Abstract
Nucleic acid polymers (NAPs) are novel, broad-spectrum antiviral compounds that use the sequence-independent properties of phosphorothioate oligonucleotides (PS-ONs) as amphipathic polymers to block amphipathic interactions involved in viral entry. Using the duck hepatitis B virus (DHBV) model of human hepatitis B virus infection, NAPs have been shown to have both entry and postentry antiviral activity against DHBV infection in vitro in primary duck hepatocytes (PDH). In the current study, various NAPs were assessed for their prophylactic activity in vivo against DHBV infection in ducks. The degenerate NAP REP 2006 prevented the development of widespread and persistent DHBV infection in 14-day-old ducks, while the acidic-pH-sensitive NAP REP 2031 had little or no prophylactic effect. REP 2006 displayed significant toxicity in ducks, which was attributed to CpG-mediated proinflammation, while REP 2031 (which has no CpG motifs) displayed no toxicity. A third NAP, REP 2055, which was designed to retain amphipathic activity at acidic pH and contained no CpG motifs, was well tolerated and displayed prophylactic activity against DHBV infection at doses as low as 1 mg/kg of body weight/day. These studies suggest that NAPs can be easily and predictably tailored to retain anti-DHBV activity and to have minimal toxic effects in vivo. Future studies are planned to establish the therapeutic efficacy of NAPs against persistent DHBV infection.
INTRODUCTION
Duck hepatitis B virus (DHBV) in its natural host, the Pekin duck (Anas domesticus platyrhynchos), has been used as an animal model in preclinical studies of antiviral drugs designed for the treatment of chronic hepatitis B virus (HBV) infection (1). Extensive information about viral replication and hepadnavirus infection outcomes has also been identified from studies of the DHBV model (1, 2). Moreover, experimental manipulation of infection outcome has been intensively studied in the DHBV model by adjusting the infection dose and age of the ducks so that the conditions required for the establishment of persistent DHBV infection are well known (3–5). The DHBV model has also been used for immunotherapeutic and antiviral studies, in particular using entecavir (ETV), a guanosine analog, now approved for the treatment of chronic HBV infection (CHB), used alone (5) or in combination with different forms of DNA and recombinant vaccines (6–8). Additionally, the activity of other nucleoside analogs such as lamivudine, penciclovir, and adefovir has also been validated in the DHBV model (9–11). These studies have established DHBV infection of ducks as a platform for examining the potential in vivo efficacy of antiviral agents for human HBV infection.
Nucleic acid polymers (NAPs) utilize the sequence-independent properties of phosphorothioated oligonucleotides (PS-ONs) to interact in a length-dependent and sequence-independent fashion with amphipathic alpha-helical protein domains in a variety of infectious agents. For example, NAPs have been shown to interact with prion proteins (12) and type 1 viral fusion glycoproteins in both human immunodeficiency virus type 1 (HIV-1) and lymphocytic choriomeningitis virus (LCMV) (13, 14). In the case of viral entry, NAP-protein interactions destroy the ability of amphipathic protein domains present in viral fusion glycoproteins to interact with each other, a process known to be important in catalyzing the entry of many enveloped viruses into their host cells (15). In HIV-1 and LCMV, the interaction of NAPs with viral fusion proteins is consistent with their ability to block viral entry, and they have little or no postentry antiviral activity (13, 14). Analogous amphipathic interactions may underlie the ability of NAPs to block entry of herpes simplex virus 2 (HSV-2), cytomegalovirus (CMV), and hepatitis C virus (HCV) (16–18).
Previous work with DHBV-infected primary duck hepatocytes (PDH) reported in our accompanying article (19) have shown that NAPs are able to block the initiation of DHBV infection in the same sequence-independent but polymer length- and phosphorothioation-dependent manner as for other enveloped viruses, suggesting the involvement of analogous amphipathic interactions. However, unlike the previously reported antiviral activity of NAPs against other viruses, NAPs also possessed postentry activity against DHBV when added 12 h after infection. This postentry activity appeared to occur in an acidic environment and inhibited DHBV infection and the accumulation of DHBV surface antigen (DHBsAg). The underlying mechanism has not yet been determined.
NAPs are long (typically 40 nucleotides [nt] in length) PS-ONs, and this class of compounds are known to concentrate (up to 40% of total dose) in the liver in mammals (20, 21), where the bulk of HBV and DHBV replication occurs. Moreover, PS-ONs are known to be readily and efficiently taken up in human cells with intracellular concentrations much higher than extracellular concentrations (22–24). In our own work, NAPs were readily taken up by PDH without the use of any transfection reagent (19). These properties of NAPs suggest that they may be well suited as anti-HBV or anti-DHBV compounds. Consistent with the known accumulation of PS-ONs in the liver in mammalian species, NAPs have been shown to be active in vivo against other viral infections with tropism in the liver: CMV, HCV, and LCMV (17, 18; A. Vaillant, unpublished observation). The current study was designed to determine if the antiviral properties of NAPs that prevent DHBV infection of PDH in vitro could provide effective protection of the liver of ducks from DHBV infection in vivo. Studies were conducted with the prototypical degenerate NAP REP 2006 and REP 2031, a NAP whose amphipathic activity is neutralized at acidic pH by the formation of tetramers (25–27), and the heteropolymeric NAP REP 2055, which was designed to combine the acid-insensitive amphipathic activity of REP 2006 with the well-tolerated nature of REP 2031. REP 2055 was used at a range of concentrations to determine the minimum effective daily dose.
MATERIALS AND METHODS
Synthesis and formulation of NAPs.
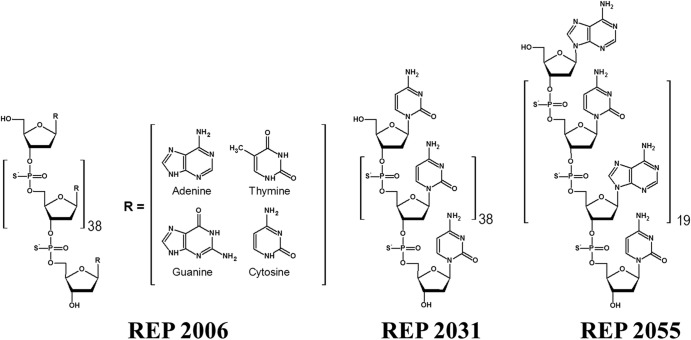
All NAPs (Fig. 1) were prepared using standard solid-phase reaction conditions for the preparation of PS-ONs. In the case of the degenerate NAP REP 2006, equal concentrations of adenosine (A), thymidine (T), guanosine (G), and cytidine (C) amidites were mixed together and used in each coupling reaction. This technique has been validated by liquid chromatography-mass spectrometry (LC-MS) to result in pools of oligonucleotides with the same length and chemistry but with a completely degenerate sequence identity, such that no sequence-dependent functionality of any kind is present (14). REP 2031 has the sequence poly(C), and REP 2055 has alternating A and C bases. All NAPs were 40 nt in length. The identity of all NAPs (as well as the degenerate nature of the NAP REP 2006) was confirmed by LC-MS. NAPs were prepared as sodium salts by salt exchange in 3 M NaCl overnight at room temperature followed by desalting by ultrahigh-pressure filtration with water for injection. NAPs were prepared as 10-mg/ml solutions in normal saline (NS) and filter sterilized prior to administration by intraperitoneal (i.p.) injection of ducks with volumes varying according to body weight and desired dose.
Fig 1.
Structures of NAPs used in this study. REP 2006 is degenerate, while REP 2031 [poly(C)] and REP 2055 [poly(AC)] have defined nucleic acid sequences. All NAPs are 40 nt in length.
Animal handling.
Pekin Aylesbury ducks (Anas platyrhynchos) obtained at day 1 posthatch from a commercial poultry supplier were used in all experiments. Ducks were held at the animal house facilities in SA Pathology (formerly the Institute of Medical and Veterinary Science [IMVS]). Animal handling protocols and standard operating procedures were approved by the Animal Ethics Committees (AEC) of the SA Pathology/Central Health Network and the University of Adelaide.
Testing the antiviral efficacy of REP 2055 in vitro.
Prior to testing REP 2055 in vivo, its antiviral activity was determined against DHBV infection of PDH. Both DHBV-positive serum and PDH were pretreated with REP 2055 for 1 h at 37°C prior to DHBV infection with 250 virus genome equivalents of DHBV per cell. DHBV was present in a pool of infected duck sera that contained 5 × 109 virus genome equivalents of DHBV per ml, as assessed by quantitative PCR (qPCR) (3). Fresh REP 2055 was added every second day during each medium change at concentrations of 0.01 to 10 μM. Antiviral activity was assessed by assaying for DHBsAg accumulation in PDH using confocal immunofluorescence microscopy, as previously described (19).
Duck infection paradigm.
Fourteen-day-old ducks were assembled into treatment groups consisting of 5 ducks and infected intravenously (i.v.) with 5 × 108 virus genome equivalents of DHBV (3), a dose known from previous experiments (5–8, 28, 29) to result in the development of persistent DHBV infection. Ducks in all experimental groups received once-daily (QD) treatment with REP 2006, REP 2031, or REP 2055 (10 mg/kg of body weight i.p.) or twice-daily (BID) treatment with REP 2055 (0.5 to 5 mg/kg i.p.). Control groups received NS via i.p. injection. In all groups, dosing was started from 1 to 3 days prior to infection as indicated until day 14 p.i. The i.p. injection was given just below the tip of the sternum, which permits an easy access to the peritoneal cavity. Due to toxicity, treatment with REP 2006 was reduced to every other day beginning at day 4 p.i.
Detection of DHBsAg by ELISA.
Serum samples collected on days 0, 5, 10, and 14 p.i. were tested to determine levels of DHBsAg by enzyme-linked immunosorbent assay (ELISA), as previously described (30). In brief, 96-well microtiter plates (Costar 3590; Corning Incorporated, USA) were coated in duplicate with 100 μl of a 1/100 dilution of test serum samples, high-titer DHBsAg-positive controls, and normal duck serum (NDS) negative controls; the plates were then wrapped in plastic film and incubated at 37°C overnight. Bound DHBsAg was detected with a 1/5,000 dilution of primary anti-DHBV preS monoclonal antibodies (31), followed by a 1/4,000 dilution horseradish peroxidase (HRP)-conjugated sheep anti-mouse polyclonal antibodies (GE Healthcare UK Limited, United Kingdom), with visualization of bound HRP using an o-phenylenediamine dihydrochloride (OPD) substrate (FastTM kit, Sigma-Aldrich, Germany). Optical density (OD) values were read at 490 nm.
Immunostaining to detect DHBsAg-positive hepatocytes.
Sections of wax-embedded, ethanol-acetic acid (EAA)-fixed biopsy and autopsy liver tissues, collected on days 4 and 14 p.i. by a standard protocol (8), were used for immunostaining to identify DHBsAg-positive hepatocytes as previously described (30). In brief, sections were blocked with 1/30 normal sheep serum (NSS) and then incubated with a 1/500 dilution of primary anti-DHBV pre-S monoclonal antibodies (31) followed by secondary HRP-conjugated goat anti-mouse polyclonal antibodies (GE Healthcare Limited, United Kingdom). Bound HRP was visualized with 0.05% diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich) before the sections were counterstained with hematoxylin. DHBsAg-positive hepatocytes were counted using an eyepiece graticule and a 250-μm by 250-μm grid. The minimum sensitivity of detection of DHBsAg-positive hepatocytes was ∼0.001% after screening ∼100,000 hepatocytes per liver section. Sections of formalin-fixed biopsy and autopsy tissues were stained by hematoxylin and eosin and assessed for liver histology.
DNA extraction and Southern blot hybridization.
Total cellular and viral DNA was extracted from ∼25-mg biopsy and autopsy liver samples using a Qiagen DNeasy kit. The amount of DNA in each sample was then determined using an ND-1000 spectrophotometer (Nanodrop, USA). Two micrograms of each DNA sample was electrophoresed in 1% agarose, and 10- and 100-pg aliquots of DHBV plasmid pBL 4.8 × 2(32), digested with PvuI and EcoRI, were run as size markers, yielding bands of 3,027, 1,708, and 1,044 bp. Following electrophoresis, DNAs were transferred to a nitrocellulose membrane, using a Southern blot hybridization procedure (33), and hybridized using a genome-length, 32P-DHBV DNA probe labeled by random primer (Roche Molecular Diagnostics catalogue no. 1585606). Hybridization was detected by exposure of the nitrocellulose membrane to X-ray film.
Drug safety and efficacy monitoring.
Ducks were assessed every day for abnormalities in feed and water intake, weight changes, gait, and behavior. Red blood cell (RBC) and white blood cell (WBC) counts were performed on a Sysmex Cooperation 2000 instrument, model XE2100, and serum samples from NAP- and NS-treated ducks were analyzed for levels of the liver enzymes gamma glutamine transferase (GGT), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) at the Diagnostic Clinical Pathology Unit at SA Pathology.
Statistical analysis.
Differences in in vivo mean body weights, RBC, WBC counts, liver enzyme levels, and percentages of DHBsAg-positive hepatocytes in liver tissues among different treatment groups were analyzed using multiple analysis of variance (ANOVA) followed by post hoc analysis. The two treatment group data and in vitro data were analyzed using Student's t test. Differences were considered to be statistically significant when the P values were <0.05.
RESULTS
Tolerability of REP 2006 and REP 2031 treatment in DHBV-infected ducks.
Ducks that were initially treated with 10 mg/kg/day of REP 2006 had reduced weight gain compared with ducks treated with REP 2031 or NS (P < 0.05) (Table 1). Moreover, ducks initially treated with 10 mg/kg/day REP 2006 showed signs of abdominal pain on i.p. injection and abdominal tenderness with palpation after i.p. injection, which was not present in ducks treated with either REP 2031 or NS. At the day 4 p.i. biopsy, the REP 2006-treated ducks bled more at the surgical incision site than ducks treated with REP 2031 or NS; however, this did not lead to any postsurgical mortality. Examination of liver histology at day 4 p.i. showed moderate levels of hepatocyte apoptosis, variability in staining of the cytoplasm of hepatocytes, and some disruption of liver architecture in the REP 2006-treated ducks compared to ducks treated with REP 2031 or NS, in which liver histology was normal. Following the biopsy on day 4 p.i., REP 2006 treatment was reduced to every other day from day 4 to day 14 p.i. In situ examination of internal organs at autopsy on day 14 p.i. showed splenomegaly, some ascites, and a white coating on the liver in the REP 2006-treated ducks. Liver histology showed low levels of hepatocyte apoptosis and some bile duct proliferation, which was absent from the livers of ducks treated with REP 2031 or NS. A moderate decrease in RBC count and a moderate rise in total WBC count were observed at day 14 p.i. in ducks treated with REP 2006 compared to ducks in the other two groups (Table 2). Although there were no statistically significant differences in levels of the liver enzymes GGT, ALT, and AST in ducks treated with REP 2006 (P > 0.05) compared with those of ducks treated with REP 2031 or NS (Table 2), the observed changes in liver histology and increased levels of hepatocyte apoptosis and bile duct proliferation suggested that daily dosing with REP 2006 was hepatotoxic.
Table 1.
Body weights of ducks treated with REP 2006, REP 2031, and NSa
| Treatmentb | Body wt (g) during growth (mean ± SD, n = 5) at indicated day posthatching |
||||
|---|---|---|---|---|---|
| 14 | 17 | 20 | 23 | 27 | |
| REP 2006c | 396 ± 7.4 | 432.2 ± 33.6 | 552.4 ± 58.2 | 758.6 ± 174.2 | 903.8 ± 200 |
| REP 2031d | 411 ± 11.4 | 493.8 ± 25.6 | 635.6 ± 54.2 | 868.2 ± 89.2 | 1,141.6 ± 152.6 |
| NS | 406 ± 11.4 | 534.2 ± 32.1 | 725.6 ± 66 | 922.2 ± 97.2 | 1,185 ± 153 |
NS, normal saline.
Once-daily (QD) treatment (10 mg/g/day) via the i.p. route from day −1 to day 14 p.i.
Differences in mean body weight versus NS-treated ducks were statistically significant (P < 0.05).
Differences in mean body weight versus NS-treated ducks were not statistically significant (P > 0.05).
Table 2.
Red and white blood cell counts and liver enzymes on day 14 p.i. in ducks treated with REP 2006, REP 2031, and NSa
| Treatmentc | Hematologyb |
Liver enzymes (U/liter)b,g |
|||
|---|---|---|---|---|---|
| RBC (1012/liter) | WBC (109/liter) | GGT | ALT | AST | |
| REP 2006 | 1.25 ± 0.6d,f | 23.59 ± 4.6d,f | 5 ± 2d | 28.6 ± 2.1d | 38.3 ± 33.3d |
| REP 2031 | 2.28 ± 0.2d | 19.95 ± 6.4d | 5.2 ± 1.8 | 43.6 ± 6.5 | 45.2 ± 39.8 |
| NS | 2.5 ± 0.2 | 16.63 ± 0.9e | 5 ± 0 | 39.4 ± 9.8 | 32.4 ± 19.3 |
NS, normal saline.
Values are means ± standard deviations (SD); n = 5 except when indicated otherwise.
Once-daily (QD) treatment (10 mg/kg/day) via the i.p. route from day −1 to day 14 p.i.
n = 3 (poor sample quality prevented analysis in two samples).
n = 4 (poor sample quality prevented analysis in one sample).
Differences versus NS-treated ducks were statistically significant (P < 0.05).
Differences versus NS-treated ducks were not statistically significant (P > 0.05).
Prophylactic efficacy of REP 2006 and REP 2031 in DHBV-infected ducks.
Treatment with REP 2006 prevented the development of detectable levels of serum DHBsAg in all ducks (Fig. 2). Results with REP 2031 and NS were more variable, and DHBsAg was not detected by ELISA in several of the ducks in these two groups, at any time point.
Fig 2.
Detection of DHBsAg by ELISA in the sera of ducks treated with REP 2006 (A), REP 2031 (B), and NS (C). Fourteen-day-old ducks were inoculated (i.v.) with 5 × 108 DHBV DNA genomes and treated with REP 2006 or REP 2031 or normal saline (NS) from day −1 to day 14 p.i., as follows: 10 mg/kg of REP 2006 i.p. once daily (QD) (A); 10 mg/kg of REP 2031 i.p. QD (B); NS i.p. QD (C). Serum samples were collected on days 1, 5, 10, and 14 p.i. and were diluted 1/100 for analysis. The normal duck serum (NDS) OD value was used as the cutoff point for the assay. Individual duck identifiers are indicated.
More-uniform results were observed upon analysis for DHBsAg-positive hepatocytes. In summary, with REP 2006 treatment, DHBsAg-positive hepatocytes on day 4 p.i. were at the lower limit of detection (∼0.005 to 0.008%) and, by day 14 p.i., were no longer detected in any of the ducks (<0.001%) (Table 3). In contrast, REP 2031 treatment slightly reduced the percentage of DHBsAg-positive hepatocytes on day 4 p.i. compared to the NS-treated ducks, but by day 14 p.i., DHBV infection had spread and >95% of hepatocytes were DHBsAg positive in both groups. Similar results were obtained when liver samples were assayed by Southern blot hybridization for accumulation of DHBV DNA replication intermediates. No DHBV DNA replication intermediates were detected at either time point in REP 2006-treated ducks, whereas high levels were detected by day 14 p.i. in both the REP 2031- and NS-treated ducks (Fig. 3).
Table 3.
Effect of treatment of ducks with REP 2006, REP 2031, and NSa on the percentage DHBsAg-positive hepatocytes in the liver on days 4 and 14 p.i.
| Treatmentc | % DHBsAg-positive hepatocytesb (mean ± SD, n = 5) |
|
|---|---|---|
| Day 4 p.i. | Day 14 p.i. | |
| REP 2006 | 0.006 ± 0.001d | <0.001 ± 0d |
| REP 2031 | 0.209 ± 0.16d | >95 ± 0 |
| NS | 1.537 ± 0.96e | >95 ± 0 |
NS, normal saline.
Lower limit of detection of DHBsAg-positive hepatocytes is 0.001%.
Once-daily (QD) treatment (10 mg/kg/day) via the i.p. route from day −1 to 14 p.i.
Differences in mean percentages of DHBsAg-positive hepatocytes versus NS-treated ducks were statistically significant (P < 0.05).
n = 4 (liver tissue not collected from one duck).
Fig 3.
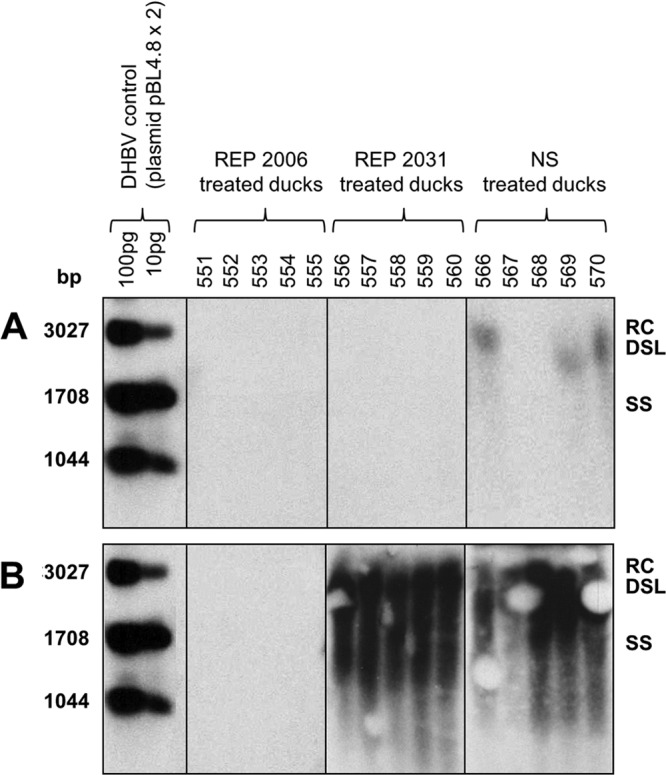
DHBV DNA levels in the liver of ducks on days 4 (A) and 14 (B) p.i. Fourteen-day-old ducks were inoculated (i.v.) with 5 × 108 DHBV genomes and treated with REP 2006 (10 mg/kg i.p. once daily [QD]) or REP 2031 (10 mg/kg i.p. QD) or normal saline (NS) (i.p. QD). Cellular and viral DNA extracts were tested for DHBV DNA by Southern blot hybridization as described in Materials and Methods. Expected positions of relaxed circular (RC), double-stranded linear (DSL), and single-stranded (SS) DHBV DNA are shown on the right. Individual duck identifiers are indicated above each lane. Note that the RC and DSL forms of DHBV DNA are occluded in some lanes in panel B due to a blotting artifact.
Previous in vitro results (19) showed that REP 2031 was able to inhibit initiation of DHBV infection, but once cells were infected, treatment with REP 2031 (from 12 h after infection) was ineffective in inhibiting subsequent DHBsAg accumulation in hepatocytes. In contrast, REP 2006 acted at both steps during DHBV infection of PDH. We suspect that the difference in activity between these two NAPs is that the amphipathic function of REP 2031 is inactive at acidic pH while that of REP 2006 is not (25–27). This difference is, at a practical level, correlated with an almost complete loss of prophylactic efficacy of REP 2031 in vivo. However, an alternative explanation of the difference between the two NAPs in vivo might lie in the fact that REP 2006 is known to have moderate proinflammatory activity, due to its degenerate nature harboring CpG motifs, while REP 2031 does not (17, 34). To test this possibility, a third NAP, REP 2055, was devised. Like REP 2031, REP 2055 is also devoid of CpG motifs, but its amphipathic activity is not neutralized at acidic pH. REP 2055 was made by “doping” the polycytidine [poly(C)] sequence of REP 2031 with adenosines at every other base to produce poly(AC) and to prevent loss of the amphipathic character at acidic pH. REP 2055 was shown to have antiviral effect in vitro comparable to that of REP 2006 and REP 2031 when added during DHBV infection of PDH (Table 4).
Table 4.
Antiviral effect of REP 2055 in comparison with REP 2006 and REP 2031 in DHBV-infected PDH in vitro
| NAPa | % of DHBsAg-positive PDHb (mean ± SD, n = 4) |
||||
|---|---|---|---|---|---|
| Concn of NAP (μM) | |||||
| 10 | 1 | 0.1 | 0.01 | Untreated | |
| REP 2006 | 2.67 ± 1.73c | 2.67 ± 1.73c | 1.33 ± 0.75c | 8.00 ± 0.58 | 7.56 ± 1.29 |
| REP 2031 | 0 ± 0c | 2.22 ± 1.25c | 3.11 ± 1.75c | 16.89 ± 2.65 | 13.78 ± 2.06 |
| REP 2055 | 0 ± 0c | 1.78 ± 1.15c | 7.56 ± 2.36 | 2.67 ± 1.29c | 7.11 ± 1.63 |
PDH were infected with 250 virus genome equivalents of DHBV per cell. NAPs were added during DHBV infection and every second day at medium change.
PDH were fixed with EAA on day 7 after DHBV infection and analyzed by confocal immunofluorescence microscopy for DHBsAg. A value of 0 means that there were no detectable DHBsAg-positive hepatocytes.
Statistically significant reduction in the percentage of DHBsAg-positive PDH compared to untreated control PDH (P < 0.05).
Tolerability of REP 2055 treatment in DHBV-infected ducks.
REP 2055 treatment in ducks at 10 mg/kg/day from day −1 to day 14 p.i. did not produce any observable changes in duck health or mean body weight (Table 5), and ducks treated with REP 2055 showed neither abdominal tenderness nor abdominal pain on i.p. injection, and no abnormalities were noted during clinical examination. Furthermore, in situ examination of internal organs at autopsy did not reveal any pathological changes, and no significant differences were noted in the mean total WBC count (P > 0.05) or in liver enzymes (GGT, ALT, AST) in ducks treated with REP 2055 compared to NS-treated ducks (Table 6); these values were similar to those observed in normal, untreated, and uninfected ducks (6).
Table 5.
Body weights of ducks treated with REP 2055 and NSa
| Treatmentb | Body wt (g) (mean ± SD, n = 5) at indicated day posthatching |
||||
|---|---|---|---|---|---|
| Day 14 | Day 17 | Day 20 | Day 23 | Day 27 | |
| REP 2055c | 403 ± 5.7 | 487 ± 25.1 | 612 ± 44.9 | 862 ± 88.5 | 1,276 ± 86.7 |
| NS | 411 ± 13.9 | 496 ± 18.8 | 709 ± 47.7 | 1,023 ± 42.1 | 1,296 ± 43.5 |
NS, normal saline.
Once-daily (QD) treatment (10 mg/kg/day) via the i.p. route from day −1 to day 14 p.i.
Differences in the mean body weights of ducks versus NS-treated ducks were not statistically significant (P > 0.05).
Table 6.
White blood cell counts and liver enzyme levels on day 14 p.i. (autopsy) in ducks treated with REP 2055 and NSa
| Treatmentb | WBC (109/liter) | Liver enzymes (U/liter) |
||
|---|---|---|---|---|
| GGT | ALT | AST | ||
| REP 2055c | 9.08 ± 1 | 3.66 ± 1.1 | 25.7 ± 5.5 | 16.53 ± 2.6 |
| NS | 9.44 ± 1.1 | 2.8 ± 0.8 | 22.6 ± 3.6 | 15.64 ± 4.2 |
NS, normal saline.
Once-daily (QD) treatment (10 mg/kg) via the i.p. route from day −1 to day 14 p.i.
Differences in mean values versus NS-treated ducks were not statistically significant (P > 0.05).
REP 2055 dose response in DHBV-infected ducks.
DHBV-infected ducks were treated with different doses of REP 2055 in order to define the minimum dose of REP 2055 with effective prophylactic efficacy. Doses ranged from 0.5 mg/kg BID to 10 mg/kg QD. As in the previous experiment, REP 2055 treatment did not produce any observable changes in health or weight during the clinical monitoring period (data not shown). Furthermore, ducks treated with REP 2055 in any of the 5 dose regimens showed neither abdominal tenderness, nor pain on i.p. injection, nor clinical and gross anatomical changes. In addition, no significant changes in liver enzymes GGT, ALT, and AST were observed (data not shown) and biopsy and autopsy liver tissues showed normal histology (data not shown). Hematological assessment was not performed for ducks in this experiment, as the highest dose of REP 2055 (10 mg/kg) was previously shown to be well tolerated (Table 6). During the course of this experiment, two ducks (ducks 502 and 519) did not recover from anesthesia following biopsy on day 4 p.i. A third duck (duck 514) was found dead on day 10 p.i., and an autopsy on this duck revealed a large perihepatic abscess, likely arising from infection at the i.p. site of drug administration. These events are known to occur sporadically in ducks, and none were judged to be drug related. Liver sections were taken from ducks 502 and 519 for DHBsAg analysis, but all three ducks were excluded from serum DHBsAg analysis by ELISA and from liver DHBV DNA analysis by Southern blot hybridization.
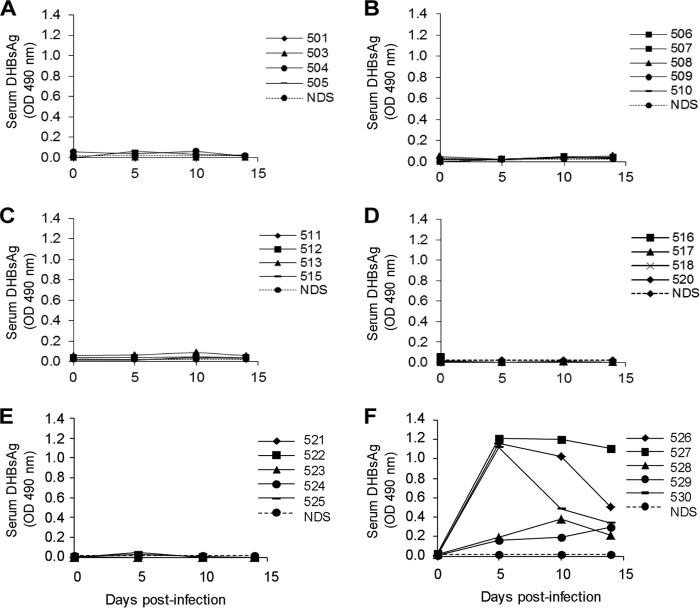
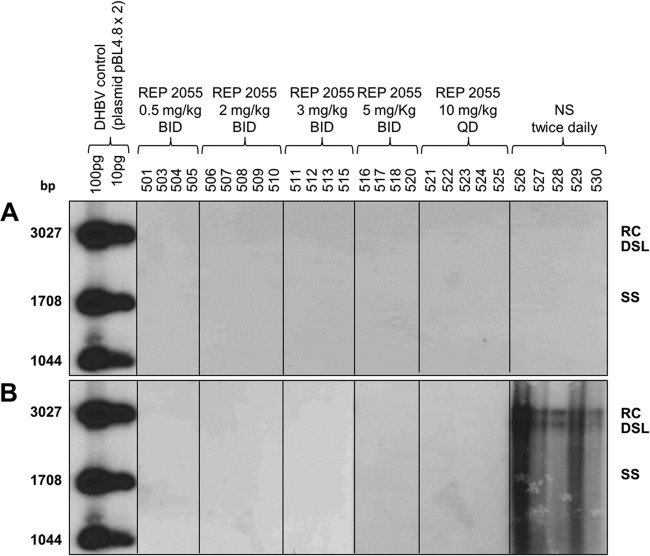
Treatment of ducks from day −3 to day 14 p.i. with REP 2055 at all 5 dose regimens prevented the development of detectable serum DHBsAg, whereas serum DHBsAg was detected in 5/5 NS-treated ducks (Fig. 4). In ducks receiving 10 mg/kg/day of REP 2055 (either 10 mg/kg QD or 5 mg/kg BID), the extent of liver infection was 0.02 to 0.17% of DHBsAg-positive hepatocytes at day 4 p.i. With the lower REP 2055 dose groups (0.5 to 3.0 mg/kg BID), the extent of liver infection at day 4 p.i. was generally higher, but still with mean values from 0.32 to 0.87% DHBsAg-positive hepatocytes, significantly below the level in the NS-treated ducks. At day 14 p.i., all REP 2055 dosing regimens reduced DHBsAg-positive hepatocytes to <0.001%, except for the 0.5-mg/kg BID group, where one duck had 4.3% and the other ducks were below 0.001%, yielding a mean of 1.07% of DHBsAg-positive hepatocytes for this group (Table 7). DHBV DNA was undetectable when tested by Southern blot hybridization in the liver of all REP 2055-treated ducks and present in the liver of all NS-treated ducks (Fig. 5).
Fig 4.
Detection of DHBsAg levels by ELISA in the sera of ducks treated with REP 2055 and normal saline (NS). Fourteen-day-old ducks were inoculated (i.v.) with 5 × 108 DHBV DNA genomes and treated with REP 2055 with 5 different dose regimens i.p. or with NS from 3 days prior to DHBV infection for 17 days: 0.5 mg/kg i.p. twice daily (BID)(A); 2 mg/kg i.p. BID (B); 3 mg/kg i.p. BID (C); 5 mg/kg i.p. BID (D); 10 mg/kg i.p. once daily (QD) (E); NS i.p. BID (F). Serum samples were collected on days 0, 5, 10, and 14 p.i. and were diluted 1/100 for analysis. The normal duck serum (NDS) OD value was used as the cutoff point for the DHBsAg assay. Individual duck identifiers are indicated. Ducks 502, 514, and 519 were excluded from analysis (see Results).
Table 7.
Effect of treatment of ducks with REP 2055 and NSa on the percentage DHBsAg-positive hepatocytes in the liver on days 4 and 14 p.i.
| Treatmentc | Dose regimen | % DHBsAg-positive hepatocytesb (mean ± SD, n = 5) |
|
|---|---|---|---|
| Day 4 p.i. | Day 14 p.i. | ||
| REP 2055d | 0.5 mg/kg, BID | 0.87 ± 0.5 | 1.07 ± 2.2e |
| 2 mg/kg, BID | 0.45 ± 0.2 | <0.001 ± 0 | |
| 3 mg/kg, BID | 0.32 ± 0.2 | <0.001 ± 0e | |
| 5 mg/kg, BID | 0.02 ± 0.0 | <0.001 ± 0e | |
| 10 mg/kg, QD | 0.17 ± 0.1 | <0.001 ± 0 | |
| NS | BID | 13.56 ± 2.8 | >95 ± 0 |
NS, normal saline.
Lower limit of detection of DHBsAg-positive hepatocytes is 0.001%.
Treatment via the i.p. route from day −3 to day 14 p.i. BID, twice daily; QD, once daily.
Differences in the mean percentages of DHBsAg-positive hepatocytes at day 4 and day 14 p.i. versus NS-treated ducks were statistically significant (P < 0.05).
n = 4 (liver tissue not collected from one duck).
Fig 5.
DHBV DNA levels in the liver of ducks on days 4 (A) and 14 (B) p.i. Fourteen-day-old ducks were inoculated i.v. with 5 × 108 DHBV genomes and treated twice daily (BID) with 0.5 to 5 mg/kg of REP 2055, once daily (QD) with 10 mg/kg of REP 2055, or with normal saline (NS). Cellular and viral DNA extracts were tested for DHBV DNA by Southern blot hybridization as described in Materials and Methods (radiographic exposure time, 24 h). The expected positions of relaxed circular (RC), double-stranded linear (DSL), and single-stranded (SS) DHBV DNA are shown on the right. Individual duck identifiers are indicated above each lane. Ducks 502, 514, and 519 were excluded from analysis (see Results).
DISCUSSION
As a model for human HBV therapy, NAP treatment of DHBV-infected ducks produced results that were comparable to the activity profiles observed in PDH in vitro (19). REP 2006 at 10 mg/kg/day was highly active in prophylaxis, preventing the detection of markers of DHBV infection either in the sera (DHBsAg) or the liver (DHBsAg and DHBV DNA) by day 14 p.i. With the same dose of REP 2031, the percentage of DHBsAg-positive hepatocytes was lower than in the NS-treated ducks at day 4 p.i. However, by day 14 p.i., DHBV infection had spread and >95% of hepatocytes were DHBsAg positive, just as in the NS-treated ducks.
REP 2006 was shown to have antiviral activity whether added during or after infection in vitro (19). In contrast, REP 2031 forms tetramers at acidic pH, which neutralizes its amphipathic activity (25–27). This NAP was able to block entry of DHBV into PDH in vitro, but it had little effect when added 12 h after infection (19). The lack of postentry activity of REP 2031 in vitro and the lack of any significant antiviral activity in vivo suggests that the acidic-pH-dependent postentry mechanism of NAPs observed in vitro also occurs in vivo. This further suggests that the targeting of this postentry mechanism by NAPs is providing an important antiviral effect in preventing the establishment and/or spread of DHBV infection in ducks. However, the mechanism targeted by NAPs that underlies these postentry effects remains unclear. Furthermore, the absence of postentry antiviral activity of REP 2031 in DHBV infection in vitro (19), and its inability to block DHBV infection in vivo, strongly suggests that NAPs do not exert their antiviral effect by an immunostimulatory mechanism.
The dramatic effect of preventing the accumulation of DHBsAg both in the blood and the liver of DHBV-infected ducks seen with the NAPs REP 2006 and REP 2055 is similar to effects of ETV treatment in vivo in DHBV-infected ducks (5). However, the mechanisms involved are likely to be significantly different. It is possible that the antiviral activities of NAPs REP 2006 and REP 2055 are in some way tied to the suppression of DHBsAg synthesis or its secretion into the blood, thus restricting the spread of DHBV infection and allowing immune control and clearance of DHBV infection.
Notwithstanding the as-yet-undefined antiviral mechanism of NAPs, the well-tolerated prophylactic activity of REP 2055 against DHBV infection in vivo suggests that this NAP may be immediately useful in the prevention of de novo liver infection in human HBV-positive liver transplant recipients. Additionally, the postentry activity of NAPs warrants a more in-depth investigation into the therapeutic antiviral activity of REP 2055 in established persistent DHBV infection, as this NAP could potentially be a novel therapeutic agent for the treatment of chronic HBV infection in human subjects.
ACKNOWLEDGMENTS
This research was supported by REPLICor Inc., Quebec, Canada.
A.V. is an employee of REPLICor with financial interests.
We thank Behzad Baradaran for help in animal experiments. We thank Catherine Scougall for assistance in immunostaining of tissue sections, Southern blot hybridization, and help in preparing PDH and thank William Mason for his critical reading of the manuscript. We also thank the staff in the SA Pathology Veterinary Services Division for the animal care and also the SA Pathology Tissue Pathology Division for tissue embedding and section preparation.
Footnotes
Published ahead of print 12 August 2013
REFERENCES
- 1.Zoulim F, Saadi F, Buronfosse T, Abdul F, Cova L. 2008. Animal models for the study of infection, p 6.1–6.20 In Locarnini S, Lai CL. (ed), Hepatitis B virus, vol 1 International Medical Press, London, United Kingdom [Google Scholar]
- 2.Schultz U, Grgacic E, Nassal M. 2004. Duck hepatitis B virus: an invaluable model system for HBV infection. Adv. Virus Res. 63:1–70 [DOI] [PubMed] [Google Scholar]
- 3.Jilbert AR, Miller DS, Scougall CA, Turnbull H, Burrell CJ. 1996. Kinetics of duck hepatitis B virus infection following low dose virus inoculation: one virus DNA genome is infectious in neonatal ducks. Virology 226:338–345 [DOI] [PubMed] [Google Scholar]
- 4.Jilbert AR, Botten JA, Miller DS, Bertram EM, Hall PM, Kotlarski J, Burrell CJ. 1998. Characterization of age- and dose-related outcomes of duck hepatitis B virus infection. Virology 244:273–282 [DOI] [PubMed] [Google Scholar]
- 5.Foster WK, Miller DS, Scougall CA, Kotlarski I, Colonno RJ, Jilbert AR. 2005. Effect of antiviral treatment with entecavir on age- and dose-related outcomes of duck hepatitis B virus infection. J. Virol. 79:5819–5832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster WK, Miller DS, Marion PL, Colonno RJ, Kotlarski I, Jilbert AR. 2003. Entecavir therapy combined with DNA vaccination for persistent duck hepatitis B virus infection. Antimicrob. Agents Chemother. 47:2624–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller DS, Boyle D, Feng F, Reaiche GY, Kotlarski I, Colonno R, Jilbert AR. 2008. Antiviral therapy with entecavir combined with post-exposure “prime-boost” vaccination eliminates duck hepatitis B virus-infected hepatocytes and prevents the development of persistent infection. Virology 373:329–341 [DOI] [PubMed] [Google Scholar]
- 8.Feng F, Boyle CQ, Qiao Q, Boyle D, Jilbert AR. 2010. The development of a persistent duck hepatitis B virus infection can be prevented using antiviral therapy combined with a DNA or recombinant fowlpoxvirus vaccines. Vaccine 28:7436–7443 [DOI] [PubMed] [Google Scholar]
- 9.Thermet A, Buronfosse T, Werle-Lapostolle B, Chevallier M, Pradat P, Trepo C, Zoulim F, Cova L. 2008. DNA vaccination in combination or not with lamivudine treatment breaks humoral immune tolerance and enhances cccDNA clearance in the duck model of chronic hepatitis B virus infection. J. Gen. Virol. 89:1192–1201 [DOI] [PubMed] [Google Scholar]
- 10.Lin E, Luscombe C, Colledge D, Wang YY, Locarnini S. 1998. Long-term therapy with the guanine nucleoside analog penciclovir controls chronic duck hepatitis B virus infection in vivo. Antimicrob. Agents Chemother. 42:2132–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicoll AJ, Colledge DL, Toole JJ, Angus PW, Smallwood RA, Locarnini SA. 1998. Inhibition of duck hepatitis B virus replication by 9-(2-phosphonylmethoxyethyl)adenine, an acyclic phosphonate nucleoside analogue. Antimicrob. Agents Chemother. 42:3130–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kocisko DA, Vaillant A, Lee KS, Arnold KM, Bertholet N, Race RE, Olsen EA, Juteau JM, Caughey B. 2006. Potent antiscrapie activities of degenerate phosphorothioate oligonucleotides. Antimicrob. Agents Chemother. 50:1034–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee AM, Rojek JM, Gundersen A, Stroher U, Juteau JM, Vaillant A, Kunz S. 2008. Inhibition of cellular entry of lymphocytic choriomeningitis virus by amphipathic DNA polymers. Virology 372:107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaillant A, Juteau JM, Lu H, Liu S, Lackman-Smith C, Ptak R, Jiang S. 2006. Phosphorothioate oligonucleotides inhibit human immunodeficiency virus type 1 fusion by blocking gp41 core formation. Antimicrob. Agents Chemother. 50:1393–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weissenhorn W, Dessen A, Calder LJ, Harrison SC, Skehel JJ, Wiley DC. 1999. Structural basis for membrane fusion by enveloped viruses. Mol. Membr. Biol. 16:3–9 [DOI] [PubMed] [Google Scholar]
- 16.Bernstein DI, Goyette N, Cardin R, Kern ER, Boivin G, Ireland J, Juteau JM, Vaillant A. 2008. Amphipathic DNA polymers exhibit antiherpetic activity in vitro and in vivo. Antimicrob. Agents Chemother. 52:2727–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardin RD, Bravo FJ, Sewell AP, Cummins J, Flamand L, Juteau JM, Bernstein DI, Vaillant A. 2009. Amphipathic DNA polymers exhibit antiviral activity against systemic murine Cytomegalovirus infection. Virology J. 6:214. 10.1186/1743-422X-6-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumura T, Hu Z, Kato T, Dreux M, Zhang YY, Imamura M, Hiraga N, Juteau JM, Cosset FL, Chayama K, Vaillant A, Liang TJ. 2009. Amphipathic DNA polymers inhibit hepatitis C virus infection by blocking viral entry. Gastroenterology 137:673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noordeen F, Vaillant A, Jilbert AR. 2013. Nucleic acid polymers inhibit duck hepatitis B virus infection in vitro. Antimicrob. Agents Chemother. 57:5291–5298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu RZ, Kim TW, Hong A, Watanabe TA, Gaus HJ, Geary RS. 2007. Cross-species pharmacokinetic comparison from mouse to man of a second-generation antisense oligonucleotide, ISIS 301012, targeting human apolipoprotein B-100. Drug Metab. Dispos. 35:460–468 [DOI] [PubMed] [Google Scholar]
- 21.Yu RZ, Lemonidis KM, Graham MJ, Matson JE, Crooke RM, Tribble DL, Wedel MK, Levin AA, Geary RS. 2009. Cross-species comparison of in vivo PK/PD relationships for second-generation antisense oligonucleotides targeting apolipoprotein B-100. Biochem. Pharmacol. 77:910–919 [DOI] [PubMed] [Google Scholar]
- 22.Iversen PL, Zhu S, Meyer S, Zon G. 1992. Cellular uptake and subcellular distribution of phosphorothioate oligonucleotides into cultured cells. Antisense Res. Dev. 2:211–222 [DOI] [PubMed] [Google Scholar]
- 23.Gao WY, Storm C, Egan W, Cheng YC. 1993. Cellular pharmacology of phosphorothioate homooligodeoxynucleotides in human cells. Mol. Pharmacol. 43:45–50 [PubMed] [Google Scholar]
- 24.Temsamani J, Kubert M, Tang J, Padmapriya A, Agrawal S. 1994. Cellular uptake of oligodeoxynucleotide phosphorothioates and their analogs. Antisense Res. Dev. 4:35–42 [DOI] [PubMed] [Google Scholar]
- 25.Kanaori K, Yasumura A, Tajima K, Makino K. 2004. 1H NMR study on equilibrium between parallel G-quartet structures. Nucleic Acids Symp. Ser. (Oxf.) 48:233–234 [DOI] [PubMed] [Google Scholar]
- 26.Kanehara H, Mizuguchi M, Tajima K, Kanaori K, Makino K. 1997. Spectroscopic evidence for the formation of four-stranded solution structure of oligodeoxycytidine phosphorothioate. Biochemistry 36:1790–1797 [DOI] [PubMed] [Google Scholar]
- 27.Manzini G, Yathindra N, Xodo LE. 1994. Evidence for intramolecularly folded i-DNA structures in biologically relevant CCC-repeat sequences. Nucleic Acids Res. 22:4634–4640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller DS, Kotlarski I, Jilbert AR. 2006. DNA vaccines expressing the duck hepatitis B virus surface proteins lead to reduced numbers of infected hepatocytes and protect ducks against the development of chronic infection in a virus dose-dependent manner. Virology 351:159–169 [DOI] [PubMed] [Google Scholar]
- 29.Miller DS, Halpern M, Kotlarski I, Jilbert AR. 2006. Vaccination of ducks with a whole-cell vaccine expressing duck hepatitis B virus core antigen elicits antiviral immune responses that enable rapid resolution of de novo infection. Virology 348:297–308 [DOI] [PubMed] [Google Scholar]
- 30.Miller DS, Bertram EM, Scougall CA, Kotlarski I, Jilbert AR. 2004. Studying host immune responses against duck hepatitis B virus infection. Methods Mol. Med. 96:3–25 [DOI] [PubMed] [Google Scholar]
- 31.Pugh JC, Di Q, Mason WS, Simmons H. 1995. Susceptibility to duck hepatitis B virus infection is associated with the presence of cell surface receptor sites that efficiently bind viral particles. J. Virol. 69:4814–4822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Triyatni M, Ey P, Tran T, Le Mire M, Qiao M, Burrell C, Jilbert A. 2001. Sequence comparison of an Australian duck hepatitis B virus strain with other avian hepadnaviruses. J. Gen. Virol. 82:373–378 [DOI] [PubMed] [Google Scholar]
- 33.Whal GM, Stern M, Stark GR. 1979. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hydridization by using dextran sulfate. Proc. Natl. Acad. Sci. U. S. A. 76:3683–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krieg AM. 2000. Immune effects and mechanisms of action of CpG motifs. Vaccine 19:618–622 [DOI] [PubMed] [Google Scholar]