Abstract
Pharmacodynamic (PD) studies with triazoles in the neutropenic murine disseminated candidiasis model have been performed extensively for Candida albicans. They have consistently shown that the pharmacodynamic index most closely correlated with efficacy is the ratio of the 24-h area under the concentration-time curve (AUC) to the MIC, and a target 24-h free-drug AUC/MIC ratio near 25 is associated with 50% of maximal microbiologic efficacy. We utilized this model to investigate the pharmacodynamics of isavuconazole. Isavuconazole pharmacokinetics were linear over the dose range studied. Oral-gastric doses of 640, 160, 40, and 10 mg of prodrug/kg of body weight produced peak levels of 0.51 to 25.4 mg/liter, an elimination half-life of 1 to 5 h, and an AUC from 0 h to infinity (AUC0-∞) of 0.9 to 287 mg · h/liter. The AUC/MIC ratio was the pharmacodynamic index that correlated best with efficacy (R2, 0.84). Pharmacodynamic target studies were performed using 4 C. albicans isolates with both a 24-h and a 96-h treatment duration. The strains were chosen to include previously characterized fluconazole-resistant strains. The mean 50% effective doses (ED50) (expressed in mg/kg of body weight/12 h) and associated 24-h free-drug AUC/MIC ratios were 89.3 ± 46.7 and 67.7 ± 35 for the 24-h treatment and 59.6 ± 22 and 33.3 ± 25.5 for the 96-h treatment. These differences were not statistically significant. Pharmacodynamic targets for two non-albicans Candida species were also explored. The mean ED50 (expressed in mg/kg/12 h) and associated 24-h free-drug AUC/MIC ratios were 31.2 and 6.2 for Candida tropicalis (n = 1) and 50.5 and 1.6 for Candida glabrata (n = 2). These PD targets were significantly different from C. albicans targets (P, 0.04). Isavuconazole PD targets for C. albicans are similar to those observed in this model with other triazoles. However, the PD targets for non-albicans Candida species were more than 10-fold lower than those for C. albicans (P, 0.04). This difference is similar to the species-specific PD relationships for the echinocandins. The lower PD targets for these species in this model will be important to consider in the analysis of clinical trial data and during the development of susceptibility breakpoints.
INTRODUCTION
Candida species are the fourth most common cause of nosocomial bloodstream infections in the United States (1, 2). Unfortunately, despite medical advances, morbidity and mortality remain unacceptably high (2–5). One strategy to improve outcomes is to utilize pharmacokinetic/pharmacodynamic (PK/PD) approaches to optimize antifungal therapy.
Mechanistically, PK/PD studies integrate the pharmacokinetic properties of a drug, in vitro potency (MIC), and treatment efficacy. The goal of PK/PD studies is to maximize the clinical outcome through the optimization of dosing design, minimize toxicity, prevent the emergence of resistance, and assist with the setting of susceptibility breakpoints (6–8). These investigations have been integral in the rational use of triazoles in mucosal and invasive candidiasis (9). Isavuconazole (BAL4815) is a new triazole compound with broad activity and potency, including Candida spp. (10–12). The active drug is generated after plasma esterases hydrolyze the prodrug, isavuconazonium sulfate (BAL8557), to the active form, isavuconazole (BAL4815), and an inactive cleavage product, BAL8728 (13, 14).
In the current study, we (i) characterized the PK/PD properties of isavuconazole, including predictive PK/PD indices, in the neutropenic murine model of invasive candidiasis (IC) and (ii) determined the PK/PD targets against eight clinical isolates of Candida species, including Candida albicans, C. glabrata, and C. tropicalis, in order to provide a framework for the development of optimal dosing regimens and in vitro susceptibility breakpoints.
MATERIALS AND METHODS
Organisms.
Eight clinical isolates were used for the in vivo studies, including 5 C. albicans, 2 C. glabrata, and 1 C. tropicalis isolate. The isolates were chosen and screened to have similar fitness in the in vivo model but to differ in susceptibility to triazoles (see Table 1). The organisms were maintained, grown, subcultured, and quantified on Sabouraud dextrose agar (SDA; Difco Laboratories, Detroit, MI). Twenty-four hours prior to the study, the organisms were subcultured at 35°C.
Table 1.
In vitro susceptibilities of select C. albicans, C. glabrata, and C. tropicalis isolates to isavuconazole (BAL4815), fluconazole, voriconazole, and posaconazole
| Species | Isolate | MIC (mg/liter)a of: |
||||
|---|---|---|---|---|---|---|
| Isavuconazole |
Fluconazole | Voriconazole | Posaconazole | |||
| CLSI | EUCAST | |||||
| C. albicans | K1 | 0.004 | 0.004 | 0.25 | 0.007 | 0.03 |
| 580 | 0.008 | 0.015 | 4 | 0.03 | 0.06 | |
| 98-210 | 0.03 | 0.12 | 16 | 0.03 | 0.125 | |
| 2183 | 0.008 | 0.008 | >128 | 0.06 | 0.06 | |
| 98-17 | 0.03 | 0.06 | 16 | 0.125 | 0.125 | |
| C. tropicalis | 98-234 | 0.016 | 0.008 | 32 | 0.25 | 0.125 |
| C. glabrata | 35315 | 0.125 | 0.06 | 0.25 | <0.03 | 0.06 |
| 14378 | 0.25 | 0.25 | 2 | |||
The MICs of fluconazole, voriconazole, and posaconazole were determined by the CLSI method. The isavuconazole MIC was determined both by the CLSI method and by the EUCAST method.
Drug.
The prodrug isavuconazonium sulfate (BAL8557) and isavuconazole powder (BAL4815) were provided by the sponsor (Astellas, Northbrook, IL) for in vivo and in vitro studies, respectively. The prodrug was dissolved in sterile water and was buffered to a pH of 4.0 with sodium hydroxide prior to oral administration. Isavuconazole powder was dissolved in dimethyl sulfoxide (DMSO) according to the sponsor's instructions prior to in vitro experiments. A conversion factor was necessary to convert the prodrug dose to an equivalent in vivo isavuconazole dose for the mice. This was determined based on a prodrug/drug equivalency ratio of 1.863 (provided by the sponsor). Additionally, the purity of the prodrug powder was 89%. Thus, the resulting conversion factor for converting the prodrug to the active moiety was 2.07 (i.e., a 100-mg oral prodrug dose was equivalent to 48.3 mg of active isavuconazole drug). The purity of isavuconazole powder for susceptibility testing was >99%.
In vitro susceptibility testing.
All isolates were tested in accordance with the both the CLSI and EUCAST methods (15). MICs were determined on three separate occasions, in duplicate. Final results are expressed as the medians of these results.
Animals.
Six-week-old specific-pathogen-free female ICR/Swiss mice (Harlan Sprague-Dawley, Indianapolis, IN) weighing 23 to 27 g were used for all studies. Animals were housed in groups of five and were allowed access to food and water ad libitum. Animals were maintained in accordance with the criteria of the American Association for Accreditation of Laboratory Animal Care (16).
Infection model.
A neutropenic murine disseminated candidiasis model was used for treatment studies (17–20). Animals were rendered neutropenic (polymorphonuclear leukocyte count, <100/mm3) by intraperitoneal injection of cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, IN) 4 days (150 mg/kg of body weight) and 1 day (100 mg/kg) before infection.
Organisms were subcultured on SDA 24 h prior to infection. The inoculum was prepared by placing three to five colonies in 5 ml of sterile 0.15 M NaCl warmed to 35°C. The final inoculum was adjusted to an absorbance at 530 nm of 0.6. Fungal counts of the inoculum, determined by viable counts on SDA, were 6.28 ± 0.19 log10 CFU/ml.
Disseminated infection was produced by lateral tail vein injection of 0.1 ml of the final inoculum 2 h prior to the start of antifungal therapy. At the end of the study period, animals were euthanized by CO2 asphyxiation. The kidneys of each mouse were immediately removed and were placed in sterile 0.15 M NaCl at 4°C. The organs were homogenized and were serially diluted 1:10. Aliquots were plated onto SDA for viable fungal colony counts after incubation for 24 h at 35°C. The lower limit of detection was 100 CFU/kidneys. The results were expressed as the mean log10 CFU/kidneys from three mice and standard deviations.
Pharmacokinetic studies.
Single-dose pharmacokinetics of isavuconazole (BAL4815) were determined in individual ICR/Swiss mice following oral administration of the prodrug (BAL8557) at 10, 40, 160, and 640 mg/kg in 0.2-ml volumes by oral-gastric (OG) gavage. Plasma from groups of three isoflurane-anesthetized mice was collected at each of 7 time points (0.5, 1, 2, 4, 8, 12, and 24 h). The plasma was stored at −80°C until the day of drug assay measurement. Drug concentrations were measured by the sponsor using liquid chromatography-tandem mass spectrometry (LC–MS-MS). The limit of assay quantification was 10 ng/ml.
A noncompartmental model was used in the PK analysis. Pharmacokinetic parameters, including the elimination half-life and the concentration of the drug in serum at time zero (C0), were calculated via nonlinear least-squares techniques. The area under the concentration-time curve (AUC) was calculated by the trapezoidal rule. For treatment doses for which kinetics were not directly determined, pharmacokinetic parameters were estimated by linear interpolation for those doses between two measured doses and by linear extrapolation for doses above or below the highest and lowest doses measured. Protein binding (99%) was based on previous studies with mice by the sponsor (personal communication).
Pharmacodynamic index determination.
Neutropenic mice were infected with C. albicans K1 2 h prior to the start of therapy. Twenty dosing regimens were chosen for determination of the impact of dose level and interval on isavuconazole efficacy. These 20 regimens comprised five total 24-h prodrug (BAL8557) dosing levels (640, 320, 160, 80, and 40 mg/kg) divided into one, two, three, or four doses (i.e., the prodrug was administered every 24, 12, 8, or 6 h, respectively). The prodrug was administered by the OG route in 0.2-ml volumes. The wide range of dosing levels and intervals was chosen to minimize interdependence among the three PD indices studied (the ratio of the maximum concentration of the drug in serum [Cmax] to the MIC, the AUC/MIC ratio, and the percentage of the dosing interval for which the concentration of free drug exceeded the MIC [%T>MIC]) and to vary the effect from no effect to maximal effect. For each respective dosing regimen, groups of three mice were treated over a 24-h period. Mice were sacrificed at the end of therapy, and kidneys were removed for CFU determination, as described above. The burden of organisms in kidneys at the end of therapy was the efficacy endpoint.
Pharmacodynamic index magnitude determination.
By use of procedures similar to those used for the PD index determination described in the preceding section, additional Candida isolates, including 4 C. albicans strains and 1 C. tropicalis strain, were studied over a 24-h treatment period. In a comparable fashion, we performed a 96-h treatment experiment with 2 C. glabrata isolates, because in general, this species exhibits a lower growth rate than other Candida species in the mouse model (21–23). Finally, we also explored the impact of treatment duration for C. albicans by the addition of a 96-h endpoint. Treatment began 2 h after infection. Dosing regimens consisted of 320, 160, 80, 40, and 20 mg/kg of prodrug (BAL8557) administered by the OG route every 12 h for the 24-h or 96-h treatment period. For the 96-h treatment experiments, an additional intraperitoneal dose of cyclophosphamide (100 mg/kg) was administered at day +2 to ensure neutropenia throughout the study period (24). Groups of three mice were used for each dosing regimen. At the end of the treatment period, animals were sacrificed and CFU determined as described above.
Data analysis.
A sigmoid dose-effect model was used to measure the in vivo potency of isavuconazole. The model was derived from the Hill equation: E = (Emax × DN)/(ED50N + DN), where E is the observed effect, D is the total dose, Emax is the maximum effect, ED50 is the 50% effective dose (i.e., the dose required to achieve 50% of Emax), and N is the slope of the dose-response curve. The correlation between efficacy and each of the three PD indices (AUC/MIC ratio, Cmax/MIC ratio, and %T>MIC) was determined by nonlinear least-squares regression analysis using SigmaStat (Systat Software Inc., Chicago, IL). The coefficient of determination (R2) was used to estimate the variance that could be due to regression with each of the three PK/PD indices. Calculations were done using both total-drug and free-drug concentrations.
PD targets were determined by identifying the ED50 for each isolate and determining the corresponding AUC/MIC ratio. The ED50 was chosen in order to allow comparison with other triazole PD studies in this model (17–20). Again, both total-drug and free-drug concentrations were utilized. PD targets, including the mean ED50 and AUC/MIC ratio results, for 24-h and 96-h C. albicans experiments were compared using Student's t test. The mean ED50s and AUC/MIC ratios for C. albicans and non-albicans Candida species were compared using the Mann-Whitney U test. A two-tailed P value of <0.05 was considered statistically significant.
RESULTS
In vitro susceptibility testing and in vivo fitness.
The 24-h isavuconazole (BAL4815) MICs for the isolates studied differed 64-fold, and except for C. albicans 98-210, MICs determined by use of the CLSI and EUCAST methodologies were similar (Table 1). Decreased susceptibility to other triazoles did not reliably predict isavuconazole susceptibility. However, a single C. glabrata isolate (strain 14378) with an elevated fluconazole MIC also exhibited a higher isavuconazole MIC. Candida albicans and C. tropicalis exhibited similar levels of fitness, with 2 to 4 log10 CFU/kidneys over 24 h. C. glabrata demonstrated lower growth rates, with 0.71 to 1.24 log10 CFU/kidneys at the end of a 96-h in vivo growth period. This difference in growth is similar to the findings of previous analyses demonstrating slower growth in the animal model for this species (17–22, 25).
Pharmacokinetics.
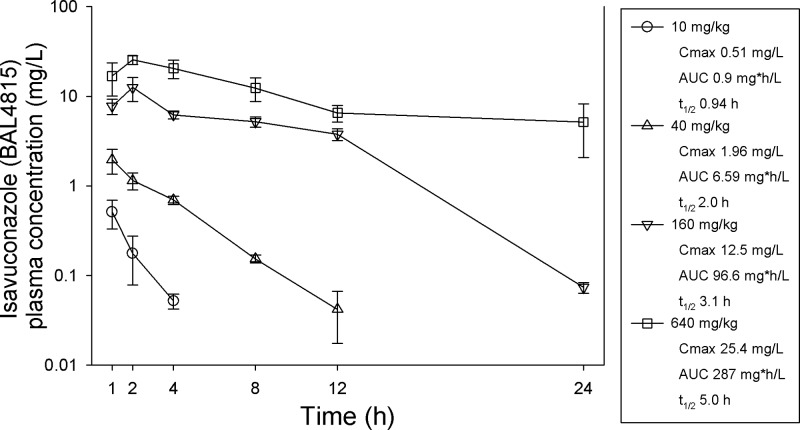
The time course for isavuconazole (BAL4815) in the plasma of mice following OG doses of 640, 160, 40, and 10 mg/kg of the prodrug (BAL8557) is shown in Fig. 1. Peak levels were achieved within 2 h for each dosing regimen and ranged from 0.51 to 25.4 mg/liter. The elimination half-life in serum increased in a dose-dependent fashion from 1 to 5 h. The AUC from 0 h to infinity (AUC0-∞), as determined by the trapezoidal rule, ranged from 0.9 to 287 mg · h/liter. The AUC was linear over the dose range (R2, 0.98).
Fig 1.
Concentrations of isavuconazole (BAL4815) in plasma after administration of the oral prodrug (BAL8557) at 640, 160, 40, or 10 mg/kg. Each symbol represents the geometric mean ± standard deviation for three mice. The peak concentration (Cmax), 24-h AUC0-∞ (AUC), and elimination half life (t1/2) are shown for each dose.
Pharmacodynamic index determination.
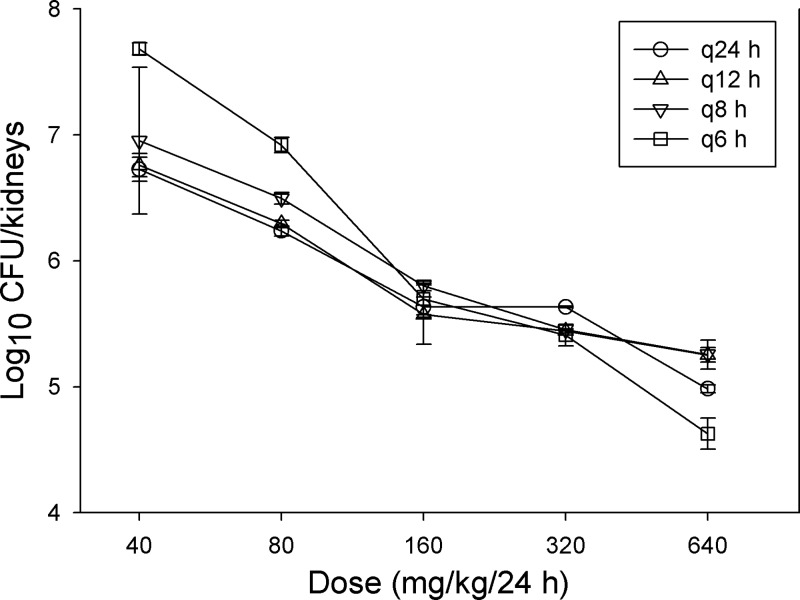
The dose-response relationship for C. albicans K1 after oral administration of the prodrug (BAL8557) at total doses of 640, 320, 160, 80, and 40 mg/kg fractionated into one, two, three, or four doses is shown in Fig. 2. At the start of therapy, after tail vein injection, mice had 3.36 ± 0.02 CFU/kidneys. The dose-response curves are similar for each of the fractionated regimens, suggesting the importance of the AUC/MIC PD index. The relationship between antifungal effect and each of the PD indices (Cmax/MIC ratio, AUC/MIC ratio, and %T>MIC) is shown in Fig. 3. Each PD index fit the data well; however, the AUC/MIC ratio provided the highest correlation (R2, 0.84).
Fig 2.
Impact of dose fractionation on the in vivo efficacy of isavuconazole prodrug (BAL8557) against C. albicans K1. Groups of three mice were treated with one of five 2-fold-increasing total doses of the oral prodrug. The doses were fractionated into one, two, three, or four doses over a 24-h treatment period. Each symbol represents the mean organism burden in the kidneys of three mice. Error bars, standard deviations.
Fig 3.
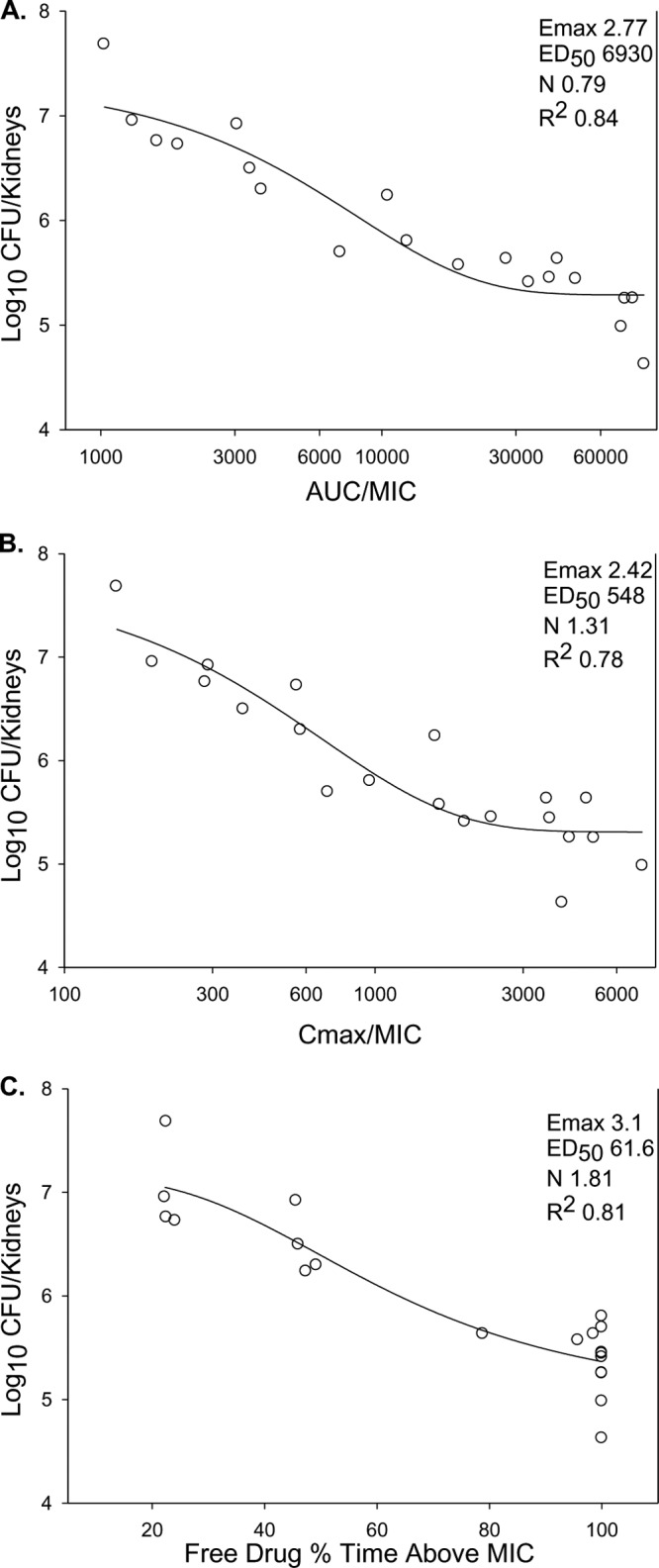
Relationship between isavuconazole (BAL4815) PD indices and in vivo efficacy against C. albicans K1. (A) Twenty-four-hour AUC/MIC ratio; (B) Cmax/MIC ratio; (C) percentage of time for which free-drug concentrations exceed the MIC (%T>MIC). Each symbol represents the mean organism burden in the kidneys of three mice. The lines through the data points represent the best-fit curves based on the Hill equation. The PD parameters Emax, ED50, and slope (N), as well as the coefficient of determination (R2), are shown for each PD index.
Pharmacodynamic index magnitude determination.
The PD index magnitude associated with the ED50 (PD target) was explored for three Candida spp. (5 C. albicans, 2 C. glabrata, and 1 C. tropicalis isolate). The AUC/MIC PD index was utilized for magnitude determination, and dosing regimens consisted of 2-fold-increasing concentrations of prodrug, from 20 to 320 mg/kg, given by the OG route every 12 h (q12 h). As noted above, a 96-h treatment period was utilized for the two C. glabrata isolates. This treatment period was also assessed for C. albicans in order to investigate the impact of treatment duration on the PD target.
The dose-response curves for C. albicans, C. tropicalis, and C. glabrata are shown in Fig. 4A to C. At the start of therapy, mice had 4.1 ± 0.4 log10 CFU of C. albicans or C. tropicalis/kidneys. For these species, growth was rapid, and their numbers increased to 6.7 ± 0.6 log10 CFU/kidneys in untreated controls after only 24 h. In general, the dose-response curves, including those for the 24-h and 96-h treatment periods, were similar among the C. albicans strains. For C. glabrata, the mice had 3.5 ± 0.4 log10 CFU/kidneys, increasing to 4.3 ± 0.2 log10 CFU/kidneys in untreated controls. In general, the dose-response curves for the two C. glabrata isolates were similar.
Fig 4.
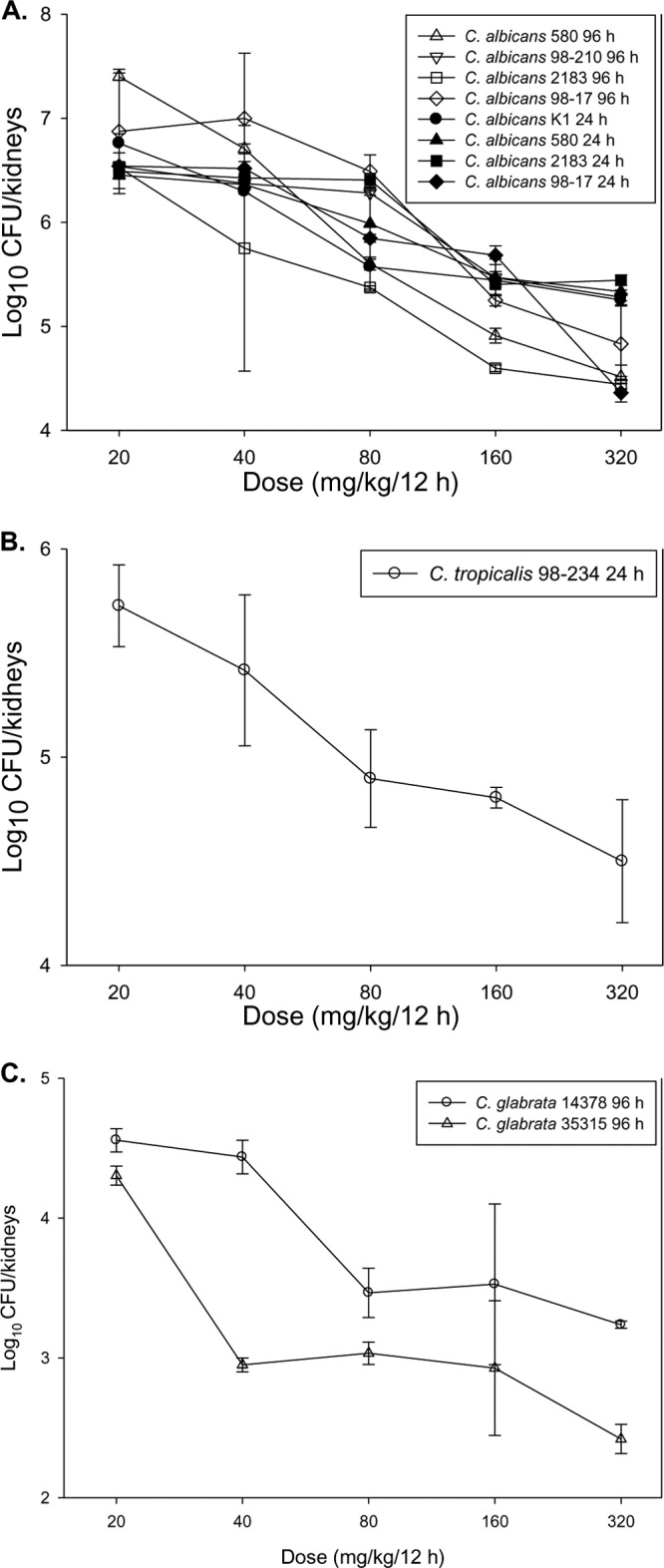
In vivo dose-response curves for 5 C. albicans (A), 1 C. tropicalis (B), and 2 C. glabrata (C) isolates. Each symbol represents the geometric mean of organism burdens in the kidneys of three mice. Error bars, standard deviations.
The ED50 determined for each Candida strain is shown in Table 2. The mean ED50 for 4 C. albicans isolates with a 96-h treatment period was 59.6 ± 22 mg/kg/12 h. In comparison, the mean ED50 for 4 C. albicans isolates with a 24-h treatment period was numerically higher, at 89.3 ± 46.7 mg/kg/12 h. This difference was not statistically significant (P, 0.30). The corresponding total-drug and free-drug AUC/MIC ratios for each group are shown in Table 2. The mean free-drug AUC/MIC ratios that corresponded to the ED50 endpoint for C. albicans were 33.3 ± 25.5 and 67.7 ± 35.0 for the 96-h and 24-h treatments. The difference observed between the 96-h and 24-h C. albicans experimental groups was not statistically significant (P, 0.16). The relationship between treatment efficacy and the corresponding AUC/MIC ratio for each C. albicans isolate is shown in Fig. 5A. The 24-h and 96-h treatment studies for C. albicans exhibited similar AUC/MIC regressions. Regression with the AUC/MIC PD index resulted in a good fit based on the coefficient of determination (R2, 0.71).
Table 2.
In vivo activities of isavuconazole against C. albicans, C. tropicalis, and C. glabrata in a neutropenic disseminated candidiasis model
| Speciesa | Isolate | Treatment duration (h) | ED50 (mg/kg/12 h) | MIC (mg/liter)b | 24-h AUC/MIC ratio |
|
|---|---|---|---|---|---|---|
| Total drug | Free drug | |||||
| C. albicans | 580 | 96 | 65.6 | 0.008 | 5,983 | 59.8 |
| 98-210 | 30.7 | 0.03 | 306 | 3.1 | ||
| 2183 | 58.5 | 0.008 | 4,787 | 47.9 | ||
| 98-17 | 83.7 | 0.031 | 2,258 | 22.6 | ||
| Mean ± SD, 96-h treatment | 3,334 ± 2,546 | 33.4 ± 25.4 | ||||
| 580 | 24 | 85.5 | 0.008 | 9,335 | 93.4 | |
| 2183 | 89.7 | 0.008 | 10,043 | 100 | ||
| 98-17 | 148 | 0.03 | 4,966 | 49.7 | ||
| K1 | 33.8 | 0.004 | 2,747 | 27.5 | ||
| Mean ± SD, 24-h treatment | 6,773 ± 3,499 | 67.7 ± 34.9 | ||||
| Mean ± SD, all C. albicans isolates | 5,053 ± 3,377 | 50.5 ± 33.7 | ||||
| C. tropicalis | 98-234 | 24 | 31.2 | 0.016 | 624 | 6.2 |
| C. glabrata | 14378 | 96 | 52.6 | 0.25 | 119 | 1.2 |
| 35315 | 48.4 | 0.125 | 193 | 1.9 | ||
| Mean ± SD, non-C. albicans isolates | 312 ± 273 | 3.1 ± 2.7 | ||||
P, 0.04 for comparison of means between all C. albicans species and non-albicans Candida species.
Determined by the CLSI method.
Fig 5.
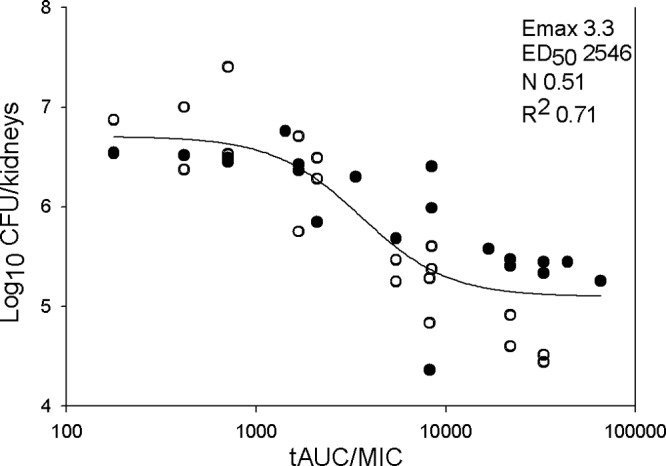
Relationship between the AUC/MIC PD index and the efficacy of isavuconazole treatment against five C. albicans isolates. Filled and open circles represent treatment durations of 24 and 96 h, respectively. Each data point represents the geometric mean of organism burdens in three mice. A best-fit line based on the Hill equation is shown. The PD parameters Emax, ED50, and slope (N), as well as the coefficient of determination (R2), are shown at the top right. tAUC, total area under the curve.
The isavuconazole ED50 for C. tropicalis was 31.2 mg/kg/12 h, and the ED50s against 2 C. glabrata isolates were 52.6 and 48.4 mg/kg/12 h. The mean free-drug AUC/MIC ratio associated with this endpoint for these non-albicans Candida isolates was 3.1 ± 2.7. The ED50s and corresponding free-drug AUC/MIC PD targets for the different C. albicans treatment periods, and for the C. albicans and non-albicans Candida isolates, were compared by the Mann-Whitney U test. The AUC/MIC target for the non-albicans Candida strains was more than 10-fold lower than that for C. albicans (P, 0.04).
DISCUSSION
Pharmacodynamic evaluation of antimicrobial agents has led to the optimization of dosing design, improved clinical outcome, decreased toxicity, prevention of the emergence of drug resistance, and appropriate application of susceptibility breakpoints (6–8, 26, 27). The goal of the current study was to characterize the pharmacokinetics and pharmacodynamics of isavuconazole (BAL4815) against multiple Candida species in a neutropenic murine disseminated candidiasis model. This infection model and study approach have been undertaken with each of the other FDA-approved triazoles (7). Previous studies by several other groups have demonstrated potent in vitro activity of isavuconazole against Candida species (11, 12), and this was confirmed in the current study, which utilized a select collection of clinical isolates of C. albicans, C. glabrata, and C. tropicalis. MICs were lowest, and similar, for C. albicans and C. tropicalis; however, the MICs for C. glabrata were 32- to 64-fold higher. This trend toward decreased in vitro potency against C. glabrata, observed in our current study, is congruent with the findings of previous larger in vitro studies (11, 12).
Pharmacokinetic and pharmacodynamic studies of isavuconazole are limited. One previous study has examined drug exposure and the efficacy of isavuconazole in a murine model of invasive candidiasis (28). As in our study, the PK of isavuconazole were linear over the dose range studied, and in general, consistent PK parameters were observed. Treatment results were also found to be relatively congruent by comparison of the two investigations.
Dose fractionation studies are critical in defining the PD index that is predictive of therapeutic efficacy. Studies with four other triazoles in this model have demonstrated that the treatment outcome is dependent on the total amount of drug (AUC), independently of the dosing interval (17–20, 29, 30). In the current study, we similarly found the AUC/MIC ratio to be the PD index most closely predictive of efficacy (R2, 0.84). This is also consistent with the results of a study of isavuconazole by Warn and colleagues (28).
In vivo experimental and clinical pharmacodynamic studies with triazoles have demonstrated the ED50, and the associated free-drug AUC/MIC ratio, to be a clinically relevant endpoint for this model that is associated with treatment outcome for patients with mucosal and invasive C. albicans infections (17–20, 31–37). These studies have consistently demonstrated that a free-drug AUC/MIC ratio between 25 and 50 is associated with the ED50 endpoint for both drug-susceptible and drug-resistant organisms. The mean free-drug AUC/MIC ratio for C. albicans isolates in the current study was approximately 50.
The majority of previous triazole PK/PD investigations have been limited to C. albicans. Recent PK/PD investigation with drugs from the echinocandin class suggest species-specific relationships (21, 23, 38). Specifically, the AUC/MIC target associated with efficacy against C. albicans was higher than that observed with non-albicans Candida species. In the current studies, we explored the impact of Candida species with two additional species, C. tropicalis and C. glabrata. The PD target for the single C. tropicalis isolate, a free-drug AUC/MIC ratio of approximately 6, was approximately 8-fold lower than that for the C. albicans isolates. The two C. glabrata isolates demonstrated an even lower PD target: a free-drug AUC/MIC ratio of 1.6 associated with the ED50 endpoint. To our knowledge, this is the first in vivo PD target study of triazoles utilizing C. glabrata isolates, and interestingly, the results are similar to those of studies of the echinocandin class, in which C. glabrata isolates had a significantly lower PD target than other Candida species (21). It will be intriguing to explore the relevance of these observations in clinical data sets.
An additional area of investigation in the current studies was the effect of treatment duration on PD targets. Previous PD studies in animal models with triazoles for therapy of invasive candidiasis have been limited predominantly to 24 h of therapy. In the current investigations, we found that a longer experimental period (96 h) resulted in PD targets slightly lower than those obtained with the more commonly studied 24-h experimental period (although this difference was not statistically significant). The longer-duration experiment resulted in a steeper exposure-effect curve and particularly identified greater differences in organism burdens for animals on the extremes of drug exposure curves. This finding may not be unexpected if one considers the impacts of effective and ineffective therapy over time. In mice receiving low and suboptimal doses, one may anticipate the growth of an organism to increase over time. Conversely, effective therapy may further reduce organism burdens with a longer-duration experiment. The clinical relevance of this observation is not clear. However, one might hypothesize the potential for resistance emergence with the marginally effective therapy. Further studies should test for the presence of resistant subpopulations among viable organisms.
PK characterization of isavuconazole in humans has been reported recently (13, 14, 39). A current phase III trial is examining the efficacy of a 200-mg loading dose three times daily for 2 days followed by 200-mg oral maintenance dosing once daily thereafter (http://clinicaltrials.gov; registration no. NCT00413218). The steady-state 24-h AUC for this regimen is approximately 90 mg · h/liter (free-drug AUC, approximately 1.8 mg · h/liter) (13). If one divides the free-drug AUC by the PD target identified for each species, a MIC threshold (i.e., ceiling) can be estimated. This would result in tentative PD-centered, species-specific breakpoints of 0.04 mg/liter for C. albicans, 1.125 mg/liter for C. glabrata, and 0.3 mg/liter for C. tropicalis based on the current animal model study. The in vitro potency of isavuconazole against various fungal pathogens, including Candida species, has also been examined in relatively large and geographically diverse surveillance series (11, 12, 40). If one integrates the animal-model ED50 and AUC/MIC target, human PK data, and surveillance MIC data, the isavuconazole dosing regimen in use for clinical trials would be predicted to achieve the PD target for 99% of Candida isolates (10). When population PK data are available, a more-robust Monte Carlo simulation should be more informative.
In summary, we have shown that isavuconazole has PK/PD characteristics similar to those described for other triazoles, including the predictive PD index and PD target, in the neutropenic murine disseminated candidiasis model. Specifically, the AUC/MIC ratio correlated best with therapeutic efficacy, and the C. albicans PD target was a free-drug AUC/MIC ratio of approximately 50. A novel finding was numerically lower PD targets for two non-albicans Candida species. It will be important to explore the clinical relevance of these findings in clinical studies.
ACKNOWLEDGMENTS
The studies reported in this article were funded by Astellas. The authors designed, completed, and interpreted the studies. Astellas was provided the opportunity to review the manuscript before submission.
Footnotes
Published ahead of print 3 September 2013
REFERENCES
- 1.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309–317 [DOI] [PubMed] [Google Scholar]
- 3.Todeschini GT. 1997. Treatment of candidiasis: a perspective on recent advances and future challenges. Int. J. Infect. Dis. 1(Suppl):S37–S41 [Google Scholar]
- 4.Falagas ME, Apostolou KE, Pappas VD. 2006. Attributable mortality of candidemia: a systematic review of matched cohort and case-control studies. Eur. J. Clin. Microbiol. Infect. Dis. 25:419–425 [DOI] [PubMed] [Google Scholar]
- 5.Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, Herwaldt L, Pfaller M, Diekema D. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37:1172–1177 [DOI] [PubMed] [Google Scholar]
- 6.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–10 [DOI] [PubMed] [Google Scholar]
- 7.Andes D. 2003. In vivo pharmacodynamics of antifungal drugs in treatment of candidiasis. Antimicrob. Agents Chemother. 47:1179–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79–86 [DOI] [PubMed] [Google Scholar]
- 9.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson GR, III, Wiederhold NP. 2010. Isavuconazole: a comprehensive review of spectrum of activity of a new triazole. Mycopathologia 170:291–313 [DOI] [PubMed] [Google Scholar]
- 11.Seifert H, Aurbach U, Stefanik D, Cornely O. 2007. In vitro activities of isavuconazole and other antifungal agents against Candida bloodstream isolates. Antimicrob. Agents Chemother. 51:1818–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guinea J, Pelaez T, Recio S, Torres-Narbona M, Bouza E. 2008. In vitro antifungal activities of isavuconazole (BAL4815), voriconazole, and fluconazole against 1,007 isolates of zygomycete, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob. Agents Chemother. 52:1396–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitt-Hoffmann A, Roos B, Maares J, Heep M, Spickerman J, Weidekamm E, Brown T, Roehrle M. 2006. Multiple-dose pharmacokinetics and safety of the new antifungal triazole BAL4815 after intravenous infusion and oral administration of its prodrug, BAL8557, in healthy volunteers. Antimicrob. Agents Chemother. 50:286–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitt-Hoffmann A, Roos B, Heep M, Schleimer M, Weidekamm E, Brown T, Roehrle M, Beglinger C. 2006. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrob. Agents Chemother. 50:279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute (CLSI) 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 3rd ed. CLSI document M27–A3 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 16.National Research Council Committee on the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, and Commission on Life Sciences 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 17.Andes D, Marchillo K, Conklin R, Krishna G, Ezzet F, Cacciapuoti A, Loebenberg D. 2004. Pharmacodynamics of a new triazole, posaconazole, in a murine model of disseminated candidiasis. Antimicrob. Agents Chemother. 48:137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andes D, Marchillo K, Stamstad T, Conklin R. 2003. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:3165–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andes D, Marchillo K, Stamstad T, Conklin R. 2003. In vivo pharmacodynamics of a new triazole, ravuconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:1193–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andes D, van Ogtrop M. 1999. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine disseminated candidiasis infection model. Antimicrob. Agents Chemother. 43:2116–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andes D, Diekema DJ, Pfaller MA, Bohrmuller J, Marchillo K, Lepak A. 2010. In vivo comparison of the pharmacodynamic targets for echinocandin drugs against Candida species. Antimicrob. Agents Chemother. 54:2497–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andes D, Diekema DJ, Pfaller MA, Prince RA, Marchillo K, Ashbeck J, Hou J. 2008. In vivo pharmacodynamic characterization of anidulafungin in a neutropenic murine candidiasis model. Antimicrob. Agents Chemother. 52:539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andes DR, Diekema DJ, Pfaller MA, Marchillo K, Bohrmueller J. 2008. In vivo pharmacodynamic target investigation for micafungin against Candida albicans and C. glabrata in a neutropenic murine candidiasis model. Antimicrob. Agents Chemother. 52:3497–3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andes D. 2005. Use of an animal model of disseminated candidiasis in the evaluation of antifungal therapy. Methods Mol. Med. 118:111–128 [DOI] [PubMed] [Google Scholar]
- 25.Andes D, Marchillo K, Lowther J, Bryskier A, Stamstad T, Conklin R. 2003. In vivo pharmacodynamics of HMR 3270, a glucan synthase inhibitor, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:1187–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drusano GL. 2007. Pharmacokinetics and pharmacodynamics of antimicrobials. Clin. Infect. Dis. 45(Suppl 1):S89–S95 [DOI] [PubMed] [Google Scholar]
- 27.Hope WW, Drusano GL. 2009. Antifungal pharmacokinetics and pharmacodynamics: bridging from the bench to bedside. Clin. Microbiol. Infect. 15:602–612 [DOI] [PubMed] [Google Scholar]
- 28.Warn PA, Sharp A, Parmar A, Majithiya J, Denning DW, Hope WW. 2009. Pharmacokinetics and pharmacodynamics of a novel triazole, isavuconazole: mathematical modeling, importance of tissue concentrations, and impact of immune status on antifungal effect. Antimicrob. Agents Chemother. 53:3453–3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klepser ME, Malone D, Lewis RE, Ernst EJ, Pfaller MA. 2000. Evaluation of voriconazole pharmacodynamics using time-kill methodology. Antimicrob. Agents Chemother. 44:1917–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louie A, Drusano GL, Banerjee P, Liu QF, Liu W, Kaw P, Shayegani M, Taber H, Miller MH. 1998. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob. Agents Chemother. 42:1105–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baddley JW, Patel M, Bhavnani SM, Moser SA, Andes DR. 2008. Association of fluconazole pharmacodynamics with mortality in patients with candidemia. Antimicrob. Agents Chemother. 52:3022–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clancy CJ, Yu VL, Morris AJ, Snydman DR, Nguyen MH. 2005. Fluconazole MIC and the fluconazole dose/MIC ratio correlate with therapeutic response among patients with candidemia. Antimicrob. Agents Chemother. 49:3171–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SC, Fung CP, Huang JS, Tsai CJ, Chen KS, Chen HY, Lee N, See LC, Shieh WB. 2000. Clinical correlates of antifungal macrodilution susceptibility test results for non-AIDS patients with severe Candida infections treated with fluconazole. Antimicrob. Agents Chemother. 44:2715–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pai MP, Turpin RS, Garey KW. 2007. Association of fluconazole area under the concentration-time curve/MIC and dose/MIC ratios with mortality in nonneutropenic patients with candidemia. Antimicrob. Agents Chemother. 51:35–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rex JH, Pfaller MA, Galgiani JN, Bartlett MS, Espinel-Ingroff A, Ghannoum MA, Lancaster M, Odds FC, Rinaldi MG, Walsh TJ, Barry AL. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Clin. Infect. Dis. 24:235–247 [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Tudela JL, Almirante B, Rodriguez-Pardo D, Laguna F, Donnelly JP, Mouton JW, Pahissa A, Cuenca-Estrella M. 2007. Correlation of the MIC and dose/MIC ratio of fluconazole to the therapeutic response of patients with mucosal candidiasis and candidemia. Antimicrob. Agents Chemother. 51:3599–3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takakura S, Fujihara N, Saito T, Kudo T, Iinuma Y, Ichiyama S. 2004. Clinical factors associated with fluconazole resistance and short-term survival in patients with Candida bloodstream infection. Eur. J. Clin. Microbiol. Infect. Dis. 23:380–388 [DOI] [PubMed] [Google Scholar]
- 38.Andes D, Ambrose PG, Hammel JP, Van Wart SA, Iyer V, Reynolds DK, Buell DN, Kovanda LL, Bhavnani SM. 2011. Use of pharmacokinetic-pharmacodynamic analyses to optimize therapy with the systemic antifungal micafungin for invasive candidiasis or candidemia. Antimicrob. Agents Chemother. 55:2113–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Desai A, Zadeikis N, Pearlman H, Kowalski D, Townsend R. 2012. Effect of multiple doses of isavuconazole on the pharmacokinetics of CYP3A4 substrate midazolam in healthy volunteers, abstr A-1936 Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]
- 40.Yamazaki T, Inagaki Y, Fujii T, Ohwada J, Tsukazaki M, Umeda I, Kobayashi K, Shimma N, Page MG, Arisawa M. 2010. In vitro activity of isavuconazole against 140 reference fungal strains and 165 clinically isolated yeasts from Japan. Int. J. Antimicrob. Agents 36:324–331 [DOI] [PubMed] [Google Scholar]