Abstract
Cell extracts of uric acid-grown Clostridium acidurici catalyzed the coupled reduction of NAD+ and ferredoxin with formate at a specific activity of 1.3 U/mg. The enzyme complex catalyzing the electron-bifurcating reaction was purified 130-fold and found to be composed of four subunits encoded by the gene cluster hylCBA-fdhF2.
TEXT
Clostridium acidurici ferments uric acid to acetate, CO2, and ammonium (1, 2) in a process involving a selenium- and tungsten-containing formate dehydrogenase (3, 4). In cell extracts, formate dehydrogenase activity requires both NAD+ and ferredoxin (5) but the purified enzyme is active with neither NAD(P)+ nor ferredoxin (Fd) (6). We report here that the cytoplasmic enzyme catalyzes the coupled reduction of NAD+ and ferredoxin with formate and propose that coupling proceeds via the mechanism of flavin-based electron bifurcation (7) in this reaction, as follows: 2 HCOO− + Fdox + NAD+ ⇋ 2 CO2 + Fdred2− + NADH + H+.
Flavin-based electron-bifurcating enzymes were first discovered in 2008, when it was shown that the butyryl-coenzyme A (butyryl-CoA)/EtfAB complex in Clostridium kluyveri couples the exergonic reduction of crotonyl-CoA with NADH with the endergonic reduction of ferredoxin with NADH (8, 9). Since then, five other types of electron-bifurcating enzyme complexes have been reported, namely, the NAD+-specific [FeFe]-hydrogenase complex HydABC(D) from Thermotoga maritima (10), Acetobacterium woodii (11), and Moorella thermoacetica (12), the NADH-dependent reduced ferredoxin:NADP+ oxidoreductase complex NfnAB from C. kluyveri (13) and M. thermoacetica (14), the [NiFe]-hydrogenase/heterodisulfide reductase complex MvhADG/HdrABC from methanogenic archaea (15, 16), the caffeyl-CoA reductase complex CarCDE from A. woodii (17), and the NADP+-specific [FeFe]-hydrogenase/formate dehydrogenase complex HytA-E/FdhA from Clostridium autoethanogenum (18). All these complexes have the following characteristics in common: they are present in the cytoplasm of anaerobic bacteria or archaea, they are flavoproteins (flavin mononucleotide [FMN] or flavin adenine dinucleotide [FAD]), they simultaneously use two different electron acceptors or donors, and one of the electron acceptors or donors is a ferredoxin. Formate dehydrogenase from C. acidurici is the seventh enzyme to share these properties and is the first identified electron-bifurcating formate dehydrogenase.
C. acidurici (strain 9a; DSM 604) (19) was grown at 37°C in three fermentors, each containing 10 liters of uric acid medium supplemented with tungstate and selenite (3, 4). The cells (15 g [wet mass]) from the 30-liter culture were harvested by centrifugation and stored under 100% N2 at −80°C. Cell extracts containing 53 mg protein per ml were prepared by passing a suspension of 5 g cells (wet mass) in 10 ml 100 mM potassium phosphate (pH 7) through a French press three times, followed by removal of cell debris and membranes by centrifugation at 65,000 × g for 40 min. In all steps, strictly anoxic conditions were employed.
Enzymes were assayed at 37°C in 1.5-ml cuvettes (optical path = 1 cm) containing 0.8 ml assay mixture and 0.7 ml N2 or H2 as gas phase. The reduction of NAD+ was monitored at 340 nm (ε = 6.3 mM−1 cm−1), that of ferredoxin (from Clostridium pasteurianum) (12) was monitored at 430 nm (Δεox-red ≈ 13.1 mM−1 cm−1), and that of methyl viologen was monitored at 578 nm (ε = 9.8 mM−1 cm−1).
Cell extracts catalyzed the coupled reduction of ferredoxin from Clostridium pasteurianum (25 μM) and NAD+ (1 mM) with formate (20 mM) at a specific activity of 1.3 U/mg protein (in 100 mM potassium phosphate, pH 7.5) (Table 1). Ferredoxin was not reduced in the absence of NAD+, and NAD+ was not reduced in the absence of ferredoxin. NADP+ could not substitute for NAD+. The reduction of methyl viologen (10 mM) with formate (20 mM) was independent of NAD(P)+. The proposed function of the ferredoxin- and NAD+-dependent formate dehydrogenase in uric acid metabolism (19, 20) is shown in Fig. 1.
Table 1.
Specific activities of oxidoreductases involved in acetic acid formation in uric acid-grown C. aciduriciabcd
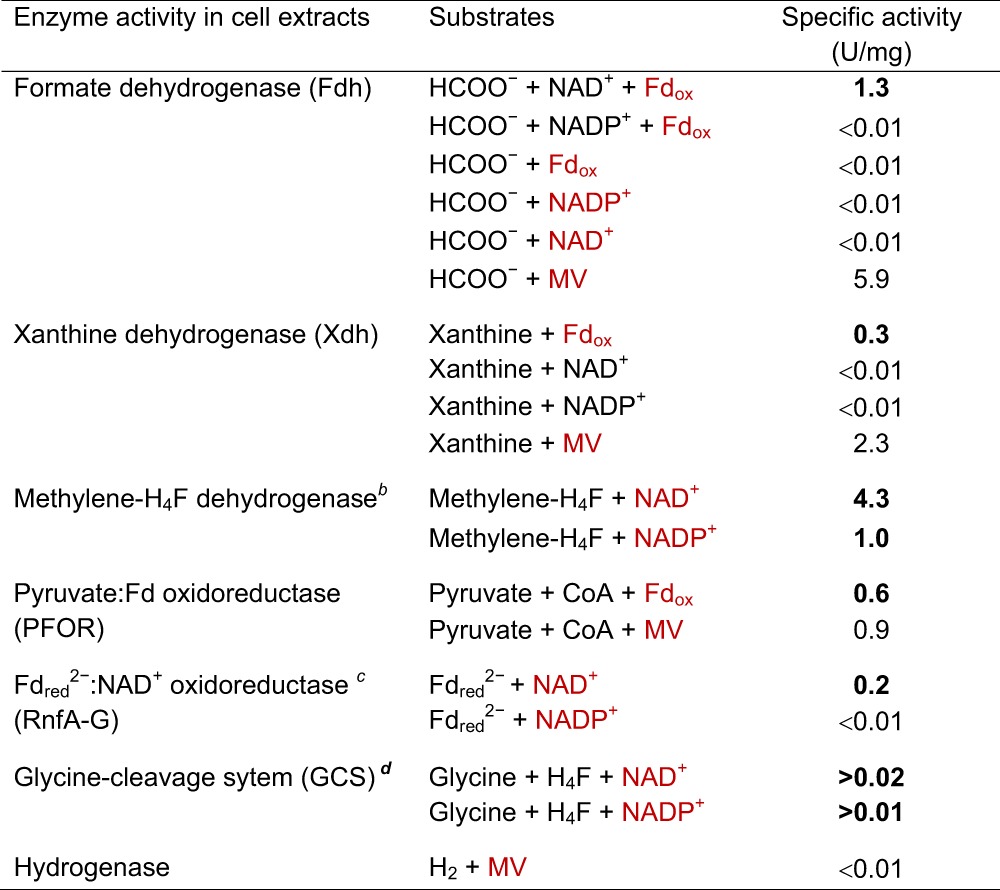
Red lettering indicates substrates or products whose reductions were monitored; boldface indicates physiologically relevant specific activities. MV, methyl viologen; Fd, ferredoxin from C. pasteurianum; H4F, tetrahydrofolate. One unit equals 2 μmol electrons transferred per min. For assay conditions, see the text.
The genome harbors two genes for methylene-H4F dehydrogenase. The NADP-specific enzyme has been purified (22).
Reduced ferredoxin was regenerated by H2 and [FeFe]-hydrogenase from C. pasteurianum (9).
Specific activity increases exponentially with the protein concentration, indicating a multicomponent enzyme system (23). The specific activity was found at a protein concentration of 4.5 mg per 0.8 ml assay mixture.
Fig 1.
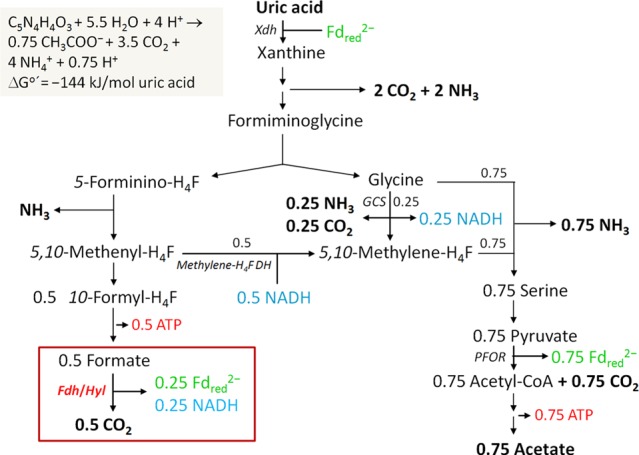
Scheme of the energy metabolism of C. acidurici growing on uric acid. The scheme highlights the proposed role of the electron-bifurcating formate dehydrogenase. Fd, ferredoxin; H4F, tetrahydrofolate; Xdh, xanthine dehydrogenase; Fhd/Hyl, electron-bifurcating formate dehydrogenase; methylene-H4F DH, 5,10-methylene-H4F dehydrogenase; GCS, glycine cleavage system; PFOR, pyruvate:Fd oxidoreductase. The reduced ferredoxin required for uric acid reduction is regenerated in the Fdh/Hyl and PFOR reactions.
We also determined the specific activities of the other oxidoreductases involved in purine metabolism (Table 1; Fig. 1): xanthine dehydrogenase was tested in 100 mM Tris-HCl (pH 8.5) containing 2 mM xanthine and 25 μM ferredoxin from C. pasteurianum (21); 5,10-methylenetetrahydrofolate dehydrogenase was tested in 100 mM MOPS-KOH (pH 6.5) (where MOPS is mospholinepropanesulfonic acid) containing 50 mM 2-mercaptoethanol, 0.5 mM tetrahydrofolate, 10 mM formaldehyde, and 0.5 mM NAD+ (22); the glycine cleavage system was tested in 100 mM potassium phosphate (pH 7.5) containing 2 mM glycine, 0.1 mM pyridoxal phosphate, 0.5 mM tetrahydrofolate, 0.1 mM d,l-alpha lipoic acid, and 1 mM NAD+ (23); pyruvate:ferredoxin oxidoreductase was tested in 100 mM potassium phosphate (pH 7.5) containing 10 mM pyruvate, 25 μM ferredoxin from C. pasteurianum, 0.1 mM thiamine pyrophosphate, and 1 mM CoA (18); and reduced ferredoxin:NAD+ oxidoreductase (RnfA-G) was tested in 100 mM potassium phosphate (pH 7.5) containing 25 μM ferredoxin, 1 mM NAD+, 1 U monomeric [FeFe]-hydrogenase from C. pasteurianum, and 100% H2 as gas phase (18). The genes for these oxidoreductases are found in the genome of C. acidurici (GenBank accession number CP003326; chromosome) (19).
To characterize the electron-bifurcating formate dehydrogenase, we purified the enzyme from 12 ml cell extract under anoxic conditions essentially as described by Kearny and Sagers (6). All the buffers were supplemented with 5 μM FAD, 5 μM FMN, and 2 mM dithiothreitol (DTT). The procedure involved centrifugation at 115,000 × g for 40 min, ammonium sulfate fractionation (the precipitate between 50% and 55% contained most of the activity), hydrophobic chromatography on a phenyl Sepharose high-performance column (2.6 cm by 12 cm), and size exclusion chromatography on a Superdex G200 column (26 cm by 60 cm). The enzyme was purified 130-fold to a specific activity of 168 U/mg. The enzyme preparation was found to contain FMN rather than FAD, which was identified by thin-layer chromatography on an RP-18 F254 aluminum sheet (Merck, Darmstadt, Germany) with FAD and FMN as the standards (12). It catalyzed the coupled reduction of NAD+ and ferredoxin from C. pasteurianum with formate (apparent Km, 0.5 mM) in an almost 1:1 stoichiometry (Fig. 2). NADP+ could not substitute for NAD+. Methyl viologen reduction with formate was not dependent on the presence of NAD(P)+. Ferredoxin from C. acidurici was not tested.
Fig 2.
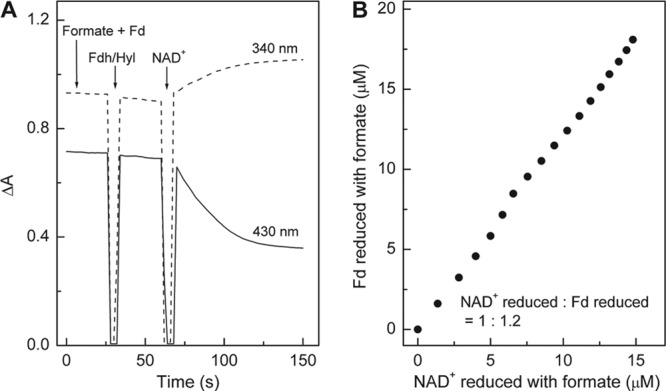
NAD+-dependent ferredoxin (Fd) reduction with formate catalyzed by electron-bifurcating ferredoxin- and NAD+-dependent formate dehydrogenase (Fdh/Hyl) from C. acidurici. (A) Kinetics of NAD+ and Fd reduction with formate monitored simultaneously at 340 nm (for NAD+ reduction) and 430 nm (for Fd reduction). (B) Stoichiometry of NAD+ and Fd reduction. The amounts of NADH (ε340 nm = 6.3 mM−1 cm−1) and reduced ferredoxin (Δεox-red, 430 nm = 13.1 mM−1 cm−1) were calculated based on their molar extinction coefficients. The absorbance change at 340 nm caused by ferredoxin reduction was very small and could therefore be disregarded. The data for panel B were taken from panel A.
SDS-PAGE of the preparation revealed the presence of mainly four proteins (Fig. 3), which were sequenced via matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF/MS) analysis after digestion with trypsin to identify the encoding genes (12). The four encoding genes were found in a cluster that may form a transcription unit (Fig. 4). The four genes have already been annotated by Hartwich et al. (19) as hydC, hydB, hydA, and fdhF2. The genes hydA, hydB, and hydC were at that time predicted to encode a heterotrimeric electron-bifurcating [FeFe]-hydrogenase, and the gene fdhF2 was predicted to encode a molybdopterin- and selenocysteine- containing formate dehydrogenase.
Fig 3.
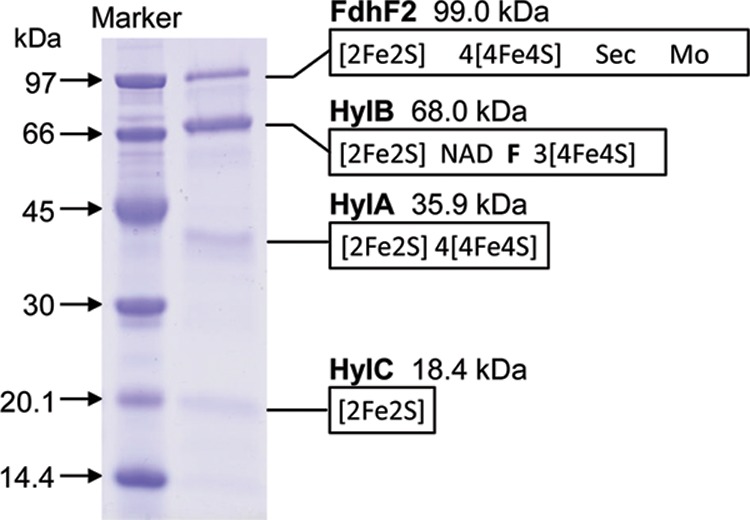
SDS-PAGE of purified electron-bifurcating ferredoxin- and NAD+-dependent formate dehydrogenase from C. acidurici. The molecular masses and cofactor binding sites of the four subunits were deduced from the amino acid sequences predicted from the DNA data (19) (see Fig. 4). Fdh, formate dehydrogenase; Hyl, [FeFe]-hydrogenase-like protein; Sec, selenocysteine; Mo, molybdopterin, to which either molybdate or tungstate can be bound; F, flavin. There is clearly more than one HylB per FdhF2.
Fig 4.

The hylCBA-fdhF2-fdhF1-rbr-mogA-mobA-fdhD gene cluster (gene numbers Curi_c29410 to Curi_c29330) in C. acidurici. Hyl, [FeFe]-hydrogenase-like protein; FdhF2, formate dehydrogenase with an active-site selenocysteine; P, promoter; Ω, terminator. Bioinformatics analysis was performed with Softberry software (Softberry, Inc., NY) and the software provided by the ARNold finding terminators at IGM-Web Server (http://rna.igmors.u-psud.fr/toolbox/arnold/index.php). The gene products Rbr (rubredoxin), MogA, MobA, FdhD, and FdhF1 are not found in the purified enzyme complex. MogA, MobA, and FdhD are involved in molybdopterin cofactor synthesis and formate dehydrogenase maturation. FdhF1 is a formate dehydrogenase with an active-site cysteine and is probably formed only under selenium-deficient growth conditions.
However, the purified formate dehydrogenase preparation, like the cell extracts (Table 1), did not show hydrogenase activity, neither with viologen dyes nor with NAD(P)+ and/or ferredoxin as electron acceptors. Closer inspection of the sequences revealed the lack of typical amino acid residues involved in the H cluster (active site of [FeFe]-hydrogenase) formation in HydA. These residues are also not present in the homologues HydA1 and HydA2 encoded by hydA1 and hydA2 located elsewhere in the genome. Consistently, the genes hydE, hydF, and hydG, which are required for H cluster biosynthesis and assembly (24, 25), were not found in the genome of C. acidurici. Growing cultures of C. acidurici neither form nor consume H2 (20). Because of all these findings, it is very unlikely that the hydCBA genes in C. acidurici code for an electron-bifurcating [FeFe]-hydrogenase (HydABC). We therefore renamed the hydCBA genes in C. acidurici the hylCBA genes (hyl for hydrogenase-like). HylB from C. acidurici shows high sequence identity (51% to 66%) to HydB and HytB from organisms with FMN-based electron-bifurcating [FeFe]-hydrogenases. Apparently, this FMN-containing, electron-bifurcating module can be combined with both formate dehydrogenases and [FeFe]-hydrogenases, just as the EtfAB module can be combined with butyryl-CoA dehydrogenase and caffeyl-CoA reductase (17).
The oxidation of protein-bound flavoquinol in two one-electron steps at different redox potentials is the mechanistic basis of flavin-based electron bifurcation (7). The two-electron reduction and twice-occurring one-electron oxidation at different potentials are inherent properties of flavins (26). It is therefore not surprising that several electron-bifurcating flavoproteins have evolved independently (27, 28), namely, HydB/HytB/HylB (10–12, 18), HdrA (15, 29), NfnB (13, 14), and EtfAB (9, 17). Genes encoding these flavoproteins—neighbors of genes with quite different annotated functions—are found in many anaerobic microorganisms, which indicates that the presently known flavin-based electron-bifurcating enzyme complexes are only the tip of an iceberg.
ACKNOWLEDGMENTS
This work was supported by the Max Planck Society and the Fonds der Chemischen Industrie.
We thank Johanna Moll for growing C. pasteurianum and purifying its ferredoxin, Yaning Zheng for helping to grow C. acidurici, and Wolfgang Buckel for helpful suggestions.
Footnotes
Published ahead of print 19 July 2013
REFERENCES
- 1.Barker HA, Beck JV. 1941. The fermentative decomposition of purines by Clostridium acidi-urici and Clostridium cylindrosporum. J. Biol. Chem. 141:3–27 [Google Scholar]
- 2.Barker HA, Beck JV. 1942. Clostridium acidi-urici and Clostridium cylindrosporum, organisms fermenting uric acid and some other purines. J. Bacteriol. 43:291–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner R, Andreesen JR. 1977. Differentiation between Clostridium acidiurici and Clostridium cylindrosporum on the basis of specific metal requirements for formate dehydrogenase formation. Arch. Microbiol. 114:219–224 [DOI] [PubMed] [Google Scholar]
- 4.Wagner R, Andreesen JR. 1987. Accumulation and incorporation of 185W-tungsten into proteins of Clostridium acidiurici and Clostridium cylindrosporum. Arch. Microbiol. 147:295–299 [Google Scholar]
- 5.Thauer RK. 1973. CO2 reduction to formate in Clostridium acidi-urici. J. Bacteriol. 114:443–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kearny JJ, Sagers RD. 1972. Formate dehydrogenase from Clostridium acidiurici. J. Bacteriol. 109:152–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckel W, Thauer RK. 2013. Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation. Biochim. Biophys. Acta 1827:94–113 [DOI] [PubMed] [Google Scholar]
- 8.Herrmann G, Jayamani E, Mai G, Buckel W. 2008. Energy conservation via electron-transferring flavoprotein in anaerobic bacteria. J. Bacteriol. 190:784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li F, Hinderberger J, Seedorf H, Zhang J, Buckel W, Thauer RK. 2008. Coupled ferredoxin and crotonyl coenzyme a (CoA) reduction with NADH catalyzed by the butyryl-CoA dehydrogenase/Etf complex from Clostridium kluyveri. J. Bacteriol. 190:843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schut GJ, Adams MW. 2009. The iron-hydrogenase of Thermotoga maritima utilizes ferredoxin and NADH synergistically: a new perspective on anaerobic hydrogen production. J. Bacteriol. 191:4451–4457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuchmann K, Müller V. 2012. A bacterial electron-bifurcating hydrogenase. J. Biol. Chem. 287:31165–31171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Huang H, Kahnt J, Thauer RK. 2013. A reversible electron-bifurcating ferredoxin- and NAD-dependent [FeFe]-hydrogenase (HydABC) in Moorella thermoacetica. J. Bacteriol. 195:1267–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Huang H, Moll J, Thauer RK. 2010. NADP+ reduction with reduced ferredoxin and NADP+ reduction with NADH are coupled via an electron-bifurcating enzyme complex in Clostridium kluyveri. J. Bacteriol. 192:5115–5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang H, Wang S, Moll J, Thauer RK. 2012. Electron bifurcation involved in the energy metabolism of the acetogenic bacterium Moorella thermoacetica growing on glucose or H2 plus CO2. J. Bacteriol. 194:3689–3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaster AK, Moll J, Parey K, Thauer RK. 2011. Coupling of ferredoxin and heterodisulfide reduction via electron bifurcation in hydrogenotrophic methanogenic archaea. Proc. Natl. Acad. Sci. U. S. A. 108:2981–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thauer RK, Kaster AK, Seedorf H, Buckel W, Hedderich R. 2008. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 6:579–591 [DOI] [PubMed] [Google Scholar]
- 17.Bertsch J, Parthasarathy A, Buckel W, Müller V. 2013. An electron-bifurcating caffeyl-CoA reductase. J. Biol. Chem. 288:11304–11311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Huang H, Kahnt J, Mueller AP, Köpke M, Thauer RK. 26 July 2013. An NADP-specific electron-bifurcating [FeFe]-hydrogenase in a functional complex with formate dehydrogenase in Clostridium autoethanogenum grown on CO. J. Bacteriol. 10.1128/JB.00678-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartwich K, Poehlein A, Daniel R. 2012. The purine-utilizing bacterium Clostridium acidurici 9a: a genome-guided metabolic reconsideration. PLoS One 7:e51662. 10.1371/journal.pone.0051662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogels GD, Van der Drift C. 1976. Degradation of purines and pyrimidines by microorganisms. Bacteriol. Rev. 40:403–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner R, Cammack R, Andreesen JR. 1984. Purification and characterization of xanthine dehydrogenase from Clostridium acidiurici grown in the presence of selenium. Biochim. Biophys. Acta 791:63–74 [Google Scholar]
- 22.Odonoghue GV, Rabinowitz JC. 1985. Purification of NADP-dependent 5,10-methylenetetrahydrofolate dehydrogenase from Clostridium acidi-urici. Fed. Proc. 44:1076–1076 [Google Scholar]
- 23.Gariboldi RT, Drake HL. 1984. Glycine synthase of the purinolytic bacterium, Clostridium acidiurici. Purification of the glycine-CO2 exchange system. J. Biol. Chem. 259:6085–6089 [PubMed] [Google Scholar]
- 24.Kuchenreuther JM, Britt RD, Swartz JR. 2012. New insights into [FeFe] hydrogenase activation and maturase function. PLoS One 7:e45850. 10.1371/journal.pone.0045850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulder DW, Shepard EM, Meuser JE, Joshi N, King PW, Posewitz MC, Broderick JB, Peters JW. 2011. Insights into [FeFe]-hydrogenase structure, mechanism, and maturation. Structure 19:1038–1052 [DOI] [PubMed] [Google Scholar]
- 26.Alagaratnam S, Van Pouderoyen G, Pijning T, Dijkstra BW, Cavazzini D, Rossi GL, Van Dongen WMAM, Van Mierlo CPM, Van Berkel WJH, Canters GW. 2005. A crystallographic study of Cys69Ala flavodoxin II from Azotobacter vinelandii: Structural determinants of redox potential. Protein Sci. 14:2284–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin WF. 2012. Hydrogen, metals, bifurcating electrons, and proton gradients: the early evolution of biological energy conservation. FEBS Lett. 586:485–493 [DOI] [PubMed] [Google Scholar]
- 28.Nitschke W, Russell MJ. 2012. Redox bifurcations: mechanisms and importance to life now, and at its origin: a widespread means of energy conversion in biology unfolds. Bioessays 34:106–109 [DOI] [PubMed] [Google Scholar]
- 29.Costa KC, Wong PM, Wang TS, Lie TJ, Dodsworth JA, Swanson I, Burn JA, Hackett M, Leigh JA. 2010. Protein complexing in a methanogen suggests electron bifurcation and electron delivery from formate to heterodisulfide reductase. Proc. Natl. Acad. Sci. U. S. A. 107:11050–11055 [DOI] [PMC free article] [PubMed] [Google Scholar]


