Abstract
Lactococcus lactis MG1363 was found to be unable to grow at temperatures above 37°C in a defined medium without riboflavin, and the cause was identified to be dissolved oxygen introduced during preparation of the medium. At 30°C, growth was unaffected by dissolved oxygen and oxygen was consumed quickly. Raising the temperature to 37°C resulted in severe growth inhibition and only slow removal of dissolved oxygen. Under these conditions, an abnormally low intracellular ratio of [ATP] to [ADP] (1.4) was found (normally around 5), which indicates that the cells are energy limited. By adding riboflavin to the medium, it was possible to improve growth and oxygen consumption at 37°C, and this also normalized the [ATP]-to-[ADP] ratio. A codon-optimized redox-sensitive green fluorescent protein (GFP) was introduced into L. lactis and revealed a more oxidized cytoplasm at 37°C than at 30°C. These results indicate that L. lactis suffers from heat-induced oxidative stress at increased temperatures. A decrease in intracellular flavin adenine dinucleotide (FAD), which is derived from riboflavin, was observed with increasing growth temperature, but the presence of riboflavin made the decrease smaller. The drop was accompanied by a decrease in NADH oxidase and pyruvate dehydrogenase activities, both of which depend on FAD as a cofactor. By overexpressing the riboflavin transporter, it was possible to improve FAD biosynthesis, which resulted in increased NADH oxidase and pyruvate dehydrogenase activities and improved fitness at high temperatures in the presence of oxygen.
INTRODUCTION
Lactococcus lactis is a mesophilic bacterium with an optimum growth temperature around 30°C (1) that is widely used in the dairy industry for production of cheese and buttermilk, where it plays a crucial role in flavor and texture formation. During cheese production, L. lactis is frequently exposed to a variety of stresses, such as heat stress (2). Superoptimal temperatures cause the denaturation of macromolecules and induce chaperone proteins, such as DnaK, GroEL, and GroES, which then help proteins to fold correctly (2). The chaperones are essential for growth at elevated temperatures, and mutants with defects in chaperone activity display a pronounced temperature-sensitive phenotype (3). The opposite effect is sometimes seen when overexpressing chaperones, and in L. lactis this results in improved fitness and lactic acid production at high temperatures (4, 5). The viability of some L. lactis strains at high temperatures is also improved by an incorporation of high concentrations of NaCl into the medium or a preadaptation prior to inoculation (6), and this phenomenon was demonstrated to be correlated with an induction of several chaperones (7). Meanwhile, metabolic responses are regarded as important factors in handling heat stress. It has been demonstrated that genes in several metabolic pathways are important for handling heat stress (8), and a recently recognized c-di-AMP-specific phosphodiesterase in L. lactis was also found to be important for heat tolerance (9).
Besides heat stress, there has also been a lot of focus on oxidative stress in L. lactis. As a facultative anaerobic bacterium that generally is unable to respire and that is catalase negative, L. lactis is more vulnerable to oxidative stresses imposed by reactive oxygen species. The damage from oxidative stress can be alleviated when heme, which enables respiration in L. lactis, is added or heterologous catalase is introduced (10, 11). Several disulfide-reducing systems, such as the thioredoxin or glutathione systems, have been demonstrated to be important for handling oxygen stress (12, 13), where, albeit glutathione is not self-synthesized by L. lactis, the incorporation of glutathione into the medium improves fitness under oxidative conditions.
The defense mechanisms for handling heat and oxygen stress are often overlapping, e.g., a recA disruption mutant is also sensitive to oxidative stress derived from hydroxyl radicals (14). It has been demonstrated that the intracellular phosphate and guanine nucleotide pools, such as ppGpp, play a central role in the multiple stress response in L. lactis (15).
Lactic acid bacteria inhabit nutritionally rich environments, which often results in accumulation of various auxotrophies (16), and a factor that generally has not been studied in depth is the effect from limitations in nutrients on heat tolerance, such as those observed for Escherichia coli starved for methionine (17). For this study, we report that L. lactis starves for riboflavin, which is the precursor of the biologically important cofactor FAD, at superoptimal temperatures in the presence of oxygen, and we characterize how growth and important enzyme activities are affected. By adapting a previously described GFP redox sensor (18) to L. lactis, we were able to substantiate the existence of a more oxidized cytoplasm at high temperatures. Finally, we have demonstrated that riboflavin starvation can be alleviated by overexpressing the riboflavin transporter (19).
MATERIALS AND METHODS
Strains and growth conditions.
E. coli strains were grown aerobically in Luria-Bertani (LB) broth (20). L. lactis MG1363 (21) was cultivated in 300-ml flasks, 1-liter bioreactors, or 96-well microplates on a Tecan Infinite 200 Pro microplate reader in M17 (22) or in a modified SALN medium (23) without riboflavin, containing 0.2% glucose and an extra 40 mM morpholinepropanesulfonic acid (MOPS). When needed, 15 mM acetate was added.
Under static conditions in bioreactors, atmospheres were kept sterile by using 0.22-μm filters (Millipore). When required, stirring was used, and the speed of stirring was 100 rpm. Aerated cultures were obtained by shaking in flasks or by airflow and stirring in fermentors (dissolved oxygen > 90%). Dissolved oxygen was monitored using a Hamilton Oxyferm FDA 160 oxygen sensor, calibrated using airflow with stirring at 100 rpm (100%) and a saturated sodium sulfite anhydrous solution (0%). The totally anaerobic condition was maintained using N2 in the headspace of the bioreactors.
Analytical methods. (i) End product formation and glucose consumption.
The concentrations of glucose and lactate were determined using an Ultimate 3000 high-pressure liquid chromatography system (Dionex, Sunnyvale, CA) equipped with an Aminex HPX-87H column (Bio-Rad, Hercules, CA) and a Shodex RI-101 detector (Showa Denko KK, Tokyo, Japan). The column oven temperature was set to 30°C, and the mobile phase consisted of 5 mM H2SO4 with a flow rate of 0.5 ml/min. For flux calculations, the cell density was correlated to the corresponding dry cell mass of L. lactis MG1363 at 30°C (1 optical density unit at 600 nm [OD600] = 0.35 g/liter) and 37°C (1 OD600 unit = 0.34 g/liter), respectively.
(ii) Hydrogen peroxide.
For the supernatant assays, statically grown cultures were collected at an OD600 of 0.5 and subsequently filtered using 0.22-μm filters.
The assay was carried out as previously described (24). In a 1.5-ml cuvette, 1 ml sample, 5 μl 2,2′-azinobis (3-ethylbenzthiazoline sulfonic acid) (35 g/liter), and 5 μl of horseradish peroxidase (500 U/ml) were mixed thoroughly, and the absorption was immediately measured at 433 nm (ε = 17.1 mM/cm). No background in the extraction buffer was detected.
(iii) Enzyme assays.
Statically grown cultures were collected at an OD600 of 0.5 and centrifuged at 5,000 rpm for 10 min at 4°C. Then, pellets were washed twice in 0.2% cold KCl and resuspended in the extraction buffer (45 mM Tris, 15 mM tricarballylate, 20% glycerol, 4.5 mM MgCl2, 1 mM dithiothreitol [DTT]; pH = 7.2) and kept at −80°C until needed.
Cells were disrupted using FastPrep with acid-washed glass beads (106 μm; Sigma). Supernatants were separated from debris by centrifuging at 20,000 rpm for 5 min at 4°C. The supernatant was directly used in following enzyme activity assays. The protein concentration was measured using the Bradford method (25).
The reaction mixture for fructose-1,6-P2 aldolase contained triethanolamine-HCl buffer (100 mM; pH 7.2), KCl (200 mM), NADH (0.3 mM), glycerophosphate dehydrogenase (2 U), and triosephosphate isomerase (5 U) and was initiated by the addition of fructose-1,6-P2 (30 mM) (26). The reaction mixture for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) contained triethanolamine-HCl buffer (125 mM; pH 7.2), NAD+ (2 mM), sodium arsenate (5 mM) and cysteine-HCl (5 mM) and was initiated by the addition of dl-glyceraldehyde-3-phosphate (2 mM) (27). The reaction mixture for pyruvate dehydrogenase (PDH) contained phosphate buffer (100 mM; pH 7.2), MgCl2 (5 mM), 2-(p-iodophenyl)-3-p-nitrophenyltetrazolium chloride (0.6 mM), bovine serum albumin (1 g/liter), lipoamide dehydrogenase (0.1 mg/ml), dithiothreitol (0.3 mM), coenzyme A (CoA) (0.2 mM), thiamine pyrophosphate (0.2 mM), and NAD+ (2 mM) and was initiated by the addition of pyruvate (5 mM) (27). The reaction mixture for pyruvate kinase contained Tris-HCl buffer (100 mM; pH 7.2), MnSO4 (5 mM), KCl (10 mM), NADH (0.3 mM), lactate dehydrogenase (10 U), and GDP (3 mM) was initiated by the addition of phosphoenolpyruvate (PEP; 6 mM) (26).
For the lactate dehydrogenase assay, a special low-pH extraction buffer was used (50 mM triethanolamine, 10 mM KH2PO4, 10 mM EDTA, and 20% glycerol; pH 4.7). The reaction mixture for lactate dehydrogenase contained triethanolamine-HCl (90 mM; pH 6.9) and NADH (2 mM) and was initiated by the addition of fructose-1,6-biphosphate (10 mM) (28).
When NADH oxidase activity was measured, the cells were resuspended in potassium phosphate buffer (50 mM; pH 7.2) and disrupted as mentioned above. The reaction mixture for NADH oxidase contained potassium phosphate buffer (50 mM; pH 7.2) and EDTA (0.3 mM) and was initiated by the addition of NADH (0.3 mM) (29).
All enzyme assays were carried out at 30°C, and the reaction was followed spectrophotometrically at either 340 nm, for the assays involving NADH (ε = 6,220 M−1 · cm−1), or at 500 nm, for the PDH assay. For the latter assay, reduction of the electron acceptor 2-(p-iodophenyl)-3-p-nitrophenyltetrazolium chloride (INT) (ε = 12,400 M−1 · cm−1) was monitored. One unit of enzyme activity is defined as the amount of enzyme which catalyzes the oxidation of 1 μmol NADH or reduction of INT per hour at 30°C.
(iv) ATP/ADP.
Statically grown cultures (5 ml) at an OD600 of 0.5 were quenched with 1 volume 80°C phenol. After being centrifuged at 4,000 × g for 10 min at room temperature, the water phase was transferred into a new tube and extracted with 1 volume of chloroform twice. ATP was measured using a BioThema ATP kit HS, and ADP was converted into ATP by 1 mM PEP and 1 U pyruvate kinase as previously described (30). The luminescence was subsequently measured on a Tecan Infinite 200 Pro microplate reader.
(v) Flavin adenine dinucleotide.
Aerobically grown cells were collected at an OD600 of 0.3 at 4°C. After washing two times with cold 0.9% NaCl, FAD was extracted using 1 ml 10% trichloracetic acid on ice for 60 min. After centrifugation at 20,000 rpm for 5 min at 4°C, 400 μl supernatant was immediately neutralized by adding 100 μl 4 M K2HPO4, and a second 400-μl supernatant was incubated at 37°C overnight away from light. After the FAD in the second supernatant had been completely hydrolyzed to flavin mononucleotide (FMN), it was neutralized by adding 100 μl 4 M K2HPO4 as well. Measurements were based on the different fluorescences (F) of FAD, FMN, and riboflavin (FFAD = 0.15 FFMN = 0.15 Friboflavin) under a high-salt condition (31). Riboflavin was used as the standard, so the content of FAD was defined as the amount of riboflavin with the same intensity of fluorescence. FAD was calculated using the following equation: FAD = (R2 − R1)/0.85, where R1 was the resulting amount as riboflavin from the first supernatant and R2 was from the second one. Fluorescence was measured on a Tecan Infinite 200 Pro microplate reader at an excitation wavelength (λex) of 450 nm and an emission wavelength (λem) of 530 nm. No background fluorescence was detected in this method.
Construction of redox-sensitive GFP and measurement of fluorescence.
A codon-optimized redox-sensitive GFP, roGFP1-R12 (18), was used. The gapB promoter region in L. lactis MG1363 was amplified using the following primers: JC0013, CCGGAATTCGAATAAAAATTACTGACAGC; JC0014, CGCGGATCCTAGTAGTTTCCTCCTTATAG. The groESL terminator region from L. lactis MG1363 was amplified using the following primers: JC0009, CTAGTCTAGATAAAAAAAAGAACCCGAGTG; JC0010, AAAACTGCAGTTTGGGACACTTAAGTCTAA. These three fragments were ligated into the multiple cloning site of the vector pCI372 (32) and transformed into L. lactis MG1363, which led to the roGFP-containing strain JC013R12.
JC013R12 was cultivated in SALN medium with 5 μg/ml chloramphenicol, where all the nucleotides and riboflavin had been removed and an additional 120 mM MOPS and 0.5% glucose had been added, with shaking at 30°C or 37°C. At the end of the exponential phase, 50-ml samples were centrifuged at 7,000 rpm for 5 min at room temperature. The pellet was resuspended in 1 to 2 ml prewarmed fresh medium. The excitation spectrum was determined using a Shimadzu RF-5301PC spectrofluorometer, where the emission wavelength was fixed at 508 nm (slit widthex = 10 nm; slit widthem = 5 nm). The background reduction was carried out using cells not expressing roGFP.
Construction of the strain with site-specific integration of an additional ribU allele on the chromosome.
The gene encoding the riboflavin transporter, ribU, was fused to a library of synthetic promoters using a previously published method (33) and the following primers: forward, CTAGACTAGTGGATGCATNNNNNAGTTTATTCTTGACANNNNNNNNNNNNNNTGRTATAATNNNNAAGTAATAAAATATTCGGAGGAATTTTGAAATGTCTAAAACACGTCGGATG; reverse, ACGCGTCGACTTAAGCATTGTAAAATTTTAC. The resulting PCR product was digested with SpeI and SalI and ligated to pLB85 digested with the same enzymes and electroporated into L. lactis expressing the TP901-1 integrase, which allows pLB85-type plasmids to site-specifically integrate into the chromosome (34). After electroporation, cells were plated on GM17 plates containing 5 μg/ml of erythromycin and 100 μg/ml 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid (X-Gluc). In this way, strains with different expression levels of RibU were obtained (33). Strain JC034 expresses RibU strongly, as estimated from the intensity of the blue-green color. Growth appeared to be unaffected by the overexpression of RibU.
The original ribU promoter region of L. lactis MG1363 was also inserted into the chromosome by using the same approach and the following primers: forward (XbaI), CTAGTCTAGATGCGCAAGGAAAATAGTTTA; reverse (PstI), AAAACTGCAGAATTAATACCATCCGACGTG. This resulted in strain JC045.
β-Glucuronidase activity assay.
β-Glucuronidase activities were determined by using the procedure described by Miller (35) and modified by Israelsen et al. (36), except that para-nitro-β-glucuronic acid (Biosynth AG) was used as the substrate (37). The activity is given in Miller units per mg cell dry weight and calculated as described by Miller (35).
RESULTS
Effect of riboflavin on growth and fluxes at high temperatures.
Riboflavin was discovered to have a positive effect on growth of L. lactis at high temperatures in static cultures (with dissolved oxygen introduced during preparation but without further aeration). A thorough characterization of growth at various temperatures was carried out, where several parameters deemed to be important were investigated. First, L. lactis was grown at different temperatures in defined medium with or without riboflavin. At 30°C, the dissolved oxygen was rapidly consumed, irrespective of the presence of riboflavin (Fig. 1A and B). Raising the temperature to 37°C resulted in a modest decrease in the specific growth rate (∼15%) with riboflavin (Fig. 1C), but in its absence growth was severely hampered and dissolved oxygen was consumed only slowly (Fig. 1D). It was found that riboflavin-containing medium could support growth up to 38°C.
Fig 1.
The effect of exogenous riboflavin on growth and oxygen consumption of L. lactis MG1363 under static conditions at different temperatures. (A) Growth at 30°C with exogenous riboflavin. (B) Growth at 30°C without exogenous riboflavin. (C) Growth at 37°C with exogenous riboflavin. (D) Growth at 37°C without exogenous riboflavin.
At 37°C, two growth phases were apparent, coinciding with the presence and absence of oxygen, respectively, and it was clear that oxygen affected growth negatively (Fig. 1C and D). After rapid depletion of oxygen, the specific growth rate accelerated in the one with riboflavin and approached that at 30°C (Table 1).
Table 1.
Specific growth rate, glucose and lactate fluxes, and ratio of ATP to ADP obtained for cultures grown under static (first four columns), fully aerated, or anaerobic conditions
| Parameter | Value for conditionb |
|||||
|---|---|---|---|---|---|---|
| 30°C | 30°C, R− | 37°C | 37°C, R− | 37°C, Aera | 37°C, R−, Ana | |
| μ (h−1)a | 1.12 ± 0.01 | 1.11 ± 0.01 | 0.95 ± 0.06 | 0.31 ± 0.05 | 0.61 ± 0.00 | 1.13 ± 0.09 |
| Glucose flux (mmol · g−1 · h−1) | 22.3 ± 0.7 | 23.7 ± 0.5 | 26.0 ± 0.6 | 13.0 ± 1.4 | 17.5 ± 1.1 | 31.9 ± 0.9 |
| Lactate flux (mmol · g−1 · h−1) | 40.6 ± 2.7 | 43.6 ± 0.0 | 47.6 ± 0.1 | 25.6 ± 3.0 | 29.8 ± 1.0 | 57.7 ± 0.6 |
| ATP/ADP | ND | ND | 4.9 ± 0.5 | 1.4 ± 0.2 | ND | 5.1 ± 0.8 |
The specific growth rates are the averages obtained from at least two independent experiments. The regression lines are shown in Fig. 1.
Abbreviations: R−, without exogenous riboflavin; Aera, with aeration; Ana, anaerobic condition with N2; ND, not determined.
Two additional growth experiments were carried out at 37°C to analyze the effects from oxygen in the medium. It was found that under anaerobic conditions, without riboflavin, growth was unaffected by the high temperature (Table 1). Adding riboflavin did not affect growth. To further assess the effect of oxygen, a fully aerobic growth experiment with riboflavin was carried out. The result was a 30% reduction in the specific growth rate (Table 1) compared to that with static cultivation, but the growth rate was still two times higher than that without riboflavin.
In addition to the growth rate, we also determined the glucose consumption rate and product formation rates. Compared to results for cultures at 30°C, intriguingly, at 37°C with riboflavin, the static cultures showed increases in the glucose and lactate fluxes of 17% (Table 1). Under anaerobic conditions at 37°C, the effect was even larger, and the glucose and lactate fluxes had increased by 42%.
The intracellular ratio of [ATP] to [ADP] was also determined as an indication of the energy state of the cells. Under static conditions at 37°C with riboflavin, the [ATP]/[ADP] ratio was around 5, which is close to what is normally found at 30°C for L. lactis (38), whereas at 37°C without riboflavin, the ratio was found to be 1.4. Under anaerobic conditions at 37°C, where growth was fast, a ratio around 5 was found.
Comparison of key enzyme activities at 37°C.
Due to the strong effect of riboflavin and oxygen on the metabolic fluxes and [ATP]/[ADP] ratio at 37°C, the activities of a selection of glycolytic enzymes and pyruvate dehydrogenase (PDH) were determined in cells grown with and without riboflavin. PDH was included because this enzyme is required for formation of acetyl-CoA when oxygen is present (39). The relative activities found can be seen in Fig. 2. No significant differences in glycolytic activities were found, but the PDH activity was about 1.5 times higher with riboflavin.
Fig 2.
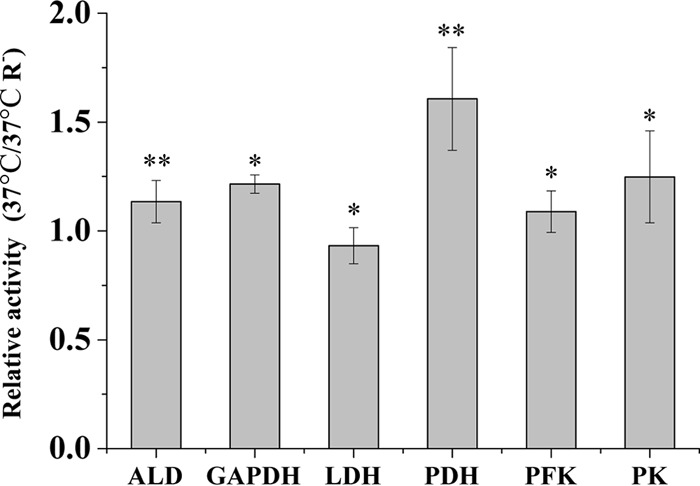
The effect of riboflavin on selected central enzyme activities. The ratio of the activity found in the presence of riboflavin to that without riboflavin is shown. ALD, aldolase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LDH, lactate dehydrogenase; PDH, pyruvate dehydrogenase; PFK, phosphofructokinase; PK, pyruvate kinase. All activities were determined using three independent samples. ∗, P > 0.05; ∗∗, P < 0.01.
Incorporation of exogenous acetate.
The lower activity of PDH without riboflavin could limit the supply of acetyl-CoA, which is essential for fatty acid biosynthesis, and thereby limit growth. One way to overcome this limitation is to add acetate to the medium, which can be taken up and converted into acetyl-CoA (40). Indeed, by adding acetate (15 mM), it was possible to stimulate growth in the absence of riboflavin at 37°C (Fig. 3). Faster growth and a higher final OD600 were observed when acetate was added, and the [ATP]/[ADP] ratio was found to be 2.5 ± 0.7 here.
Fig 3.
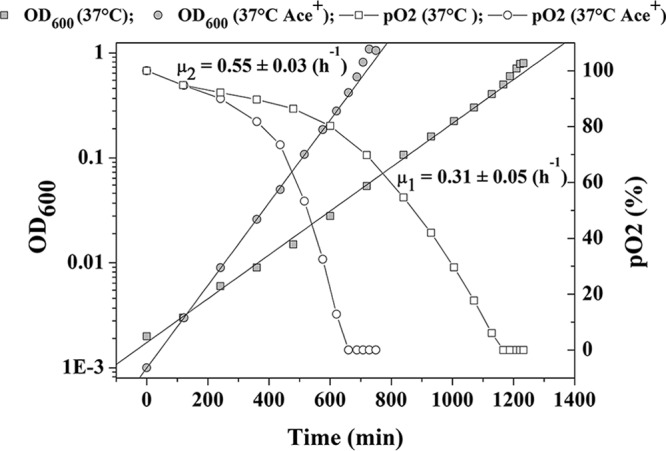
The effect from adding acetate on growth at 37°C without riboflavin. Ace+, with exogenous acetate; μ1, the specific growth rate without acetate; μ2, the specific growth rate with acetate.
Using a redox-sensitive GFP to assess redox status at high temperature in L. lactis MG1363.
The deleterious effects that oxygen has on L. lactis at high temperatures could be the result of a change in the redox status of the cytoplasm. To determine whether the redox status had changed, a redox-sensitive GFP, roGFP1-R12, was constructed and introduced into L. lactis MG1363 (resulting in strain JC013R12), thus allowing real-time intracellular assessment of the redox status using fluorescence. Since a long folding time is needed for GFP (41), all the nucleotides were removed from the medium to slow down the growth. By treating the live bacteria with either 10 mM H2O2 or 10 mM DTT, it was possible to get excitation spectra corresponding to the fully oxidized and reduced conditions (Fig. 4A). The ratio of emission intensities at the two excitation wavelengths 400 nm and 475 nm are used to estimate the redox state (4), and this ratio was found to be 1.45 ± 0.05 and 1.93 ± 0.25 at 30°C and 37°C, respectively, while the fully oxidized and reduced standards were 3.09 and 0.52 (Fig. 4B). Thus, by comparing spectra from bacteria at 30 and 37°C, it was possible to conclude that the cytoplasm was more reduced at 30°C than at 37°C.
Fig 4.
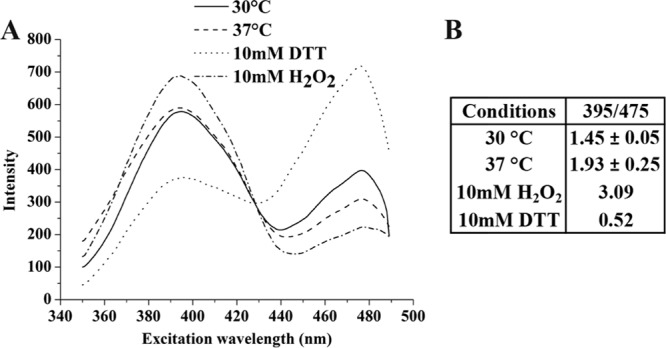
The excitation spectra of redox-sensitive GFP in L. lactis MG1363 at the different temperatures. (A) excitation spectrum; (B) excitation ratio for 395 nm/475 nm. The standard deviations were calculated from three independent samples.
Analysis of temperature- and riboflavin-dependent FAD, NADH oxidase, and pyruvate dehydrogenase activities.
Riboflavin does not have biological functions per se but is the precursor for FAD, which is an important cofactor involved in several flavoproteins, such as NADH oxidase and pyruvate dehydrogenase (42, 43, 56). To substantiate whether riboflavin and heat stress affect the biosynthesis of FAD, the intracellular FAD content was measured (Fig. 5). At 30°C, the incorporation of riboflavin hardly affected the total intracellular FAD content, which was 66.9 ± 1.2 (μg/g wet weight) with riboflavin and 63.5 ± 0.8 (μg/g wet weight) without riboflavin. However, when the temperature was raised to 37°C, there was a significant reduction in the FAD content to 33.2 ± 2 (μg/g wet weight) with riboflavin in the medium. If riboflavin was removed, the FAD content dropped to 19.6 ± 1.1 (μg/g wet weight). A direct correlation between FAD content and the activities of NADH oxidase and pyruvate dehydrogenase was observed (Fig. 5). Increasing the temperature or removing riboflavin both lead to reduced activities, and temperature especially had a profound effect. Exogenous FAD was added to see if it could relieve the intracellular FAD deficiency and thereby recover the activity of NADH oxidase, but this was not found to be the case.
Fig 5.

The intracellular FAD contents and NoxE and PDH activities from aerobically grown MG1363 under different conditions. The data shown are the averages obtained from at least three independent samples. R−, without exogenous riboflavin; F+, with exogenous FAD. PDH activity was measured only at 30°C and 37°C with riboflavin.
Overexpression of the riboflavin transporter RibU in L. lactis MG1363.
At high temperatures, a low riboflavin transport activity may limit formation of FAD, since riboflavin is a precursor for FAD. To substantiate this, we constructed an L. lactis strain (JC034) overexpressing the riboflavin transporter, RibU, using a strong synthetic promoter (SP). In JC034, the overexpressed ribU gene is transcriptionally fused to the reporter gene gusA, thus allowing easy estimation of expression. In order to be able to compare the strength of the synthetic promoter with that of the native ribU promoter, a strain containing the ribU promoter in front of gusA was also constructed. The synthetic promoter resulted in an almost 15-fold increase in RibU expression at 38°C (β-glucuronidase activity of JC034 was 70.08 ± 2.98 [Miller units per mg cell dry weight], whereas the strain with the ribU promoter resulted in an activity of 5.12 ± 0.31 [Miller units per mg cell dry weight]). Under fully aerated conditions at 38°C, an increase in the specific growth rate of more than 20% was observed when RibU was overexpressed. More specifically, the specific growth rate of JC034 was 0.62 ± 0.02 (h−1), compared to 0.52 ± 0.03 (h−1) for the wild type. At 37°C, the FAD content of this strain was found to be 47.3 ± 1.44 (μg/g wet weight), and the NoxE and PDH activity were 11.32 ± 1.59 (U/mg) and 11.07 ± 0.63 (U/mg), respectively. Compared to the wild-type strain grown under the same conditions, this is an increase of 50% in FAD content and NoxE activity and a 30% increase in PDH activity.
Importance of riboflavin for high-temperature growth of other L. lactis strains.
L. lactis can be found in different niches and consists of two major subspecies, namely, L. lactis subsp. lactis and L. lactis subsp. cremoris, that display different phenotypes (e.g., temperature and salt tolerance and substrate utilization) (44). L. lactis subsp. cremoris MG1363, which was used in this study, is a well-characterized plasmid-free derivative of a dairy strain. It is possible that the effect of riboflavin at high temperatures is specific to MG1363 which is why we decided to investigate its effect on a selection of other L. lactis strains. Both subspecies as well as two plant isolates (KF147 and IO-1) were investigated. It was found that riboflavin was important for all the strains investigated (Table 2). With the exception of IO-1 and KF147, all L. lactis strains were affected at 37°C, and FHCY-1 and 337 were unable to grow without riboflavin at this temperature. The latter two strains were even affected at 30°C. The plant isolates KF147 and IO-1 were not affected at 37°C but were strongly affected at 40°C, where IO-1 grew more slowly and KF147 was unable to grow without riboflavin. We also tested another commonly used laboratory strain, IL1403 (L. lactis subsp. lactis) (61), and this strain was unable to grow without riboflavin at 30°C (data not shown).
Table 2.
Effect of riboflavin on high-temperature growth for diverse L. lactis strains
| Strain (source or reference) | L. lactis subspecies | Origin | Growth at indicated temp (°C)a |
||
|---|---|---|---|---|---|
| 30 | 37 | 40 | |||
| IO-1 (45) | lactis | Nondairy | NDb | + | − |
| KF147 (46) | lactis | Nondairy | ND | + | − − |
| FHCY-1 (CHCC)c | lactis | Dairy | − | − − | ND |
| C2 (47) | lactis | Dairy | + | − | ND |
| TC-1 (CHCC) | cremoris | Dairy | + | − | ND |
| MB324 (CHCC) | cremoris | Dairy | + | − | ND |
| 337 (CHCC) | Uncharacterized | Dairy | − | − − | ND |
“+,” normal growth without riboflavin; “−”, reduced growth without riboflavin; “− −,” no growth without riboflavin. The detailed growth profiles are shown in Fig. S1 in the supplemental material.
ND, not determined.
CHCC, Chr. Hansen Culture Collection.
DISCUSSION
In this study, the importance of riboflavin for growth of L. lactis at high temperatures in the presence of oxygen was demonstrated. An inability to grow without exogenous riboflavin at 38°C exactly indicates its necessity for survival at high temperatures, even though a sufficient biosynthesis capacity has been proven to exist at lower temperatures (48). When riboflavin was removed at 37°C, the bacteria had difficulties removing the dissolved oxygen, and this caused a dramatic decline in the specific growth rate, and only close to stationary-phase anaerobic conditions were reached. With supplementation of riboflavin, a slow-growth phase was also observed, but it was shortened drastically, and after the oxygen had been depleted, the growth rate accelerated and approached that at 30°C, whereas at 30°C under static conditions, the presence of dissolved oxygen did not have any effects on growth. This demonstrates that high temperatures can induce oxidative stress that is seen even in the presence of riboflavin, but riboflavin apparently can alleviate the stress by enabling rapid consumption of oxygen. In lactic acid bacteria, oxidative stress is generally derived from hydrogen peroxide, which can result in production via the Fenton reaction of reactive hydroxyl radicals, which are considered far more toxic and that can damage cell components such as DNA, protein, and the cell membrane (49). However, the accumulation of H2O2 was not observed here (data not shown). It was speculated that the oxidative stress was derived from oxygen itself, which is known to be able to cause less-reactive oxidative damage (49). The fact that oxygen was indeed at play was further substantiated by the observation that growth was accelerated after depletion of oxygen (Fig. 1C and 1D) and that an anaerobic fermentation without riboflavin at 37°C was unaffected by the high temperature (Table 1).
On the other hand, it was observed that the glucose consumption and lactate production fluxes were enhanced by the elevated growth temperature (Table 1). Indeed, several studies have shown increased glycolytic flux at high temperatures for L. lactis, while growth is uncoupled (50, 51). It is not surprising that heat stress imposes an energy burden and stimulates ATP recycling via glycolysis to fulfil the energy demand for survival (52). In this study, due to the presence of oxygen at 37°C, however, a negative effect on glycolysis was observed, and the fluxes were below what was found at 30°C. This is similar to what happens under acid stress, where inhibition of glycolytic enzymes leads to a catabolic repression and decreased energy production (53). Because L. lactis lacks oxidative phosphorylation and depends on substrate phosphorylation to fulfil the ATP demand via glycolysis (54), the response is expected to be particularly severe. The low glycolytic flux was reflected in a low ATP-to-ADP ratio of only 1.4, which is known to be insufficient for normal growth of L. lactis (38). However, under anaerobic conditions, the ratio returned to a normal value around 5. This indicates that glycolysis might be affected by oxygen. It is known that some glycolytic enzymes, such as glucokinase (GK), GAPDH, and aldolase (ALD), are targets for oxidative attack (24, 49), and lactate dehydrogenase is also strongly inhibited by oxidative stress rather than heat stress (55). However, measuring the activities of several glycolytic enzymes did not reveal any obvious differences (Fig. 2). The PDH activity was also measured, and here a large drop in activity was found. In the presence of oxygen, L. lactis relies on PDH rather than pyruvate formate lyase (PFL) to supply the acetyl-CoA which is needed for biosynthesis of lipids (40) and thereby also for growth. Acetyl-CoA can, however, be derived from acetate, and it has previously been shown that under conditions where PDH is inhibited, adding acetate can help recover growth (40). We also found that growth at 37°C without riboflavin was partially improved by adding acetate. In this case, lack of riboflavin may cause the deficiency of FAD, which is involved in the pyruvate dehydrogenase complex E3 (dihydrolipoyl dehydrogenase) as a cofactor (42), which in turn will result in reduced PDH activity. That acetate has this beneficial effect clearly demonstrates that PDH is affected severely.
To verify the deficiency of FAD, the intracellular FAD content at different growth conditions was measured (Fig. 5), and some interesting phenomena were uncovered. First, at 30°C, we found that adding riboflavin did not affect the intracellular FAD content significantly, which confirms that riboflavin biosynthesis indeed is sufficient to support optimal growth (48). However, at 37°C, this seemed not to be the case, and biosynthesis of FAD was dependent on exogenous riboflavin. Second, even with riboflavin in the medium, the intracellular FAD content was much lower than that at 30°C, and it appears that either the riboflavin transporter or FAD biosynthesis is less active at 37°C. The PDH and NADH oxidase activities, which also depend on FAD (42, 56), correlated nicely with the intracellular FAD content (Fig. 5). NADH oxidase, which converts O2 into H2O using NADH, is the main enzyme involved in oxygen consumption in L. lactis (57). The higher FAD and NoxE activity in the presence of riboflavin at 37°C could apparently provide the explanation for the difference in oxygen consumption (Fig. 1C and 1D). It has been shown that a general NADH oxidase activity, which efficiently consumes dissolved oxygen, is not important for L. lactis as a protection against oxidative stress under normal growth conditions (57). Indeed, this was confirmed in our study, since the residual O2 did not affect exponential growth at 30°C. At the higher temperature, on the contrary, there were notable effects on growth caused by the presence of oxygen in the medium, and under these conditions a high NADH oxidase activity is definitely an advantage (Fig. 1C).
The results obtained strongly indicate that oxygen has negative effects on growth at high temperatures, but additional indicators of the intracellular redox state are useful for substantiating this conclusion. For this purpose, we adapted a redox-sensitive GFP (roGFP1-R12, a fast-responding roGFP mutant), enabling real-time assessment of the intracellular redox state (18). This system, to our best knowledge, has never been used in L. lactis before and did indeed reveal a more oxidized cytoplasm at high temperatures. An oxidized cytoplasm could have multiple consequences, and formation of disulfide bonds in various proteins is inevitable. This will most likely affect the activities of many enzymes, and for instance phosphofructokinase is inactivated rapidly in the presence of oxygen (data not shown). Maintaining a reduced cytoplasm, via glutathione and thioredoxin reductase (flavoprotein disulfide reductase [FDR]), is probably essential for L. lactis in order to survive aerobic conditions (12, 13). The activities of FDR depend on the incorporation of FAD during enzyme synthesis, and the bound FAD is involved in transfer of electrons from NADPH to oxidized glutathione or thioredoxin. This step is essential for the recycling of these thiol-containing molecules so that they can be functional in the thioredoxin-thioredoxin reductase and glutathione-glutathione reductase systems, which maintain the reduced state of the cytoplasm (58). Previous research indicates that the activities of FDR depend on the FAD concentration during cofactor incorporation (59). In this study, the low FAD content at the high temperature could result in a less-active FDR, which causes the oxidative stress.
In principle, it could be either riboflavin biosynthesis or FAD biosynthesis that was affected by the high temperature. Overexpression of riboflavin transporter rather than biosynthesis was chosen here to avoid an additional metabolic load. Overexpressing the riboflavin transporter was proven to alleviate FAD starvation and improve aerobic growth at high temperatures. As expected, both NADH oxidase and PDH activities were increased.
The current work was carried out using L. lactis subsp. cremoris MG1363 as a model organism, and in order be able to generalize the findings, additional L. lactis strains had been tested. We found that riboflavin had beneficial effects on all the strains examined at elevated temperatures (Table 2) and for some even at 30°C. The effect was found to be independent of subspecies and therefore cannot explain the better thermotolerance displayed generally by L. lactis subsp. lactis (44). Some of the dairy strains examined were found to be quite temperature sensitive without riboflavin, and this may be a result of adaptation to growth in milk which is rich in riboflavin (43).
To sum up, at high temperatures L. lactis suffers from oxidative stress primarily caused by riboflavin starvation, which reduces NADH oxidase and pyruvate dehydrogenase activities. The findings could have industrial interest because growth at high temperatures leads to a larger lactate flux, which could speed up the fermentation process or alternatively reduce starter culture costs. If dissolved oxygen is removed prior to inoculation, the benefits would probably be even greater and lead to a more consistent fermentation, since we have demonstrated that different L. lactis strains, and in particular dairy strains, can indeed be very sensitive to high-temperature oxidative stress. The advantage of high-temperature cultivation of lactic acid bacteria has also been stated in the literature previously (60). Although this study has identified the importance of riboflavin for growth at high temperatures, further research is needed in order to be able to fully understand the molecular basis of heat-induced riboflavin starvation and oxidative stress.
Supplementary Material
Footnotes
Published ahead of print 2 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01953-13.
REFERENCES
- 1.Lee DA, Collins EB. 1976. Influences of temperature on growth of Streptococcus cremoris and Streptococcus lactis. J. Dairy Sci. 59:405–409 [Google Scholar]
- 2.van de Guchte M, Serror P, Chervaux C, Smokvina T, Ehrlich SD, Maguin E. 2002. Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek 82:187–216 [PubMed] [Google Scholar]
- 3.Paek KH, Walker GC. 1987. Escherichia coli DnaK null mutants are inviable at high temperature. J. Bacteriol. 169:283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdullah-Al-Mahin Sugimoto S, Higashi C, Matsumoto S, Sonomoto K. 2010. Improvement of multiple-stress tolerance and lactic acid production in Lactococcus lactis nz9000 under conditions of thermal stress by heterologous expression of Escherichia coli dnaK. Appl. Environ. Microbiol. 76:4277–4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desmond C, Fitzgerald GF, Stanton C, Ross RP. 2004. Improved stress tolerance of GroESL-overproducing Lactococcus lactis and probiotic Lactobacillus paracasei NFBC 338. Appl. Environ. Microbiol. 70:5929–5936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilstrup M, Hammer K. 2000. Short communication: salt extends the upper temperature limit for growth of Lactococcus lactis ssp. cremoris on solid M17 medium. J. Dairy Sci. 83:1448–1450 [DOI] [PubMed] [Google Scholar]
- 7.Kilstrup M, Jacobsen S, Hammer K, Vogensen FK. 1997. Induction of heat shock proteins DnaK, GroEl, and GroES by salt stress in Lactococcus lactis. Appl. Environ. Microbiol. 63:1826–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duwat P, Ehrlich SD, Gruss A. 1999. Effects of metabolic flux on stress response pathways in Lactococcus lactis. Mol. Microbiol. 31:845–858 [DOI] [PubMed] [Google Scholar]
- 9.Smith WM, Pham TH, Lei L, Dou J, Soomro AH, Beatson SA, Dykes GA, Turner MS. 2012. Heat resistance and salt hypersensitivity in Lactococcus lactis due to spontaneous mutation of llmg_1816 (gdpP) induced by high-temperature growth. Appl. Environ. Microbiol. 78:7753–7759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duwat P, Sourice S, Cesselin B, Lamberet G, Vido K, Gaudu P, Le Loir Y, Violet F, Loubiere P, Gruss A. 2001. Respiration capacity of the fermenting bacterium Lactococcus lactis and its positive effects on growth and survival. J. Bacteriol. 183:4509–4516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rochat T, Miyoshi A, Gratadoux JJ, Duwat P, Sourice S, Azevedo V, Langella P. 2005. High-level resistance to oxidative stress in Lactococcus lactis conferred by Bacillus subtilis catalase KatE. Microbiology 151:3011–3018 [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Hugenholtz J, Abee T, Molenaar D. 2003. Glutathione protects Lactococcus lactis against oxidative stress. Appl. Environ. Microbiol. 69:5739–5745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vido K, Diemer H, Van Dorsselaer A, Leize E, Juillard V, Gruss A, Gaudu P. 2005. Roles of thioredoxin reductase during the aerobic life of Lactococcus lactis. J. Bacteriol. 187:601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duwat P, Ehrlich SD, Gruss A. 1995. The recA gene of Lactococcus lactis: characterization and involvement in oxidative and thermal stress. Mol. Microbiol. 17:1121–1131 [DOI] [PubMed] [Google Scholar]
- 15.Rallu F, Gruss A, Ehrlich SD, Maguin E. 2000. Acid-and multistress-resistant mutants of Lactococcus lactis: identification of intracellular stress signals. Mol. Microbiol. 35:517–528 [DOI] [PubMed] [Google Scholar]
- 16.Makarova K, Slesarev A, Wolf Y, Sorokin A, Mirkin B, Koonin E, Pavlov A, Pavlova N, Karamychev V, Polouchine N, Shakhova V, Grigoriev I, Lou Y, Rohksar D, Lucas S, Huang K, Goodstein DM, Hawkins T, Plengvidhya V, Welker D, Hughes J, Goh Y, Benson A, Baldwin K, Lee JH, Díaz-Muñiz I, Dosti B, Smeianov V, Wechter W, Barabote R, Lorca G, Altermann E, Barrangou R, Ganesan B, Xie Y, Rawsthorne H, Tamir D, Parker C, Breidt F, Broadbent J, Hutkins R, O'Sullivan D, Steele J, Unlu G, Saier M, Klaenhammer T, Richardson P, Kozyavkin S, Weimer B, Mills D. 2006. Comparative genomics of the lactic acid bacteria. Proc. Natl. Acad. Sci. U. S. A. 103:15611–15616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ron EZ, Davis BD. 1971. Growth rate of Escherichia coli at elevated temperatures—limitation by methionine. J. Bacteriol. 107:391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannon MB, Remington SJ. 2006. Re-engineering redox-sensitive green fluorescent protein for improved response rate. Protein Sci. 15:45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess CM, Slotboom DJ, Geertsma ER, Duurkens RH, Poolman B, van Sinderen D. 2006. The riboflavin transporter RibU in Lactococcus lactis: molecular characterization of gene expression and the transport mechanism. J. Bacteriol. 188:2752–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook J, Russell D. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 21.Gasson MJ. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terzaghi BE, Sandine W. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michelsen O, Hansen FG, Albrechtsen B, Jensen PR. 2010. The MG1363 and IL1403 laboratory strains of Lactococcus lactis and several dairy strains are diploid. J. Bacteriol. 192:1058–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Niel EW, Hofvendahl K, Hahn-Hagerdal B. 2002. Formation and conversion of oxygen metabolites by Lactococcus lactis subsp. lactis ATCC 19435 under different growth conditions. Appl. Environ. Microbiol. 68:4350–4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 26.Even S, Lindley ND, Cocaign-Bousquet M. 2001. Molecular physiology of sugar catabolism in Lactococcus lactis IL1403. J. Bacteriol. 183:3817–3824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garrigues C, Loubiere P, Lindley ND, Cocaign-Bousquet M. 1997. Control of the shift from homolactic acid to mixed-acid fermentation in Lactococcus lactis: predominant role of the NADH/NAD+ ratio. J. Bacteriol. 179:5282–5287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crow VL, Pritchard GG. 1977. Fructose 1,6-diphosphate-activated l-lactate dehydrogenase from Streptococcus lactis: kinetic properties and factors affecting activation. J. Bacteriol. 131:82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tachon S, Brandsma JB, Yvon M. 2010. NoxE NADH oxidase and the electron transport chain are responsible for the ability of Lactococcus lactis to decrease the redox potential of milk. Appl. Environ. Microbiol. 76:1311–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koebmann BJ, Westerhoff HV, Snoep JL, Nilsson D, Jensen PR. 2002. The glycolytic flux in Escherichia coli is controlled by the demand for ATP. J. Bacteriol. 184:3909–3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bessey OA, Lowry OH, Love RH. 1949. The fluorometric measurement of the nucleotides of riboflavin and their concentration in tissues. J. Biol. Chem. 180:755–769 [PubMed] [Google Scholar]
- 32.Hayes F, Daly C, Fitzgerald GF. 1990. Identification of the minimal replicon of Lactococcus lactis subsp. lactis UC317 plasmid pCI305. Appl. Environ. Microbiol. 56:202–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solem C, Jensen PR. 2002. Modulation of gene expression made easy. Appl. Environ. Microbiol. 68:2397–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brøndsted L, Hammer K. 1999. Use of the integration elements encoded by the temperate lactococcal bacteriophage tp901-1 to obtain chromosomal single-copy transcriptional fusions in Lactococcus lactis. Appl. Environ. Microbiol. 65:752–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller JH. 1972. Experiments in molecular genetics, p 352–359 Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 36.Israelsen H, Madsen SM, Vrang A, Hansen EB, Johansen E. 1995. Cloning and partial characterization of regulated promoters from Lactococcus lactis Tn917-lacZ integrants with the new promoter probe vector, pAK80. Appl. Environ. Microbiol. 61:2540–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Platteeuw C, Simons G, de Vos WM. 1994. Use of the Escherichia coli beta-glucuronidase (gusA) gene as a reporter gene for analyzing promoters in lactic acid bacteria. Appl. Environ. Microbiol. 60:587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koebmann BJ, Solem C, Pedersen MB, Nilsson D, Jensen PR. 2002. Expression of genes encoding F1-ATPase results in uncoupling of glycolysis from biomass production in Lactococcus lactis. Appl. Environ. Microbiol. 68:4274–4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jensen NB, Melchiorsen CR, Jokumsen KV, Villadsen J. 2001. Metabolic behavior of Lactococcus lactis MG1363 in microaerobic continuous cultivation at a low dilution rate. Appl. Environ. Microbiol. 67:2677–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nordkvist M, Jensen NB, Villadsen J. 2003. Glucose metabolism in Lactococcus lactis MG1363 under different aeration conditions: requirement of acetate to sustain growth under microaerobic conditions. Appl. Environ. Microbiol. 69:3462–3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heim R, Cubitt AB, Tsien RY. 1995. Improved green fluorescence. Nature 373:663–664 [DOI] [PubMed] [Google Scholar]
- 42.de Kok A, Hengeveld AF, Martin A, Westphal AH. 1998. The pyruvate dehydrogenase multi-enzyme complex from gram-negative bacteria. Biochim. Biophys. Acta 1385:353–366 [DOI] [PubMed] [Google Scholar]
- 43.Massey V. 2000. The chemical and biological versatility of riboflavin. Biochem. Soc. Trans. 28:283–296 [PubMed] [Google Scholar]
- 44.Rademaker JLW, Herbet H, Starrenburg MJC, Naser SM, Gevers D, Kelly WJ, Hugenholtz J, Swings J, van Hylckama Vlieg JET. 2007. Diversity analysis of dairy and nondairy Lactococcus lactis isolates, using a novel multilocus sequence analysis scheme and (GTG)5-PCR fingerprinting. Appl. Environ. Microbiol. 73:7128–7137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishizaki A. 1990. Biochemical characterization of Lactococcus lactis IO-1 whose optimal temperature is as high as 37°C. J. Gen. Appl. Microbiol. 36:1–6 [Google Scholar]
- 46.Kelly WJ, Davey GP, Ward LJH. 1998. Characterization of lactococci isolated from minimally processed fresh fruit and vegetables. Int. J. Food Microbiol. 45:85–92 [DOI] [PubMed] [Google Scholar]
- 47.Huggins AR, Sandine WE. 1977. Incidence and properties of temperate bacteriophages induced from lactic streptococci. Appl. Environ. Microbiol. 33:184–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burgess C, O'Connell-Motherway M, Sybesma W, Hugenholtz J, van Sinderen D. 2004. Riboflavin production in Lactococcus lactis: potential for in situ production of vitamin-enriched foods. Appl. Environ. Microbiol. 70:5769–5777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cesselin B, Derré-Bobillot A, Fernandez A, Lamberet G, Lechardeur D, Yamamoto Y, Pedersen MB, Garrigues C, Gruss A, Gaudu P. 2011. Responses of lactic acid bacteria to oxidative stress, stress responses of lactic acid bacteria, p 111–127 In Tsakalidou E, Papadimitriou K. (ed), Stress responses of lactic acid bacteria. Springer, New York, NY [Google Scholar]
- 50.Adamberg K, Kask S, Laht TM, Paalme T. 2003. The effect of temperature and pH on the growth of lactic acid bacteria: a pH-auxostat study. Int. J. Food Microbiol. 85:171–183 [DOI] [PubMed] [Google Scholar]
- 51.Franks P, Hall R, Linklater P. 1980. Mechanistic model of the growth of Streptococcus cremoris HP at super optimal temperatures. Biotechnol. Bioeng. 22:1465–1487 [Google Scholar]
- 52.Piper PW. 1995. The heat shock and ethanol stress responses of yeast exhibit extensive similarity and functional overlap. FEMS Microbiol. Lett. 134:121–127 [DOI] [PubMed] [Google Scholar]
- 53.Even S, Lindley ND, Loubiere P, Cocaign-Bousquet M. 2002. Dynamic response of catabolic pathways to autoacidification in Lactococcus lactis: transcript profiling and stability in relation to metabolic and energetic constraints. Mol. Microbiol. 45:1143–1152 [DOI] [PubMed] [Google Scholar]
- 54.Kandler O. 1983. Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 49:209–224 [DOI] [PubMed] [Google Scholar]
- 55.Gosslau A, Ruoff P, Mohsenzadeh S, Hobohm U, Rensing L. 2001. Heat shock and oxidative stress-induced exposure of hydrophobic protein domains as common signal in the induction of hsp68. J. Biol. Chem. 276:1814–1821 [DOI] [PubMed] [Google Scholar]
- 56.Sakamoto M, Komagata K. 1996. Aerobic growth of and activities of NADH oxidase and NADH peroxidase in lactic acid bacteria. J. Ferment. Bioeng. 82:210–216 [Google Scholar]
- 57.Tachon S, Chambellon E, Yvon M. 2011. Identification of a conserved sequence in flavoproteins essential for the correct conformation and activity of the NADH oxidase NoxE of Lactococcus lactis. J. Bacteriol. 193:3000–3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arner ES, Holmgren A. 2000. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 267:6102–6109 [DOI] [PubMed] [Google Scholar]
- 59.Knapp KG, Swartz JR. 2004. Cell-free production of active E. coli thioredoxin reductase and glutathione reductase. FEBS Lett. 559:66–70 [DOI] [PubMed] [Google Scholar]
- 60.Budde-Niekielw A, Geis A, Hassan M, Heller K. July 2005. Method of improving food fermentation procedures. U.S. patent US20050158423 A1
- 61.Chopin A, Chopin MC, Moillo-Batt A, Langella P. 1984. Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid 11:260–263 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



