Abstract
Previous studies examining combination therapy for invasive pulmonary aspergillosis (IPA) have revealed conflicting results, including antagonism, indifference, and enhanced effects. The most commonly employed combination for this infection includes a mold-active triazole and echinocandin. Few studies have evaluated combination therapy from a pharmacodynamic (PD) perspective, and even fewer have examined combination therapy against both wild-type and azole-resistant Cyp51 mutant isolates. The current studies aim to fill this gap in knowledge. Four Aspergillus fumigatus isolates were utilized, including a wild-type strain, an Fks1 mutant (posaconazole susceptible and caspofungin resistant), and two Cyp51 mutants (posaconazole resistant). A neutropenic murine model of IPA was used for the treatment studies. The dosing design included monotherapy with posaconazole, monotherapy with caspofungin, and combination therapy with both. Efficacy was determined using quantitative PCR, and results were normalized to known quantities of conidia (conidial equivalents [CE]). The static dose, 1-log kill dose, and associated PD target area under the curve (AUC)/MIC ratio were determined for monotherapy and combination therapy. Monotherapy experiments revealed potent activity for posaconazole, with reductions of 3 to 4 log10 Aspergillus CE/ml with the two “low”-MIC isolates. Posaconazole alone was less effective for the two isolates with higher MICs. Caspofungin monotherapy did not produce a significant decrease in fungal burden for any strain. Combination therapy with the two antifungals did not enhance efficacy for the two posaconazole-susceptible isolates. However, the drug combination produced synergistic activity against both posaconazole-resistant isolates. Specifically, the combination resulted in a 1- to 2-log10 decline in burden that would not have been predicted based on the monotherapy results for each drug. This corresponded to a reduction in the free-drug posaconazole AUC/MIC ratio needed for stasis of up to 17-fold. The data suggest that combination therapy using a triazole and an echinocandin may be a beneficial treatment strategy for triazole-resistant isolates.
INTRODUCTION
Invasive pulmonary aspergillosis (IPA) is a leading cause of morbidity and mortality in immunosuppressed patients (1, 2). Despite advances in the antifungal armamentarium, including the development of new triazoles with potent Aspergillus activity and echinocandins, outcomes remain suboptimal, with mortality rates near 50% (3). One treatment strategy that has been proposed to improve outcomes is the use of combinations of two or more antifungals with distinct mechanisms of action. This approach has proven useful for a number of other infectious diseases, such as HIV, tuberculosis, Gram-negative bacterial sepsis, enterococcal endocarditis, and cryptococcal meningitis (4–8). However, combination studies against Aspergillus, both in vitro and in vivo, have produced conflicting results for different infection models and drug combinations (9–12). One combination of interest is a mold-active triazole and echinocandin.
Aspergillus-active triazoles are considered first-line therapy for IPA and have proven efficacy in initial and salvage therapy (13–16). However, the recent emergence of A. fumigatus isolates exhibiting reduced susceptibility to triazoles is a threat to this class (17–21). We theorized that consideration of the pharmacokinetics (PK) and pharmacodynamics (PD) of the triazole-echinocandin interaction would advance our understanding of the utility of this combination strategy. We specifically posited that (i) the in vivo potency of the mold-active triazoles makes identification of synergistic interactions between a triazole and echinocandin difficult to demonstrate for Cyp51 wild-type organisms and (ii) when there is inadequate triazole drug exposure or triazole drug resistance, beneficial interactions would be observed.
MATERIALS AND METHODS
Organisms.
Four A. fumigatus isolates (DPL, EMFR S678P, F16216, and F11628) were chosen for the current study, including two with wild-type Cyp51 (one wild-type strain and one Fks1 mutant) and two Cyp51 mutants. Isolates DPL, F16216, and F11628 are clinical isolates, whereas EMFR S678P is a laboratory engineered mutant. The two Cyp51 mutants were chosen based upon varying posaconazole MICs. An isolate with a moderately elevated MIC (isolate F16216) (posaconazole MIC, 2 mg/liter) and an isolate with a highly elevated MIC (isolate F11628) (posaconazole MIC, 8 mg/liter) were utilized. Organisms were grown and subcultured on potato dextrose agar (PDA) (Difco Laboratories, Detroit, MI). The organisms were chosen to include similar fitness as determined by growth in lungs and mortality in untreated mice over 7 days (Table 1).
Table 1.
In vitro susceptibility and in vivo fitness of selected A. fumigatus isolates
| A. fumigatus isolate | Caspofungin MEC (mg/liter) | Posaconazole MIC (mg/liter) | In vivo growth (log10 CE/ml lung homogenate) in untreated control (mean ± SD) | Mortality (%) in untreated control at day 7 | Description |
|---|---|---|---|---|---|
| DPL | 0.25 | 0.25 | 1.7 ± 0.4 | 100 | Wild type |
| EMFR S678P | 16 | 0.25 | 1.6 ± 0.4 | 100 | Fks1 S678P |
| F16216 | 0.5 | 2 | 2.0 ± 0.6 | 100 | Cyp51 L98H+TR |
| F11628 | 0.5 | 8 | 1.9 ± 0.5 | 100 | Cyp51 G138C |
Drugs.
Posaconazole solution and caspofungin powder were obtained from the University of Wisconsin Hospital and Clinics pharmacy. Posaconazole drug solutions were prepared on the day of use with sterile saline as the diluent and vortexed vigorously prior to administration by oral-gastric (OG) gavage. Caspofungin was similarly prepared on the day of use with sterile saline as the diluent and was administered by intraperitoneal injection.
In vitro susceptibility.
The posaconazole MIC and caspofungin minimal effective concentration (MEC) were determined by broth microdilution using the CLSI M38-A2 method (22). MICs were determined in duplicate three times; the median values are reported in Table 1.
Animals.
Six-week-old Swiss/ICR specific-pathogen-free female mice weighing 23 to 27 g were used for all studies (Harlan Sprague-Dawley, Indianapolis, IN). Animals were housed in groups of five and allowed access to food and water ad libitum. Animals were maintained in accordance with the American Association for Accreditation of Laboratory Care criteria (23). The Animal Research Committee of the William S. Middleton Memorial VA Hospital and University of Wisconsin—Madison approved the animal studies.
Infection model.
Mice were rendered neutropenic (<100 polymorphonuclear cells/mm3) by injection of 150 mg/kg cyclophosphamide subcutaneously (s.c.) on days 4 and 1 before infection and 3 days after infection. Prior studies have shown that this protocol sustains neutropenia (<100 CFU/mm3) for the 7-day experiment (24–26). Additionally, cortisone acetate (250 mg/kg s.c.) was administered on day 1 before infection as previously described (24, 27, 28). Mice were also given ceftazidime (50 mg/kg/day s.c.) to prevent opportunistic bacterial infection.
Organisms were subcultured on PDA 5 days prior to infection and incubated at 37°C. On the day of infection, the inoculum was prepared by flooding the culture plate with 5 ml of normal saline with 0.05% Tween 20. The conidial suspension was collected and quantitated with a hemacytometer (Bright-Line; Hausser Scientific, Horsham PA). The suspension was diluted to a final concentration of 1 × 107 to 2 × 107/ml. Viability was assessed by viable plate counts on PDA.
Prior to induction of infection, mice were anesthetized with a 50-μl intramuscular (i.m.) injection of ketamine (37.5 mg/ml) and xylazine (5 mg/ml). Fifty microliters of a conidial suspension of 1 ×107 to 2 ×107/ml was pipetted into the anterior nares and aspirated into the lungs. This procedure produced invasive aspergillosis in over 90% of animals and 100% mortality in untreated infected mice.
Antifungal dosing design.
Each antifungal drug was administered alone and in combination in the in vivo model. Posaconazole was administered by the OG route using five 4-fold-increasing doses from 0.156 mg/kg to 40 mg/kg once daily. Similarly, caspofungin was administered by the intraperitoneal (i.p.) route using five 4-fold-increasing doses from 0.156 mg/kg to 40 mg/kg once daily. A checkerboard design of combination therapy was utilized, resulting in 25 different combination regimens (Table 2). The doses were selected to vary the expected result from zero effect to maximal effect or the highest tolerated dose. Controls were utilized for each experiment and included a start and an end of therapy. Four mice were used for each treatment regimen and control group. Therapy was initiated 2 h after infection. At the time of sacrifice of moribund animals or at the study endpoint (7 days), lungs were aseptically harvested and processed for quantitative PCR (qPCR) as described below.
Table 2.
Checkerboard dosing design for monotherapy and combination therapy
| Caspofungin dose (mg/kg/day) | No. of mice in group given the following posaconazole dose (mg/kg/day): |
|||||
|---|---|---|---|---|---|---|
| 40 | 10 | 2.5 | 0.625 | 0.156 | 0 | |
| 40 | 4 | 4 | 4 | 4 | 4 | 4 |
| 10 | 4 | 4 | 4 | 4 | 4 | 4 |
| 2.5 | 4 | 4 | 4 | 4 | 4 | 4 |
| 0.625 | 4 | 4 | 4 | 4 | 4 | 4 |
| 0.156 | 4 | 4 | 4 | 4 | 4 | 4 |
| 0 | 4 | 4 | 4 | 4 | 4 | 4 |
Lung processing and organism quantitation.
Processing of lungs and quantitation of lung burdens were performed based upon previously described protocols (29, 30). Briefly, at the time of sacrifice for moribund animals or at the end of therapy (7 days), lungs were aseptically harvested and placed in 2 ml of sterile saline in a 2-ounce sterile polyethylene Whirl-Pak bag (Nasco, Fort Atkinson, WI). The lungs were manually homogenized using direct pressure (31). One milliliter of the primary homogenate was placed in a sterile bead beating tube (Sarstedt, Newton, NC) with 700 μl of 425- to 600-μm acid-washed glass beads (Sigma-Aldrich, St. Louis, MO). The primary homogenate was bead beaten in a mini-bead beater (BioSpec, Bartlesville, OK) for 90 s at 4,200 rpm to yield a secondary homogenate. One hundred microliters of the secondary homogenate was mixed with 100 μl of ATL buffer and 20 μl of proteinase K (Qiagen, Valencia, CA) and incubated overnight at 56°C with gentle agitation. DNA was then isolated following the DNeasy blood and tissue protocol (Qiagen, Valencia, CA). A final elution step was carried out with 100 μl of AE elution buffer (Qiagen, Valencia, CA) placed over the column twice to maximize DNA isolation. The DNA was stored at −20°C until the day of qPCR.
qPCR plates were prepared on the day of assay. Defined quantities of conidia (conidial equivalents) were used for standard curves. Samples were assayed in triplicate using a CFX96 real-time system (Bio-Rad, Hercules, CA). A single-copy gene, Fks1, was chosen for quantitation (32). Primer sequences were forward primer 5′GCCTGGTAGTGAAGCTGAGCGT-3′, reverse primer 5′CGGTGAATGTAGGCATGTTGTCC-3′, and probe 6-carboxyfluorescein (FAM)-AGCCAGCGGCCCGCAAATG-MGB-3′ (Integrated DNA Technologies, Coralville, IA). The Fks1 mutation (S678P) was not located in the primer-probe area of the genome. The primer-probe set was validated for all isolates by comparing the kinetics and quantitative results for known quantities of conidia over the dynamic range (102 to 108) (data not shown). Prior studies in our lab have also shown that there is an absence of inhibitors that may adversely affect the qPCRs as determined by spiking lung homogenate with known quantities of conidia. The organism burden was reported as conidial equivalents (CE) per ml of primary lung homogenate (log10 CE/ml lung homogenate).
PK.
Murine posaconazole and caspofungin pharmacokinetic (PK) data, including area under the concentration-time curve (AUC) and protein binding, were derived from our previous studies (33, 34).
Outcome measure and PD index exploration.
The effect of a particular dosing regimen (monotherapy or combination therapy) was determined by comparing the mean change in log10 CE/ml lung homogenate at the end of therapy or time of sacrifice to the initial starting log10 CE/ml lung homogenate at time zero. The dose-response graphs were fit to a sigmoid Hill-type dose-response curve. The AUC/MIC was used as the pharmacodynamic (PD) index for exploration of exposure-response relationships based upon previous PK/PD investigations for posaconazole and caspofungin (33–37). Both total and free (non-protein-bound) drug concentrations were considered.
Monotherapy analysis.
The qPCR data were modeled according to a Hill-type dose response equation: log10 D = log10 (E/Emax − E)/N + log10 ED50, where D is the drug dose, E is the growth (as measured by qPCR and represented as CE/ml of lung homogenate) in untreated control mice, Emax is the maximal effect, N is the slope of the dose-response relationship, and ED50 is the dose needed to achieve 50% of the maximal effect. The posaconazole and caspofungin static doses (i.e., no change in fungal burden from the start of therapy) and doses associated with a net 1-log decrease in burden (1-log kill), when achieved, were determined for all isolates. The PD target total and free-drug AUC/MIC for each endpoint were also calculated. The coefficient of determination (r2) was used to estimate the percent variance in the change of log10 CE/ml of lung homogenate over the treatment period for the different dosing regimens that could be attributed to the PD index AUC/MIC. The static dose and 1-log kill dose and the associated free-drug AUC/MIC for the two Cyp51 wild-type isolates and the Cyp51 mutants were compared by using the Mann-Whitney rank sum test.
Combination therapy analysis.
The potential for combination therapy to confer beneficial microbiological effects compared to monotherapy was explored in two manners. First, we compared the drug doses associated with two endpoints (static dose and 1-log kill dose) for each drug and isolate in monotherapy to those for the drug in combination therapy using the Student t test. When monotherapy resulted in a static dose above the highest dose used (40 mg/kg/24 h), the static dose was set to 40 mg/kg/24 h for comparison against combination therapy using the Student t test.
The second analysis was employed to determine the presence of traditionally defined antagonism, indifference, or synergy by Bliss interaction analysis (38). Specifically, Bliss independence is described by the formula Ec = (EA + EB) − (EA × EB), where Ec is the expected (i.e., calculated) fractional effect of a particular combination therapy regimen consisting of drug A and drug B, EA is the fractional effect of monotherapy with drug A, and EB is the fractional effect of monotherapy with drug B. Fractional effects for monotherapy were determined by comparing the observed monotherapy effect of a particular dosing regimen to the maximal effect and is represented by the equation EA = (Emax − Emono A)/Emax, where Emono A is the observed monotherapy effect in relation to no effect for drug A and Emax is the maximum effect. The same equation was used to calculate EB: EB = (Emax − Emono B)/Emax. Therefore, for each monotherapy dosing regimen, a fractional (or percent) effect compared to the maximal effect was determined and then utilized to calculate the Ec for each combination dosing regimen using the formula for Ec given above. Observed combination effects (Eobs) were then compared to the calculated effect (Ec) for each isolate for each combination dosing regimen. The difference between the observed combination effect and the calculated combination effect, including 95% confidence intervals (CI), was calculated by the equation ΔE = Eobs − Ec, where Eobs is the observed combination effect in relation to no effect and Ec is the calculated (i.e., predicted) combination effect. An enhanced effect or synergy was suggested if ΔE, including the 95% CI for Eobs and Ec, was greater than 0. Antagonism was determined if ΔE, including the 95% CI for Eobs and Ec, was less than 0. For all other cases, where the 95% CI for ΔE would include 0, the conclusion was indifference (Bliss independence).
To account for the potential for small and likely clinically insignificant synergistic or antagonistic interactions to be found mathematically based on the Bliss interaction analysis, we defined a biologically meaningful change a priori. Specifically, a fractional change leading to a 1-log10 increase or decrease in fungal burden or approximately 0.2 (or 20%) was considered relevant. Therefore, only observed effects (including 95% CI) that did not overlap calculated effects (including 95% CI) and where the difference between the two was 20% or more were deemed significantly synergistic or antagonistic.
RESULTS
Antifungal susceptibility and in vivo fitness.
Posaconazole and caspofungin in vitro susceptibility testing (with each drug alone), genetic mutations where applicable, and the relative fitness in the in vivo murine model of each isolate are shown in Table 1. The posaconazole MIC was elevated for strain F16216 at 2 mg/liter and was further elevated for strain F11628 at 8 mg/liter, whereas the MIC was lower (0.25 mg/liter) for the two organisms that did not have Cyp51 mutations. The isolate containing an Fks1 hot spot mutation (EMFR S678P) exhibited a higher MEC for caspofungin (16 mg/liter) than the three isolates without a mutation (range, 0.25 to 0.5 mg/liter). The organisms exhibited similar in vivo fitness as exhibited by growth and mortality in untreated animals. At the start of therapy, mice had 5.60 ± 0.4 log10 CE/ml of lung homogenate, and the burden increased to 7.41 ± 0.44 log10 CE/ml of lung homogenate in untreated animals. Each isolate produced 100% mortality prior to the end of the study in untreated animals.
Pharmacokinetics.
Data from our previous pharmacokinetic studies of posaconazole and caspofungin in this mouse model were used for the current study (33, 34). The AUC over the dose range was linear for both drugs. Thus, for dose levels that were not directly measured, the AUC was estimated using linear extrapolation or interpolation. The posaconazole total-drug AUC range was 1.78 to 351 mg · h/liter over the dose range of 0.156 to 40 mg/kg/24 h. The caspofungin total-drug AUC range was 5.21 to 452 mg · h/liter over the dose range of 0.156 to 40 mg/kg/24 h. Protein binding was 99% and 97% for posaconazole and caspofungin, respectively.
Monotherapy analysis.
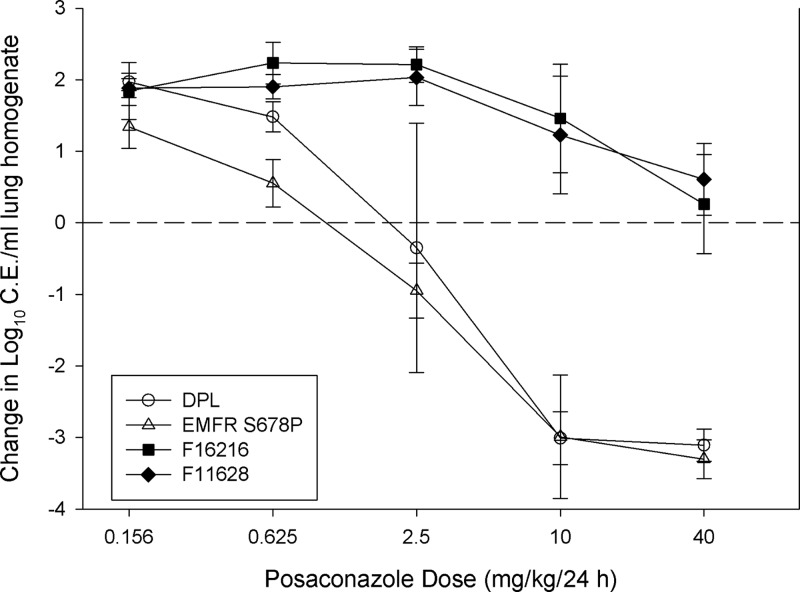
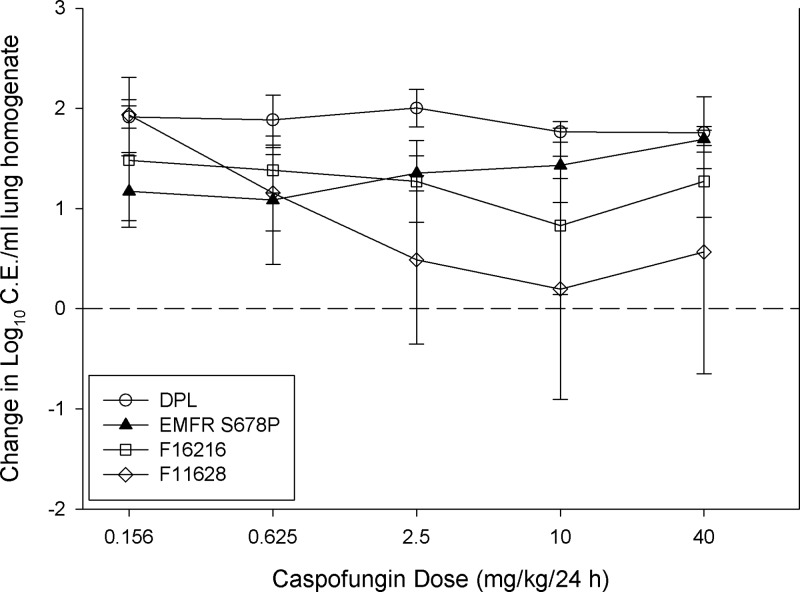
A sigmoid dose-response relationship for posaconazole was observed for each isolate studied. As expected, higher doses were necessary to achieve similar microbiologic outcomes against organisms with elevated posaconazole MICs (Fig. 1). Posaconazole treatment against the more susceptible strains resulted in a reduction in lung burden of more than 3 log10 CE/ml. However, as shown in Fig. 2, caspofungin monotherapy exhibited only a modest in vivo effect in this animal model for each of the strains. The posaconazole static dose and 1-log kill dose (when achieved) were determined for each isolate and are shown in Table 3. Doses of 1.87 and 1.09 mg/kg/24 h of posaconazole were associated with net stasis for Cyp51 wild-type strains DPL and EMFR S678P, respectively. Comparatively, a similar level of efficacy for the Cyp51 mutant strains required more than 30-fold more drug (static dose, >40 mg/kg/24 h). The dose-response curve was steep for Cyp51 wild-type isolates, and only 1.19 to 1.26 mg/kg/24 h were needed to produce a 1-log reduction in burden compared to that at the start of therapy. Caspofungin therapy did not produce the stasis or 1-log-reduction endpoints for any of the four isolates.
Fig 1.
Posaconazole monotherapy dose-response curves for each isolate. Open symbols represent Cyp51 wild-type organisms, and closed symbols represent Cyp51 mutants. Each data point is the mean (± standard deviation [SD]) log10 CE/ml of lung homogenate for four mice. The horizontal dashed line represents net stasis or infectious burden at the start of therapy. Points above the line represent an increase in burden (i.e., net growth), whereas those below the line represent a decrease in burden.
Fig 2.
Caspofungin monotherapy dose-response curves for each isolate. Open symbols represent Cyp51 wild-type organisms, and closed symbols represent Cyp51 mutants. Each data point is the mean (± SD) log10 CE/ml of lung homogenate for four mice. The horizontal dashed line represents net stasis or infectious burden at the start of therapy. Points above the line represent an increase in burden (i.e., net growth), whereas those below the line represent a decrease in burden.
Table 3.
Posaconazole monotherapy doses and total- and free-drug AUC/MIC ratios needed to achieve net stasis and 1-log kill endpoints (when achieved) for each isolate
| Isolate | Stasis |
1-log kill |
|||||
|---|---|---|---|---|---|---|---|
| Dose (mg/kg/24 h) | MIC (mg/liter) | 24-h total-drug AUC/MIC | 24-h free-drug AUC/MIC | Dose (mg/kg/24 h) | 24-h total-drug AUC/MIC | 24-h free-drug AUC/MIC | |
| DPL | 1.87 | 0.25 | 85.1 | 0.85 | 3.12 | 142 | 1.42 |
| EMFR S678P | 1.09 | 0.25 | 49.7 | 0.50 | 2.28 | 104 | 1.04 |
| F16216 | >40 | 2 | Xa | X | >40 | X | X |
| F11628 | >40 | 8 | X | X | >40 | X | X |
X, not attainable.
The posaconazole free-drug AUC/MIC exposures were determined for each isolate and fit to a Hill sigmoid dose-response model. The posaconazole exposure response data fit the model well (Fig. 3), with an r2 of 0.79. Taking into account free drug concentrations, the posaconazole AUC/MIC values associated with the stasis endpoints for the susceptible strains DPL and EMFR S678P were 0.85 and 0.50, respectively. The static-dose AUC/MIC targets could not be determined for the two Cyp51 mutants, F16216 and F11628, as net stasis was not achieved over the dose range studied. The posaconazole free-drug AUC/MIC associated with the 1-log-reduction endpoint was only slightly higher for the Cyp51 wild-type isolates. As noted above, caspofungin monotherapy pharmacodynamic targets could not be determined, as the stasis and 1-log-reduction endpoints were not achieved in the monotherapy.
Fig 3.
The relationship between posaconazole monotherapy AUC/MIC and microbiological effect is plotted for each of the 4 A. fumigatus isolates. Free (not protein-bound) concentrations were used. Open symbols denote results from Cyp51 wild-type organisms and closed symbols those from Cyp51 mutants. The horizontal dashed line represents net stasis or infectious burden at the start of therapy. Points above the line represent an increase in burden (i.e., net growth), whereas those below the line represent a decrease in burden.
Combination analysis.
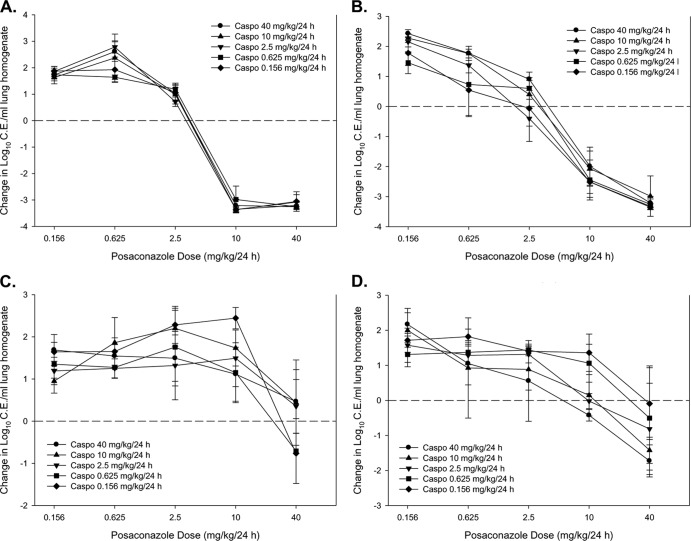
The dose-response results for combination therapy are shown in Fig. 4. The impacts of the addition of caspofungin to the posaconazole stasis and 1-log-reduction dose levels are shown in Table 4. There were no significant changes in the posaconazole static dose or 1-log reduction dose for the Cyp51 wild-type DPL isolate. However, the posaconazole dose endpoints were somewhat higher for the wild-type EMFR S678P strain in combination with two caspofungin dose levels, 40 and 10 mg/kg/24 h. Conversely, the posaconazole dose-response curves were distinctly shifted to the left for the Cyp51 mutants across many of the caspofungin exposures (Fig. 4C and D). The enhancement in efficacy was most evident for the more highly resistant F11628 isolate (Table 4). For instance, the posaconazole static dose for the three highest caspofungin additions was 7- to 13-fold lower in combination therapy than in monotherapy (P ≤ 0.004). If one examines the posaconazole monotherapy free-drug AUC/MIC associated with net stasis for the susceptible isolates (mean = 0.68), the posaconazole free-drug AUC/MIC target associated with net stasis in these three combination therapy regimens against the azole-resistant isolate F11628 was 8.5- to 17-fold lower (P < 0.001).
Fig 4.
Dose-response curves for combination posaconazole and caspofungin therapy against isolates DPL (wild type) (A), EMFR S678P (Cyp51 wild type, Fks mutant) (B), F16216 (Cyp51 mutant) (C), and F11628 (Cyp51 mutant) (D). Each graph represents the microbiological effect of varied posaconazole doses (shown on x axis) with addition of each of the 5 dosing regimens of caspofungin (represented by each of the 5 curves). In total, there are 25 different combination data points on each graph. Each data point represents the mean (± SD) log10 CE/ml of lung homogenate for four mice. The horizontal dashed line represents net stasis or infectious burden from the start of therapy. Points above the line represent an increase in burden (i.e., net growth), whereas those below the line represent a decrease in burden.
Table 4.
Posaconazole combination therapy static doses, 1-log-kill doses, and associated AUC/MIC PD targets (when achieved) in combination therapy with five different doses of caspofungin against two Cyp51 wild-type and two Cyp51 mutant A. fumigatus isolates
| Isolate | Caspofungin dose (mg/kg/24 h) | Posaconazole static dose (mg/kg/24 h) | MIC (mg/liter) | Posaconazole static-dose 24-h total-drug AUC/MIC | Posaconazole static-dose 24-h free-drug AUC/MIC | Posaconazole 1-log-kill dose (mg/kg/24 h) | Posaconazole 1-log-kill total-drug 24-h AUC/MIC | Posaconazole 1-log-kill free-drug 24-h AUC/MIC |
|---|---|---|---|---|---|---|---|---|
| DPL | 40 | 3.00 | 0.25 | 137 | 1.37 | 3.27 | 149 | 1.49 |
| 10 | 3.00 | 0.25 | 137 | 1.37 | 3.29 | 150 | 1.50 | |
| 2.5 | 2.86 | 0.25 | 130 | 1.30 | 3.14 | 143 | 1.43 | |
| 0.625 | 3.98 | 0.25 | 182 | 1.82 | 4.79 | 218 | 2.18 | |
| 0.156 | 3.07 | 0.25 | 140 | 1.40 | 3.38 | 154 | 1.54 | |
| EMFR S678P | 40 | 3.88a | 0.25 | 177 | 1.77a | 6.44a | 294 | 2.94a |
| 10 | 2.85a | 0.25 | 130 | 1.30a | 5.51a | 251 | 2.51a | |
| 2.5 | 1.55 | 0.25 | 70.1 | 0.70 | 3.47 | 158 | 1.58 | |
| 0.625 | 1.36 | 0.25 | 62.2 | 0.62 | 3.98 | 182 | 1.82 | |
| 0.156 | 1.03 | 0.25 | 47.3 | 0.47 | 3.57 | 163 | 1.63 | |
| F16216 | 40 | >40 | 2 | Xb | X | X | X | X |
| 10 | >40 | 2 | X | X | X | X | X | |
| 2.5 | >40 | 2 | X | X | X | X | X | |
| 0.625 | 24.5c | 2 | 128 | 1.28c | X | X | X | |
| 0.156 | 28.6c | 2 | 140 | 1.40c | X | X | X | |
| F11628 | 40 | 3.11c | 8 | 4.43 | 0.04c | 8.48 | 12.1 | 0.12c |
| 10 | 4.07c | 8 | 5.80 | 0.06c | 13.8 | 19.6 | 0.20c | |
| 2.5 | 5.75c | 8 | 8.19 | 0.08c | 21.0 | 29.2 | 0.29c | |
| 0.625 | 11.0c | 8 | 15.7 | 0.16c | >40 | |||
| 0.156 | 17.6c | 8 | 25.1 | 0.25c | >40 |
The static dose, 1-log-kill dose, and associated PD targets total- and free-drug AUC/MIC were significantly higher (P < 0.001) for these combinations than for monotherapy.
X, not attained.
The static dose, 1-log-kill dose, and associated PD targets total- and free-drug AUC/MIC were significantly lower (P < 0.001) for these combinations than for monotherapy.
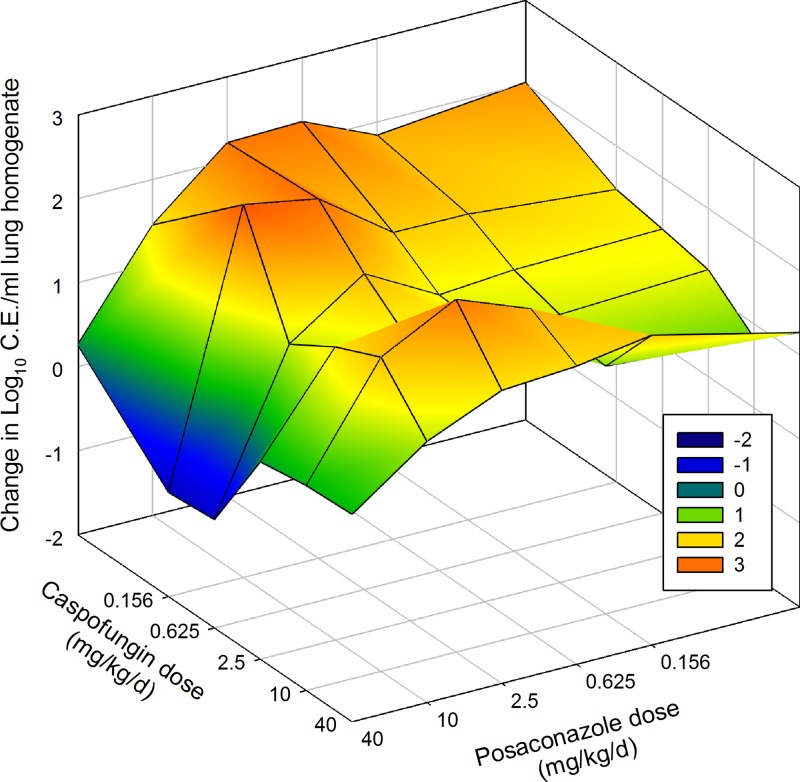
Bliss independence analysis found no synergistic or antagonistic combinations against the wild-type isolate DPL. There were also no synergistic combinations for Cyp51 wild-type, Fks1 mutant isolate EMFR S678P. However, similar to the case for the static-dose analysis, there were three antagonistic combinations (Table 5). Synergistic combinations were noted for both Cyp51 mutants. Three combination regimens exhibited 22 to 25% more effect in combination than would have been expected if the two drugs were acting independently for the resistant F16216. Seven combination regimens exhibited synergy against the isolate with the highest posaconazole MIC (F11628). The seven synergistic combinations (Table 5) exhibited 22 to 49% more microbiological effect than would have been expected if the two drugs were acting independently. These combinations produced a statistically significant 1- to 2.5-log10 decrease in observed infectious burden compared to the predicted combination effect. Surface response three-dimensional plots for the two posaconazole-resistant isolates are shown in Fig. 5 and 6.
Table 5.
Posaconazole and caspofungin combination regimens exhibiting synergy or antagonism by Bliss independence analysisa
| Isolate | Posaconazole dose (mg/kg/24 h) | Caspofungin dose (mg/kg/24 h) | Ec (95% CI) | Eobs (95% CI) | ΔE | Synergy or antagonism |
|---|---|---|---|---|---|---|
| F16216 | 40 | 0.625 | 0.40 (0.32–0.49) | 0.65 (0.56–0.71) | 0.25 | Synergy |
| 2.5 | 40 | 0.13 (0.09–0.16) | 0.35 (0.29–0.41) | 0.22 | Synergy | |
| 2.5 | 40 | 0.13 (0.09–0.17) | 0.38 (0.29–0.47) | 0.25 | Synergy | |
| F11628 | 40 | 40 | 0.37 (0.21–0.53) | 0.86 (0.81–0.91) | 0.49 | Synergy |
| 40 | 10 | 0.43 (0.28–0.58) | 0.80 (0.76–0.84) | 0.37 | Synergy | |
| 40 | 2.5 | 0.38 (0.25–0.52) | 0.67 (0.54–0.79) | 0.29 | Synergy | |
| 40 | 0.625 | 0.27 (0.19–0.35) | 0.61 (0.46–0.75) | 0.34 | Synergy | |
| 40 | 0.156 | 0.19 (0.13–0.24) | 0.55 (0.43–0.67) | 0.36 | Synergy | |
| 10 | 40 | 0.31 (0.12–0.50) | 0.61 (0.59–0.63) | 0.30 | Synergy | |
| 10 | 2.5 | 0.32 (0.15–0.49) | 0.54 (0.51–0.56) | 0.22 | Synergy | |
| EMFR S678P | 2.5 | 40 | 0.49 (0.46–0.52) | 0.14 (0.11–0.17) | −0.35 | Antagonism |
| 2.5 | 10 | 0.50 (0.45–0.55) | 0.24 (0.18–0.29) | −0.26 | Antagonism | |
| 2.5 | 0.625 | 0.53 (0.46–0.60) | 0.20 (0.16–0.24) | −0.33 | Antagonism |
Ec, predicted fractional effect based on the Bliss independence equation [Ec = (EA + EB) − (EA × EB); see Materials and Methods]; Eobs, observed fractional effect in the combination dosing experiment; ΔE, difference between Ec and Eobs (Eobs − Ec).
Fig 5.
Three-dimensional surface-response plot of combination posaconazole and caspofungin therapy and microbiological effect against F16216 (Cyp51 mutant; posaconazole MIC = 2 mg/liter). The vertical axis represents the change in burden from the start of therapy. Each data point is the mean change in log10 CE/ml of lung homogenate from four mice. Areas above zero (green, yellow, and orange) represent an increase in burden (i.e., net growth). Areas below zero (blue and dark blue) represent a decrease in burden.
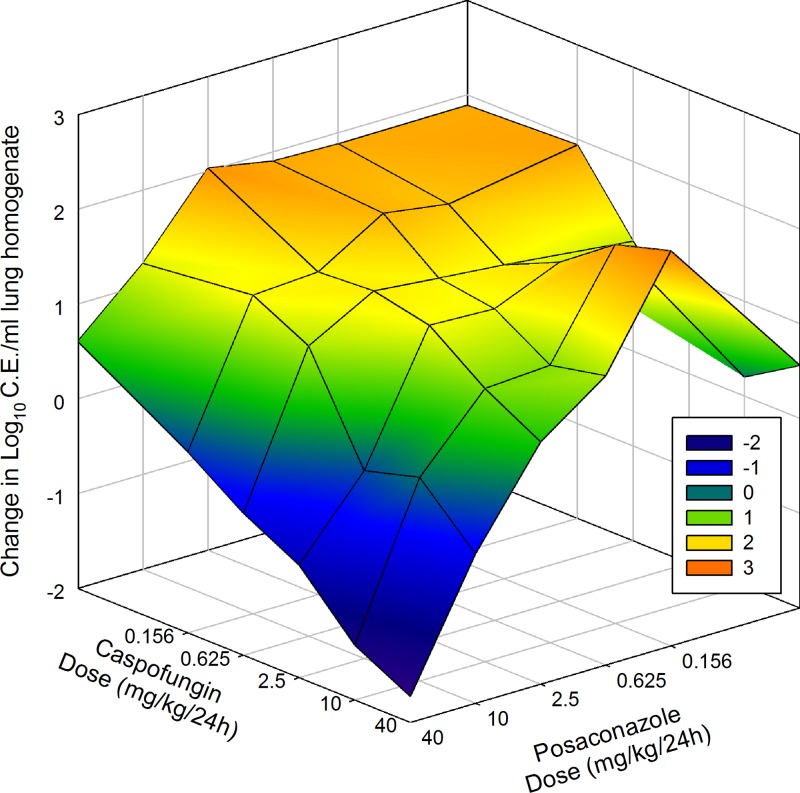
Fig 6.
Three-dimensional surface-response plot of combination posaconazole and caspofungin therapy and microbiological effect against F11628 (Cyp51 mutant; posaconazole MIC = 8 mg/liter). The vertical axis represents the change in burden from the start of therapy. Each data point is the mean change in log10 CE/ml of lung homogenate from four mice. Areas above zero (green, yellow, and orange) represent an increase in burden (i.e., net growth). Areas below zero (blue and dark blue) represent a decrease in burden.
DISCUSSION
Combination anti-infective therapy with two or more drugs that act at different sites has been considered in situations when outcomes in monotherapy are suboptimal. This strategy has been recently popular in the study of therapy for invasive Aspergillus infections. The mold-active triazoles and echinocandins are two of the commonly studied classes due to their efficacy and relative safety (13, 14, 16, 39, 40). Many in vitro assays have demonstrated additive or synergistic interactions for the two drug classes. However, evidence from in vivo models has been conflicting (9–12). Clinically, a number of small, nonrandomized trials have suggested potential benefits of this drug class combination (11, 40–44). One potential explanation for differences among previous in vivo study results relates to variation in drug exposure. Most in vivo combination studies have utilized minimal if any dose ranging and often only a single regimen with each antifungal. This is understandable given the large number of animals and cost of these studies. However, it is possible or even likely that not all dosing regimen combinations will be optimal for detection of enhanced efficacy. For example, the few or single dose levels chosen are often based upon optimal efficacy in the infection model or the maximally tolerated dose. This may be particularly problematic for the echinocandin class given the possibility of a paradoxical effect. We attempted to overcome this limitation by examining a wide dose range (256-fold for each compound) to include a full sigmoid effect (no effect to maximal effect) concentration range from monotherapy experiments. This approach results in a checkerboard design similar to that in most in vitro studies, but it was costly and did utilize a large number of mice. The in vivo efficacy of posaconazole monotherapy against Cyp51 wild-type strains was marked, with a maximal kill of 3 to 4 log10 CE/ml. This potency has been confirmed in other in vivo studies (35, 36, 45, 46). Caspofungin monotherapy, however, was less effective in this model. The reasons for this are not clear; however, previous in vivo studies have shown limited microbiological effectiveness in terms of reducing fungal burden (26, 47). A recent in vitro microcolony study observed slowing but not the halt of Aspergillus growth in the presence of echinocandins, and this phenomenon may be reflected in our in vivo study (48). Additionally, a previous study has suggested that the primary means of echinocandin effectiveness may be related to beta-glucan unmasking and resultant increased recognition and killing by polymorphonuclear cells (49). We utilized a neutropenic model, and therefore this could explain why only a modest microbiological effect was noted in the absence of polymorphonuclear cells.
Another goal of the present study was to consider the impact of MIC variation and drug resistance on the drug class interaction. While echinocandin resistance in Aspergillus is at this point a laboratory phenomenon, triazole resistance is an emerging clinical threat in many regions of the world (17–21). To our knowledge, this is the first in vivo, dose-ranging pharmacodynamic study examining the effects of combination triazole and echinocandin therapy against Cyp51 wild-type and mutant isolates.
An additional study factor that can impact interpretation of drug interaction experiments is the analysis model. Among the numerous potential approaches, we chose two analyses. The first was a simple and practical analysis of the impact of the combination on the antifungal dose associated with meaningful treatment endpoints, in this case both stasis and killing. The second method utilized the Bliss independence model (50). This model operates on the assumption that two drugs act at different, independent, and mutually nonexclusive sites. Given the distinct sites of drug action and, more importantly, the relatively modest activity of the echinocandins in this infection model, we felt that this was the most biologically relevant method. We were encouraged to observe congruence with the two analytical approaches.
Similar to those of previous in vivo studies, our results were in some manner conflicting in that the interactions were not consistent for each of the four Aspergillus strains. However, we were not entirely surprised to find difficulty in demonstrating beneficial combination effects against Cyp51 wild-type isolates given the extreme potency of posaconazole in previous monotherapy experiments (46). This is similar to results from a clinical study of this combination, in which most patients were likely to be infected with wild-type, triazole-susceptible isolates and an enhanced effect and improvement in patient outcomes were not observed (51). Unfortunately, susceptibility data were not tracked in that clinical study, and therefore it is unknown whether enhanced effects would be observed based on triazole susceptibility. It is interesting to note the observation of a statistically antagonistic effect against the echinocandin-resistant strain EMFR S678P. A significantly higher posaconazole static dose was observed in combination therapy, and specifically, three combinations exhibited antagonistic effects based on Bliss analysis. The basis for this antagonistic interaction is unclear but is an area for future mechanistic investigation. The clinical relevance of this observation is unclear, since this isolate was a laboratory-engineered mutant strain and echinocandin resistance in Aspergillus appears to be an incredibly rare clinical event. Further studies, especially with a clinical echinocandin-resistant isolate, will be important to further understand this finding.
In contrast, we were intrigued to find a quite large enhancement of efficacy of the drug combination for Cyp51 mutants. For both Cyp51 mutant isolates, the combinations resulted in a 1- to 2.5-log10-enhanced microbiological effect compared to monotherapy. Based on our previous demonstration of a strong correlation between qPCR results and animal mortality (46), this enhanced effect would correspond to an increase in survival of 17 to 43%. For each mutant isolate there was at least one combination regimen that resulted in net cidal activity, whereas in posaconazole monotherapy stasis was not achieved. The addition of the echinocandin seemed to restore the cidal activity for the triazole. It is further interesting that the impact of this combination was most profound for the least triazole-susceptible isolate, where the observed fractional effect was over 200% greater than that predicted by monotherapy. This corresponds to approximately a 2-log10 increase in microbiological activity over what would have been predicted. The posaconazole static dose for this combination was reduced by almost 13-fold compared to that for monotherapy, which is both highly statistically significant and, one might expect, clinically important (P ≤ 0.001). Previous in vivo studies have demonstrated similar enhanced effects with voriconazole and each of the three licensed echinocandins (52–57), although this is the first to utilize posaconazole and to examine the results from a pharmacodynamic perspective.
There are limitations to the current study that deserve consideration. The complexity and size of the experiment using a checkerboard technique made it difficult to study a larger number of isolates. Second, we did not consider sequential combination therapy, which is commonly used as salvage therapy for patients failing monotherapy. Study with the triazole-polyene combination has identified differences when this approach has been explored. Finally, we did not evaluate other triazole-echinocandin combinations. While one might expect similar results for drugs with similar mechanisms of action, a previous in vitro pharmacodynamic study against Cyp51 mutants did not demonstrate significant enhancement with combination voriconazole and anidulafungin (58).
In summary, we did not observe an enhanced in vivo effect for combination posaconazole and caspofungin therapy against Cyp51 wild-type organisms. This suggests that combination therapy may not offer further benefit over triazole monotherapy as long as drug concentrations against a triazole-susceptible isolate are sufficient. In contrast, treatment efficacy was enhanced for Aspergillus isolates with elevated posaconazole MICs. The mechanisms that underlie this phenomenon are unknown but are an intriguing area for further research. These findings challenge our therapeutic strategy when dealing with a drug-resistant isolate. In many situations when drug resistance is encountered, the approach employed is to abandon the drug to which the organism is resistant and use an alternative class to which the organism is susceptible. However, our in vivo results contest this paradigm. We found the combination of a posaconazole and caspofungin in the setting of posaconazole resistance not only can outperform echinocandin monotherapy but can rescue cidal activity that is typical for the triazoles against susceptible strains. This finding suggests that the optimal strategy when encountering triazole resistance in IPA may be combination therapy with a triazole and echinocandin. However, it will be important to verify these observations with a larger set of triazole-resistant isolates. These results, though, provide a basis for further study of combination therapy, with the focus on triazole-resistant isolates.
ACKNOWLEDGMENTS
We thank David Perlin for providing isolates DPL and EMFR S678P.
This study was funded by a grant from Merck.
Footnotes
Published ahead of print 19 August 2013
REFERENCES
- 1.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) database. Clin. Infect. Dis. 50:1091–1100 [DOI] [PubMed] [Google Scholar]
- 2.Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, Anaissie EJ, Brumble LM, Herwaldt L, Ito J, Kontoyiannis DP, Lyon GM, Marr KA, Morrison VA, Park BJ, Patterson TF, Perl TM, Oster RA, Schuster MG, Walker R, Walsh TJ, Wannemuehler KA, Chiller TM. 2010. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 50:1101–1111 [DOI] [PubMed] [Google Scholar]
- 3.Baddley JW, Andes DR, Marr KA, Kontoyiannis DP, Alexander BD, Kauffman CA, Oster RA, Anaissie EJ, Walsh TJ, Schuster MG, Wingard JR, Patterson TF, Ito JI, Williams OD, Chiller T, Pappas PG. 2010. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin. Infect. Dis. 50:1559–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panel on Antiretroviral Guidelines for Adults and Adolescents 22 November 2012, accession date Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, Washington, DC: http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf [Google Scholar]
- 5.Centers for Disease Control and Prevention 2003. Treatment of tuberculosis, American Thoracic Society, CDC, and Infectious Diseases Society of America. MMWR Recomm. Rep. 52(RR-11):1–77 [PubMed] [Google Scholar]
- 6.Abad CL, Kumar A, Safdar N. 2011. Antimicrobial therapy of sepsis and septic shock—when are two drugs better than one? Crit. Care Clin. 27:e1–27 [DOI] [PubMed] [Google Scholar]
- 7.Baddour LM, Wilson WR, Bayer AS, Fowler VG, Jr, Bolger AF, Levison ME, Ferrieri P, Gerber MA, Tani LY, Gewitz MH, Tong DC, Steckelberg JM, Baltimore RS, Shulman ST, Burns JC, Falace DA, Newburger JW, Pallasch TJ, Takahashi M, Taubert KA. 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111:e394–e434 [DOI] [PubMed] [Google Scholar]
- 8.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 50:291–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson MD, Perfect JR. 2010. Use of antifungal combination therapy: agents, order, and timing. Curr. Fungal Infect. Rep. 4:87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segal BH, Steinbach WJ. 2007. Combination antifungals: an update. Expert Rev. Anti Infect. Ther. 5:883–892 [DOI] [PubMed] [Google Scholar]
- 11.Steinbach WJ, Stevens DA, Denning DW. 2003. Combination and sequential antifungal therapy for invasive aspergillosis: review of published in vitro and in vivo interactions and 6281 clinical cases from 1966 to 2001. Clin. Infect. Dis 37(Suppl 3):S188–S224 [DOI] [PubMed] [Google Scholar]
- 12.Vazquez JA. 2008. Clinical practice: combination antifungal therapy for mold infections: much ado about nothing? Clin. Infect. Dis. 46:1889–1901 [DOI] [PubMed] [Google Scholar]
- 13.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327–360 [DOI] [PubMed] [Google Scholar]
- 14.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408–415 [DOI] [PubMed] [Google Scholar]
- 15.Patterson TF, Boucher HW, Herbrecht R, Denning DW, Lortholary O, Ribaud P, Rubin RH, Wingard JR, DePauw B, Schlamm HT, Troke P, Bennett JE. 2005. Strategy of following voriconazole versus amphotericin B therapy with other licensed antifungal therapy for primary treatment of invasive aspergillosis: impact of other therapies on outcome. Clin. Infect. Dis. 41:1448–1452 [DOI] [PubMed] [Google Scholar]
- 16.Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, Greene RE, Hachem R, Hadley S, Herbrecht R, Langston A, Louie A, Ribaud P, Segal BH, Stevens DA, van Burik JA, White CS, Corcoran G, Gogate J, Krishna G, Pedicone L, Hardalo C, Perfect JR. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin. Infect. Dis. 44:2–12 [DOI] [PubMed] [Google Scholar]
- 17.Chandrasekar PH. 2005. Antifungal resistance in aspergillus. Med. Mycol. 43(Suppl 1):S295–S298 [DOI] [PubMed] [Google Scholar]
- 18.Denning DW, Perlin DS. 2011. Azole resistance in Aspergillus: a growing public health menace. Future Microbiol. 6:1229–1232 [DOI] [PubMed] [Google Scholar]
- 19.Howard SJ, Arendrup MC. 2011. Acquired antifungal drug resistance in Aspergillus fumigatus: epidemiology and detection. Med. Mycol. 49(Suppl 1):S90–S95 [DOI] [PubMed] [Google Scholar]
- 20.Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg. Infect. Dis. 15:1068–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snelders E, van der Lee HA, Kuijpers J, Rijs AJ, Varga J, Samson RA, Mellado E, Donders AR, Melchers WJ, Verweij PE. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 5:e219. 10.1371/journal.pmed.0050219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute 2008. Refence method for broth dilution antifungal susceptbility testing of filamentous fungi. Approved standard, 2nd ed. CLSI document M38-A2 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 23.National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 24.Lepak A, Sanchez H, Marchillo K, Andes D. 2010. Comparative pharmacodynamics of a triazole and echinocandin for invasive pulmonary aspergillosis. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother. http://www.icaac.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andes D, Craig WA. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis RE, Liao G, Hou J, Prince RA, Kontoyiannis DP. 2011. Comparative in vivo dose-dependent activity of caspofungin and anidulafungin against echinocandin-susceptible and -resistant Aspergillus fumigatus. J. Antimicrob. Chemother. 66:1324–1331 [DOI] [PubMed] [Google Scholar]
- 27.Lewis RE, Albert ND, Kontoyiannis DP. 2008. Efficacy of single-dose liposomal amphotericin B or micafungin prophylaxis in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 52:4178–4180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiederhold NP, Tam VH, Chi J, Prince RA, Kontoyiannis DP, Lewis RE. 2006. Pharmacodynamic activity of amphotericin B deoxycholate is associated with peak plasma concentrations in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 50:469–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowman JC, Abruzzo GK, Anderson JW, Flattery AM, Gill CJ, Pikounis VB, Schmatz DM, Liberator PA, Douglas CM. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallor AC, Kirkpatrick WR, Najvar LK, Bocanegra R, Kinney MC, Fothergill AW, Herrera ML, Wickes BL, Graybill JR, Patterson TF. 2008. Assessment of Aspergillus fumigatus burden in pulmonary tissue of guinea pigs by quantitative PCR, galactomannan enzyme immunoassay, and quantitative culture. Antimicrob. Agents Chemother. 52:2593–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh TJ, McEntee C, Dixon DM. 1987. Tissue homogenization with sterile reinforced polyethylene bags for quantitative culture of Candida albicans. J. Clin. Microbiol. 25:931–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrera ML, Vallor AC, Gelfond JA, Patterson TF, Wickes BL. 2009. Strain-dependent variation in 18S ribosomal DNA copy numbers in Aspergillus fumigatus. J. Clin. Microbiol. 47:1325–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andes D, Marchillo K, Conklin R, Krishna G, Ezzet F, Cacciapuoti A, Loebenberg D. 2004. Pharmacodynamics of a new triazole, posaconazole, in a murine model of disseminated candidiasis. Antimicrob. Agents Chemother. 48:137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andes D, Diekema DJ, Pfaller MA, Bohrmuller J, Marchillo K, Lepak A. 2010. In vivo comparison of the pharmacodynamic targets for echinocandin drugs against Candida species. Antimicrob. Agents Chemother. 54:2497–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howard SJ, Lestner JM, Sharp A, Gregson L, Goodwin J, Slater J, Majithiya JB, Warn PA, Hope WW. 2011. Pharmacokinetics and pharmacodynamics of posaconazole for invasive pulmonary aspergillosis: clinical implications for antifungal therapy. J. Infect. Dis. 203:1324–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mavridou E, Bruggemann RJ, Melchers WJ, Mouton JW, Verweij PE. 2010. Efficacy of posaconazole against three clinical Aspergillus fumigatus isolates with mutations in the cyp51A gene. Antimicrob. Agents Chemother. 54:860–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Louie A, Deziel M, Liu W, Drusano MF, Gumbo T, Drusano GL. 2005. Pharmacodynamics of caspofungin in a murine model of systemic candidiasis: importance of persistence of caspofungin in tissues to understanding drug activity. Antimicrob. Agents Chemother. 49:5058–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bliss CI. 1939. The toxicity of poisons applied jointly. Ann. Appl. Biol. 26:585–615 [Google Scholar]
- 39.Denning DW, Marr KA, Lau WM, Facklam DP, Ratanatharathorn V, Becker C, Ullmann AJ, Seibel NL, Flynn PM, van Burik JA, Buell DN, Patterson TF. 2006. Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis. J. Infect. 53:337–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kontoyiannis DP, Ratanatharathorn V, Young JA, Raymond J, Laverdiere M, Denning DW, Patterson TF, Facklam D, Kovanda L, Arnold L, Lau W, Buell D, Marr KA. 2009. Micafungin alone or in combination with other systemic antifungal therapies in hematopoietic stem cell transplant recipients with invasive aspergillosis. Transplant Infect. Dis. 11:89–93 [DOI] [PubMed] [Google Scholar]
- 41.Maertens J, Glasmacher A, Herbrecht R, Thiebaut A, Cordonnier C, Segal BH, Killar J, Taylor A, Kartsonis N, Patterson TF, Aoun M, Caillot D, Sable C. 2006. Multicenter, noncomparative study of caspofungin in combination with other antifungals as salvage therapy in adults with invasive aspergillosis. Cancer 107:2888–2897 [DOI] [PubMed] [Google Scholar]
- 42.Marr KA, Boeckh M, Carter RA, Kim HW, Corey L. 2004. Combination antifungal therapy for invasive aspergillosis. Clin. Infect. Dis. 39:797–802 [DOI] [PubMed] [Google Scholar]
- 43.Singh N, Limaye AP, Forrest G, Safdar N, Munoz P, Pursell K, Houston S, Rosso F, Montoya JG, Patton P, Del Busto R, Aguado JM, Fisher RA, Klintmalm GB, Miller R, Wagener MM, Lewis RE, Kontoyiannis DP, Husain S. 2006. Combination of voriconazole and caspofungin as primary therapy for invasive aspergillosis in solid organ transplant recipients: a prospective, multicenter, observational study. Transplantation 81:320–326 [DOI] [PubMed] [Google Scholar]
- 44.Thomas A, Korb V, Guillemain R, Caruba T, Boussaud V, Billaud E, Prognon P, Begue D, Sabatier B. 2010. Clinical outcomes of lung-transplant recipients treated by voriconazole and caspofungin combination in aspergillosis. J. Clin. Pharm. Ther. 35:49–53 [DOI] [PubMed] [Google Scholar]
- 45.Mavridou E, Bruggemann RJ, Melchers WJ, Verweij PE, Mouton JW. 2010. Impact of cyp51A mutations on the pharmacokinetic and pharmacodynamic properties of voriconazole in a murine model of disseminated aspergillosis. Antimicrob. Agents Chemother. 54:4758–4764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lepak AJ, Marchillo K, Vanhecker J, Andes DR. 2013. Posaconazole pharmacodynamic target determination against wild-type and Cyp51 mutant isolates of Aspergillus fumigatus in an in vivo model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 57:579–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petraitiene R, Petraitis V, Groll AH, Sein T, Schaufele RL, Francesconi A, Bacher J, Avila NA, Walsh TJ. 2002. Antifungal efficacy of caspofungin (MK-0991) in experimental pulmonary aspergillosis in persistently neutropenic rabbits: pharmacokinetics, drug disposition, and relationship to galactomannan antigenemia. Antimicrob. Agents Chemother. 46:12–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ingham CJ, Schneeberger PM. 2012. Microcolony imaging of Aspergillus fumigatus treated with echinocandins reveals both fungistatic and fungicidal activities. PLoS One 7:e35478. 10.1371/journal.pone.0035478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lamaris GA, Lewis RE, Chamilos G, May GS, Safdar A, Walsh TJ, Raad II, Kontoyiannis DP. 2008. Caspofungin-mediated beta-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae. J. Infect. Dis. 198:186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greco WR, Bravo G, Parsons JC. 1995. The search for synergy: a critical review from a response surface perspective. Pharmacol. Rev. 47:331–385 [PubMed] [Google Scholar]
- 51.Marr KA, Schlamm H, Rottinghaus ST, Jagannatha S, Bow EJ, Wingard JR, Pappas P, Herbrecht R, Walsh TJ, Maertens J. 2012. A randomised, double-blind study of combination antifungal therapy with voriconazole and anidulafungin versus voriconazole monotherapy for primary treatment of invasive aspergillosis, abstr LB 2812 Abstr. 22nd Eur. Congr. Clin. Microbiol. Infect. Dis [Google Scholar]
- 52.Chandrasekar PH, Cutright JL, Manavathu EK. 2004. Efficacy of voriconazole plus amphotericin B or micafungin in a guinea-pig model of invasive pulmonary aspergillosis. Clin. Microbiol. Infect. 10:925–928 [DOI] [PubMed] [Google Scholar]
- 53.Kirkpatrick WR, Perea S, Coco BJ, Patterson TF. 2002. Efficacy of caspofungin alone and in combination with voriconazole in a Guinea pig model of invasive aspergillosis. Antimicrob. Agents Chemother. 46:2564–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacCallum DM, Whyte JA, Odds FC. 2005. Efficacy of caspofungin and voriconazole combinations in experimental aspergillosis. Antimicrob. Agents Chemother. 49:3697–3701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petraitis V, Petraitiene R, Hope WW, Meletiadis J, Mickiene D, Hughes JE, Cotton MP, Stergiopoulou T, Kasai M, Francesconi A, Schaufele RL, Sein T, Avila NA, Bacher J, Walsh TJ. 2009. Combination therapy in treatment of experimental pulmonary aspergillosis: in vitro and in vivo correlations of the concentration- and dose-dependent interactions between anidulafungin and voriconazole by Bliss independence drug interaction analysis. Antimicrob. Agents Chemother. 53:2382–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van de Sande WW, Mathot RA, ten Kate MT, van Vianen W, Tavakol M, Rijnders BJ, Bakker-Woudenberg IA. 2009. Combination therapy of advanced invasive pulmonary aspergillosis in transiently neutropenic rats using human pharmacokinetic equivalent doses of voriconazole and anidulafungin. Antimicrob. Agents Chemother. 53:2005–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seyedmousavi S, Bruggemann RJ, Melchers WJ, Rijs AJ, Verweij PE, Mouton JW. 2012. Efficacy and pharmacodynamics of voriconazole combined with anidulafungin in azole-resistant invasive aspergillosis. J. Antimicrob. Chemother. [DOI] [PubMed] [Google Scholar]
- 58.Jeans AR, Howard SJ, Al-Nakeeb Z, Goodwin J, Gregson L, Warn PA, Hope WW. 2012. Combination of voriconazole and anidulafungin for treatment of triazole-resistant Aspergillus fumigatus in an in vitro model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 56:5180–5185 [DOI] [PMC free article] [PubMed] [Google Scholar]