Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is a well-known public health concern. However, the means by which methicillin resistance genes are transferred among staphylococci in nature remains unknown. Older scientific literature suggests transduction as a means of mecA transfer, but the optimal conditions are reported to require plasmids and potentially a lysogenic phage. These reports preceded discovery of the staphylococcal cassette chromosome mec (SCCmec) elements. We undertook studies to confirm and clarify the conditions promoting transduction of SCCmec in S. aureus populations using well-characterized donor and recipient strains primarily of the USA300 lineage. Both bacteriophages 80α and 29 were capable of transducing SCCmec type IV and SCCmec type I to recipient strains of S. aureus. Pulsed-field gel electrophoresis and mec-associated dru typing were used to confirm the identity of the transductants. Transfer of mecA via transduction occurred at low frequency and required extended selection times for mecA gene expression and the presence of a penicillinase plasmid in the recipient. However, interference with the process by clavulanic acid and the necessity of lysogeny with ϕ11 in the recipient or the presence of a small (4-kb) tetracycline resistance plasmid, as previously reported, were not confirmed. SCCmec transduction was occasionally associated with substantial deletions or truncation of SCCmec and the arginine catabolic metabolic element in USA300 recipients. Overall, these data clarify the conditions required for SCCmec transduction and document that rearrangements may occur during the process.
INTRODUCTION
Staphylococcus aureus is a ubiquitous human pathogen responsible for greater than 50% of all skin and soft tissue infections as well as more serious systemic infections, including sepsis, toxic shock syndrome, and necrotizing pneumonia (1). Since the early 1960s, increasing numbers of S. aureus infections have been caused by methicillin-resistant S. aureus (MRSA). MRSA infections have traditionally been associated with hospitalized patients; however, in recent years, a steadily increasing number of MRSA infections have been community associated (CA-MRSA infections) (2–7).
Methicillin resistance is mediated by a novel penicillin binding protein designated PBP2a, which has lower affinity for the β-lactam antibiotics than does the native PBP2 enzyme (8, 9). The mecA gene is harbored on one of several mobile genetic elements, which are designated staphylococcal cassette chromosome mec (SCCmec). Eleven variations of SCCmec (I to XI) have been reported (10–13). The global health problems caused by MRSA have generated significant interest in understanding the movement of SCCmec among staphylococci. SCCmec is known to reside at the orfX insertion site in the S. aureus genome. In some strains (e.g., USA300) SCCmec is adjacent to a region named the arginine catabolic metabolic element (ACME) (14). While SCCmec transfer can likely occur via homologous recombination, in at least some instances, transfer and site-specific insertion are facilitated by the ccr recombinase gene products encoded within SCCmec (15). However, an overall understanding of SCCmec movement in staphylococcal populations suffers, in part, from conflicting early studies conducted prior to current knowledge of SCCmec structure and function. In the early 1970s, Cohen and Sweeney suggested that transduction of methicillin resistance in S. aureus required the presence of both a prophage (ϕ11) and a penicillinase plasmid (pI524) in the recipient (16, 17). Conversely, Stewart and Rosenblum found no lysogenic requirement for transduction of methicillin resistance but did report the inhibitory effect of clavulanic acid on the process (18). A related study by Shafer and Iandolo reported cotransduction of methicillin with a small (4-kb) tetracycline resistance plasmid (19).
The goal of this study was to clarify the conditions required for transduction of SCCmec elements to recipient strains of S. aureus. To this end, we revisited the requirements for both donor and recipient cells by using a variety of genetically characterized strains.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Characteristics of the bacterial strains used in the study are summarized in Table 1. Donor strains for transduction experiments were S. aureus COL (20), USA300 FPR3757 (14), and recent USA300 clinical isolates RG1786 and RG1710. Recipients were all methicillin-susceptible USA300 strains but differed in the presence or absence of ACME. This included a derivative of USA300 FPR3757 that had lost SCCmec after repeated subculture. For some experiments, recipients were transduced to novobiocin resistance (using S. aureus U9NO [21] as a donor) as an additional strain marker. Additional recipients were also transduced or natively carried USA300 penicillinase plasmid pCRG1308 or pCRG1306B, both of which are ∼30 kb in size, or one of the well-characterized plasmids pI258, pI524, and pII147 (22). In some instances, plasmids were cured from host cells by repeated subculture under nonselective conditions at 37°C with replica plating to detect loss of resistance.
Table 1.
Characteristics of bacterial strains
| Strain | Relevant characteristics | Reference |
|---|---|---|
| Methicillin-resistant donors | ||
| FPR3757a | SCCmec IVd, dru type 9g, ACME+ tetc | 14 |
| RG1710a | USA300 SCCmec IV, dru type 9i, ACME+ tet | This study |
| RG1786a | USA300 SCCmec IV, dru type 9af, ACME+ tet | This study |
| COLb | SCCmec I,d dru type 10a, ACME− tet | 20 |
| Methicillin-sensitive recipients | ||
| RG1439 | RG1308 USA300, ACME+ (pCRG1308) nov | This study |
| RG1933 | RG1439 (ΔpCRG1308) | This study |
| RG2150 | RG1933(pCRG1308) | This study |
| RG2373 | RG1933(pI258) | This study |
| RG2374 | RG1933(pI524) | This study |
| RG1438 | RG1306B USA300, ACME+ (pCRG1306B) nov | This study |
| RG1171 | USA300 ACME− nov | This study |
| RG2151 | RG1171(pCRG1308) | This study |
| RG2367 | RG1171(pII147) | This study |
| RG2392 | FPR3757 ΔSCCmec nov | This study |
| RG2393 | RG2392(pCRG1308) nov | This study |
| 8325-4 | 8325 cured of prophages | 40 |
| RG2319 | 8325-4(pCRG1308) | This study |
| RN4220 | Restriction-deficient mutant of 8325-4 | 40 |
| RG2323 | RN4220(pCRG1308) | This study |
| GS451 | 8325-4 (ϕ11) | 19 |
Donor propagated with phage 80α.
Donor propagated with phage 29.
Encoded by a small (∼4-kb) plasmid.
SCCmec I and SCCmec IV are 34 kb and 24 kb in size, respectively.
Transduction and selection.
Transductions were performed essentially following the method of Cohen and Sweeney (16) using donor and recipient strains and either phage 80α or 29 as indicated in Table 1. Methicillin-resistant transductants were selected at 30°C or 37°C for 24 to 72 h on brain heart infusion (BHI) agar (Becton, Dickinson, and Company, Sparks, MD) containing cefoxitin (6 μg/ml) and, for some experiments, clavulanic acid (4 μg/ml). Transductants were further purified by subculture on BHI agar containing both cefoxitin (6 μg/ml) and novobiocin (7 μg/ml) (Sigma-Aldrich, St. Louis, MO).
Molecular characterization. (i) Pulsed-field gel electrophoresis (PFGE).
Chromosomal DNA was prepared in agarose plugs, digested with SmaI restriction endonuclease, and analyzed as previously described (23).
mec-associated dru typing.
DNA was harvested from overnight BHI agar cultures using the Qiagen DNeasy kit (Qiagen, Gaithersburg, MD). The direct-repeat unit (dru) region was sequenced from PCR-amplified products as previously described (24). The resulting dru types were verified using the international dru typing database (http://www.dru-typing.org).
Sequence analysis of the SCCmec-ACME junction.
Sequence analysis of the junction between SCCmec and ACME in selected transductants was performed on PCR amplification products of the regions between the similar direct-repeat sequences found in both elements (USA300_FPR3757 [NCBI accession number NC_007793.1]). Amplification, using primer sequences 5′ACCAATTCATGGTAGAGGGCTT3′ (forward) and 5′GAGGAAGGACGCTGGATAGC3′ (reverse), consisted of an initial denaturation step at 94°C for 2 min, 30 cycles of denaturing at 94°C for 1 min, annealing at 50°C for 1 min, and extension at 72°C for 3 min, and a final extension at 72°C for 7 min.
RESULTS
MRSA donor strains of USA300 dru type 9g, dru type 9i, and dru type 9af, which carry SCCmec type IV, and the related COL strain (dru type 10a, SCCmec type I) (Table 1) (25) all yielded methicillin-resistant transductants. The recipient strains were methicillin-susceptible USA300 isolates lacking SCCmec and, in some cases, ACME (Fig. 1). Transductions with S. aureus USA300 donors were successful with bacteriophage 80α only after repeated passage to improve lytic efficiency. Transductions with COL employed bacteriophage 29. Transduction of SCCmec from USA300 (ACME-positive) donors to ACME-positive methicillin-susceptible recipients was observed, while transfer to ACME-negative recipients was unsuccessful (Tables 1 and 2). Conversely, with the ACME-negative S. aureus COL donor, transduction was successful only with methicillin-susceptible USA300 ACME-negative recipients.
Fig 1.
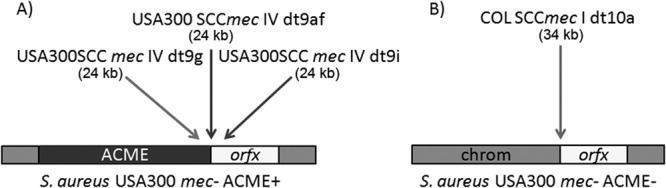
SCCmec types transduced into USA300 recipients. (A) USA300 donor SCCmec type IVa with dru types 9g, 9af, and 9i was transduced into ACME positive methicillin-susceptible USA300 recipients. (B) S. aureus COL SCCmec type I with dru type 10a was transduced into an ACME negative methicillin-susceptible USA300 recipient.
Table 2.
SCCmec transfer frequencies, selecting for cefoxitin resistance in S. aureus recipients
| Donor | Recipient | Relevant recipient characteristics |
Transduction frequencya | |
|---|---|---|---|---|
| Penicillin resistance | ACME | |||
| FPR3757 (ACME+) | RG1439 | + | + | 3.8 × 10−9 |
| RG1933 | − | + | 0 | |
| RG2150 | + | + | 1.7 × 10−9 | |
| RG2373 | + | + | 2.3 × 10−10 | |
| RG2374 | + | + | 2.3 × 10−10 | |
| RG1438 | + | + | 1.3 × 10−9 | |
| RG2392 | − | + | 0 | |
| RG2393 | + | + | 8.5 × 10−10 | |
| RG1171 | − | − | 0 | |
| RG2151 | + | − | 0 | |
| RG1786 (ACME+) | RG1439 | + | + | 1.2 × 10−9 |
| RG1933 | − | + | 0 | |
| RG1438 | + | + | 1.3 × 10−9 | |
| RG1171 | − | − | 0 | |
| RG1710 (ACME+) | RG1439 | + | + | 6.9 × 10−10 |
| RG1438 | + | + | 6.9 × 10−10 | |
| COL (ACME−) | RG1439 | + | + | 0 |
| RG2150 | + | + | 0 | |
| RG2374 | + | + | 0 | |
| RG1171 | − | − | 0 | |
| RG2151 | + | − | 1.2 × 10−9 | |
| RG2367 | + | − | 3.1 × 10−10 | |
Expressed as average number of SCCmec transductants per PFU of phage donor as a result of 2 or more replicates.
Initial attempts to optimize transduction revealed that transfer frequencies were highest with selection for methicillin resistance at 30°C for 48 to 72 h, yielding from 3.8 × 10−9 to 6.9 × 10−10 transductants per PFU, consistent with previous reports (16–19). Thus, only data obtained under these conditions are shown (Table 2).
The presence of a penicillinase-producing plasmid was required in recipients for successful transduction of methicillin resistance (Table 2), consistent with previous reports (16–18, 26). Transduction of SCCmec was observed from USA300 FPR3757 (dru type 9g) into a methicillin-susceptible ACME-positive USA300 recipient (strain RG1439) which naturally carries penicillinase plasmid pCRG1308. However, loss of the penicillinase plasmid from this strain (RG1933) resulted in concomitant loss of the ability to serve as a transduction recipient. Reintroduction of pCRG1308 into strain RG1933 (i.e., strain RG2150) by transduction restored recipient effectiveness (Table 2). Methicillin-susceptible USA300 isolates naturally carrying an alternative 30-kb penicillinase plasmid (i.e., pCRG1306B) or strains that were transduced to carry either plasmid pI258, pI524, or pII147 (22) also served as effective recipients. (Table 1 and 2) Interestingly, this requirement was also demonstrated with the USA300 FPR3757 donor and the isogenic (but methicillin-susceptible) recipient strain RG2392, where transfer was observed only after introduction of a penicillinase plasmid (pCRG1308; RG2393). A similar plasmid requirement for successful SCCmec transduction was observed with additional USA300 donors RG1710 and RG1786 (carrying SCCmec IV with dru types dt9i and 9af, respectively) and with S. aureus COL (carrying SCCmec I with dru type 10a). However, in contrast to an older report (18) the addition of clavulanic acid (4 μg/ml) to the selection medium did not reduce the frequency of transduction (data not shown).
One early study reported the successful cotransduction of methicillin resistance with a small tetracycline resistance plasmid facilitated by the presence of a penicillinase plasmid and/or prophage in the recipient (19). We examined this issue using donor strains USA300 FPR3757 and COL (used in the earlier study), both of which natively carry 4.4-kb tetracycline resistance plasmids (pUSA02 and pT181, respectively) (14, 20). With USA300 FPR3757 transductions, USA300 recipient strains RG1438 and RG1439, carrying pCRG1306B and pCRG1308, respectively, were found to effectively receive SCCmec with direct selection as noted above. With strain COL, recipients were RG2319 (8325-4), RG2323 (RN4220) (both transduced to carry pCRG1308), and GS451 (8325-4 carrying phage ϕ11). Transfer of the tetracycline resistance plasmid from USA300 FPR3757 or COL to recipients was readily observed (e.g., 1.5 × 10−7 transductants per PFU). However, detectable cotransfer of methicillin resistance was not seen (data not shown).
SCCmec transfer was confirmed by dru typing both the donor and the transduced recipients. PFGE typing provided additional confirmation (see, e.g., Fig. 2, lanes 4, 5, and 9), where the SmaI fragment containing orfX in the transductants was comparable in size to that in the donor strain. However, approximately 60% of transfers from USA300 FPR3757 donors (e.g., FPR3757 to RG1439 or RG2393 recipients) yielded an atypical PFGE profile with the orfX-containing band only slightly larger than its SCCmec-negative counterpart (Fig. 2, lanes 8 and 7, respectively). DNA sequence analysis of the SCCmec and ACME regions in USA300 FPR3757 (GenBank accession number NC_007793.1) confirmed approximately 90% sequence similarity in the 1.8-kb direct repeats found immediately downstream of IS1272 in SCCmec and approximately 5.5 kb upstream of arcA in ACME (Fig. 3). These direct repeats have been only partially characterized in previous studies, although it is known that the SCCmec repeat region contains sequence for one hypothetical protein, SAUSA300_0036, and a truncated ccrB2. Conversely, in ACME the repeat has been annotated to encode four hypothetical proteins: SAUSA300_0056, SAUSA300_0057, SAUSA300_0058, and SAUSA300_0059. The first hypothetical protein encoded in both repeats, SAUSA300_0036 and SAUSA300_0056, matches to protein family HMM PF07799 with 100% identity for 103 bp (depicted as white boxes within the SCCmec and ACME repeats in Fig. 3). Using PCR primers to amplify the region between the direct repeats, sequence analysis revealed a 22-kb deletion due to recombination between these homologous 103-bp regions. As illustrated in Fig. 3, the result is a truncated SCCmec-ACME element with a 12-kb loss of the SCCmec J1 region and ccr recombinase genes extending 10 kb into the adjacent ACME.
Fig 2.
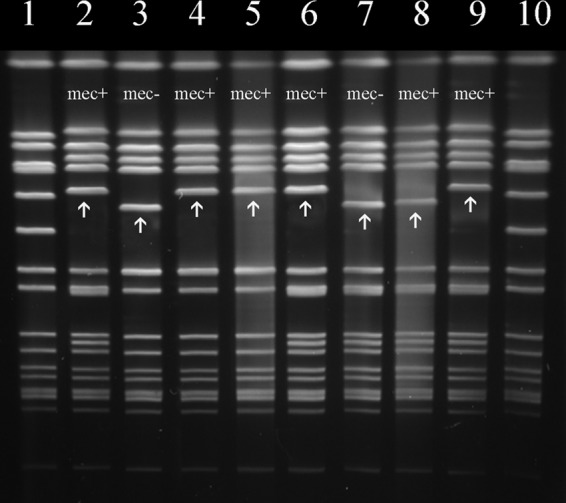
PFGE banding patterns associated with the presence or absence of SCCmec in SmaI-digested chromosomal DNA. Lanes 1 and 10 are size standard S. aureus 8325. The remaining lanes are as follows: 2, donor RG1786; 3, recipient RG1439; 4 and 5, SCCmec 1786/1439 transductants 1 and 2, respectively; 6, donor FPR3757; 7, recipient RG2393; 8, FPR3757/2393 truncated ACME-SCCmec transductant; and 9, FPR3757/2393 SCCmec transductant. Arrows indicate orfX-containing chromosomal SmaI fragments.
Fig 3.
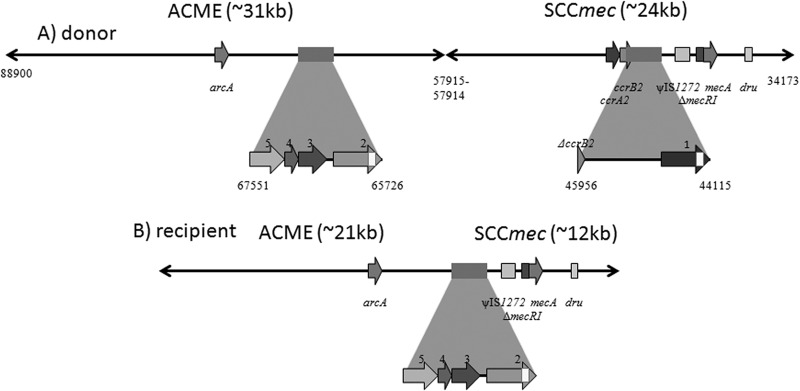
Comparison of SCCmec-ACME structure in S. aureus USA300 donors and truncated transductants due to recombination between direct-repeat sequences. The expanded boxes depict previously undescribed 1,847-bp direct repeats with ∼90% similarity in USA300 SCCmec and ACME. (A) A hypothetical protein in the SCCmec direct repeat is as follows: 1, SAUSA300_0036; truncated ccrB2 (ΔccrB2). Hypothetical proteins in the ACME direct repeat are as follows: 2, SAUSA300_0056; 3, SAUSA300_0057; 4, SAUSA300_0058; 5, SAUSA300_0059. SAUSA300_0036 and SAUSA300_0056 contain a region of 103 bp with 100% identity (highlighted as a white box) where recombination occurred. (B) SCCmec and ACME in a transductant that underwent SCCmec-ACME recombination. The majority of the direct repeat retained was from ACME.
DISCUSSION
Early reports describe conflicting requirements for successful transduction of methicillin resistance in S. aureus (16–19). Cohen and Sweeney reported successful transfer only into recipients that possessed a prophage and/or a penicillinase plasmid (16, 17), whereas Stewart and Rosenblum noted only the requirement of a penicillinase plasmid in the recipient (18). Shafer and Iandolo described plasmid-associated cotransfer of methicillin and tetracycline resistance in S. aureus strain 8325-4 lysogenized with bacteriophage ϕ11. However, these studies were conducted prior to our current understanding of staphylococcal genomics and the structure and function of SCCmec. Clarification of this issue is important for several reasons. While multiple mechanisms of horizontal gene transfer may occur in staphylococcal populations, transduction represents a plausible means by which SCCmec moves from one strain to another. Thus, increased understanding of this process may provide important insight regarding the emergence and spread of problematic (e.g., community-associated) MRSA strains. In addition, insight into the movement and potential rearrangement of SCCmec elements (discussed more fully below) has profound implications for epidemiological analysis (i.e., SCCmec typing) (10) as well as molecular diagnostics based on SCCmec architecture (27). To clarify the conditions associated with transduction of SCCmec in S. aureus, we focused primarily on characterized USA300 MRSA donors and methicillin-susceptible recipients, since the emergence and spread of community-associated MRSA is of clear clinical concern. In addition, these strains carry SCCmec IV, which is of relatively small size (e.g., 20 to 25 kb) and thus potentially more amenable to bacteriophage packaging for transfer by transduction.
Overall our results demonstrate the movement of four variations (i.e., dru types) of two different SCCmec elements (types I and IV) from donors to recipients as confirmed by using a combination of PFGE and mec-associated dru typing. However, the data underscore the important interplay of two key processes in recipient cells that are required for effective chromosomal gene transfer: chromosomal integration and expression. Regarding the former, transduction of SCCmec was observed only when donors and recipients were homologous with regard to the presence or absence of ACME. This result suggests the acquisition of SCCmec by homologous rather than ccr-mediated recombination due to the requirement for complementary SCCmec flanking sequences. However, given the low frequency of transfer (i.e., 3.8 × 10−9 to 6.9 × 10−10 transductants per PFU), the possibility that ccr-associated acquisition may naturally occur via transduction, albeit at a lower, undetectable frequency, cannot be ruled out. Low frequency of SCCmec transfer is consistent with the limited known number of phylogenetically distinct MRSA lineages (28, 29) and the possibility of host cell barriers to the acquisition of SCCmec (30). Contrary to previous reports (16, 17, 19), we did not find SCCmec cotransfer with plasmids encoding tetracycline resistance or associated with phage ϕ11 lysogeny in recipients. The reasons for these discrepancies are unclear but may be due in part to the lack of genetic characterization of the S. aureus strains employed in earlier studies. Conversely, we did confirm the extended time required for mecA gene expression in the recipient cells (48 to 72 h) at 30°C and the necessity for recipients to possess a penicillinase plasmid, which could include those naturally found in USA300 and other S. aureus lineages (22). Contrary to Stewart and Rosenblum's previous report (18), we found that the role of the penicillinase plasmid was not related to β-lactamase (BlaZ) production, since clavulanic acid, a β-lactamase inhibitor, had no effect on transduction frequency. The role of the penicillinase plasmid logically relates to plasmid blaR1-blaI regulatory genes (penicillin sensor/inducer and resistance repressor, respectively), which can regulate transcription of the blaZ gene for penicillinase production, and also transcription of mecA (31–33). However, BlaR1 and BlaI participation in regulating mecA expression is a complicated interaction that is currently far from clear. Arede et al. (34) have recently demonstrated that while the bla sensor/inducer (BlaR1) cannot substitute for the mec sensor inducer, the bla repressor (BlaI) and mec repressor (MecI) may interactively form a MecI::BlaI heterodimer with decreased affinity for the mecA promoter, likely increasing mecA expression. Although BlaRI does not appear to be directly involved in mecA expression, Arede et al. (34) demonstrated its ability to destabilize the MecI::BlaI heterodimer, leading to constitutive expression of mecA. Moreover, Ryffel et al. (33) suggested that the presence of plasmid regulatory genes allowed rapid induction of mecA expression, which otherwise could take hours to upregulate. Taken together, these data support the theory of Cohen and Sweeney that only a subset of SCCmec transductants may be able to establish early mecA transcription leading to full expression of methicillin resistance and ultimately survival (16). The potential importance of bla-mec “cross talk” in the successful transfer and expression of SCCmec is supported by the fact that MRSA typically possess blaZ-encoded β-lactamase and its regulatory genes (35). Thus, there appears to be a delicate balance between insertion of SCCmec (as investigated here) and mecA expression in recipient strains, which requires extensive additional study for clarification.
One of the most intriguing aspects of this study was the observation that SCCmec transfer can lead to significant SCCmec rearrangement (e.g., deletions). With ACME-positive donor FPR3757 or RG1710 and the FPR3757 or RG1439 methicillin-susceptible recipient, 60% of SCCmec transductants possessed a deletion of approximately 22 kb initially detected by PFGE. Sequence analysis in FPR3757 isolates revealed that the deletion was associated with 1.8-kb direct repeats of approximately 90% similarity immediately downstream of IS1272 in SCCmec and approximately 5.5 kb upstream of arcA in ACME. The first hypothetical protein encoded in both repeats, SAUSA300_0036 and SAUSA300_0056, respectively, matched to protein family HMM PF07799, with a 103-bp region of 100% identity serving as a focus for recombination leading to a 22-kb deletion of interrepeat SCCmec-ACME sequence. HMMPF07799 appears to have homologs in numerous bacteria, including, but not limited to, Yersinia ruckeri and Yersinia pestis, E. coli O157:H7, Salmonella enterica, Bacillus cereus, and several species of staphylococci, including S. aureus, S. hominis, S. epidermidis, and S. capitis. Although it appears to have no described activity at present, the sequence encoding this hypothetical protein represents a region of potential SCCmec and ACME rearrangement in staphylococci or perhaps even a potential site for SCCmec or ACME integration by homologous recombination. It is tempting to speculate that the deletion event we observed occurred during DNA packaging of the transducing bacteriophage. However, regardless of when the event occurred during the transduction process, this result demonstrates the important role that homologous sequences can play in the structural variation observed in mobile elements such as SCCmec. Bartels et al. (36) recently reported a Danish MRSA outbreak with isolates exhibiting deleted SCCmec ccr-ACME, which is related to what we report here. Additional studies of SCCmec rearrangement during transfer may provide important insight regarding how such SCCmec deletions and rearrangements may occur. Improved understanding of such events has obvious and important ramifications for epidemiological typing (i.e., SCCmec typing), where accurate analysis requires proper alignment of PCR primers and amplification to recognize different SCCmec types, which is confounded by SCCmec rearrangement (37–39). Such information is also fundamental to the accuracy of molecular diagnostics, such as MRSA detection based on SCCmec architecture, where rearrangement can directly affect identification (27, 39).
The three classical means of horizontal gene transfer are available to staphylococcal populations: conjugation, transformation, and transduction. While only the last was explored here, the results underscore the importance of such studies to increase our understanding of the conditions under which SCCmec transfer most likely occurs in nature and the events associated with SCCmec rearrangement. This knowledge has the potential to provide important insight into understanding the emergence and persistence of present and perhaps even future MRSA strains.
ACKNOWLEDGMENTS
Funding for this study was provided by the Department of Microbiology and Immunology, Creighton University Medical Center.
There are no potential conflicts of interest for any of the authors.
Footnotes
Published ahead of print 12 August 2013
REFERENCES
- 1.Chambers HF, DeLeo FR. 2009. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 7:629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herold BC, Immergluck LC, Maranan MC, Lauderdale DS, Gaskin RE, Boyle-Vavra S, Leitch CD, Daum RS. 1998. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 279:593–598 [DOI] [PubMed] [Google Scholar]
- 3.Udo EE, Pearman JW, Grubb WB. 1993. Genetic analysis of community isolates of methicillin-resistant Staphylococcus aureus in Western Australia. J. Hosp. Infect. 25:97–108 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention 2003. Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections—Los Angeles County, California, 2002-2003. MMWR Morb. Mortal. Wkly. Rep. 52:88. [PubMed] [Google Scholar]
- 5.Chambers HF. 2001. The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 7:178–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, Harriman K, Harrison LH, Lynfield R, Farley MM. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352:1436–1444 [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Liu Y, Yang Y, Huang G, Wang C, Deng L, Zheng Y, Fu Z, Li C, Shang Y, Zhao C, Sun M, Li X, Yu S, Yao K, Shen X. 2012. Multidrug-resistant clones of community-associated meticillin-resistant Staphylococcus aureus isolated from Chinese children and the resistance genes to clindamycin and mupirocin. J. Med. Microbiol. 61:1240–1247 [DOI] [PubMed] [Google Scholar]
- 8.Reynolds PE, Brown DF. 1985. Penicillin-binding proteins of beta-lactam-resistant strains of Staphylococcus aureus. Effect of growth conditions. FEBS Lett. 192:28–32 [DOI] [PubMed] [Google Scholar]
- 9.Hartman BJ, Tomasz A. 1984. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J. Bacteriol. 158:513–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements 2009. Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob. Agents Chemother. 53:4961–4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, Skov RL, Han X, Larsen AR, Larsen J, Sorum M, Wulf M, Voss A, Hiramatsu K, Ito T. 2011. Novel types of staphylococcal cassette chromosome mec elements identified in clonal complex 398 methicillin-resistant Staphylococcus aureus strains. Antimicrob. Agents Chemother. 55:3046–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Alvarez L, Holden MT, Lindsay H, Webb CR, Brown DF, Curran MD, Walpole E, Brooks K, Pickard DJ, Teale C, Parkhill J, Bentley SD, Edwards GF, Girvan EK, Kearns AM, Pichon B, Hill RL, Larsen AR, Skov RL, Peacock SJ, Maskell DJ, Holmes MA. 2011. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. Lancet Infect. Dis. 11:595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shore AC, Deasy EC, Slickers P, Brennan G, O'Connell B, Monecke S, Ehricht R, Coleman DC. 2011. Detection of staphylococcal cassette chromosome mec type XI carrying highly divergent mecA, mecI, mecR1, blaZ, and ccr genes in human clinical isolates of clonal complex 130 methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 55:3765–3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367:731–739 [DOI] [PubMed] [Google Scholar]
- 15.Katayama Y, Ito T, Hiramatsu K. 2000. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 44:1549–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen S, Sweeney HM. 1970. Transduction of methicillin resistance in Staphylococcus aureus dependent on an unusual specificity of the recipient strain. J. Bacteriol. 104:1158–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen S, Sweeney HM. 1973. Effect of the prophage and penicillinase plasmid of the recipient strain upon the transduction and the stability of methicillin resistance in Staphylococcus aureus. J. Bacteriol. 116:803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart GC, Rosenblum ED. 1980. Transduction of methicillin resistance in Staphylococcus aureus: recipient effectiveness and beta-lactamase production. Antimicrob. Agents Chemother. 18:424–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shafer WM, Iandolo JJ. 1979. Genetics of staphylococcal enterotoxin B in methicillin-resistant isolates of Staphylococcus aureus. Infect. Immun. 25:902–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill SR, Fouts DE, Archer GL, Mongodin EF, Deboy RT, Ravel J, Paulsen IT, Kolonay JF, Brinkac L, Beanan M, Dodson RJ, Daugherty SC, Madupu R, Angiuoli SV, Durkin AS, Haft DH, Vamathevan J, Khouri H, Utterback T, Lee C, Dimitrov G, Jiang L, Qin H, Weidman J, Tran K, Kang K, Hance IR, Nelson KE, Fraser CM. 2005. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 187:2426–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pattee PA, Baldwin JN. 1961. Transduction of resistance to chlortetracycline and novobiocin in Staphylococcus aureus. J. Bacteriol. 82:875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novick RP, Murphy E, Gryczan TJ, Baron E, Edelman I. 1979. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid 2:109–129 [DOI] [PubMed] [Google Scholar]
- 23.Goering RV, Ribot EM, Gerner-Smidt P. 2011. Pulsed-field gel electrophoresis: laboratory and epidemiologic considerations for interpretation of data, p 167–177 In Persing DH, Tenover FC, Tang YW, Nolte FS, Hayden RT, Van BelkumA. (ed), Molecular microbiology, 2nd ed. ASM Press, Washington, DC [Google Scholar]
- 24.Goering RV, Morrison D, Al-Doori Z, Edwards GF, Gemmell CG. 2008. Usefulness of mec-associated direct repeat unit (dru) typing in the epidemiological analysis of highly clonal methicillin-resistant Staphylococcus aureus in Scotland. Clin. Microbiol. Infect. 14:964–969 [DOI] [PubMed] [Google Scholar]
- 25.Otto M. 2013. Coagulase-negative staphylococci as reservoirs of genes facilitating MRSA infection: staphylococcal commensal species such as Staphylococcus epidermidis are being recognized as important sources of genes promoting MRSA colonization and virulence. Bioessays 35:4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiramatsu K, Suzuki E, Takayama H, Katayama Y, Yokota T. 1990. Role of penicillinase plasmids in the stability of the mecA gene in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 34:600–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Ito T, Li S, Misawa S, Kondo S, Miida T, Ohsaka A, Hiramatsu K. 2013. Analysis of staphylococcal cassette chromosome mec in BD GeneOhm MRSA assay-negative strains. Antimicrob. Agents Chemother. 57:2890–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG. 2002. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc. Natl. Acad. Sci. U. S. A. 99:7687–7692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson DA, Enright MC. 2003. Evolutionary models of the emergence of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:3926–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katayama Y, Zhang HZ, Hong D, Chambers HF. 2003. Jumping the barrier to beta-lactam resistance in Staphylococcus aureus. J. Bacteriol. 185:5465–5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveira DC, de Lencastre H. 2011. Methicillin-resistance in Staphylococcus aureus is not affected by the overexpression in trans of the mecA gene repressor: a surprising observation. PLoS One 6:e23287. 10.1371/journal.pone.0023287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hackbarth CJ, Chambers HF. 1993. blaI and blaR1 regulate beta-lactamase and PBP 2a production in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 37:1144–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryffel C, Kayser FH, Berger-Bachi B. 1992. Correlation between regulation of mecA transcription and expression of methicillin resistance in staphylococci. Antimicrob. Agents Chemother. 36:25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arede P, Ministro J, Oliveira DC. 2013. Redefining the role of the beta-lactamase locus in methicillin-resistant Staphylococcus aureus: beta-lactamase regulators disrupt the MecI-mediated strong repression on mecA and optimize the phenotypic expression of resistance in strains with constitutive mecA expression. Antimicrob. Agents Chemother. 57:3037–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livermore DM. 1995. β-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8:557–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartels MD, Boye K, Oliveira DC, Worning P, Goering R, Westh H. 2013. Associations between dru types and SCCmec cassettes. PLoS One. 8:e61860. 10.1371/journal.pone.0061860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shore A, Rossney AS, Keane CT, Enright MC, Coleman DC. 2005. Seven novel variants of the staphylococcal chromosomal cassette mec in methicillin-resistant Staphylococcus aureus isolates from Ireland. Antimicrob. Agents Chemother. 49:2070–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milheirico C, Oliveira DC, de Lencastre H. 2007. Multiplex PCR strategy for subtyping the staphylococcal cassette chromosome mec type IV in methicillin-resistant Staphylococcus aureus: ‘SCCmec IV multiplex'. J. Antimicrob. Chemother. 60:42–48 [DOI] [PubMed] [Google Scholar]
- 39.Lindqvist M, Isaksson B, Grub C, Jonassen TO, Hallgren A. 2012. Detection and characterisation of SCCmec remnants in multiresistant methicillin-susceptible Staphylococcus aureus causing a clonal outbreak in a Swedish county. Eur. J. Clin. Microbiol. Infect. Dis. 31:141–147 [DOI] [PubMed] [Google Scholar]
- 40.Novick R. 1967. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33:155–166 [DOI] [PubMed] [Google Scholar]


