Abstract
Cerebral malaria (CM) is associated with low nitric oxide (NO) bioavailability, cerebrovascular constriction, occlusion, and hypoperfusion. Administration of exogenous NO partially prevents the neurological syndrome and associated vascular pathology in an experimental CM (ECM) mouse model. In this study, we evaluated the effects of transdermal glyceryl trinitrate in preventing ECM and, in combination with artemether, rescuing late-stage ECM mice from mortality. The glyceryl trinitrate and/or artemether effect on survival and clinical recovery was evaluated in C57BL/6 mice infected with P. berghei ANKA. NO synthase (NOS) expression in mouse brain was determined by Western blots. Mean arterial pressure (MAP) and pial arteriolar diameter were monitored using a tail-cuff blood pressure system and a cranial window preparation, respectively. Preventative administration of glyceryl trinitrate at 0.025 mg/h decreased ECM mortality from 67 to 11% and downregulated inducible NOS expression in the brain. When administered as adjunctive rescue therapy with artemether, glyceryl trinitrate increased survival from 47 to 79%. The adjunctive therapy caused a sustained reversal of pial arteriolar vasoconstriction in ECM mice, an effect not observed with artemether alone. Glyceryl trinitrate induced a 13% decrease in MAP in uninfected mice but did not further affect MAP in hypotensive ECM mice. Glyceryl trinitrate, when combined with artemether, was an effective adjunctive rescue treatment for ECM. This treatment ameliorated pial arteriolar vasospasm and did not significantly affect MAP. These results indicate that transdermal glyceryl trinitrate has potential to be considered as a candidate for adjunctive therapy for CM.
INTRODUCTION
Cerebral malaria (CM) is a lethal complication of Plasmodium falciparum infection and is largely responsible for the estimated 1 million-plus malaria deaths every year (1). CM has high mortality rates of 20% even upon administration of prompt antimalarial treatment, which is based on the parenteral administration of quinine or artemisinin derivatives. In an attempt to reduce mortality, various adjunctive treatments for CM have been evaluated in clinical trials, though mostly with unfavorable outcomes (2). Human CM is a severe vasculopathy (3) and is commonly associated with acidosis and other complications (4). Postmortem studies show diffuse microhemorrhages and cerebrovascular obstruction by parasitized RBCs (pRBCs) and often leukocytes sequestered in inflamed endothelium via receptors, such as intercellular adhesion molecule 1 (ICAM-1) (5–7). In vivo studies of the retinal microcirculation of CM patients revealed vascular obstruction, hypoperfusion and intravascular filling defects (8). Endothelial dysfunction in CM has been demonstrated, with low nitric oxide (NO) bioavailability (9), elevated plasma levels of cell-free hemoglobin (10), asymmetric dimethylarginine (11), endothelin 1 (12), and angiopoietins (13), and spastic constriction of cerebral arterioles (14).
Plasmodium berghei ANKA (PbA) infection in susceptible mice induces a neurological syndrome known as experimental cerebral malaria (ECM), whose pathogenesis shares similarities with human CM (15). The relevance of this model has recently been debated (16–21). Similarly to human severe malaria, low NO bioavailability has been linked to the genesis of ECM (9, 22, 23). We have shown that exogenous NO administration in the form of NO donors such as dipropylenetriamine NONOate (DPTA-NO) and S-nitrosoglutathione (GSNO) decreases ECM incidence, as well as cerebral edema, leukocyte accumulation, and hemorrhages (22, 24, 25). Similar findings were obtained with inhaled NO (26). Using intravital microscopy of the pial microcirculation through a closed cranial window, we demonstrated that ECM is associated with cerebrovascular constriction, hypoperfusion, vessel blockage, marked decreases in cerebral blood flow, and eventually vascular collapse (27), features similar to those in human CM (8). More importantly, coadministration of the calcium channel blocker nimodipine, a potent cerebral vasodilator, with artemether markedly increased survival and recovery of mice with late-stage ECM (27), indicating that interventions to counteract cerebral vasoconstriction and improve cerebral blood flow are logical and potentially powerful approaches for CM adjunctive therapies.
Glyceryl trinitrate is used for the treatment of angina and heart failure due to its dilator activity in large veins and arteries (28). Glyceryl trinitrate induced vasodilatation occurs via a biotransformation process through denitrification to yield NO, which activates soluble guanylate cyclase and thus relaxes vascular smooth muscle (29). Besides vasodilatation, glyceryl trinitrate has also been proven to decrease inflammation (30). Since orally administered glyceryl trinitrate has very short elimination half-life and undergoes extensive gastrointestinal and hepatic first-pass metabolism, the use of transdermal administration of glyceryl trinitrate represents an interesting approach to obtain good bioavailability and to prolong the duration of action (31). Moreover, glyceryl trinitrate patches are inexpensive and generic formulations can be found for less than $1 per unit. We hypothesized that since transdermal glyceryl trinitrate can be used as a source of sustained and slow NO release, glyceryl trinitrate patches may be promising for ECM adjunctive therapy. In the present study, we report the efficacy of transdermal glyceryl trinitrate in preventing ECM and, in combination with artemether, rescuing mice from late-stage ECM. We also describe the effects of this adjunctive therapy in mouse blood pressure and cerebrovascular dilatation.
MATERIALS AND METHODS
Mice, P. berghei ANKA infection, and parasitemia follow-up.
All protocols for animal handling and care were approved by the La Jolla Bioengineering Institute's Animal Care and Use Committee. Eight- to ten-week-old female C57BL/6 mice (Jackson Laboratory, Sacramento, CA) were infected intraperitoneally with 106 PbA parasites expressing the green fluorescent protein (obtained from the MR4, Manassas, VA, reference MRA-865, deposited by C. J. Janse and A. P. Waters). Parasitemia levels were monitored by flow cytometry or by microscopy in mice under artemether treatment.
Clinical evaluation and ECM definition.
ECM was defined by the occurrence of at least one of the following clinical signs: ataxia, limb paralysis, rollover, seizures, convulsions, poor righting reflex, hypothermia, and/or coma. Body temperature was monitored by using an Acorn Series Thermocouple with a mouse rectal probe (Oakton Instruments, Vernon Hills, IL). In addition, a set of six motor behavior tests, with scores ranging from 0 (complete impairment) to 23 (maximum performance), was performed as described previously (27, 32).
Treatments.
Two different types of experimental treatments were evaluated: (i) preventative treatment to assess whether glyceryl trinitrate protects against ECM and (ii) rescue treatment to evaluate whether glyceryl trinitrate was able to increase the efficacy of artemether in rescuing mice presenting late-stage ECM.
(i) ECM preventative treatment.
Three days before infection, mice were mildly anesthetized with isoflurane, and part of the back fur was removed with hair removal cream (Nair lotion, Princeton, NJ). After PbA inoculation, a quarter of a glyceryl trinitrate patch (nitroglycerin transdermal system, 0.1 mg/h; Mylan Pharmaceuticals, Inc., Morgantown, WV) delivering 0.025 mg/h was applied to the back of the animal in cycles of 12 h to avoid the development of glyceryl trinitrate tolerance until day 8 of infection. The control group consisted of infected mice that were subjected to back fur removal under light anesthesia 3 days after infection but had no patch implanted. The lack of a placebo patch was a limitation in the experimental procedure. Parasitemia, rectal temperature, and motor behavior scores were recorded daily (32). On days 6 and 12 of infection, the hematocrit levels were measured (33). After the cessation of glyceryl trinitrate treatment on day 8, survivor mice were monitored up to day 12 of infection. Mortality rates were recorded and, at the end of the experimental protocol (day 12), mice were euthanized with sodium pentobarbital at 390 mg plus sodium phenytoin at 50 mg/ml (Euthasol; 100 mg/kg, intraperitoneally).
(ii) ECM rescue treatments.
On day 3 of infection, the mice were shaved. Beginning on day 4 and until the end of the experiment, parasitemia, rectal temperature, and motor behavior were monitored daily. Mice with at least one of the ECM clinical signs and showing body temperatures between 32 and 34°C (used as an objective, quantitative criterion for treatment and for group comparison) on days 5 to 7 were randomly assigned to different treatment groups: (i) artemether (Artesiane; Dafra Pharma, Belgium) at 25 mg/kg, administered intraperitoneally without patches; (ii) artemether at 25 mg/kg, administered intraperitoneally, plus glyceryl trinitrate patches delivering 0.025 mg/h; and (iii) artemether at 25 mg/kg, administered intraperitoneally, plus glyceryl trinitrate patches delivering 0.1 mg/h. Glyceryl trinitrate patches were left on the mouse skin for 24 h, after which they were removed. Therefore, after 24 h all mice in all groups received only artemether at 25 mg/kg, administered intraperitoneally daily, for a total course of 5 days (32). Mortality rates were recorded for all treatments. One week after the cessation of artemether, mice were euthanized with Euthasol (100 mg/kg, intraperitoneally).
Brain sample preparation and NO synthase (NOS) expression.
Uninfected mice and PbA-infected mice undergoing or not glyceryl trinitrate preventative treatment were euthanized (Euthasol, 100 mg/kg, intraperitoneally) on day 6 of infection; brains were immediately harvested, flash frozen in liquid nitrogen, and stored at −80°C. Brains were homogenized, lysates subjected to SDS-PAGE, transferred to polyvinylidene fluoride membranes, and incubated against different antibodies (for details, see the supplemental material). The following primary antibodies were used: β-tubulin, total iNOS, and nNOS (Santa Cruz, CA); total eNOS (Stressgen Bioreagents, Victoria, British Columbia, Canada); and P-eNOS (S1176; Cell Signaling Technology, Danvers, MA). Horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology) were used for detection. Band intensity was quantified on unsaturated X-ray films and quantified with ImageJ software (National Institutes of Health [NIH], Bethesda, MD). The total NOS expression levels are presented as a ratio versus the β-tubulin content.
Determination of plasma nitrite and nitrate content.
Uninfected and PbA-infected mice with ECM were treated with artemether monotherapy, artemether plus glyceryl trinitrate patch at 0.025 mg/h, or artemether plus glyceryl trinitrate patch at 0.1 mg/h. During the course of the experiment, blood (30 μl) samples were collected from the saphenous vein before (0 h) and at different time points after the treatment (3, 6, and 24 h). Plasma was recovered by centrifugation (800 × g, 10 min, 4°C) and mixed with an equal volume of methanol. After protein precipitation (5,000 × g, 10 min, 4°C), the plasma nitrite (NO2−) and nitrate (NO3−) concentrations were quantified by ion chromatography (ENO20 Analyzer; Eicom, Kyoto, Japan). The concentrations of nitrite and nitrate were estimated by assessing the peak height of the absorption compared to sodium nitrite and sodium nitrate standard solutions.
Blood pressure measurements.
The mean arterial blood pressure (MAP) was recorded in conscious mice using the CODA noninvasive tail-cuff system (Kent Instruments, Torrington, CT) (34). Mice were allowed to acclimate to the restrainer for 5 min prior to initiating the blood pressure measurement. At least 10 readings were taken from each animal at each time point. The MAP was measured on day 0, and the animals were subsequently infected with PbA. Mice showing signs of ECM on day 6 were treated with either artemether or artemether plus glyceryl trinitrate patch at 0.1 mg/h and had MAP recorded just before and at four time points after glyceryl trinitrate patch implantation (0, 3, 6, 24, and 48 h). All MAP measurements were normalized to day 0 baseline.
Cranial window preparation and intravital microscopy.
The chronic closed cranial window preparation, as previously described (35, 36), was utilized for monitoring changes in pial arteriolar diameters (see the supplemental material for details). Briefly, 2 weeks after surgery, the mouse was mildly anesthetized with isoflurane and transferred onto an intravital microscope stage (customized Leica-McBain, San Diego, CA). Pial arterioles (N = 2 to 6; baseline vessel diameters, 35 to 115 μm) were visualized by epi-illumination using a 20× water immersion objective lens, and their diameters were measured using an Image Shear device (0.213 μm/pixel; Vista Electronics, San Diego, CA). After microscopy, the mice were infected with PbA (106), and intravital procedures were repeated on day 6 of infection before and at multiple time points (0, 3, 6, and 24 h) after the rescue treatment (artemether or artemether plus glyceryl trinitrate 0.1 mg/h). Infected mice without treatment were also evaluated. All diameter measurements were normalized to the day 0 baseline.
Statistical analyses.
Data are presented as means ± the standard errors of the mean (SEM) unless otherwise indicated. Significant differences in survival rates were determined using log-rank (Mantel-Cox) tests. One-way or two-way analysis of variance, followed by Bonferroni test comparisons, was used to test the significance of differences in Western blot analysis during the preventative and rescue treatments follow-ups with regard to temperature, motor behavior score, parasitemia, hematocrit, plasma nitrite/nitrate levels, MAP, and vessel diameters. The evaluated group sizes (N) are reported in the graphs and/or figure legends. Differences were considered statistically significant at P ≤ 0.05. Statistical analyses were performed using GraphPad 5.0 (GraphPad Software, San Diego, CA).
RESULTS
Glyceryl trinitrate treatment protects against ECM.
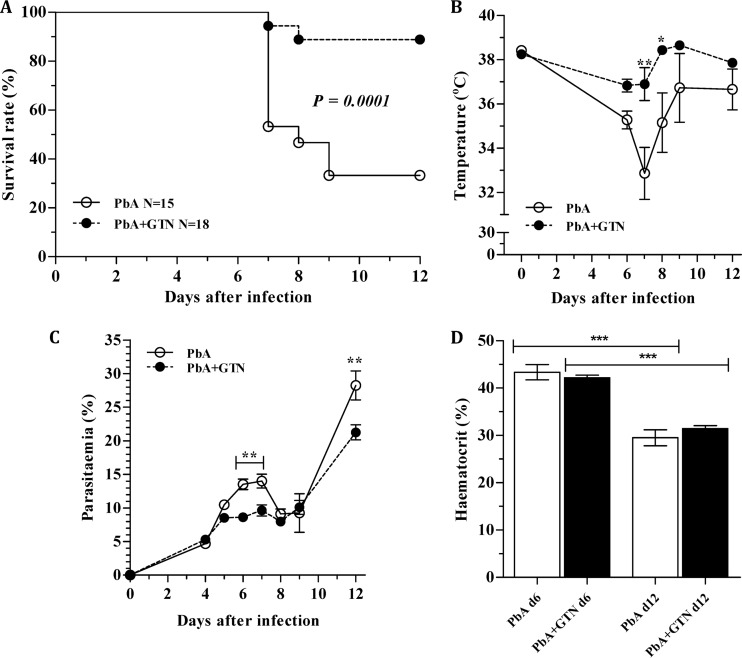
PbA infection led to the development of ECM and 67% mortality in untreated mice (Fig. 1A), with ECM manifestation starting on day 6 and most deaths occurring on day 7. Application of glyceryl trinitrate delivering patches to the skin of PbA-infected mice daily from days 0 to 8 led to a marked reduction in mortality by ECM (11%; P < 0.0001). Glyceryl trinitrate treatment positively influenced the animals' body temperature (Fig. 1B). Two mice in each group showed low body temperatures (<33°C) on day 6 or 7 but recovered. At least part of the protection could be attributed to glyceryl trinitrate effect in inhibiting parasite growth, since glyceryl trinitrate-treated animals better controlled parasitemia at a critical period for ECM development (days 6 and 7) (Fig. 1C). Despite the effect on parasitemia, hematocrit values were not different between glyceryl trinitrate treated and untreated PbA-infected mice on day 6 or 12 (Fig. 1D).
Fig 1.
Glyceryl trinitrate protects from ECM. Effect of glyceryl trinitrate at 0.025 mg/h on cumulative survival (A), temperature (B), parasitemia (C), and hematocrit (D). In panel A, the number of mice (N) is shown. In panel D, hematocrit values were recorded at day 6 (PbA, N = 15; PbA-GTN, N = 18) and day 12 (PbA, N = 4; PbA-GTN, N = 8) of infection. The data for panels B to D are derived from the same animals of the survival experiment (A) and are expressed as the means of two separate experiments ± the SEM (except for the D12 hematocrit data, which are data from one experiment). *, P < 0.05; **, P < 0.001; ***, P < 0.0001. GTN, glyceryl trinitrate; PbA, P. berghei ANKA.
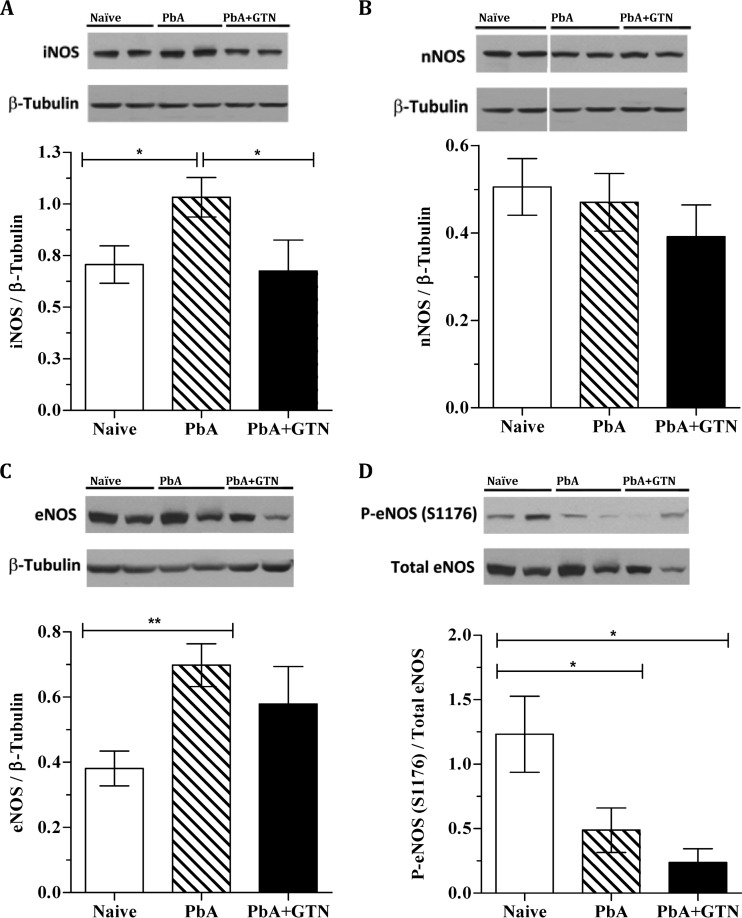
Glyceryl trinitrate treatment prevents iNOS and eNOS upregulation in PbA-infected mice.
Nitric oxide is synthesized by NO synthases (NOS) whose dysfunction was recently shown to contribute to impaired cerebroarteriolar reactivity in ECM (37). Brains of PbA-infected mice on day 6 of infection showed increased expression of both the inducible NOS (iNOS) and the endothelial specific NOS (eNOS) isoforms, but not of the neuronal NOS (nNOS) (Fig. 2A to C). eNOS activation can be induced by phosphorylation of serine 1176 (S1176) by mechanical forces of fluid shear stress on the endothelium of the blood vessel wall. As we previously reported, eNOS S1176 phosphorylation was downregulated during ECM (Fig. 2D). Glyceryl trinitrate treatment prevented iNOS and eNOS upregulation during ECM (Fig. 2A and C, P < 0.05), although the effect was stronger and more uniform in preventing iNOS than eNOS upregulation. Glyceryl trinitrate treatment did not affect the levels of phosphorylation of eNOS during infection (Fig. 2D).
Fig 2.
Glyceryl trinitrate preventative treatment limits NOS expression. (A to C) Bar graphs from Western blot analyses of NOS isoforms expression as a ratio versus the total β-tubulin. A representative blot is shown above the graph. (D) Immunoblot and bar graph showing the ratio of phosphorylation at eNOS (S1176) specific site to total amount of respective NOS. The results are derived from one experiment evaluating 8 mice treated with glyceryl trinitrate, 10 naive mice, and 10 mice infected but not treated. *, P < 0.05; **, P < 0.001. GTN, glyceryl trinitrate; PbA, P. berghei ANKA.
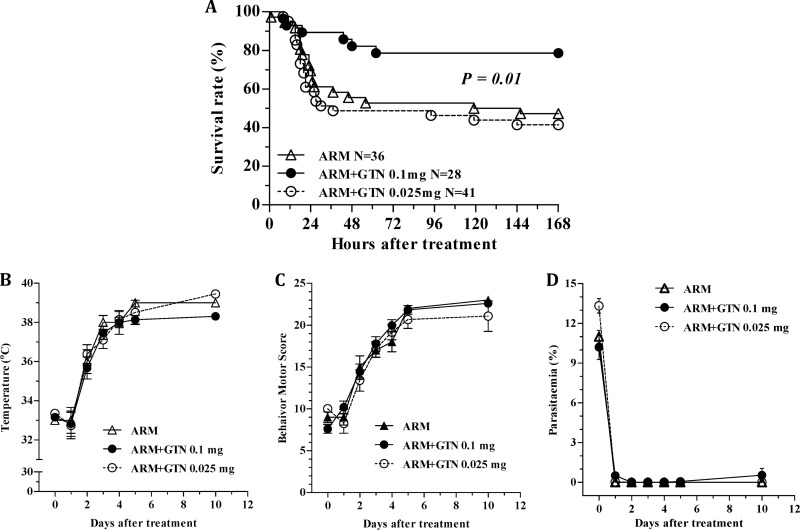
Adjunctive therapy with glyceryl trinitrate markedly increases the efficacy of artemether in rescuing mice from late-stage ECM.
Mice with ECM treated with artemether plus glyceryl trinitrate at 0.1 mg/h showed significantly increased survival rates compared to artemether monotherapy (79% versus 47%, respectively; P = 0.01) (Fig. 3A). This beneficial effect of glyceryl trinitrate on survival was not observed with the 0.025-mg/h dose. There were no significant differences in rectal temperature and motor behavior scores (Fig. 3B and C) among the different groups at the time of the first dose (time zero on day 6 of infection). There was no difference, as well in parasitemia between each glyceryl trinitrate-treated group and the artemether only treated group at the time of treatment, although the group of mice treated with artemether plus glyceryl trinitrate at 0.025 mg/h showed higher parasitemias compared to the artemether plus glyceryl trinitrate at 0.1 mg/h group (Fig. 3D). Glyceryl trinitrate treatment did not affect the recovery in body temperature and motor behavior scores, or the rate of parasite clearance after treatment, compared to the artemether-treated group (Fig. 3B to D).
Fig 3.
Efficacy of artemether plus glyceryl trinitrate rescuing mice from late stage ECM. (A to D) Cumulative survival of rescue treatments (A) and follow-up of survivors, including the restoration of temperature (B) and behavior motor score (C) and parasitemia clearance (D). All data are derived from eight individual experiments. The numbers of mice evaluated in each group (N) are shown in panel A. The data from panels B to D are expressed as means ± the SEM. *, P < 0.001. ARM, artemether; GTN, glyceryl trinitrate; PbA, P. berghei ANKA.
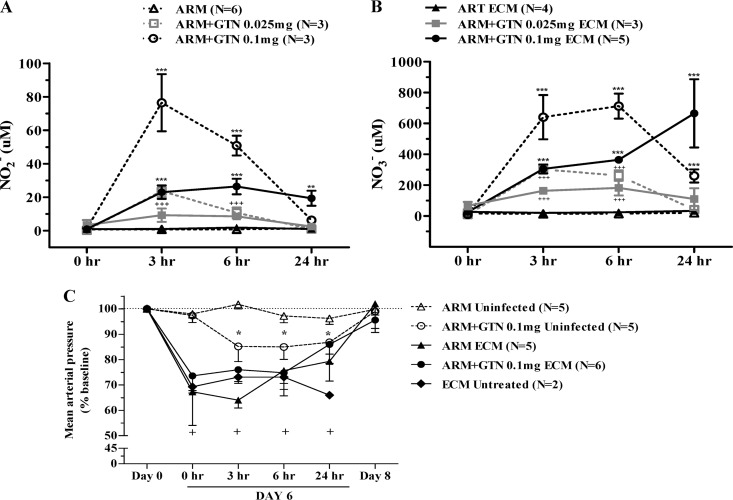
Plasma nitrite and nitrate levels are significantly increased after glyceryl trinitrate treatment.
Treatment with glyceryl trinitrate at 0.1 or 0.025 mg/h resulted in significant increases of both plasma nitrite and nitrate levels in both uninfected and ECM mice (Fig. 4A and B). Nitrite is the first oxidation product of NO and because it is rapidly oxidized to nitrate, its levels represent a reliable and real-time measure of NO production. Nitrate is the end product of NO oxidation and provides an estimate of its cumulative production. In uninfected mice, plasma nitrite levels peaked at 3 h and were still high at 6 h, but it was no longer detected at 24 h (Fig. 4A). Plasma nitrate levels followed a similar trend, but the peak was observed at 6 h and, despite a marked drop, it was still detected after 24 h (Fig. 4B), which is consistent with its cumulative nature. As expected, glyceryl trinitrate at a 0.1-mg/h dose induced higher levels of both nitrite and nitrate than did the 0.025-mg/h dose (∼4-fold higher nitrite levels overall). Interestingly, plasma nitrite levels in mice with ECM were much lower than in uninfected mice at the 3- and 6-h time points after glyceryl trinitrate patch administration but remained stable even at the 24-h time point with the 0.1-mg/h dose (Fig. 4A). Plasma nitrate levels, followed a similar trend, except for an increase at the 24-h time point with the 0.1-mg/h dose (Fig. 4B). Again, in mice with ECM glyceryl trinitrate at 0.1-mg/h dose induced higher and longer-lasting levels of both nitrite and nitrate than did the 0.025-mg/h dose. These findings indicate that release of glyceryl trinitrate is slower and longer lasting in mice with ECM.
Fig 4.
Effect of artemether plus glyceryl trinitrate treatment in plasma nitrite/nitrate levels and systemic blood pressure from uninfected and ECM mice. Uninfected mice, open symbols and dashed lines; PbA-infected mice with ECM, solid symbols and solid lines; artemether monotherapy, triangles; artemether plus glyceryl trinitrate at 0.025 mg/h, squares (gray color); artemether plus glyceryl trinitrate at 0.1 mg/h, circles. The number of mice (N) evaluated in each experiment is shown. (A and B) Kinetics changes of nitrite (A) and nitrate (B) in plasma samples collected before (0 h) and 3, 6, and 24 h after treatment. In the case of PbA-infected mice, treatment was performed as in previous experiments (mice shaved on day 4 of infection, artemether administration, and patch implantation when mice showed at least one clinical sign of ECM and rectal temperatures between 32 and 34°C); this time point is shown as “0 h” in figures. Values represent mean ± the SEM. Asterisks (PbA-infected) and crosses (uninfected) denote a significant increase in nitrite/nitrate levels at individual time points. (C) MAP was measured before infection (day 0), on day 6 of infection at the moment of the ECM manifestation as described above (“0 h”) and then 3, 6, 24, and 48 h after artemether with or without glyceryl trinitrate treatment (day 8). Asterisks represent a significant drop in MAP values from uninfected mice treated with artemether plus glyceryl trinitrate patch in relation to day 0 baseline. Crosses represent a significant drop in MAP values from PbA-infected mice with respect to day 0 baseline on day 6 of infection. The data are expressed as the mean of at least 10 MAP readings per mouse ± the SEM. Horizontal dotted line represents baseline. +, P < 0.05; ++, P < 0.01; +++ or ***, P < 0.001. ARM, artemether; GTN, glyceryl trinitrate; ECM, experimental cerebral malaria.
Glyceryl trinitrate lowers blood pressure of healthy mice but does not affect blood pressure of ECM mice.
Glyceryl trinitrate is recognized as a potent vasodilator affecting systemic blood pressure. The potential hypotensive effect of glyceryl trinitrate, especially at the high doses used in the present study, could be a major concern when considering its use in the clinical setting. Glyceryl trinitrate at 0.1 mg/h induced a 13% decrease in the MAP values 3 h after the patch implantation in control, uninfected mice (baseline, 116 ± 1.6 mm Hg); this relatively mild hypotension persisted during the 24 h of patch treatment, and the MAP returned to baseline after its removal (Fig. 4C). No changes in MAP were observed in uninfected mice receiving only artemether. PbA-infected mice with ECM signs were hypotensive, with MAP values already decreased by around 30%. Glyceryl trinitrate treatment caused no further deterioration in MAP, which remained relatively stable up to the 6-h mark examined and started to recover by 24 h, returning to preinfection baseline levels by 48 h (Fig. 4C). Conversely, PbA-infected but untreated mice remained hypotensive throughout the course of investigation and died within 24 h of ECM development.
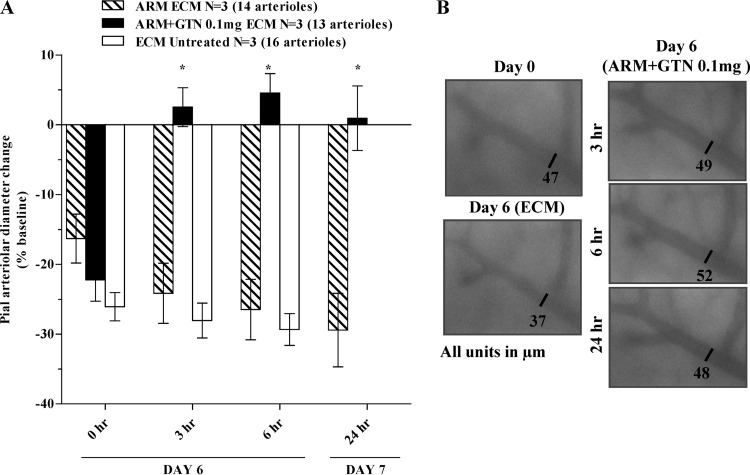
Glyceryl trinitrate dilates brain arterioles of mice with late-stage ECM.
Using intravital microscopy through a closed cranial window, the effects of artemether and artemether plus glyceryl trinitrate treatments on pial arteriolar diameters in day 6 PbA-infected mice were examined. All PbA-infected mice with ECM signs presented marked vasoconstriction of pial arterioles from baseline preinfection values (Fig. 5). Vessels in untreated mice remained constricted at 3 and 6 h after treatment (these mice succumbed to the disease before 24 h). Similarly, vessels from artemether-treated mice remained constricted throughout the course of investigation (time zero, −16%; 3 h, −24%; 6 h, −26%; and 24 h, −29%) relative to the preinfection baseline. There were no significant differences between the extent of vasoconstriction in untreated and artemether-treated mice. In contrast, mice receiving artemether plus glyceryl trinitrate at 0.1 mg/h showed reversal of vasoconstriction with vessel diameters returning to their preinfection levels after 3 h and were maintained in their recovered states at subsequent time points (Fig. 5).
Fig 5.
Beneficial effect of artemether plus glyceryl trinitrate treatment in pial arterioles from late-stage ECM mice. Changes in arteriolar diameter were recorded at day 0 and day 6 of infection before (time zero) and after dosing (3, 6, and 24 h). In panel A, groups are displayed as follows: untreated PbA-infected mice (□), PbA-infected mice treated with artemether (▧), and PbA-infected mice treated with artemether plus glyceryl trinitrate at 0.1 mg/h (■). In panel B, five sections of the same arteriole showing the effect of ECM and artemether plus glyceryl trinitrate treatment in arteriole diameters. The numbers of mice (N) and the arterioles evaluated in each group are shown. The data are expressed as means ± the SEM. *, P < 0.001. ARM, artemether; GTN, glyceryl trinitrate; ECM, experimental cerebral malaria.
DISCUSSION
Effective adjunctive therapies capable of increasing survival and decreasing incidence of sequelae in patients with cerebral malaria treated with artemisinin derivatives remain elusive (2). In the present study, we observed that glyceryl trinitrate increases the survival of mice with late-stage ECM when given in combination with artemether. The relevance of this experimental model for human CM has been heavily debated in the past years (16–21). The main criticism conveyed is that pRBC sequestration in brain vessels is the histopathological hallmark of human CM, accompanied by little inflammatory response, whereas ECM is associated with inflammation and leukocyte (rather than pRBC) sequestration in the brain (16). However, several aspects of inflammation have been demonstrated in human CM, and an inflamed endothelium has even been described as a condition for pRBC attachment and accumulation in the brain, with increased expression of major histocompatibility complex class II and ICAM-1 (the latter incriminated as the receptor for PfEMP1, and hence for pRBC, in the brain) (38, 39), the frequent presence though in low numbers of leukocytes (5), high cytokine (40) and chemokine (41) levels, and loss of endothelial protein C receptors (42), among other findings. Conversely, P. berghei pRBC accumulation in the brain has been demonstrated in a number of studies (43–46). As we have previously discussed (17), although the two syndromes show quantitative differences in the blood cell type sequestered (more pRBCs than leukocytes in human CM and the opposite in ECM), the cause of sequestration (endothelial inflammation with ICAM-1 upregulation) and its most obvious consequence (vascular obstruction of blood flow) are similar, resulting in hypoperfusion, ischemia, and hypoxia. The difference between the pathologies makes it evident that not every intervention would work in human CM compared to ECM. For instance, pRBCs bind to ICAM-1 through PfEMP-1, whereas leukocytes do it through LFA-1, and specific binding inhibitors aimed to detach adherent cells might not work in both cases. However, in both cases, restoration of vascular perfusion is expected to have a beneficial effect, and in such cases the ECM model can work as a viable and relevant surrogate for human CM. This is also the case for interventions that address mechanisms of pathogenesis and damage that have been shown to be shared by the two pathological entities (15, 40). This includes endothelial dysfunction, low NO bioavailability, and hypoperfusion-hypoxia (37). We emphasize, nevertheless, that findings in this model obviously cannot be directly translated to the human situation, this being dependent upon the performance of properly designed clinical studies.
The objective of the present study was to address whether interventions to reverse cerebral vasoconstriction and to improve cerebral blood flow would promote sustenance of life in moribund animals displaying ECM, allowing time for the full action of artemether and therefore increasing survival. We have previously shown that NO donors such as DPTA-NO and GSNO partially prevent ECM development and decrease vascular pathology, improving pial blood flow and attenuating vasoconstriction, inflammation, and hemorrhages (24, 25, 33). Glyceryl trinitrate showed similar effects and, as a drug for potential use in the clinical setting, it has a number of advantages over DPTA-NO and GSNO: (i) clinical data on glyceryl trinitrate are abundant, with a long history of efficacy and safety; (ii) several formulations are available, including transdermal patches that allow continuous delivery of sustained levels for prolonged periods of time; (iii) available formulations are stable for long periods at room temperature; and (iv) glyceryl trinitrate is inexpensive and easily accessible. Similarly to DPTA-NO and GSNO, glyceryl trinitrate-delivering patches largely protected against ECM development. Since glyceryl trinitrate showed some inhibitory effect on parasitemia at a critical time for ECM development (days 6 and 7 of infection), it is unclear whether the glyceryl trinitrate protective effect was solely secondary to this antiparasite effect or resulted from other beneficial actions on the host itself. The latter seems to be the case as glyceryl trinitrate treatment downregulated iNOS expression in the brains of PbA-infected mice, suggesting that it also displayed anti-inflammatory activity. iNOS upregulation is expected to occur in a highly inflammatory condition, such as ECM, and eNOS upregulation might occur as a tentative response to reverse the state of low NO bioavailability. Indeed, iNOS and eNOS upregulation has been shown to be triggered by an increase in oxidative stress under conditions of low NO bioavailability (47), similar to the conditions observed in ECM (37). Therefore, providing exogenous NO during PbA infection may prevent the very causes of iNOS and eNOS upregulation, decreasing inflammation and ameliorating endothelial function in ECM (22, 24, 25). Glyceryl trinitrate had a more uniform effect in preventing iNOS than eNOS upregulation, resulting in more variable expression of the latter. In addition, glyceryl trinitrate was unable to prevent S1176-P-eNOS downregulation. S1176-P-eNOS activation occurs mainly as a result of mechanical stimulation of endothelial cells by shear stress (48, 49), and decreased shear rates on the contrary promotes P-eNOS downregulation. In fact, we have recently shown that PbA infection leads to decreased microvascular wall shear rates due to decreased hematocrit and decreased RBC velocities (48). The fact that glyceryl trinitrate treatment did not prevent S1176-P-eNOS downregulation indicate that it did not prevent the PbA-induced decrease in shear rates, which is consistent with the fact that glyceryl trinitrate treatment did not prevent the PbA-induced decrease in hematocrit.
The most striking observation in the present study, however, was the demonstration of the adjunctive effect of glyceryl trinitrate in association with artemether in mice with ECM. A number of studies have recently been published describing adjunctive therapies in ECM (50–53). One disadvantage is that more often than not intervention is given early, before full development of the neurological syndrome occurs. We have previously developed a model system for testing the effect of antimalarial drugs in late-stage ECM (32). The advantage of this model is that treatment is given only after mice display clear signs of neurological derangement, therefore mimicking more closely the situations under which a CM patient will receive treatment. In mice, hypothermia precedes death by ECM (54, 55), and therefore body temperature is an objective, quantifiable parameter allowing precise staging of the disease. The relatively tight preset range for treatment (32 to 34°C) allowed better intergroup homogenization to improve comparison, reduce variability, and decrease the number of animals needed to achieve significance. At this stage, mice also generally show other signs of neurological involvement, low motor scores, and hypotension, denoting the advanced stage of the disease. Using this system, we show that glyceryl trinitrate at 0.1 mg/h, but not at 0.025 mg/h, increased the survival of mice with ECM from 47 to 79%. We have previously shown that ECM is associated with a vasospasm-like brain microcirculatory dysfunction (27). The vasodilatation effect in pial arterioles after glyceryl trinitrate administration has been well characterized in vivo, mainly in the study of migraine (56–58). Our data show that indeed the success of glyceryl trinitrate therapy in improving ECM survival was associated with a marked reversal of cerebral vasospasm. Conversely, artemether alone was unable to reverse vasospasm even 24 h after treatment, despite its dramatic effect in decreasing parasitemia (32). Although artemether did not decrease the number of vessels containing leukocytes after 24 h, it did decrease the number of leukocytes per vessel (32), indicating that vascular constriction is more resilient and harder to reverse than inflammation and perhaps vascular occlusion. The positive effects of glyceryl trinitrate in reversing vasospasm are in line with our previous findings that nimodipine, a calcium channel blocker used to prevent vasospasm in patients with subarachnoid hemorrhages, is also beneficial in late-stage ECM (27). Overall, these data support the concept that tackling vasospasm can be of great benefit in CM.
Most of the potentially severe side effects of glyceryl trinitrate overdose are related to its systemic vasodilator effect, which can cause hypotension and headaches (59, 60). We therefore used blood pressure as the indicator for glyceryl trinitrate in vivo activity and to assess severe side effects. The effective glyceryl trinitrate dose used was high, as 0.1 mg/h in a 20-g mouse is roughly equivalent to 80 μg/kg/min. Doses given to humans using glyceryl trinitrate patches usually do not exceed 0.8 mg/h in adults (28, 61), but trials with much higher doses (160 μg/min to 10 mg/h) have been performed (62). These doses are still 30 to 40 times lower than those used here, and yet the side effects associated with this high dose in the mouse seemed to be relatively mild. Indeed, the hypotensive effect of glyceryl trinitrate at 0.1 mg/h caused a drop of 13% in MAP during the 24 h of glyceryl trinitrate treatment in uninfected mice. This decrease in MAP was much lower than that observed with bolus intraperitoneal injection of DPTA-NO and GSNO in mice (22, 33) or with similar doses of glyceryl trinitrate intravenously infused in rabbits (63), which can cause acute drops in MAP of >40%. On the other hand, glyceryl trinitrate administration to mice with ECM, which already show hypotension, did not cause further decreases in blood pressure. There are a number of considerations to be made. First, ECM mice were hypothermic (32 to 34°C), and it has been shown that skin temperature changes can cause major short-term modifications in glyceryl trinitrate bioavailability (64). Indeed, the plasma levels of nitrite and nitrate, the surrogate markers for NO production, were at least 2- to 4-fold lower in mice with ECM compared to uninfected mice 3 to 6 h after patch implantation. The plasma nitrite levels achieved with the 0.025-mg/h dose were even lower and therefore, in this case, NO generation may have failed to reach the threshold needed to be effective in mice with ECM. Second, mice with ECM show high plasma levels of NO-scavenging cell-free hemoglobin (22). This means that a large fraction of the NO derived from the glyceryl trinitrate patches is diverted to react and be scavenged by cell-free hemoglobin instead of playing its expected effect on blood vessels. Third, it has been shown that the vasodilatory action of low-dose glyceryl trinitrate is partly due to NOS-dependent mechanisms (65). NOS (both eNOS and nNOS isoforms) are dysfunctional in mice with ECM (37); therefore, it is conceivable that the action of glyceryl trinitrate through this mechanism is impaired in mice with ECM, also explaining the need for higher doses. Therefore, decreased glyceryl trinitrate delivery due to hypothermia, increased NO consumption due to scavenging by plasma cell-free hemoglobin and decreased action due to NOS dysfunction may explain the need for higher doses of glyceryl trinitrate to rescue mice with ECM. Finally, the baseline vascular diameter upon which the glyceryl trinitrate-derived NO will exert its actions is markedly lower in mice with ECM than in uninfected animals due to vasoconstriction (27). This means that NO will be basically used to reverse vasoconstriction rather than to cause deleterious vasodilation in sick animals. Indeed, this conclusion is supported by Fig. 5, as well as by the absence of deleterious effects on blood pressure shown in Fig. 4C. In human CM, the required glyceryl trinitrate dose would likely be much lower than that needed in mice since hyperthermia rather than hypothermia is usually observed (4). On the other hand, high plasma levels of cell-free hemoglobin and endothelial dysfunction are also a feature of human severe malaria (11) and therefore doses higher than usually prescribed for angina would probably be necessary.
In conclusion, glyceryl trinitrate has been shown in the present study to be an effective adjunctive therapy for ECM in association with artemether, and this effect was associated with reversal of pial arteriolar vasospasm and also with limited side effects, as measured by changes in systemic blood pressure. Further studies to back the potential of glyceryl trinitrate as an adjunctive therapy for cerebral malaria are warranted.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the U.S. NIH grants R01-AI082610 (L.J.M.C.) and NIH MERIT R37HL040696 (J.A.F.).
We thank Dafra Pharma International (Turnhout, Belgium) for kindly providing the artemether (Artesiane) used in this study. We are also grateful to John Nolan from La Jolla Bioengineering Institute for allowing access to the flow cytometry facilities and to Haleigh Howard for animal care.
Footnotes
Published ahead of print 26 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00488-13.
REFERENCES
- 1.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. 2012. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379:413–431 [DOI] [PubMed] [Google Scholar]
- 2.John CC, Kutamba E, Mugarura K, Opoka RO. 2010. Adjunctive therapy for cerebral malaria and other severe forms of Plasmodium falciparum malaria. Expert Rev. Anti-Infect. Ther. 8:997–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shikani HJ, Freeman BD, Lisanti MP, Weiss LM, Tanowitz HB, Desruisseaux MS. 2012. Cerebral malaria: we have come a long way. Am. J. Pathol. 181:1484–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Seidlein L, Olaosebikan R, Hendriksen IC, Lee SJ, Adedoyin OT, Agbenyega T, Nguah SB, Bojang K, Deen JL, Evans J, Fanello CI, Gomes E, Pedro AJ, Kahabuka C, Karema C, Kivaya E, Maitland K, Mokuolu OA, Mtove G, Mwanga-Amumpaire J, Nadjm B, Nansumba M, Ngum WP, Onyamboko MA, Reyburn H, Sakulthaew T, Silamut K, Tshefu AK, Umulisa N, Gesase S, Day NP, White NJ, Dondorp AM. 2012. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin. Infect. Dis. 54:1080–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pongponratn E, Turner GD, Day NP, Phu NH, Simpson JA, Stepniewska K, Mai NT, Viriyavejakul P, Looareesuwan S, Hien TT, Ferguson DJ, White NJ. 2003. An ultrastructural study of the brain in fatal Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 69:345–359 [PubMed] [Google Scholar]
- 6.Ponsford MJ, Medana IM, Prapansilp P, Hien TT, Lee SJ, Dondorp AM, Esiri MM, Day NP, White NJ, Turner GD. 2012. Sequestration and microvascular congestion are associated with coma in human cerebral malaria. J. Infect. Dis. 205:663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark IA, Awburn MM, Whitten RO, Harper CG, Liomba NG, Molyneux ME, Taylor TE. 2003. Tissue distribution of migration inhibitory factor and inducible nitric oxide synthase in falciparum malaria and sepsis in African children. Malar. J. 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beare NA, Harding SP, Taylor TE, Lewallen S, Molyneux ME. 2009. Perfusion abnormalities in children with cerebral malaria and malarial retinopathy. J. Infect. Dis. 199:263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopansri BK, Anstey NM, Weinberg JB, Stoddard GJ, Hobbs MR, Levesque MC, Mwaikambo ED, Granger DL. 2003. Low plasma arginine concentrations in children with cerebral malaria and decreased nitric oxide production. Lancet 361:676–678 [DOI] [PubMed] [Google Scholar]
- 10.Yeo TW, Lampah DA, Tjitra E, Gitawati R, Kenangalem E, Piera K, Granger DL, Lopansri BK, Weinberg JB, Price RN, Duffull SB, Celermajer DS, Anstey NM. 2009. Relationship of cell-free hemoglobin to impaired endothelial nitric oxide bioavailability and perfusion in severe falciparum malaria. J. Infect. Dis. 200:1522–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeo TW, Lampah DA, Tjitra E, Gitawati R, Darcy CJ, Jones C, Kenangalem E, McNeil YR, Granger DL, Lopansri BK, Weinberg JB, Price RN, Duffull SB, Celermajer DS, Anstey NM. 2010. Increased asymmetric dimethylarginine in severe falciparum malaria: association with impaired nitric oxide bioavailability and fatal outcome. PLoS Pathog. 6:e1000868. 10.1371/journal.ppat.1000868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenisch C, Wenisch H, Wilairatana P, Looareesuwan S, Vannaphan S, Wagner O, Graninger W, Schonthal E, Rumpold H. 1996. Big endothelin in patients with complicated Plasmodium falciparum malaria. J. Infect. Dis. 173:1281–1284 [DOI] [PubMed] [Google Scholar]
- 13.Lovegrove FE, Tangpukdee N, Opoka RO, Lafferty EI, Rajwans N, Hawkes M, Krudsood S, Looareesuwan S, John CC, Liles WC, Kain KC. 2009. Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS One 4:e4912. 10.1371/journal.pone.0004912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polder TW, Jerusalem CR, Eling WM. 1991. Morphological characteristics of intracerebral arterioles in clinical (Plasmodium falciparum) and experimental (Plasmodium berghei) cerebral malaria. J. Neurol. Sci. 101:35–46 [DOI] [PubMed] [Google Scholar]
- 15.de Souza JB, Hafalla JC, Riley EM, Couper KN. 2010. Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology 137:755–772 [DOI] [PubMed] [Google Scholar]
- 16.White NJ, Turner GD, Medana IM, Dondorp AM, Day NP. 2010. The murine cerebral malaria phenomenon. Trends Parasitol. 26:11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalho LJ. 2010. Murine cerebral malaria: how far from human cerebral malaria? Trends Parasitol. 26:271–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt NH, Grau GE, Engwerda C, Barnum SR, van der Heyde H, Hansen DS, Schofield L, Golenser J. 2010. Murine cerebral malaria: the whole story. Trends Parasitol. 26:272–274 [DOI] [PubMed] [Google Scholar]
- 19.Stevenson MM, Gros P, Olivier M, Fortin A, Serghides L. 2010. Cerebral malaria: human versus mouse studies. Trends Parasitol. 26:274–275 [DOI] [PubMed] [Google Scholar]
- 20.Renia L, Gruner AC, Snounou G. 2010. Cerebral malaria: in praise of epistemes. Trends Parasitol. 26:275–277 [DOI] [PubMed] [Google Scholar]
- 21.Riley EM, Couper KN, Helmby H, Hafalla JC, de Souza JB, Langhorne J, Jarra WB, Zavala F. 2010. Neuropathogenesis of human and murine malaria. Trends Parasitol. 26:277–278 [DOI] [PubMed] [Google Scholar]
- 22.Gramaglia I, Sobolewski P, Meays D, Contreras R, Nolan JP, Frangos JA, Intaglietta M, van der Heyde HC. 2006. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat. Med. 12:1417–1422 [DOI] [PubMed] [Google Scholar]
- 23.Anstey NM, Weinberg JB, Hassanali MY, Mwaikambo ED, Manyenga D, Misukonis MA, Arnelle DR, Hollis D, McDonald MI, Granger DL. 1996. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J. Exp. Med. 184:557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabrales P, Zanini GM, Meays D, Frangos JA, Carvalho LJ. 2011. Nitric oxide protection against murine cerebral malaria is associated with improved cerebral microcirculatory physiology. J. Infect. Dis. 203:1454–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanini GM, Cabrales P, Barkho W, Frangos JA, Carvalho LJ. 2011. Exogenous nitric oxide decreases brain vascular inflammation, leakage, and venular resistance during Plasmodium berghei ANKA infection in mice. J. Neuroinflammation 8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serghides L, Kim H, Lu Z, Kain DC, Miller C, Francis RC, Liles WC, Zapol WM, Kain KC. 2011. Inhaled nitric oxide reduces endothelial activation and parasite accumulation in the brain, and enhances survival in experimental cerebral malaria. PLoS One 6:e27714. 10.1371/journal.pone.0027714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabrales P, Zanini GM, Meays D, Frangos JA, Carvalho LJ. 2010. Murine cerebral malaria is associated with a vasospasm-like microcirculatory dysfunction, and survival upon rescue treatment is markedly increased by nimodipine. Am. J. Pathol. 176:1306–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boden WE, Finn AV, Patel D, Peacock WF, Thadani U, Zimmerman FH. 2012. Nitrates as an integral part of optimal medical therapy and cardiac rehabilitation for stable angina: review of current concepts and therapeutics. Clin. Cardiol. 35:263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z, Zhang J, Stamler JS. 2002. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc. Natl. Acad. Sci. U. S. A. 99:8306–8311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Berrazueta JR, Sampedro I, Garcia-Unzueta MT, Llorca J, Bustamante M, Amado JA. 2003. Effect of transdermal nitroglycerin on inflammatory mediators in patients with peripheral atherosclerotic vascular disease. Am. Heart J. 146:E14. [DOI] [PubMed] [Google Scholar]
- 31.James MA, Walker PR, Papouchado M, Wilkinson PR. 1985. Efficacy of transdermal glyceryl trinitrate in the treatment of chronic stable angina pectoris. Br. Heart J. 53:631–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clemmer L, Martins YC, Zanini GM, Frangos JA, Carvalho LJ. 2011. Artemether and artesunate show the highest efficacies in rescuing mice with late-stage cerebral malaria and rapidly decrease leukocyte accumulation in the brain. Antimicrob. Agents Chemother. 55:1383–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zanini GM, Martins YC, Cabrales P, Frangos JA, Carvalho LJ. 2012. S-nitrosoglutathione prevents experimental cerebral malaria. J. Neuroimmune Pharmacol. 7:477–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng M, DiPetrillo K. 2009. Non-invasive blood pressure measurement in mice. Methods Mol. Biol. 573:45–55 [DOI] [PubMed] [Google Scholar]
- 35.Holtmaat A, Bonhoeffer T, Chow DK, Chuckowree J, De Paola V, Hofer SB, Hubener M, Keck T, Knott G, Lee WC, Mostany R, Mrsic-Flogel TD, Nedivi E, Portera-Cailliau C, Svoboda K, Trachtenberg JT, Wilbrecht L. 2009. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 4:1128–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cabrales P, Carvalho LJ. 2010. Intravital microscopy of the mouse brain microcirculation using a closed cranial window. J. Vis. Exp. 45:2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong PK, Melchior B, Martins YC, Hofer A, Orjuela-Sanchez P, Cabrales P, Zanini GM, Frangos JA, Carvalho LJ. 2013. Nitric oxide synthase dysfunction contributes to impaired cerebroarteriolar reactivity in experimental cerebral malaria. PLoS Pathog. 9:e1003444. 10.1371/journal.ppat.1003444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silamut K, Phu NH, Whitty C, Turner GD, Louwrier K, Mai NT, Simpson JA, Hien TT, White NJ. 1999. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am. J. Pathol. 155:395–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner GD, Morrison H, Jones M, Davis TM, Looareesuwan S, Buley ID, Gatter KC, Newbold CI, Pukritayakamee S, Nagachinta B, et al. 1994. An immunohistochemical study of the pathology of fatal malaria: evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am. J. Pathol. 145:1057–1069 [PMC free article] [PubMed] [Google Scholar]
- 40.van der Heyde HC, Nolan J, Combes V, Gramaglia I, Grau GE. 2006. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation, and hemostasis leading to microcirculatory dysfunction. Trends Parasitol. 22:503–508 [DOI] [PubMed] [Google Scholar]
- 41.Lucchi NW, Jain V, Wilson NO, Singh N, Udhayakumar V, Stiles JK. 2011. Potential serological biomarkers of cerebral malaria. Dis. Markers 31:327–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moxon CA, Wassmer SC, Milner DA, Jr, Chisala NV, Taylor TE, Seydel KB, Molyneux ME, Faragher B, Esmon CT, Downey C, Toh CH, Craig AG, Heyderman RS. 2013. Loss of endothelial protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria in African children. Blood 122:842–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amante FH, Stanley AC, Randall LM, Zhou Y, Haque A, McSweeney K, Waters AP, Janse CJ, Good MF, Hill GR, Engwerda CR. 2007. A role for natural regulatory T cells in the pathogenesis of experimental cerebral malaria. Am. J. Pathol. 171:548–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McQuillan JA, Mitchell AJ, Ho YF, Combes V, Ball HJ, Golenser J, Grau GE, Hunt NH. 2011. Coincident parasite and CD8 T cell sequestration is required for development of experimental cerebral malaria. Int. J. Parasitol. 41:155–163 [DOI] [PubMed] [Google Scholar]
- 45.Baptista FG, Pamplona A, Pena AC, Mota MM, Pied S, Vigario AM. 2010. Accumulation of Plasmodium berghei-infected red blood cells in the brain is crucial for the development of cerebral malaria in mice. Infect. Immun. 78:4033–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Claser C, Malleret B, Gun SY, Wong AY, Chang ZW, Teo P, See PC, Howland SW, Ginhoux F, Renia L. 2011. CD8+ T cells and IFN-gamma mediate the time-dependent accumulation of infected red blood cells in deep organs during experimental cerebral malaria. PLoS One 6:e18720. 10.1371/journal.pone.0018720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhen J, Lu H, Wang XQ, Vaziri ND, Zhou XJ. 2008. Upregulation of endothelial and inducible nitric oxide synthase expression by reactive oxygen species. Am. J. Hypertens. 21:28–34 [DOI] [PubMed] [Google Scholar]
- 48.Fisslthaler B, Dimmeler S, Hermann C, Busse R, Fleming I. 2000. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol. Scand. 168:81–88 [DOI] [PubMed] [Google Scholar]
- 49.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. 1999. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399:601–605 [DOI] [PubMed] [Google Scholar]
- 50.Bienvenu AL, Ferrandiz J, Kaiser K, Latour C, Picot S. 2008. Artesunate-erythropoietin combination for murine cerebral malaria treatment. Acta Trop. 106:104–108 [DOI] [PubMed] [Google Scholar]
- 51.Pena AC, Penacho N, Mancio-Silva L, Neres R, Seixas JD, Fernandes AC, Romao CC, Mota MM, Bernardes GJ, Pamplona A. 2012. A novel carbon monoxide-releasing molecule fully protects mice from severe malaria. Antimicrob. Agents Chemother. 56:1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Achtman AH, Pilat S, Law CW, Lynn DJ, Janot L, Mayer ML, Ma S, Kindrachuk J, Finlay BB, Brinkman FS, Smyth GK, Hancock RE, Schofield L. 2012. Effective adjunctive therapy by an innate defense regulatory peptide in a preclinical model of severe malaria. Sci. Transl. Med. 4:135ra164. [DOI] [PubMed] [Google Scholar]
- 53.Reis PA, Comim CM, Hermani F, Silva B, Barichello T, Portella AC, Gomes FC, Sab IM, Frutuoso VS, Oliveira MF, Bozza PT, Bozza FA, Dal-Pizzol F, Zimmerman GA, Quevedo J, Castro-Faria-Neto HC. 2010. Cognitive dysfunction is sustained after rescue therapy in experimental cerebral malaria, and is reduced by additive antioxidant therapy. PLoS Pathog. 6:e1000963. 10.1371/journal.ppat.1000963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grau GE, Pointaire P, Piguet PF, Vesin C, Rosen H, Stamenkovic I, Takei F, Vassalli P. 1991. Late administration of monoclonal antibody to leukocyte function-antigen 1 abrogates incipient murine cerebral malaria. Eur. J. Immunol. 21:2265–2267 [DOI] [PubMed] [Google Scholar]
- 55.Piguet PF, Kan CD, Vesin C. 2002. Role of the tumor necrosis factor receptor 2 (TNFR2) in cerebral malaria in mice. Lab. Invest. 82:1155–1166 [DOI] [PubMed] [Google Scholar]
- 56.Wei EP, Moskowitz MA, Boccalini P, Kontos HA. 1992. Calcitonin gene-related peptide mediates nitroglycerin and sodium nitroprusside-induced vasodilation in feline cerebral arterioles. Circ. Res. 70:1313–1319 [DOI] [PubMed] [Google Scholar]
- 57.Read SJ, Manning P, McNeil CJ, Hunter AJ, Parsons AA. 1999. Effects of sumatriptan on nitric oxide and superoxide balance during glyceryl trinitrate infusion in the rat. Implications for antimigraine mechanisms. Brain Res. 847:1–8 [DOI] [PubMed] [Google Scholar]
- 58.Read SJ, Smith MI, Hunter AJ, Parsons AA. 1997. Enhanced nitric oxide release during cortical spreading depression following infusion of glyceryl trinitrate in the anaesthetized cat. Cephalalgia 17:159–165 [DOI] [PubMed] [Google Scholar]
- 59.Bagdy G, Riba P, Kecskemeti V, Chase D, Juhasz G. 2010. Headache-type adverse effects of NO donors: vasodilation and beyond. Br. J. Pharmacol. 160:20–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tfelt-Hansen PC, Tfelt-Hansen J. 2009. Nitroglycerin headache and nitroglycerin-induced primary headaches from 1846 and onwards: a historical overview and an update. Headache 49:445–456 [DOI] [PubMed] [Google Scholar]
- 61.Uxa A, Thomas GR, Gori T, Parker JD. 2010. Standard versus low-dose transdermal nitroglycerin: differential effects on the development of tolerance and abnormalities of endothelial function. J. Cardiovasc. Pharmacol. 56:354–359 [DOI] [PubMed] [Google Scholar]
- 62.Elkayam U, Bitar F, Akhter MW, Khan S, Patrus S, Derakhshani M. 2004. Intravenous nitroglycerin in the treatment of decompensated heart failure: potential benefits and limitations. J. Cardiovasc. Pharmacol. Ther. 9:227–241 [DOI] [PubMed] [Google Scholar]
- 63.Agvald P, Hammar L, Gustafsson LE. 2005. Nitroglycerin-patch induced tolerance is associated with reduced ability of nitroglycerin to increase exhaled nitric oxide. Vascul. Pharmacol. 43:449–457 [DOI] [PubMed] [Google Scholar]
- 64.Klemsdal TO, Gjesdal K, Bredesen JE. 1992. Heating and cooling of the nitroglycerin patch application area modify the plasma level of nitroglycerin. Eur. J. Clin. Pharmacol. 43:625–628 [DOI] [PubMed] [Google Scholar]
- 65.Bonini MG, Stadler K, Silva SO, Corbett J, Dore M, Petranka J, Fernandes DC, Tanaka LY, Duma D, Laurindo FR, Mason RP. 2008. Constitutive nitric oxide synthase activation is a significant route for nitroglycerin-mediated vasodilation. Proc. Natl. Acad. Sci. U. S. A. 105:8569–8574 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.