Abstract
Triazoles are first-line agents for treating aspergillosis. The prevalence of azole resistance in Aspergillus fumigatus is increasing, and cross-resistance is a growing concern. In this study, the susceptibilities of 40 A. fumigatus clinical isolates were tested by using the CLSI method with amphotericin B, itraconazole, voriconazole, posaconazole, and the new triazole isavuconazole. Isavuconazole MICs were higher in strains with reduced susceptibilities to other triazoles, mirroring changes in voriconazole susceptibility. Isavuconazole MICs differed depending on the Cyp51A substitution.
TEXT
Triazoles are first-line antifungal agents for the treatment of invasive, chronic, and allergic diseases caused by Aspergillus spp. (1). There have been increasing reports of azole resistance in Aspergillus fumigatus (2–4). The clinical outcomes of infections caused by strains with reduced susceptibility to triazoles are frequently suboptimal (3, 5–8). Furthermore, the extent of a correlation (if any) between the in vitro susceptibilities of Aspergillus spp. to licensed azole compounds and newer agents, such as isavuconazole, is undefined (3, 7, 9, 10). The aim of this study was to determine the in vitro susceptibility to isavuconazole of a collection of 40 A. fumigatus isolates among which the majority had reduced susceptibility to itraconazole, voriconazole, and posaconazole.
All strains were clinical isolates obtained from the Mycology Reference Centre Manchester and were primarily isolated in the United Kingdom (but also included some strains from Canada, Denmark, United States, and France) between 1989 and 2008. The vast majority of isolates were obtained from cases following azole exposure (patients had received 1 to 30 months of azole therapy), although treatment was unknown in some cases. The majority (i.e., 33/40) of isolates had previously been demonstrated to possess an elevated MIC to at least one triazole, and a putative molecular mechanism of resistance had been defined (3, 4). The remaining 7 isolates were well-characterized clinical strains that demonstrated triazole MICs within the wild-type range, and all had wild-type cyp51A sequences except strain AF250, which had an N248K mutation.
MICs were determined using the Clinical and Laboratory Standards Institute (CLSI) broth microdilution methodology (11). Quality control strains Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 were used. MICs were estimated in three independently conducted experiments, and a consensus MIC value was established (i.e., the mode or median value; 96% were within 4-fold variation). The following compounds were tested: amphotericin B (Sigma, Poole, United Kingdom), itraconazole (Sigma), voriconazole (Pfizer Ltd., Sandwich, United Kingdom), posaconazole (Schering-Plough, New Jersey), and isavuconazole (Astellas Pharma Inc., Tokyo, Japan). The concentration range tested was 0.015 to 8 mg/liter for all compounds. For the purposes of statistical analysis, MICs of >8 mg/liter were classified as 16 mg/liter.
The following epidemiological cutoff values (ECVs) within the CLSI methodology for A. fumigatus were used in the subsequent analysis: ≤1 mg/liter for itraconazole and voriconazole, ≤0.25 mg/liter for posaconazole, and ≤4 mg/liter for amphotericin (9, 12). There are no ECVs currently available for isavuconazole.
Thirty-two strains had triazole MICs above the ECV (9). Two strains had voriconazole MICs that straddled the ECV (1 to 2 mg/liter). One of these strains (F5211) had an MIC value that fell within the wild-type range in a previous study (3) but was above the ECV in this study, as the consensus MIC was slightly higher. The opposite was true for the other strain (F17764). An additional strain (F16867) had a reproducibly high itraconazole MIC (>8 mg/liter) in a previous study (3) but was consistently within the wild-type distribution (0.5 mg/liter) in this study. Potential reasons for this difference may be related to a change in methodology between the two studies from a modified EUCAST method (the modification was the use of a lower final inoculum concentration of 0.5 × 105, as opposed to 1 × 105 to 2.5 × 105 CFU/ml) versus the CLSI method, or loss of resistance during long-term storage at −80°C (3). A molecular mechanism that could potentially account for elevated triazole MICs was not apparent for any of these isolates. The first two strains (F5211 and F17764) had a wild-type cyp51A sequence, and the latter (F16867) had a mutation known not to confer resistance (E427G), as it has been found in azole-susceptible strains. For the purposes of analysis, these strains were all classified in the wild-type group (n = 9), leaving 31 non-wild-type strains with an MIC for at least one triazole agent that was above the ECV.
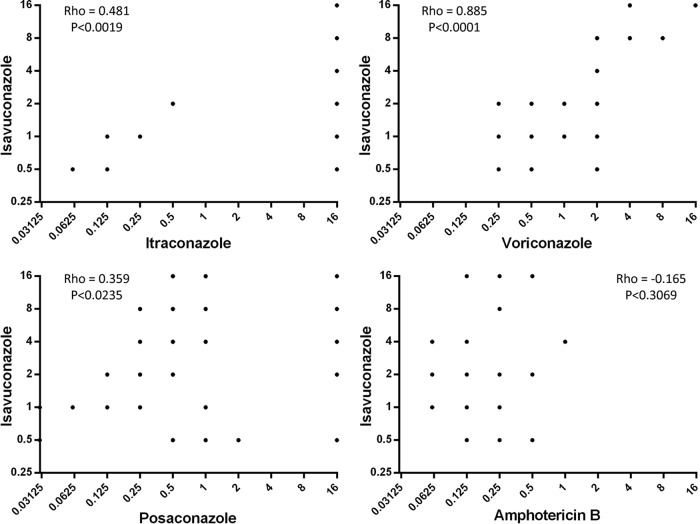
The isavuconazole MICs (geometric mean [range] of 0.93 mg/liter [0.5 to 2 mg/liter]) of the wild-type strains were consistent with previous reports (13–15). Isolates with raised MICs to one or more triazole were more likely to have higher isavuconazole MICs. The geometric mean (range) of the non-wild-type strains was 3.66 mg/liter (0.5 to >8 mg/liter). There was a high degree of correlation between isavuconazole and voriconazole MICs (r = 0.885 [Spearman's correlation coefficient]; P < 0.001) (Fig. 1). The extent of correlation between isavuconazole with itraconazole and posaconazole was less than that observed with voriconazole (Fig. 1), which corresponds with the drugs; structures (isavuconazole is similar in chemical structure to voriconazole, whereas posaconazole has a long side chain like itraconazole).
Fig 1.
Relationship between isavuconazole in vitro susceptibility and susceptibility of the other antifungal drugs tested. The Spearman's rank correlation coefficient (rho) and statistical significance for each drug pair combination are shown within each graph.
Amphotericin B susceptibility was within the wild-type range for all isolates in this study (≤1 mg/liter). The geometric mean for this drug did not significantly differ between the wild-type and non-wild-type groups (0.27 mg/liter versus 0.18 mg/liter, respectively), and the r value of −0.165 was not statistically significant (P < 0.3069), suggesting there was no correlation between amphotericin B and isavuconazole MICs.
Isolates with the alterations L98H, G138C, Y431C, G434C, and G448S showed elevated MICs to all triazoles, including isavuconazole (Table 1). Voriconazole and isavuconazole MICs were lower in isolates with amino acid substitutions at position G54, suggesting that alterations at this position only affect itraconazole and posaconazole susceptibility, as has been previously described (10). However, isavuconazole and voriconazole MICs were variable in isolates with M220 alterations. Additionally, specific amino acid substitutions can affect in vitro susceptibility (10). Therefore, isolates with a variety of different amino acid alterations were selected for this study, to investigate if there were any significant differences between substitutions. The only notable observation was that isolates with lysine substitutions at position 220 had higher posaconazole MICs than those with isoleucine, threonine, or valine alterations. However, the potential effects from other mechanisms of resistance contributing to this pattern of susceptibility cannot be discounted.
Table 1.
MICs determined via the CLSI broth microdilution method
| Susceptibility/Cyp51A alteration (n) | MIC (mg/liter) geometric mean (range) |
||||
|---|---|---|---|---|---|
| Amphotericin B | Itraconazole | Voriconazole | Posaconazole | Isavuconazole | |
| Wild-type group (9) | 0.27 (0.25–0.5) | 0.15 (0.06–0.5) | 0.58 (0.25–2) | 0.06 (0.03–0.125) | 0.93 (0.5–2) |
| Non-wild-type group (31) | 0.18 (0.06–1) | 16.00 (16) | 2.00 (0.25–16) | 1.02 (0.125–16) | 3.66 (0.5–16) |
| G54 (6) | 0.22 (0.06–0.5) | 16.00 (16) | 0.40 (0.25–2) | 1.41 (0.5–16) | 0.63 (0.5–2) |
| L98 (3) | 0.25 (0.25) | 16.00 (16) | 5.04 (4–8) | 0.63 (0.5–1) | 10.08 (8–6) |
| M220 (9) | 0.16 (0.06–1) | 16.00 (16) | 1.71 (0.5–4) | 1.71 (0.25–16) | 3.43 (1–8) |
| G138/Y431/G434/G448 (5) | 0.19 (0.125–0.5) | 16.00 (16) | 12.13 (4–16) | 2.64 (0.5–16) | 16.00 (16) |
| Others (8) | 0.16 (0.125–0.25) | 16.00 (16) | 1.83 (0.25–16) | 0.30 (0.125–1) | 4.00 (1–16) |
In summary, isavuconazole MICs were more likely to be higher in strains with reduced susceptibilities to other triazoles and had a high degree of correlation with voriconazole MICs. The extent to which diminished in vitro susceptibility has an impact on clinical outcome remains to be established. Therefore, additional in vivo, pharmacodynamic, and clinical data are required. The development of ECVs/breakpoints for isavuconazole against Aspergillus will aid in the interpretation of isavuconazole MICs.
ACKNOWLEDGMENTS
This work was sponsored by Astellas Pharma Global Development Inc.
Malcolm Richardson is a consultant for Gilead Sciences Inc. and has received lecture fees from Astellas Pharma, Pfizer, and MSD. William Hope is supported by a National Institutes of Health (NIHR) Clinician Scientist Fellowship. Susan Howard has received research grants and travel grants and has been paid for talks on behalf of Astellas. None of the other authors declare any conflicts of interest.
Isolates used in this study were provided by and are held in the clinical culture collection at The Mycology Reference Centre Manchester.
Footnotes
Published ahead of print 16 September 2013
REFERENCES
- 1.Denning DW, Hope WW. 2010. Therapy for fungal diseases: opportunities and priorities. Trends Microbiol. 18:195–204 [DOI] [PubMed] [Google Scholar]
- 2.Snelders E, van der Lee HA, Kuijpers J, Rijs AJ, Varga J, Samson RA, Mellado E, Donders AR, Melchers WJ, Verweij PE. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 5(11):e219. 10.1371/journal.pmed.0050219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg. Infect. Dis. 15:1068–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bueid A, Howard SJ, Moore CB, Richardson MD, Harrison E, Bowyer P, Denning DW. 2010. Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. J. Antimicrob. Chemother. 65:2116–2118 [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Li H, Li R, Bu D, Wan Z. 2005. Mutations in the cyp51A gene and susceptibility to itraconazole in Aspergillus fumigatus serially isolated from a patient with lung aspergilloma. J. Antimicrob. Chemother. 55:31–37 [DOI] [PubMed] [Google Scholar]
- 6.Dannaoui E, Garcia-Hermoso D, Naccache JM, Meneau I, Sanglard D, Bouges-Michel C, Valeyre D, Lortholary O. 2006. Use of voriconazole in a patient with aspergilloma caused by an itraconazole-resistant strain of Aspergillus fumigatus. J. Med. Microbiol. 55:1457–1459 [DOI] [PubMed] [Google Scholar]
- 7.Verweij PE, Mellado E, Melchers WJ. 2007. Multiple-triazole-resistant aspergillosis. N. Engl. J. Med. 356:1481–1483 [DOI] [PubMed] [Google Scholar]
- 8.Bellete B, Raberin H, Morel J, Flori P, Hafid J, Sung RT. 2010. Acquired resistance to voriconazole and itraconazole in a patient with pulmonary aspergilloma. Med. Mycol. 48:197–200 [DOI] [PubMed] [Google Scholar]
- 9.Pfaller MA, Diekema DJ, Ghannoum A, Rex JH, Alexander BD, Andes D, Brown SD, Chaturvedi V, Espinel-Ingroff A, Fowler CL, Johnson EM, Knapp CC, Motyl MR, Ostrosky-Zeichner L, Sheehan DJ, Walsh TJ. 2009. Wild-type MIC distribution and epidemiological cutoff values for Aspergillus fumigatus and three triazoles as determined by the Clinical and Laboratory Standards Institute broth microdilution methods. J. Clin. Microbiol. 47:3142–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howard SJ, Arendrup MC. 2011. Acquired antifungal drug resistance in Aspergillus fumigatus: epidemiology and detection. Med. Mycol. 49(Suppl 1):S90–S95 [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed. Document M38-A2 CLSI, Wayne, PA [Google Scholar]
- 12.Espinel-Ingroff A, Cuenca-Estrella M, Fothergill A, Fuller J, Ghannoum M, Johnson E, Pelaez T, Pfaller MA, Turnidge J. 2011. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B and Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). Antimicrob. Agents Chemother. 55:5150–5154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warn PA, Sharp A, Denning DW. 2006. In vitro activity of a new triazole BAL4815, the active component of BAL8557 (the water-soluble prodrug), against Aspergillus spp. J. Antimicrob. Chemother. 57:135–138 [DOI] [PubMed] [Google Scholar]
- 14.Perkhofer S, Lechner V, Lass-Florl C. 2009. In vitro activity of isavuconazole against Aspergillus species and zygomycetes according to the methodology of the European Committee on Antimicrobial Susceptibility Testing. Antimicrob. Agents Chemother. 53:1645–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guinea J, Pelaez T, Recio S, Torres-Narbona M, Bouza E. 2008. In vitro antifungal activities of isavuconazole (BAL4815), voriconazole, and fluconazole against 1,007 isolates of zygomycete, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob. Agents Chemother. 52:1396–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]