significant, since the sample size of 6 animals per group did not provide sufficient power. The gross necropsy analysis confirmed that all animals that died during the study observation period had lesions consistent with anthrax.
Fig 6.
Survival results (Kaplan-Meier plot) of therapeutic efficacy study in NHPs (trigger-based intervention). Animals were challenged with a target dose of 200 LD50 of B. anthracis Ames spores.
Human safety and PK study.
In the clinical safety and PK study, a putative target AIGIV dose of 7.1 mg/kg of anti-PA IgG was selected, based on an anthrax immune globulin dose of 500 mg of anti-PA IgG proposed by a CDC panel of experts for therapy of anthrax for an adult (7.1 mg/kg for a 70-kg person) (C. Dykewicz, presented at an external review committee of anthrax and immune globulin experts of CDC, NIH, and USAMRID, 2003). Doses 50% lower and 2-fold higher were also evaluated.
The PK analysis, based on measuring postinfusion serum TNA levels, demonstrated dose proportionality with respect to AUC0-∞ and Cmax (Table 3). The mean t1/2 ranged from 22.3 to 25.3 days, and the average mean residence time (MRT) ranged from 25.0 to 30.2 days. Both t1/2 and MRT were independent of dose.
The overall incidence of adverse events in subjects who received a single IV infusion of AIGIV was comparable to that in subjects who received a single IV infusion of Gamunex. In most subjects that exhibited treatment-emergent adverse events (TEAEs), these were mild or moderate in severity (74% of AIGIV subjects and 84% of Gamunex subjects). Increased blood pressure and respiratory rate were the most common TEAEs and were possibly related to AIGIV in 29% and 2% of AIGIV-infused subjects, respectively, and 17% and 3% of Gamunex-infused subjects, respectively. Grade 3 increases in blood pressure postinfusion occurred in 13% of AIGIV subjects and 6% of Gamunex subjects. All cases were resolved within 24 h postinfusion.
A low absolute neutrophil count (ANC) was observed in three subjects in cohort A (870 cells/mm3, 990 cells/mm3, and 970 cells/mm3), all of whom had relatively low baseline ANCs (1,170 cells/mm3, 2,420 cells/mm3, and 2,550 cells/mm3, respectively). Entry criteria were modified after the completion of dosing in cohort A to exclude subjects with low ANCs at baseline, and a more balanced racial distribution (African American versus other races) was also employed for enrollment in cohorts B and C, since African Americans are more likely to have low normal ANCs (28–30). In these higher-dose cohorts, no subjects had grade 3 ANCs (<1,000 cells/mm3), three subjects (2 AIGIV and 1 Gamunex) had grade 2 ANCs (1,000 to 1,490 cells/mm3), and two subjects (1 AIGIV and 1 Gamunex) had grade 1 ANCs (500 to 990 cell/mm3). All subjects normalized their ANCs by 2 weeks following infusion.
Other reported TEAEs included a transient increase in alanine aminotransferase (ALT) levels at 24 h (196 U/liter) and 48 h (113 U/liter) in cohort B (n = 1), an increased aspartate transaminase (AST) level on day 5 (123 U/liter) in cohort C (n = 1), and proteinuria (100 mg/dl) in cohort C (n = 2).
DISCUSSION
Anti-PA antibody-based therapeutics can neutralize anthrax toxins and, therefore, may play a role in controlling the morbidity and mortality associated with exposure to aerosolized anthrax spores (4). In particular, they can be beneficial if a delay in the initiation of antimicrobial intervention results in onset of fulminant toxemic disease. Because clinical efficacy studies with these types of products are not feasible or ethical, efficacy must be evaluated in animal models and the human dose derived based on comparison of the PK parameters in animals and humans (17, 18).
AIGIV PK analysis demonstrated that the decay of the human anti-PA antibody is more rapid in animals than in humans (Table 3). As a result, a higher dose has to be administered to animals to mimic the overall exposure to the drug in humans. Indeed, comparison of the animal and human PK data suggests that a human dose of 7 mg/kg is similar to a dose of 21 mg/kg in rabbits and NHPs with respect to the observed AUC0-∞ (see Table 3).
The efficacy studies in rabbits and NHPs indicated that AIGIV can increase survival of the anthrax-infected animals, even when administered after the onset of clinical disease. Furthermore, the results of trigger-based intervention studies also indicated that AIGIV reduced the bacterial burden in the surviving animals, suggesting that although only a limited number of animals survived the challenge, therapeutic intervention with AIGIV is biologically relevant in improving survival outcome in animals subjected to lethal anthrax aerosol challenge. Importantly, the studies highlighted substantial animal-to-animal variability with regard to the timing of onset of bacteremia and toxemia, which has been reported previously (21). This variability, in combination with small group sizes, likely contributed to the variable survival outcome in both animal models and to the lack of a clear dose response.
Animal survival data suggest that AIGIV provides a higher level of protection from death when administered earlier rather than later in the fulminant stage of the disease, which is consistent with data reported for other anthrax antitoxins (31). The animal studies described here suggest that AIGIV neutralizes anthrax toxin and as such has the potential to reduce morbidity and mortality associated with anthrax. Further studies are warranted to investigate efficacy of AIGIV in symptomatic animals (i.e., bacteremic, antigenemic, and/or febrile), as well as its interaction with other anthrax countermeasures, as described in the accompanying article (32).
ACKNOWLEDGMENTS
We thank Gregory Stark, Bill Blackwelder, and Diane Sweeney for statistical support; Virginia Johnson, Weila Wang, Jeffrey Smith, Xiaomi Tong, Stephanie Banach, Devinder Poonian, Dave Main, Martina Hemmer, Shannon Cureton, Ron Aimes, Christine Valencia, Bryan Fortson, and Tyler Laudenslager for programmatic and technical support; and Louise Pitt, Paul Dabisch, and Tony Alves for conducting initial proof-of-concept studies.
This work was supported by contract no. HHSN272200800034C and grant no. U01AI070486 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services (DHHS), as well as contract no. HHSO100200700037C from the Biomedical Advanced Research and Development Authority, Office of the Assistant Secretary for Preparedness and Response, DHHS.
All the authors are either current or former employees or contractors of Emergent BioSolutions, the developer of AIGIV and manufacturer of BioThrax.
Footnotes
Published ahead of print 26 August 2013
REFERENCES
- 1.Brachman PS, Friedlander AM, Grabenstein JD. 2004. Anthrax vaccine, 3rd ed, p 887–903 Saunders, Philadelphia, PA [Google Scholar]
- 2.Grabenstein JD. 2008. Countering anthrax: vaccines and immunoglobulins. Clin. Infect. Dis. 46:129–136 [DOI] [PubMed] [Google Scholar]
- 3.Inglesby TV, O'Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Gerberding J, Hauer J, Hughes J, McDade J, Osterholm MT, Parker G, Perl TM, Russell PK, Tonat K. 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236–2252 [DOI] [PubMed] [Google Scholar]
- 4.Holty JE, Bravata DM, Liu H, Olshen RA, McDonald KM, Owens DK. 2006. Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann. Intern. Med. 144:270–280 [DOI] [PubMed] [Google Scholar]
- 5.Dixon TC, Meselson M, Guillemin J, Hanna PC. 1999. Anthrax. N. Engl. J. Med. 341:815–826 [DOI] [PubMed] [Google Scholar]
- 6.Beedham RJ, Turnbull PC, Williamson ED. 2001. Passive transfer of protection against Bacillus anthracis infection in a murine model. Vaccine 19:4409–4416 [DOI] [PubMed] [Google Scholar]
- 7.Little SF, Ivins BE, Fellows PF, Pitt ML, Norris SL, Andrews GP. 2004. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine 22:422–430 [DOI] [PubMed] [Google Scholar]
- 8.Little SF, Ivins BE, Webster WM, Fellows PF, Pitt ML, Norris SL, Andrews GP. 2006. Duration of protection of rabbits after vaccination with Bacillus anthracis recombinant protective antigen vaccine. Vaccine 24:2530–2536 [DOI] [PubMed] [Google Scholar]
- 9.Pitt ML, Little SF, Ivins BE, Fellows P, Barth J, Hewetson J, Gibbs P, Dertzbaugh M, Friedlander AM. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19:4768–4773 [DOI] [PubMed] [Google Scholar]
- 10.Reuveny S, White MD, Adar YY, Kafri Y, Altboum Z, Gozes Y, Kobiler D, Shafferman A, Velan B. 2001. Search for correlates of protective immunity conferred by anthrax vaccine. Infect. Immun. 69:2888–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jernigan JA, Stephens DS, Ashford DA, Omenaca C, Topiel MS, Galbraith M, Tapper M, Fisk TL, Zaki S, Popovic T, Meyer RF, Quinn CP, Harper SA, Fridkin SK, Sejvar JJ, Shepard CW, McConnell M, Guarner J, Shieh WJ, Malecki JM, Gerberding JL, Hughes JM, Perkins BA. 2001. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg. Infect. Dis. 7:933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barakat LA, Quentzel HL, Jernigan JA, Kirschke DL, Griffith K, Spear SM, Kelley K, Barden D, Mayo D, Stephens DS, Popovic T, Marston C, Zaki SR, Guarner J, Shieh WJ, Carver HW, Meyer RF, Swerdlow DL, Mast EE, Hadler JL. 2002. Fatal inhalational anthrax in a 94-year-old Connecticut woman. JAMA 287:863–868 [DOI] [PubMed] [Google Scholar]
- 13.Huntington R, Thompson W, Gordon H. 1937. The treatment of tetanus with antitoxin: an analysis of the outcome in six-hundred forty-two cases. Ann. Surg. 105:93–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogunrin OA. 2009. Tetanus—a review of current concepts In management. Benin J. Postgraduate Med. 11:46–61 [Google Scholar]
- 15.Mayers CN, Holley JL, Brooks T. 2001. Antitoxin therapy for botulinum intoxication. Rev. Med. Microbiol. 12:29–37 [Google Scholar]
- 16.Goossens PL. 2009. Animal models of human anthrax: the quest for the holy grail. Mol. Aspects Med. 30:467–480 [DOI] [PubMed] [Google Scholar]
- 17.Bergman KL. 2009. The animal rule and emerging infections: the role of clinical pharmacology in determining an effective dose. Clin. Pharmacol. Ther. 86:328–331 [DOI] [PubMed] [Google Scholar]
- 18.FDA 2009. Guidance for industry animal models—essential elements to address efficacy under the animal rule. FDA, Washington, DC [Google Scholar]
- 19.Quinn CP, Dull PM, Semenova V, Li H, Crotty S, Taylor TH, Steward-Clark E, Stamey KL, Schmidt DS, Stinson KW, Freeman AE, Elie CM, Martin SK, Greene C, Aubert RD, Glidewell J, Perkins BA, Ahmed R, Stephens DS. 2004. Immune responses to Bacillus anthracis protective antigen in patients with bioterrorism-related cutaneous or inhalation anthrax. J. Infect. Dis. 190:1228–1236 [DOI] [PubMed] [Google Scholar]
- 20.Quinn CP, Semenova VA, Elie CM, Romero-Steiner S, Greene C, Li H, Stamey K, Steward-Clark E, Schmidt DS, Mothershed E, Pruckler J, Schwartz S, Benson RF, Helsel LO, Holder PF, Johnson SE, Kellum M, Messmer T, Thacker WL, Besser L, Plikaytis BD, Taylor TH, Jr, Freeman AE, Wallace KJ, Dull P, Sejvar J, Bruce E, Moreno R, Schuchat A, Lingappa JR, Martin SK, Walls J, Bronsdon M, Carlone GM, Bajani-Ari M, Ashford DA, Stephens DS, Perkins BA. 2002. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen. Emerg. Infect. Dis. 8:1103–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comer JE, Ray BD, Henning LN, Stark GV, Barnewall RE, Mott JM, Meister GT. 2012. Characterization of a therapeutic model of inhalational anthrax using an increase in body temperature in New Zealand white rabbits as a trigger for treatment. Clin. Vaccine Immunol. 19:1517–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henning LN, Comer JE, Stark GV, Ray BD, Tordoff KP, Knostman KA, Meister GT. 2012. Development of an inhalational Bacillus anthracis exposure therapeutic model in cynomolgus macaques. Clin. Vaccine Immunol. 19:1765–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasconcelos D, Barnewall R, Babin M, Hunt R, Estep J, Nielsen C, Carnes R, Carney J. 2003. Pathology of inhalation anthrax in cynomolgus monkeys (Macaca fascicularis). Lab. Invest. 83:1201–1209 [DOI] [PubMed] [Google Scholar]
- 24.Zaucha GM, Pitt LM, Estep J, Ivins BE, Friedlander AM. 1998. The pathology of experimental anthrax in rabbits exposed by inhalation and subcutaneous inoculation. Arch. Pathol. Lab. Med. 122:982–992 [PubMed] [Google Scholar]
- 25.Li H, Soroka SD, Taylor TH, Jr, Stamey KL, Stinson KW, Freeman AE, Abramson DR, Desai R, Cronin LX, Oxford JW, Caba J, Pleatman C, Pathak S, Schmidt DS, Semenova VA, Martin SK, Wilkins PP, Quinn CP. 2008. Standardized, mathematical model-based and validated in vitro analysis of anthrax lethal toxin neutralization. J. Immunol. Methods 333:89–106 [DOI] [PubMed] [Google Scholar]
- 26.Omland KS, Brys A, Lansky D, Clement K, Lynn F. 2008. Interlaboratory comparison of results of an anthrax lethal toxin neutralization assay for assessment of functional antibodies in multiple species. Clin. Vaccine Immunol. 15:946–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Twenhafel NA, Leffel E, Pitt ML. 2007. Pathology of inhalational anthrax infection in the African green monkey. Vet. Pathol. 44:716–721 [DOI] [PubMed] [Google Scholar]
- 28.Haddy TB, Rana SR, Castro O. 1999. Benign ethnic neutropenia: what is a normal absolute neutrophil count? J. Lab. Clin. Med. 133:15–22 [DOI] [PubMed] [Google Scholar]
- 29.Hsieh MM, Everhart JE, Byrd-Holt DD, Tisdale JF, Rodgers GP. 2007. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann. Intern. Med. 146:486–492 [DOI] [PubMed] [Google Scholar]
- 30.Reich D, Nalls MA, Kao WH, Akylbekova EL, Tandon A, Patterson N, Mullikin J, Hsueh WC, Cheng CY, Coresh J, Boerwinkle E, Li M, Waliszewska A, Neubauer J, Li R, Leak TS, Ekunwe L, Files JC, Hardy CL, Zmuda JM, Taylor HA, Ziv E, Harris TB, Wilson JG. 2009. Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS Genet. 5:e1000360. 10.1371/journal.pgen.1000360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Migone TS, Subramanian GM, Zhong J, Healey LM, Corey A, Devalaraja M, Lo L, Ullrich S, Zimmerman J, Chen A, Lewis M, Meister G, Gillum K, Sanford D, Mott J, Bolmer SD. 2009. Raxibacumab for the treatment of inhalational anthrax. N. Engl. J. Med. 361:135–144 [DOI] [PubMed] [Google Scholar]
- 32.Malkevich NV, Basu S, Rudge TL, Jr, Clement KH, Chakrabarti AC, Aimes RT, Nabors GS, Skiadopoulos MH, Ionin B. 2013. Effect of anthrax immune globulin on response to BioThrax (anthrax vaccine adsorbed) in New Zealand White rabbits. Antimicrob. Agents Chemother. 57:5693–5696 [DOI] [PMC free article] [PubMed] [Google Scholar]