Abstract
Noninferiority trial design and analyses are commonly used to establish the effectiveness of a new antimicrobial drug for treatment of serious infections such as complicated urinary tract infection (cUTI). A systematic review and meta-analysis were conducted to estimate the treatment effects of three potential active comparator drugs for the design of a noninferiority trial. The systematic review identified no placebo trials of cUTI, four clinical trials of cUTI with uncomplicated urinary tract infection as a proxy for placebo, and nine trials with reports of treatment effect estimates for doripenem, levofloxacin, or imipenem-cilastatin. In the meta-analysis, the primary efficacy endpoint of interest was the microbiological eradication rate at the test-of-cure visit in the microbiological intent-to-treat population. The estimated eradication rates and corresponding 95% confidence intervals (CI) were 31.8% (26.5% to 37.2%) for placebo, 81% (77.7% to 84.2%) for doripenem, 79% (75.9% to 82.2%) for levofloxacin, and 80.5% (71.9% to 89.1%) for imipenem-cilastatin. The treatment effect estimates were 40.5% for doripenem, 38.7% for levofloxacin, 34.7% for imipenem-cilastatin, and 40.8% overall. These treatment effect estimates can be used to inform the design and analysis of future noninferiority trials in cUTI study populations.
INTRODUCTION
Complicated urinary tract infections (cUTI) occur in persons with structural or functional abnormalities of the urinary tract and in hospitalized patients with significant medical or surgical comorbidities (1, 2). These infections are a major cause of hospital admission, morbidity, mortality, and excess health care costs (3–6). National and international treatment guidance for urinary tract infections includes recommendations for targeted and empirical treatment of the major causative pathogens, including Escherichia coli, Klebsiella pneumoniae, and non-Enterobacteriaceae organisms such as Pseudomonas aeruginosa (7–9).
Of notable concern for the clinical care of patients with cUTI and other serious infections is effective antimicrobial treatment amid the emergence and spread of drug-resistant pathogens (10, 11). To demonstrate efficacy of a new or test antimicrobial agent for the treatment of adult subjects with cUTI, randomized parallel-group noninferiority phase 3 trials have traditionally been used to meet regulatory requirements for approval of the test drug (12). An active-comparator, noninferiority study design is generally used in cUTI trials given the ethical implications of placebo treatment (13, 14). In order to conduct a noninferiority trial, an estimate of the treatment effect of the planned antimicrobial comparator must be obtained from historical data (12). Once an estimate of the treatment effect of the antimicrobial comparator is determined, the largest clinically acceptable difference of the test drug compared to the comparator must be decided. This decision is critical to the design, analysis, and interpretation of a noninferiority trial.
We conducted a systematic review and meta-analysis of antimicrobial cUTI treatment effects to inform the future design of noninferiority trials. Although aminopenicillins, cephalosporins, other fluoroquinolones, and carbapenems are prescribed as cUTI treatment, we a priori restricted our systematic review to doripenem, levofloxacin, and imipenem-cilastatin as drugs representative of potential active comparators for hospitalized adults with cUTI for a global clinical development program of phase 3 registration trials. The systematic review included published clinical trials for placebo, doripenem, levofloxacin, and imipenem-cilastatin; source documents were restricted to published historical clinical studies and, if relevant, Summary Basis of Approval documents from the U.S. Food and Drug Administration (FDA). The systematic review incorporated the principles and recommendations for standardized data quality assessment and reporting of results (15–17). A predefined meta-analysis plan defined the efficacy variables, endpoints of interest, and computational methods to estimate the treatment effects of the three antimicrobial agents identified in cUTI clinical trials over the past 2 decades.
MATERIALS AND METHODS
Identification of studies.
We conducted computer-based literature searches and systematic reviews to identify published historical placebo-controlled and antimicrobial comparator trials of cUTI, inclusive of hospitalized acute pyelonephritis. The searches were conducted using the MEDLINE search engine (PubMed; U.S. National Library of Medicine, National Institutes of Health [http://www.ncbi.nlm.nih.gov]) and EMBASE (Biomedical and Pharmacologic Database; Elsevier). The search for publications of placebo treatment effect was defined as all publications prior to 30 November 2011. In contrast, the search for publications of the antimicrobial treatment effect was restricted to the time interval from 1 January 1978 through 30 November 2011 to reflect the execution of contemporary trials.
The initial search identified no placebo-controlled trials with cUTI, and hence, the search was extended to include placebo-controlled trials with uncomplicated urinary tract infections (uUTI) and randomized-controlled trials (RCTs) of cranberry (juice or extract) for the treatment of uUTI. For placebo treatment effect in uUTI trials, the search terms were “urinary tract infection,” “placebo,” and “clinical trial.” For cranberry treatment effect in uUTI trials, the search terms were “cranberry juice,” “cranberry extract,” or “Vaccinium macrocarpon,” “urinary tract infection,” and “placebo-controlled clinical trial.” For trials of antimicrobial treatment effect, the search terms were “doripenem,” “levofloxacin,” or “imipenem-cilastatin,” “complicated urinary tract infection” or “acute pyelonephritis,” and “randomized clinical trial.” An additional search was conducted to identify antimicrobial treatment effect in postmarketing studies, with the search terms “postmarketing studies,” “complicated urinary tract infection,” “clinical trial,” and “doripenem,” “levofloxacin,” or “imipenem-cilastatin.” In each search, Boolean operators (AND, OR) were used in succession to narrow or widen the respective search, with restrictions to studies conducted in human adults. Additionally, relevant articles from the reference listings and abstracts, and from Summary Basis of Approval documents, were reviewed to minimize ascertainment and publication bias.
Subjects in trials who received inappropriate antimicrobial treatment were recommended for potential inclusion in the assessment of placebo treatment effect of noninferiority cUTI trials by the FDA (12, 18–20). Inappropriate or inadequate antimicrobial treatment has generally been defined as treatment with an antimicrobial agent to which the bacteria causing the infection were resistant (12). We conducted a systematic literature search for such reports of inappropriate or inadequate treatment of uUTI or cUTI in PubMED and EMBASE, using combinations of the following search terms: “inappropriate treatment,” “inadequate treatment,” “delayed treatment,” and “urinary tract infection”; no publications were identified. For assessment of inappropriate antimicrobial treatment in cUTI, trials were restricted to three publications reported in the FDA guidance for noninferiority trial assessment of cUTI and the final selection of trials included in the meta-analysis from our systematic review (12, 18–34).
Eligibility criteria.
Eligible studies were original placebo-controlled trials that either compared the efficacy of an antimicrobial agent to placebo in uUTI or the efficacy of an antimicrobial agent in cUTI and the registration trials and postmarketing studies for doripenem, levofloxacin, and imipenem-cilastatin. Studies were limited to adults of ≥18 years of age and excluded for one or more of the following criteria: (i) pediatric studies, (ii) duplicate studies, (iii) review papers, (iv) studies with incomplete data, (v) observational studies, and (vi) studies among outpatient populations. Observational studies were not eligible for inclusion in the assessment of treatment effect, given the heterogeneity of such study populations relative to subjects enrolled in clinical trials. In addition, three trials of levofloxacin with initial oral therapy, reported in four publications, were excluded from the primary meta-analysis but included in the sensitivity analyses (28–31).
Data extraction.
Two reviewers independently assessed the identified publications and studies for trial eligibility, data quality, and efficacy data. For each trial, the following data were systematically extracted: (i) general study characteristics, including the author, publication date, study drug, comparator, study population description, study years, countries or regions where study was conducted, study design, number of study centers, number enrolled, intent-to-treat (ITT) population, microbiological ITT population, modified microbiological ITT population, microbiologically evaluable population, and clinically evaluable population; (ii) baseline demographics, including age, gender, geographic regions, and baseline diagnosis; (iii) microbiological eradication rates in the microbiologically evaluable population at test of cure (TOC), late follow-up, and end of treatment; (iv) microbiological eradication rate in the modified microbiological ITT population at TOC; (v) microbiological eradication rates in the microbiological ITT population at end of treatment and posttherapy; (vi) clinical cure rates in the clinically evaluable population, as well as at TOC, late follow-up, end of treatment, and posttherapy; (vii) study methodology, including blinding, randomization, duration of therapy, eligibility criteria, inclusion criteria, exclusion criteria, primary outcome variable, secondary outcome variable, urine collection methods, threshold for bacterial quantification in the urine culture (number of CFU/ml), and noninferiority margin used; (viii) study definitions and time point definitions of early and late follow-up, end of treatment, posttherapy, and poststudy; and (ix) subgroup cure rates stratified by cUTI and acute pyelonephritis. Assessment of risk of bias in the final selected studies was performed in duplicate by two independent assessors, using a three-item metric scale which allows for the assessment of potential sources of bias due to methodological quality of study design, randomization, allocation concealment, and the handling of patient attrition or loss to follow-up (35). Discrepancies in the assessment of risk of bias using this scale were resolved by further discussion until consensus between the two assessors was reached.
Meta-analysis and statistical methods.
The primary efficacy endpoint of interest was the microbiological eradication rate assessed at the TOC visit (5 to 9 days after the last dose of study medication) in the microbiological ITT population. If the eradication rate was not available or not reported for the TOC visit, the response rate at the late follow-up visit or end-of-treatment visit was substituted based on proximity in time to the TOC visit window. Additionally, sensitivity analyses of the microbiological eradication in the microbiologically evaluable population and the clinical response in the clinically evaluable population were conducted when data allowed. A meta-regression was also conducted to adjust for the route of administration in the five levofloxacin trials.
The treatment effect of an antimicrobial treatment was calculated as the difference of the eradication rate for the antimicrobial treatment and the eradication rate for the placebo treatment. Ideally, a treatment effect estimate was obtained from historical randomized placebo-controlled trials of the antimicrobial treatment. Since no historical placebo-controlled studies involving doripenem, levofloxacin, or imipenem-cilastatin were available, sequential indirect comparisons were necessary to estimate the antimicrobial treatment effect. First, the placebo rate was estimated from studies that involved placebo in the treatment of uUTI, which was used as a proxy for the placebo rate in cUTI. Second, the treatment effect rates of doripenem, levofloxacin, and imipenem-cilastatin were determined from cUTI studies. Lastly, the treatment effects of doripenem, levofloxacin, and imipenem-cilastatin versus placebo were estimated through cross-trial comparisons. The cross-trial comparison to estimate the antimicrobial treatment effect was calculated as the difference between the lower bound of the 95% confidence interval (CI) for each comparator and the upper bound of the 95% CI for the proxy of placebo (12). This methodology was also used by the FDA in their guidance document on design of noninferiority trials (12, 13). To account for interstudy variability in the analysis of overall treatment effect, a weighted, noniterative, random-effects model was utilized, using R (metaphor) software, to obtain an estimate of the microbiological eradication rate and corresponding 95% CI for each treatment group (36). This method is commonly used for meta-analysis of clinical trials, as it accounts for the heterogeneity of studies through a statistical parameter that represents the interstudy variation of the trials in the model (36). To examine the sensitivity of results to the meta-analysis method, sensitivity analyses were conducted using fixed-effects models. When four or more studies were included in the meta-analysis, the interstudy heterogeneity was assessed, and the P value and I2 value were reported (37). The P values were computed based on Cochran's chi-square test for interstudy heterogeneity, and I2 values were measures of the proportion of total variation in the study estimates that was due to heterogeneity.
RESULTS
Placebo treatment effect in urinary tract infections.
No publications were identified in the systematic search for cUTI placebo-controlled trials. Two subsequent searches were conducted to estimate a proxy estimate for the placebo response rate (Fig. 1). In one systematic search of cranberry juice or cranberry extract treatment in clinical trials for urinary tract infection, 116 publications were identified in PubMed, and 348 publications were identified in EMBASE. The screening process resulted in the exclusion of all publications identified in each search; no publications were eligible for inclusion. In another systematic search for placebo-controlled clinical trials of urinary tract infection (presumed uncomplicated), 459 publications were identified in PubMed, and 2,303 publications were identified in EMBASE (Fig. 1). The screening process resulted in the exclusion of 453 of the publications from PubMed and 2,296 searches from EMBASE, resulting in seven nonduplicate, eligible publications (21–24, 38–40). Four of these seven eligible publications met the final inclusion criteria (Table 1), with full review of the study by Dubi et al. after certified translation of the publication from French into English (21–24). The estimated microbiological eradication was 31.8% (95% CI, 26.5% to 37.2%) among 291 subjects in four uUTI trials. No study heterogeneity was detected (P value = 0.68; I2 = 0), the funnel plot did not suggest publication bias (data not shown), and a forest plot of the meta-analysis is depicted in Fig. 2a.
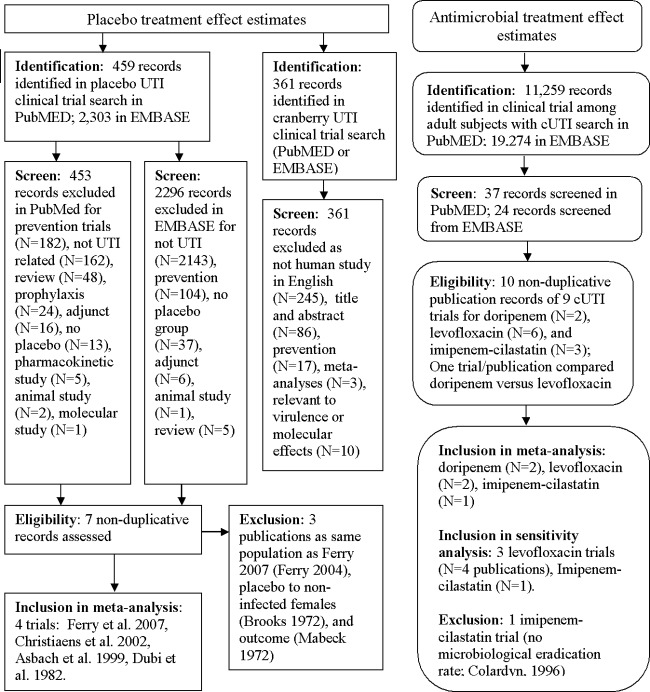
Fig 1.
Flow chart depicting the systematic literature search for trials of placebo and antimicrobial treatment of complicated urinary tract infection (cUTI), including study identification, screening, eligibility, and inclusion.
Table 1.
Microbiological eradication rates for 291 subjects assigned to placebo in four placebo-controlled trials of uncomplicated urinary tract infectiona
| Study author(s), yr (reference) | Quantitative urine culture (no. of CFU/ml) |
Assessment window | Microbiological eradication in MITT (no. of subjects with eradication/total no. of subjects [%]) | |
|---|---|---|---|---|
| Entry | Eradication | |||
| Ferry et al., 2007 (24) | ≥103 | <103 | Days 8–10 | 71/227 (31.3)b |
| Christiaens et al., 2002 (23) | ≥105 | <105 | Day 7 | 9/27 (33.3) |
| Asbach, 1991 (22) | NA | NA | Days 14–17 | 5/19 (26.3) |
| Dubi et al., 1982 (21) | ≥105 | <105 | Week 1 | 8/18 (44.4) |
MITT, microbiological intention-to-treat population. NA, not available.
Subjects lost to follow-up were considered failures for the MITT population.
Fig 2.
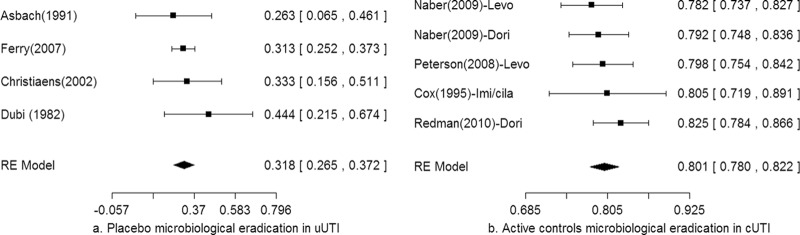
Forest plots of the primary meta-analysis for microbiological eradication of the proxy placebo treatment (a) and the three active control treatments (b).
Inappropriate antimicrobial therapy treatment effects.
Among the 14 publications meeting inclusion criteria for the meta-analysis as either placebo (n = 4) or antimicrobial (n = 10) trials, 4 were identified as reports with cases meeting criteria for assessment of inappropriate antimicrobial treatment effects (20, 21, 26, 27). Microbiological eradication was different among 76 case subjects in four trials of uUTI, acute pyelonephritis, and cUTI (Table 2). The random-effects weighted estimate of treatment response for these cases was 28.6% (95% CI, 18.7% to 38.5%).
Table 2.
Inappropriate antimicrobial treatment after assignment to study drug in 76 subjects from trials identified in the systematic review for inclusion in the meta-analysis
| Infection | Assigned antimicrobial agent | Microbiological eradication (no. of subjects with eradication/total no. of subjects [%]) | Author(s) of trial, yr (reference) |
|---|---|---|---|
| Complicated urinary tract infection, inclusive of acute pyelonephritis | Levofloxacin | 6/21 (28.6) | Redman et al., 2010 (26) |
| Levofloxacin | 4/12 (33.3) | Peterson, 2008 (27) | |
| Ciprofloxacin | 4/21 (19) | Peterson, 2008 (27) | |
| Acute pyelonephritis | Trimethoprim-sulfamethoxazole | 7/14 (50) | Talan et al., 2000 (20) |
| Urinary tract infection | Amoxicillin | 2/8 (25) | Dubi et al., 1982 (21) |
Antimicrobial treatment effects in cUTI.
In the systematic search for clinical trials of adults with cUTI, the identification process yielded 11,259 publications in PubMed and 19,274 publications in EMBASE (Fig. 1). No postmarketing trials meeting the search term criteria were identified. The screening process resulted in 37 publications from PubMed and 24 publications from EMBASE, resulting in 10 nonduplicate, eligible publications (25–34). Among these eligible publications, two reported on doripenem, six reported on levofloxacin, and three reported on imipenem-cilastatin; one of the doripenem trials also reported on levofloxacin (Table 3). The funnel plot for these publications did not detect a publication bias (data not shown), and the risk-of-bias scores ranged from 0 to 5 based on a three-item metric assessment (Table 3). The Dori-06 trial was an open-label study that used the same inclusion criteria as the Dori-05 trial and, by design, had the same duration of therapy, outcome assessments, and endpoints (25, 26). The five levofloxacin clinical trials, reported in six publications, were partitioned as two trials of initial intravenous (i.v.) levofloxacin and three trials with initial oral levofloxacin (as reported in four publications). Given that the meta-analysis focus was a hospitalized adult target population with cUTI, only the two levofloxacin trials with initial i.v. therapy were included in the primary meta-analysis (25, 27). One of the three eligible imipenem-cilastatin trials met final inclusion criteria for the meta-analysis (33). The study by Colardyn and Faulkner reported serious bacterial infections, including cUTI as a subgroup, but the microbiological eradication rate for imipenem-cilastatin was not available (34). The study by Naber et al. was excluded because the sample size of the microbiological eradication rate for imipenem-cilastatin was not reported (32). The estimated microbiological eradication rates were 81% (95% CI, 77.7% to 84.2%) for doripenem, 79% (95% CI, 75.9% to 82.2%) for levofloxacin, and 80.5% (95% CI, 71.9% to 89.1%) for imipenem-cilastatin. The pooled microbiological eradication rate was 80.1% (95% CI, 78% to 82.2%); no study heterogeneity was identified (P value = 0.7; I2 = 0). The results of the meta-analyses are summarized in Table 4, and a forest plot of the primary meta-analyses is depicted in Fig. 2b.
Table 3.
Heterogeneity and consistency of data in 9 trials reporting treatment effect of doripenem, levofloxacin, or imipenem-cilastatin for cUTI inclusive of acute pyelonephritisa
| Drug (no. of trials) and trial or publication identifiers | Study design, treatment | Quantitative urine culture (no. of CFU/ml) |
Proportions of subjects by type of cUTI and by gender (%) | Risk–of–bias scoreb | |
|---|---|---|---|---|---|
| Entry | TOC | ||||
| Doripenem (n = 2) | |||||
| Dori–05 (Naber et al., 2009 [25]) | RDB, i.v. to oral | ≥105 | <104 | cUTI:AP, 49:51 | 5 |
| Male:female, 38:62 | |||||
| Dori-06 (Redman et al., 2010 [26]) | Open label, i.v. to oral | ≥105 | <104 | NA | 0 |
| Levofloxacin (n = 5) | |||||
| Dori-05 (Naber et al., 2009 [25]) | RDB, i.v. to oral | ≥105 | <104 | cUTI:AP, 47:53 | 5 |
| Male:female, 39:61 | |||||
| Peterson, 2008 (27) | RDB, i.v. or oral | ≥105 | <104 | cUTI:AP, 73:27 | 5 |
| Male:female, 39:61 | |||||
| L91-058 (Richard et al., 1998 [28, 29]) | RDB, oral only | ≥105 | <104 | cUTI:AP:uUTI, 69:24:7 | 4 |
| Male:female, 41:59 | |||||
| L91-059 (Richard et al., 1998; Klimberg et al., 1998 [28–30]) | Randomized open label, oral only | ≥105 | <104 | cUTI:AP:uUTI, 74:16:10 | 4 |
| Male:female, 38:62 | |||||
| Peng, 1999 (31) | RDB, oral only | >104 | NS | AP:cystitis, 80:20 | 2 |
| Male:female, 65:35 | |||||
| Imipenem-cilastatin (n = 3) | |||||
| Naber et al., 2002 (32) | RDB, i.v. | Variedc | Variedc | cUTI:AP, 89:11 | 3 |
| Male:female, 56:44 | |||||
| Cox et al., 1995 (33) | i.v. | NA | ≤104 | cUTI:AP, 73:27 | 2 |
| Male:female, 39:61 | |||||
| Colardyn and Faulkner, 1996 (34 | Randomized, i.v. | NA | <104 | 3 cUTI subjects in imipenem-cilastatin arm | 2 |
AP, acute pyelonephritis; i.v., intravenous; RDB, randomized, double blind; NA, not available; TOC, test of cure; uUTI, uncomplicated urinary tract infection.
Three-item composite categorical score from 0 (high bias) to 5 (low bias) due to study design, randomization, allocation concealment, and the handling of patient attrition or loss to follow-up (35).
Varied by sex and method of urine collection.
Table 4.
Summary of meta-analysis results for active comparator treatments
| Treatment | Efficacy endpoint | Analysis (population)a | Meta-analysis treatment effect estimate (mean % [95% CI]) | Reference(s) |
|---|---|---|---|---|
| Doripenem | Microbiological eradication | Primary (MITT) | 81.0 (77.7, 84.2) | 25, 26 |
| Microbiological eradication | Sensitivity (ME) | 82.9 (79.6, 86.1) | 25, 26 | |
| Clinical response | Sensitivity (CE) | 94.3 (92.2, 96.3) | 25, 26 | |
| Levofloxacin | Microbiological eradication | Primary (MITT) | 79.0 (75.9, 82.2) | 25, 27 |
| Microbiological eradication | Sensitivity (ME) | 84.8 (81.8, 87.9) | 25, 27 | |
| Microbiological eradication | Sensitivity (MITT) | 79.0 (75.1, 82.9)b | 25, 27–31 | |
| Microbiological eradication | Sensitivity (ME) | 84.8 (81.8, 87.9)b | 25, 27–31 | |
| Imipenem-cilastatin | Microbiological eradication | Primary (MITT) | 80.5 (71.9, 89.1) | 33 |
| Clinical response | Sensitivity (CE) | 89.6 (71.0, 100) | 32, 33 | |
| Overall | Microbiological eradication | Primary (MITT) | 80.1 (78.0, 82.2) | 25–27, 33 |
| Microbiological eradication | Sensitivity (MITT) | 80.1 (78.0, 82.2)b | 25–27, 33 |
MITT, microbiological intention-to-treat (primary analyses) population; ME, microbiologically evaluable population; CE, clinically evaluable population.
Initial administration (i.v. or oral) was adjusted in the meta-regression; only the estimates and 95% confidence intervals for i.v. treatment are presented.
Estimation of antimicrobial treatment effects for complicated urinary tract infection.
In the cross-study comparisons to estimate the antimicrobial treatment effects for cUTI, the difference between the lower bound of the 95% CI for each antimicrobial agent and the upper bound of the 95% CI for placebo was calculated (12). The treatment effect estimates were 40.5% for doripenem, 38.7% for levofloxacin, and 34.7% for imipenem-cilastatin. The overall treatment effect estimate was 40.8% when historical data for all three antimicrobial drugs were considered.
Sensitivity analyses.
For each antimicrobial treatment, the microbiological eradication rate in the microbiologically evaluable population and the clinical response rate in the clinically evaluable population were estimated to check the sensitivity of results to either different endpoints or different analysis populations (Table 4). The microbiological eradication rates and clinical response rates in these evaluable populations were generally higher than the rates in the microbiological ITT population. For levofloxacin treatment, a meta-regression analysis was conducted, with initial route of administration as a study-level covariate for all five levofloxacin trials. The estimate of microbiological eradication rate for levofloxacin was 79.0% (95% CI, 75.1% to 82.9%) in the microbiological ITT population, which approximated the 79.0% (95% CI, 75.9% to 82.2%) eradication rate estimated in the two levofloxacin trials with initial i.v. therapy. Overall, there were no marked differences between the treatment effect estimates determined by the fixed-effects models versus the random-effects models.
DISCUSSION
The major finding from this systematic review and meta-analysis is the highly consistent cUTI treatment effect estimates for doripenem (40.5%), levofloxacin (38.7%), and imipenem-cilastatin (34.7%). These estimates, together with the pooled treatment effect (40.8%), suggest a low level of heterogeneity among well-designed and well-executed clinical trials in cUTI study populations. There was little evidence of between-study variance among the three antimicrobial agents or significant bias based on the different sample sizes for each comparator (Fig. 2b), with consequent enhanced objective evidence of antimicrobial treatment estimates for future design and analysis of cUTI noninferiority trials. The methodology in this meta-analysis to estimate the treatment effect, as the difference between the lower bound of the 95% CI for each comparator and the upper bound of the 95% CI for the placebo proxy, has frequently been used by the FDA (12). This approach generally yields a conservative estimate, which can have a significant impact on sample size and the feasibility of conducting noninferiority trials. One could argue that an alternate approach which estimates the treatment effect as the difference in the point estimates of the response rates between the treatment groups may be more realistic. Application of this metric for the estimation of treatment effect would result in higher and more consistent estimates for doripenem (49.2%), levofloxacin (47.2%), and imipenem-cilastatin (48.7%). Debate exists in the literature for use of other alternative methods to estimate the antimicrobial treatment effect (41).
The systematic review identified historical clinical trials of treatment effect as a proxy of placebo treatment effect. The placebo treatment effect estimate of 31.8% (95% CI, 26.5% to 37.2%) was similar to the estimate of 28.6% (95% CI, 18.7% to 38.5%) for inappropriate antimicrobial treatment response. Together, these proxies for placebo treatment effect likely overestimate the true placebo treatment effect of subjects with cUTI, given the lower severity of infection in subjects with uUTI than that in subjects with cUTI. The systematic review also identified historical clinical trials of three antimicrobial drugs. These drugs were a priori selected as being representative of proposed comparator drugs in future global cUTI antimicrobial development programs. Ten trials were eligible, among which five were included in the primary meta-analysis, four were included in the sensitivity analysis, and one trial was excluded due to a lack of necessary data. Notably, the bias scores for these trials ranged from low to high, given potential bias related to quality of design, randomization, blinding, and the handling of patient attrition (35). Nonetheless, all were included in the meta-analysis because of the required available data necessary to estimate the treatment effect. Most notably, a higher bias score occurred for one of the two pivotal doripenem trials that lacked allocation concealment, reflecting the secular changes in optimized robust trial design since the creation of the three-item metric in 1996 (26, 35).
We acknowledge several limitations associated with this meta-analysis. First, and perhaps most notably, there were ascertainment and reporting biases inherent in the study design given the recognized heterogeneity of and inconsistencies within the reported study measures, endpoints, and gaps in available information (15–17). Nonetheless, the three antimicrobial drugs had very similar treatment effect estimates. Second, the placebo treatment effect estimate was likely overestimated given the use of proxy data for placebo treatment effect in uUTI, a less severe infection. Third, as noted in the U.S. FDA guidance for noninferiority trial design, the use of data representing inadequate antimicrobial therapy may result in a conservative estimate of placebo rate, because even inappropriate or inadequate therapy may have some effect on the treatment outcome (12). Given the challenges in the search of such studies, we restricted the identification of inappropriate antimicrobial treatment to studies identified in this systematic review, which resulted in a heterogeneous group of subjects with uUTI, acute pyelonephritis, and cUTI (Table 2). Fourth, we did not exclude any studies based on risk of bias assessment. Despite risk for high bias based on the three-item Jadad metric, all included trials had necessary data to estimate the cUTI treatment effect (35). Lastly, we were unable to incorporate data regarding development of resistance to the administered antimicrobial agents into the meta-analysis; this could perhaps be a subject of future investigations.
With respect to study implications and future trials, the meta-analysis results were robust in regard to analysis models and indicative of little interstudy heterogeneity (41). The primary efficacy endpoint in cUTI clinical trials has historically been the microbiological response at the follow-up visit, typically 5 to 9 days after the last treatment dose in the microbiological ITT population, which consists of all assigned subjects with microbiologically evaluable uropathogens at the baseline visit (13, 14). In a recent addendum to the guidance on bacterial infections, the European Medical Agency (EMA) still recommended to assess primary endpoints at a TOC visit occurring approximately 7 days after the last possible day of treatment and requested the microbiological ITT and microbiologically evaluable populations as the co-primary analysis populations (14). In contrast, the FDA recently defined different time points for assessing the primary endpoint for an investigational i.v.-only drug with a switch to an FDA-approved oral drug. In trials for an investigational i.v. drug with a minimum of 5 days of i.v. therapy and a planned switch to another oral drug, the clinical and microbiological response at day 5 was the primary endpoint, and maintenance of resolution of the core symptoms of cUTI and microbiological success 7 days after the completion of antimicrobial therapy were recommended as the primary endpoints (13). However, for an investigational i.v. drug with an oral switch, the recommended time point for assessment of the primary efficacy endpoint remains 7 days posttherapy, or the traditional TOC visit, which is the same as the EMA's guidance.
In summary, the results of this meta-analysis for treatment effect estimates from clinical trial populations with cUTI will inform the design, execution, and analysis of future cUTI trials. The proportion of successfully treated subjects with antimicrobial treatment suggests that additional information regarding emergence of resistance will further inform treatment effect estimates in future trials.
ACKNOWLEDGMENTS
We acknowledge Sara Hughes, Michael Irizarry, and Ohad Amit for their critical reviews of this manuscript.
Footnotes
Published ahead of print 12 August 2013
REFERENCES
- 1.Foxman B. 2010. The epidemiology of urinary tract infection. Nat. Rev. Urol. 7:653–660 [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez CM, Schaeffer AJ. 1999. Treatment of urinary tract infection: what's old, what's new, and what works. World J. Urol. 17:372–382 [DOI] [PubMed] [Google Scholar]
- 3.Brown P, Ki M, Foxman B. 2005. Acute pyelonephritis among adults: cost of illness and considerations for the economic evaluation of therapy. Pharmacoeconomics 23:1123–1142 [DOI] [PubMed] [Google Scholar]
- 4.Klevens RM, Edwards JR, Gaynes RP. 2008. The impact of antimicrobial-resistant, health care-associated infections on mortality in the United States. National Nosocomial Infections Surveillance System. Clin. Infect. Dis. 47:927–930 [DOI] [PubMed] [Google Scholar]
- 5.Neal DE. 2008. Complicated urinary tract infections. Urol. Clin. N. Am. 35:13–22 [DOI] [PubMed] [Google Scholar]
- 6.Magill SS, Hellinger W, Cohen J, Kay R, Bailey C, Boland B, Carey D, de Guzman J, Dominguez K, Edwards J, Goraczewski L, Horan T, Miller M, Phelps M, Saltford R, Seibert J, Smith B, Starling P, Viergutz B, Walsh K, Rathore M, Guzman N, Fridkin S. 2012. Prevalence of healthcare-associated infections in acute care hospitals in Jacksonville, Florida. Infect. Control Hosp. Epidemiol. 33:283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naber KG, Bergman B, Bishop MC, Bjerklund-Johansen TE, Botto H, Lobel B, Jinenez Cruz F, Selvaggi FP, Urinary Tract Infection Working Group of the Health Care Office of the European Association of Urology 2001. EAU guidelines for the management of urinary and male genital tract infections. Eur. Urol. 40:576–588 [DOI] [PubMed] [Google Scholar]
- 8.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, Saint S, Schaeffer AJ, Tambyah PA, Tenke P, Nicolle LE. 2010. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin. Infect. Dis. 50:625–663 [DOI] [PubMed] [Google Scholar]
- 9.Nicolle LE, Evans G, Laverdieve M, Phillips P, Quan C, Rotstein C. 2005. Complicated urinary tract infection in adults. Can. J. Infect. Dis. Med. Microbiol. 16:349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrell DJ, Morrissey I, DeRubeis D, Robbins M, Felmingham D. 2003. A UK multicentre study of the antimicrobial susceptibility of bacterial pathogens causing urinary tract infection. J. Infect. 46:94–100 [DOI] [PubMed] [Google Scholar]
- 11.Tadesse DA, Zhao S, Tong E, Ayers S, Singh A, Bartholomew MJ, McDermott PF. 2012. Antimicrobial drug resistance in Escherichia coli from humans and food animals, United States, 1950–2002. Emerg. Infect. Dis. 18:741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration 2010. Guidance for industry non-inferiority clinical trials. US Food and Drug Administration, Silver Spring, MD [Google Scholar]
- 13.US Food and Drug Administration 2012. Guidance for industry: complicated urinary tract infections: developing drugs for treatment. US Food and Drug Administration, Silver Spring, MD [Google Scholar]
- 14.European Medicines Agency/Committee for Medicine Products for Human Use (EMA/CHMP) 2012. Addendum to the note for guidance on evaluation of medicinal products indicated for treatment of bacterial infections (CPMP/EWP/558/95 REV 2) to address indication-specific clinical data. EMA/CHMP, London, United Kingdom [Google Scholar]
- 15.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. 1999. Improving the quality of reports of meta-analyses of randomized controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet 354:1896–1900 [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Simera I, Schulz KF, Hoey J, Altman DG. 2008. Helping editors, peer reviewers and authors improve the clarity, completeness, and transparency of reporting health research. BMC Med. 6:13. 10.1186/1741-7015-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, Clarke M, Devereauz PJ, Kleijnen Moher D. 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanations and elaboration. Ann. Intern. Med. 151:W65–W94 [DOI] [PubMed] [Google Scholar]
- 18.Allais JM, Preheim LC, Cuevas TA, Roccaforte JS, Mellencamp MA, Bittner MJ. 1988. Randomized, double-blind comparison of ciprofloxacin and trimethoprim-sulfamethoxazole for complicated urinary tract infections. Antimicrob. Agents Chemother. 32:1327–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang GD, Brennen C, Wagener M, Swanson D, Hilf M, Zadecky L, DeVine J, Yu VL. 1991. Use of ciprofloxacin versus use of aminoglycosides for therapy of complicated urinary tract infection: prospective, randomized clinical and pharmacokinetic study. Antimicrob. Agents Chemother. 35:1849–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talan DA, Stamm WE, Hooton TM, Moran GJ, Burke T, Iravani A, Reuning-Scherer J, Church DA. 2000. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis in women. JAMA 283:1583–1590 [DOI] [PubMed] [Google Scholar]
- 21.Dubi J, Chappuis PH, Darioli R. 1982. Traitement de l'infection urinaire por un doce unique do co-trimoxazole compare a une dose unique d'amoxycilline et a un placebo. Schweiz. Med. Wochenschr. 112:90–92 [PubMed] [Google Scholar]
- 22.Asbach HW. 1991. Single dose oral administration of cefixime 400 mg in the treatment of acute uncomplicated cystitis and gonorrhoeae. Drugs 42:10–13 [DOI] [PubMed] [Google Scholar]
- 23.Christiaens TC, De Meyere M, Verschraegen G, Peersman W, Heytens S, De Maeseneer JM. 2002. Randomised controlled trial of nitrofurantoin versus placebo in the treatment of uncomplicated urinary tract infection in adult women. Br. J. Gen. Pract. 52:729–734 [PMC free article] [PubMed] [Google Scholar]
- 24.Ferry S, Holm SE, Stenlund H, Lundholm R, Monsen TJ. 2007. Clinical and bacteriological outcome of different doses and duration of pivmecillinam compared with placebo therapy of uncomplicated lower urinary tract infection in women: the LUTIW project. Scand. J. Prim. Health Care 25:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naber KG, Llorens L, Kaniga K, Kotey P, Hedrich D, Redman R. 2009. Intravenous doripenem at 500 milligrams versus levofloxacin at 250 milligrams, with an option to switch to oral therapy, for treatment of complicated lower urinary tract infection and pyelonephritis. Antimicrob. Agents Chemother. 53:3782–3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redman R, Damiao R, Kotey P, Kaniga K, Davies T, Naber KG. 2010. Safety and efficacy of intravenous doripenem for the treatment of complicated urinary tract infections and pyelonephritis. J. Chemother. 22:384–391 [DOI] [PubMed] [Google Scholar]
- 27.Peterson J. 2008. A double-blind, randomized comparison of levofloxacin 750 mg once daily for five days with ciprofloxacin 400/500 mg twice-daily for 10 days for the treatment of complicated urinary tract infections and acute pyelonephritis. Urology 71:17–22 [DOI] [PubMed] [Google Scholar]
- 28.Richard GA, Klimberg IN, Fowler CL, Callery-D'Amico S, Kim SS. 1998. Levofloxacin versus ciprofloxacin versus lomefloxacin in acute pyelonephritis. Urology 52:51–55 [DOI] [PubMed] [Google Scholar]
- 29.Richard GA, Pittman W, Childs SJ, Nicolle LE, Fowler CL, Callery-D'Amico S. 1998. Safety and efficacy of levofloxacin versus ciprofloxacin in complicated urinary tract infections in adults. Pharm. Ther. 23:534–540 [Google Scholar]
- 30.Klimberg IW, Cox CE, Fowler CL, King W, Kim SS, Callery-D'Amico S. 1998. A controlled trial of levofloxacin and lomefloxacin in the treatment of complicated urinary tract infection. Urology 51:610–615 [DOI] [PubMed] [Google Scholar]
- 31.Peng MY. 1999. Randomized, double-blind, comparative study of levofloxacin and ofloxacin in the treatment of complicated urinary tract infections. J. Microbiol. Immunol. Infect. 32:33–39 [PubMed] [Google Scholar]
- 32.Naber KG, Savov O, Salmen HC. 2002. Piperacillin 2g/tazobactam 0.5g is as effective as imipenem 0.5g/cilastatin 0.5g for the treatment of acute uncomplicated pyelonephritis and complicated urinary tract infections. Int. J. Antimicrob. Agents 19:95–103 [DOI] [PubMed] [Google Scholar]
- 33.Cox CE, Holloway WJ, Geckler RW. 1995. A multicenter comparative study of meropenem and imipenem/cilastatin in the treatment of complicated urinary tract infections in hospitalized patients. Clin. Infect. Dis. 21:86–92 [DOI] [PubMed] [Google Scholar]
- 34.Colardyn F, Faulkner KL. 1996. Intravenous meropenem versus imipenem/cilastatin in the treatment of serious bacterial infections in hospitalized patients. J. Antimicrob. Chemother. 38:523–537 [DOI] [PubMed] [Google Scholar]
- 35.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan D, McQuay HJ. 1996. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control. Clin. Trials 17:1–12 [DOI] [PubMed] [Google Scholar]
- 36.DerSimonian R, Laird N. 1986. Meta-analysis in clinical trials. Control. Clin. Trials 7:177–188 [DOI] [PubMed] [Google Scholar]
- 37.Higgins JP, Thompson SG. 2002. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21:1539–1558 [DOI] [PubMed] [Google Scholar]
- 38.Ferry SA, Holm SE, Stenlund H, Lundholm R, Monsen TJ. 2004. The natural course of uncomplicated lower urinary tract infection in women illustrated by a randomized placebo controlled study. Scand. J. Infect. Dis. 36:296–301 [DOI] [PubMed] [Google Scholar]
- 39.Brooks D, Garrett G, Hollihead R. 1972. Sulphadimidine, cotrimoxazole, and a placebo in the management of symptomatic urinary tract infection in general practice. J. R. Coll. Gen. Practitioners 22:695–703 [PMC free article] [PubMed] [Google Scholar]
- 40.Mabeck CE. 1972. Treatment of uncomplicated urinary tract infection in non-pregnant women. Postgrad. Med. J. 48:69–75 [PMC free article] [PubMed] [Google Scholar]
- 41.Everson-Stewart S, Emerson SS. 2010. Bio-creep in non-inferiority clinical trials. Stat. Med. 29:2769–2780 [DOI] [PubMed] [Google Scholar]