Abstract
The activity of bacteriophages and phage-related mobile elements is a major source for genome rearrangements and genetic instability of their bacterial hosts. The genome of the industrial amino acid producer Corynebacterium glutamicum ATCC 13032 contains three prophages (CGP1, CGP2, and CGP3) of so far unknown functionality. Several phage genes are regularly expressed, and the large prophage CGP3 (∼190 kbp) has recently been shown to be induced under certain stress conditions. Here, we present the construction of MB001, a prophage-free variant of C. glutamicum ATCC 13032 with a 6% reduced genome. This strain does not show any unfavorable properties during extensive phenotypic characterization under various standard and stress conditions. As expected, we observed improved growth and fitness of MB001 under SOS-response-inducing conditions that trigger CGP3 induction in the wild-type strain. Further studies revealed that MB001 has a significantly increased transformation efficiency and produced about 30% more of the heterologous model protein enhanced yellow fluorescent protein (eYFP), presumably as a consequence of an increased plasmid copy number. These effects were attributed to the loss of the restriction-modification system (cg1996-cg1998) located within CGP3. The deletion of the prophages without any negative effect results in a novel platform strain for metabolic engineering and represents a useful step toward the construction of a C. glutamicum chassis genome of strain ATCC 13032 for biotechnological applications and synthetic biology.
INTRODUCTION
In recent years, the reduction or redesign of genomes of selected microbial model species has become a major focus in synthetic biology. These efforts aim at the construction of chassis organisms with genomes reduced in their complexity and background noise and hence with improved predictability and controllability (1, 2). These strains are supposed to be advantageous both for basic research and for application in industrial biotechnology. There are basically two strategies for the genome reduction process: the bottom-up strategy, where a minimal genome is redesigned from scratch by de novo synthesis and assembly of essential genes, and the top-down strategy, where nonessential genes are deleted starting from native genomes (3).
In our study, we concentrated on the nonpathogenic, Gram-positive soil bacterium Corynebacterium glutamicum. Originally isolated because of its natural ability to excrete l-glutamate (4), C. glutamicum is now used for the large-scale industrial production of amino acids, in particular l-glutamate and l-lysine (about 2.2 million and 1.5 million tons per year, respectively) (5). Since the world market for amino acids is continuously increasing, there are ongoing efforts to improve production strains and processes (6). While Escherichia coli and Bacillus subtilis have been the object of several genome reduction studies in the last decade (7–13), not much has been published with respect to C. glutamicum, representing one of the most important platform species used in industrial biotechnology. Suzuki et al. successfully deleted 11 distinct genomic regions ranging from 11 to 56 kbp with no effect on growth under standard laboratory conditions (14). These regions had been identified by comparative genomics, and in a follow-up study, eight of those were combined in a single strain, leading to a total genome reduction of 190 kbp with no negative effect under standard conditions (15).
The sequencing of many bacterial genomes revealed that the appearance of prophages is rather common, since the majority of genomes contain at least one prophage. In some cases, prophages, phage remnants, or orphan phage genes constitute up to 20% of the whole bacterial genome (16). Temperate phages frequently provide their host with new biological properties, such as genes involved in virulence, toxin-encoding genes, or resistance to further phage infection (16, 17). Nevertheless, the activity of prophages and phage remnants is a major source of genetic variation and horizontal gene transfer (16, 18, 19) and may have a significant negative impact on the performance of bioprocesses. Therefore, prophages represent ideal targets for genome reduction projects (10, 11).
Three prophages have been identified in the genome of C. glutamicum ATCC 13032, CGP1 (cg1507-cg1524), CGP2 (cg1746-cg1752), and CGP3 (cg1890-cg2071) (20, 21) (see Fig. 1A). The C. glutamicum R strain is lacking the CGP2 and CGP3 regions and contains only some phage remnants (22). In ATCC 13032, CGP1 and CGP2 are rather small (13.5 kbp and 3.9 kbp, respectively) and appear to be highly degenerated. CGP3 (187.3 kbp) is one of the largest known prophages, constituting almost 6% of the entire C. glutamicum genome, and previous studies revealed spontaneous induction of CGP3 in a small fraction of wild-type cells even under standard cultivation conditions (23). A mutant lacking the master regulator of iron homeostasis (C. glutamicum ΔdtxR) also exhibited increased CGP3 gene expression (24). However, until now not much has been known with respect to the functionality of the prophages and their impact on the physiology of C. glutamicum. Why do these large prophage elements remain in the genome of ATCC 13032? One would assume that selective pressure under laboratory conditions should favor strains that have lost this genomic burden. Is deletion of all three prophages possible, and what is the impact on host fitness?
Fig 1.
Locations of the three prophages (CGP1, CGP2, and CGP3) in the genome of C. glutamicum (A) and expression patterns of CGP3 genes (B). (A) Circular representation of the C. glutamicum ATCC 13032 chromosome. The concentric circles denote (from outward to inward) coding sequences (CDS) transcribed clockwise and counterclockwise, locations of the three prophages, relative G/C content, and GC skew. A positive deviation in G/C content from the average is shown by bars pointing outward and a negative deviation by bars pointing inward. The same holds for the GC skew plot, where positive skew values are shown in red and negative values in blue. (B) Presentation of the fluorescent output of CGP3 (cg1890-cg2071) and adjacent genes in the genome of C. glutamicum out of more than 100 different microarray experiments stored in our in-house database. Presented are all experiments performed with the current array series. Several prophage genes appear to be regularly expressed (red color). In some of the experiments, the whole prophage region is upregulated, e.g., 1, overexpression of a transcriptional regulator; or 2, induction of the SOS response by addition of mitomycin C. Labeled are the first and the last genes of CGP3 (cg1890 and cg2071) and some examples of frequently expressed prophage genes.
In this study, we addressed these questions, and we present the successful construction of MB001, a prophage-free C. glutamicum strain based on ATCC 13032. Phenotypic characterization revealed no observable negative effect in comparison to the wild type. On the contrary, this strain shows an improved fitness under conditions triggering CGP3 activity and a significantly increased production of the heterologous model protein enhanced yellow fluorescent protein (eYFP).
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
The bacterial strains used in this study are listed in Table 1; the plasmids are listed in Table 2. For growth experiments, 20 ml of brain heart infusion broth (BHI) (Difco Laboratories, Detroit, MI, USA) was inoculated with a colony from a fresh BHI agar plate and incubated overnight at 30°C and 120 rpm in shaking flasks. Cells of this preculture were washed once in phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, and 1.4 mM KH2PO4, pH 7.3) and used to inoculate the main culture to an optical density at 600 nm (OD600) of about 1. Main cultures of 700 to 750 μl CGXII minimal medium (25) were incubated at 30°C, 95% humidity, and 1200 rpm in a BioLector system (m2p-labs, Baesweiler, Germany) using 48-well Flowerplates. The medium was supplemented with 3,4-dihydroxybenzoate (30 mg liter−1) as an iron chelator, as well as glucose (56 to 111 mM), sodium gluconate (100 mM), sodium acetate (122 mM), or sodium lactate (100 mM) as a carbon source, as needed. BioLector cultivations were monitored for backscatter, eYFP, and Crimson fluorescence with gains of 17, 20, and 50. Bioreactor cultivations in CGXII medium with 56 mM glucose were performed in 1-liter DASGIP reactors (Eppendorf, Hamburg, Germany) with automated process control for pH, temperature, and dissolved oxygen (DO). The pH was regulated to 7.0 with 4 M NaOH and HCl. The DO was kept constant at 30% by increasing the stirrer speed (two Rushton turbines) from 200 to 1,200 rpm. Expression of eyfp or crimson under the control of the Ptac promoter was induced by the addition of 0.1 to 1.0 mM isopropyl β-d-1-thiogalactopyranoside (IPTG). For the quantitative Western blot analysis, the main culture was grown in 500 ml baffled shake flasks containing 50 ml CGXII medium. When appropriate, the medium for C. glutamicum was supplemented with 25 μg ml−1 kanamycin, 10 μg ml−1 chloramphenicol, or 5 μg ml−1 tetracycline.
Table 1.
Strains used in this study
| Strain | Relevant characteristics | Source or reference |
|---|---|---|
| E. coli | ||
| DH5α | F− ϕ80dlac Δ(lacZ)M15 Δ(lacZYA-argF) U169 endA1 recA1 hsdR17 (rK− mK+) deoR thi-1 phoA supE44 λ− gyrA96 relA1; strain used for cloning procedures | 28 |
| C. glutamicum | ||
| ATCC 13032 | Biotin-auxotrophic wild type | 4 |
| ATCC 13032 ΔCGP3 | ATCC 13032 with in-frame deletion of prophage CGP3 (cg1890-cg2071) | This work |
| ATCC 13032 ΔCGP13 | ATCC 13032 with in-frame deletion of prophages CGP1 (cg1507-cg1524) and CGP3 (cg1890-cg2071) | This work |
| MB001 | ATCC 13032 with in-frame deletion of prophages CGP1 (cg1507-cg1524), CGP2 (cg1746-cg1752), and CGP3 (cg1890-cg2071) | This work |
| ATCC 13032 ΔcglMRR | ATCC 13032 with in-frame deletion of restriction-modification system cglMRR (cg1996-cg1998), located within prophage CGP3 | This work |
| ATCC 13032::Ptac-eyfp | ATCC 13032 with eyfp under control of Ptac promoter integrated into intergenic region of cg1121-cg1122 | This work |
| ATCC 13032::Ptac-crimson | ATCC 13032 with crimson under control of Ptac promoter integrated into intergenic region of cg1121-cg1122 | This work |
| MB001::Ptac-eyfp | MB001 with eyfp under control of Ptac promoter integrated into intergenic region of cg1121-cg1122 | This work |
| MB001::Ptac-crimson | MB001 with crimson under control of Ptac promoter integrated into intergenic region of cg1121-cg1122 | This work |
Table 2.
Plasmids used in this study
| Plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| pK19mobsacB | Kanr; plasmid for allelic exchange in C. glutamicum (pK18 oriVE.c. sacB lacZα) | 56 |
| pK19mobsacB-ΔCGP1 | Kanr; pK19mobsacB derivative containing PCR product covering up- and downstream regions of prophage CGP1 | This work |
| pK19mobsacB-ΔCGP2 | Kanr; pK19mobsacB derivative containing PCR product covering up- and downstream regions of prophage CGP2 | This work |
| pK19mobsacB-ΔCGP3 | Kanr; pK19mobsacB derivative containing PCR product covering up- and downstream regions of prophage CGP3 | This work |
| pK19mobsacB-ΔcglMRR | Kanr; pK19mobsacB derivative containing PCR product covering up- and downstream regions of restriction-modification system cglMRR | This work |
| pK18mobsacB-int1 | Kanr; plasmid for integration of foreign DNA into intergenic region between cg1121 and cg1122 (oriVE.c sacB lacZα) | This work |
| pK18mobsacB-int-Ptac-eyfp | Kanr; plasmid for integration of Ptac-eyfp into intergenic region of cg1121-cg1122 | This work |
| pK18mobsacB-int-Ptac-crimson | Kanr; plasmid for integration of Ptac-crimson into intergenic region of cg1121-cg1122 | This work |
| pK18-lacZ | pK18 harboring coding sequence for lacZ; used as PCR template | B. Kleine, unpublished |
| pACYC184 | Tetr Cmr; E. coli cloning vector with p15A ori; not replicable in C. glutamicum | 57 |
| pACYC-cglM-Lac | Tetr Cmr; pACYC184 derivative containing lacZ, lacI, and a 500-bp fragment of cglM | This work |
| pEKEx3 | Specr, pEKEx2 derivative containing spectinomycin resistance gene instead of kanamycin resistance gene | 23 |
| pEKEx2-eyfp | Kanr; pEKEx2 containing eyfp with artificial RBS, under control of Ptac | 58 |
| pE2-crimson | Ampr; encodes E2-Crimson | Clontech |
| pAN6 | Kanr; C. glutamicum/E. coli shuttle vector for regulated gene expression, derivative of pEKEx2 | 59 |
| pAN6-crimson | Kanr; pAN6 derivative for expression of E2-crimson under control of Ptac promoter | This work |
| pMBX1 | Specr, pEKEx3 derivative lacking 1,256-bp fragment between the two NdeI sites and carrying an RBS | This work |
| pMBX1-eyfp | Specr; pMBX1 derivative for expression of eyfp under control of Ptac promoter | This work |
| pZ8-1 | Kanr; C. glutamicum/E. coli shuttle vector containing tac promoter, mcs, E. coli ori from pACYC177, C. glutamicum ori from pHM1519 | 36 |
| pCR003d | pK18mobsacB carrying cg0961del | 37 |
oriVE.c., E. coli oriV; RBS, ribosome binding site.
Recombinant DNA work.
The enzymes for recombinant DNA work were obtained from New England BioLabs (NEB) (Frankfurt, Germany) or Fermentas (St. Leon-Rot, Germany). The oligonucleotides used in this study were obtained from Eurofins MWG Operon (Ebersberg, Germany) and are listed in Table S1 in the supplemental material. Routine methods, such as PCR, DNA restriction, and ligation, were performed using standard protocols (26). Chromosomal DNA of C. glutamicum was prepared as described previously (27). E. coli plasmids were isolated using the QIAprep spin miniprep kit (Qiagen, Hilden, Germany). E. coli was transformed using the RbCl method (28). C. glutamicum was transformed by electroporation (29). DNA sequencing was performed by Eurofins MWG Operon (Ebersberg, Germany). A detailed description of the construction of plasmids used in this study is included in the supplemental material.
Microfluidic cultivation.
The PDMS microfluidic system used in this study was designed for single-cell studies of microcolonies under constant environmental conditions. For fabrication of the chip system and experimental setup, the reader is referred to reference 30. Prior to seeding and trapping of single cells within the microfluidic device, the strains were precultivated in BHI medium before inoculation of CGXII minimal medium. Cells were harvested at an OD600 of 0.5 to 1 and perfused with CGXII minimal medium containing 222 mM glucose during the microfluidic growth experiments.
Construction of deletion and insertion strains.
Transfer of the sequenced plasmid pK19mobsacB-ΔCGP1 into C. glutamicum and screening for the first and second recombination events were performed as described previously (31). Kanamycin-sensitive and sucrose-resistant clones were tested by colony PCR analysis with the oligonucleotide pair CGP1-Dfw/CGP1-Drv for the deletion of CGP1. The other deletion mutants were constructed analogously, except for the ΔCGP3 mutant. The deletion of CGP3 was not successful using the straightforward approach described above, and the following modifications were necessary. After integration of the deletion plasmid pK19mobsacB-ΔCGP3, a second plasmid was integrated into the cglM gene to inactivate the restriction-modification system organized in one operon (32). For this purpose, the plasmid pACYC184 was chosen because it contains two genes for resistance against chloramphenicol and tetracycline. Selections were made using both but in separate experiments. This approach revealed that both are appropriate resistance markers for chromosomal integration in C. glutamicum. After transformation of pACYC184-cglM-Lac, it took 4 to 5 days until a few tiny colonies appeared. Clones carrying this plasmid could easily be identified on agar plates containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) and IPTG. After the second recombination event, leading to the excision of the pK19 plasmid backbone, kanamycin-sensitive and sucrose-resistant clones were tested by colony PCR analysis with the oligonucleotide pair CGP3-Dfw/CGP3-Drv for the deletion of CGP3. Absence of the stationary-phase chromosomal circular DNA molecule of CGP3 was tested using the primer pairs prophage-1611-for/prophage-1816-rev (circular form) and D_cg2040_for/D_cg2040_rev (prophage gene cg2040). A DNA microarray analysis with chromosomal DNA of the ΔCGP3 strain as the template was used to confirm the deletion.
For competitive growth experiments, C. glutamicum wild type and MB001 (ΔCGP123) containing chromosomally integrated fluorescence markers were constructed. The markers contained the coding sequence of a fluorescent protein (eyfp or crimson) under the control of the tac promoter. The procedure was analogous to the two-step homologous recombination method described above. The markers were integrated into the intergenic region of cg1121-cg1122 and allowed optical differentiation of two strains after induction with 100 μM IPTG. Cultivation was performed in 48-well Flowerplates in a BioLector system at 30°C and 1,200 rpm. In control experiments, fluorescent markers were switched between the strains to exclude an influence due to expression of the respective reporter gene.
Western blot analysis.
The amount of eYFP was quantified as described in reference 33 with the following modifications. Blocking of unspecific binding was performed with 2% (wt vol−1) ECL advance blocking agent (GE Healthcare) in PBST buffer (PBS plus Tween 20). Both primary antibodies (anti-eYFP and anti-GltA) were used in a 1:10,000 dilution; the secondary antibody was a goat anti-rabbit immunoglobulin G Cy5 conjugate (GE Healthcare) and was used in a 1:2,500 dilution. After washing, the blots were scanned using a Typhoon TrioTM scanner (GE Healthcare). Bands were quantified using ImageQuant software (GE Healthcare).
Plasmid copy number determination using qPCR.
The copy number of the pEKEx2-eyfp plasmid was determined by quantitative PCR (qPCR) as described previously (23, 34) with the following modifications. Each sample contained 100 ng template DNA. The pEKEx2-specific oligonucleotides (qPCR-pEKEx-5/qPCR-pEKEx-6) were designed to amplify a 234-bp fragment located within the kanamycin resistance gene of the plasmid, and the oligonucleotides specific for a nonprophage part of the genome (ddh-LC-for/ddh-LC-rev) were designed to amplify a 234-bp fragment of the ddh gene (cg2900). Serial 10-fold dilutions of pEKEx2-eyfp plasmid DNA (5 ng μl−1 to 5 pg μl−1) and a PCR product covering the ddh fragment (50 pg μl−1 to 5 fg μl−1) were used to establish a standard curve. The plasmid copy number per genome was calculated for each sample by using the following equation: [amount of plasmid DNA (pg)/amount of ddh DNA (pg)] × [size of DNA fragment (234 bp)/size of plasmid (8,885 bp)].
Determination of transformation efficiency.
Preparation of electrocompetent C. glutamicum cells was performed as described previously (35), with the following modifications. Cells were grown in 50 ml fresh BHIS (BHI plus sorbitol) medium in 250-ml shaking flasks at 300 rpm in an Innova orbital shaker (New Brunswick). The final cell suspension in 10% glycerol was adjusted to an OD600 of 10. Stock solutions of plasmids pZ8-1 (36) and pCR003d (37) (100 ng μl−1) were prepared and used for all tests. To determine the electroporation efficiencies, 500 ng plasmid DNA (5-μl solution) was added to the 150-μl cell suspension on ice. After regeneration, 10-μl and 300-μl aliquots were plated on BHIS agar plates containing 25 mg liter−1 kanamycin. CFU were counted after 2 days of incubation at 30°C, and the number of transformants was calculated as CFU per μg DNA.
Nucleotide sequence accession number.
The nucleotide sequence of the ΔCGP123 strain (MB001) was deposited in GenBank under accession number CP005959.
RESULTS
Expression of phage genes: mining the microarray database.
The genome of C. glutamicum contains three prophages, termed CGP1, CGP2, and CGP3 (Fig. 1A). Expression of phage genes has been observed in numerous microarray experiments under different laboratory conditions during the last decade (Fig. 1B). While the majority of the CGP3 genes are not expressed (or are expressed at very low level) under standard cultivation conditions (glucose minimal medium), there are some examples which appear to be transcribed regularly, for example, a putative kinase (cg1905), gntR2 (cg1935), a restriction-modification (RM) system (cglMRR, cg1996-cg1998), and the phage integrase (cg2071). Remarkably, in some experiments, the whole CGP3 cluster is upregulated, for example, upon treatment with mitomycin C, which induces the cellular SOS response, or by the overexpression of a transcriptional regulator (Fig. 1B). Thus, phage gene expression may significantly interfere with the host physiology depending on the growth condition or the genetic background of the strain. Based on these observations, removal of the phage regions was expected to have a positive impact on the genome stability and the biomass-specific productivity of C. glutamicum and moreover could provide interesting insights into the influence of the prophages on host physiology.
Deletion of prophage elements.
Initially, several trials to delete CGP3 with the standard pK19mobsacB method failed; after the final recombination event, all clones showed the wild-type situation. The cg1996-cg1998 operon (within the CGP3 region) encoding the RM system was the prime candidate to prevent deletion because this cluster is significantly expressed in most of the microarray experiments (Fig. 1B) and such systems are known to act as selfish genes (38). Therefore, the RM system was inactivated by integration of a second plasmid into the coding region of the first gene of the operon, cglM (for details, see Materials and Methods). After inactivation of the RM system, it took 4 to 5 days until a few tiny colonies appeared, which were used for the second recombination step. This growth behavior is a result of the presence of two antibiotics in the growth medium and the low copy number of the resistance genes due to the chromosomal integration. After the second recombination, several clones lacking the CGP3 prophage were identified by colony PCR. As control, different primer pairs were used to test for the chromosomally integrated CGP3 prophage and for the presence of the excised circular CGP3 DNA. Subsequently, the two small prophages CGP1 and CGP2 were deleted with the standard double-crossover method (31). Finally, the genome of the ΔCGP123 strain, lacking about 6% of the genome compared to the parental strain, ATCC 13032, was sequenced and designated MB001 (accession number CP005959). The genome sequence confirmed the absence of all prophage elements, and no other conspicuous mutations were observed. In comparison to the published genome of ATCC 13032 (accession number BX927147), the genome of MB001 contains 71 small nucleotide polymorphisms (SNPs), 28 insertions, and 25 deletions (for details, see Table S2 in the supplemental material). All but two of these variations were also found in the resequenced progenitor strain, C. glutamicum ATCC 13032. Whether the shared variations are due to errors in the published sequence or represent mutations accumulated in the wild-type genome over the last 10 years remains to be elucidated.
Characterization of prophage deletion strains under standard conditions.
To analyze the impact of the prophage deletion on host physiology, we characterized the ΔCGP3 and MB001 strains in growth experiments with different standard media and cultivation scales (Table 3). No significant difference between the wild type and the prophage-deleted strains were observed during growth in microtiter plates in minimal medium with glucose (Fig. 2A) and all other carbon sources tested (sodium gluconate, sodium acetate, and sodium lactate; see Fig. S1 in the supplemental material). To test for an effect of the prophage deletion at higher cell densities, the wild type, the ΔCGP3 strain, and MB001 were cultivated in 1-liter bioreactors under defined conditions (DO, 30%; pH 7.0) until an OD600 of 20 was reached. Again, the growth rate as well as oxygen uptake, off-gas composition, pH profile (amounts of base and acid used for titration), and respiratory quotient (RQ) were identical for the two strains. Even growth under optimal conditions in a microfluidic system ensuring a continuous supply of fresh medium and removal of by-products showed no significant difference between the tested strains (wild type and MB001) in terms of growth and morphology (data not shown).
Table 3.
Comparison of C. glutamicum ATCC 13032 and MB001a
| Type of expt | Conditions | Resultc |
|---|---|---|
| Growth | ||
| Different C sourcesb; see Fig. S1 in the supplemental material | CGXII minimal medium with glucose, gluconate, acetate, or lactate as carbon source | x |
| Bioreactor scale | CGXII minimal medium with 56 mM glucose, 1-liter bioreactor, 200–1,200 rpm; DO, 30%; pH 7.0 | x |
| Microfluidic scale | CGXII minimal medium with 222 mM glucose, array of picoliter bioreactors, continuous medium supply, 100-nl/min flow rate | x |
| Stress resistance | ||
| Iron excessb | CGXII minimal medium with 56 mM glucose, 25–3,125 μM FeSO4 | x |
| Osmotic stressb | CGXII minimal medium with 56 mM glucose, 1–2,000 mM NaCl | x |
| Phosphate starvationb | CGXII minimal medium with 56 mM glucose, 13–0.65 mM phosphate | x |
| Mitomycin C treatment; see Fig. 2 | CGXII minimal medium with 111 mM glucose and 1 μM mitomycin C | Growth rates, ATCC 13032 vs MB001: 7–12 h, 0.15 ± 0.01 vs 0.17 ± 0.00; 15–17 h, 0.18 ± 0.02 vs 0.27 ± 0.01 |
| Agar diffusion assay; see Fig. S2 | 6 M NaOH, 6 M HCl, 30% H2O2, ethambutol, penicillin, vancomycin, Tween 40 | x |
| Plate assays; see Fig. S3 | UV radiation (0.5–5 min), mitomycin C added (10–1,000 nM), iron starvation (0 μM), iron excess (100 μM), heat shock (42°C, 30–60 min), osmotic stress (1 M NaCl) | x |
| Heterologous protein production | ||
| Production of eYFPb (Western blot); see Fig. 4 | Plasmid pEKEx2-eyfp, CGXII minimal medium with 56 mM glucose and 1 mM IPTG | 40% increased eYFP signal and increased amount of eYFP protein in strain MB001 |
| Plasmid copy no.b (qPCR); see Fig. 4C | Plasmid pEKEx2-eyfp, CGXII minimal medium with 56 mM glucose and 1 mM IPTG | 2- to 3-fold-increased plasmid copy no. in MB001 |
| Other | ||
| Transcriptomics, ATCC 13032 ΔCGP3 vs ATCC 13032 (see Table S3) | CGXII minimal medium with 111 mM glucose, harvested in exponential growth phase at OD600 of 5 | x |
| Transformation efficiency; see Fig. 3 | Tested for integrative and replicative plasmids | 2- to 3-fold increase for integrative plasmid and 5- to 8-fold increase for replicative plasmid in strain MB001 |
Overview of all experiments for comparison of wild-type ATCC 13032 and the prophage-free strain MB001 performed in this study.
Cultivation in the BioLector system in 48-well Flowerplates (m2p-labs, Germany).
x, no significant difference was observed under the chosen conditions.
Fig 2.

Growth of C. glutamicum ATCC 13032 and the prophage-free strain MB001 on minimal medium with glucose in the presence of mitomycin C (A) or in direct competition (B). (A) The strains were precultivated in BHI medium without mitomycin and inoculated to an OD600 of 1 in CGXII minimal medium with or without 1 μM mitomycin C. Each graph shows the average values and standard deviations resulting from four independent cultures. (B) The strains carrying chromosomal fluorescence markers were precultivated in BHI medium, mixed in a 1:1 ratio, and used to inoculate the main cultures in CGXII minimal medium with 2% (wt vol−1) glucose. Production of fluorescence marker proteins was induced with 100 μM IPTG after 2 h and monitored online. On the following day, 100 μl of these cultures was used to inoculate 750 μl fresh CGXII minimal medium. This procedure was repeated three times. Presented is the ratio of fluorescence signals in the stationary phase of each culture (n = 3).
It was hypothesized that there might be very small differences in growth or fitness, which become apparent only when wild-type and prophage-deleted strains have to compete for nutrients. To test this, chromosomal fluorescence markers (eyfp and crimson under the control of the tac promoter) were constructed and integrated into the genomes of MB001 and the wild type. This allowed optical discrimination of the two strains and direct monitoring of the growth behavior during cocultivation in the BioLector system. Four rounds of repetitive cocultivations were performed. The ratio of the two strains, represented by the respective fluorescence signals, did not change over 4 days, proving that the strains exhibit no difference in growth behavior under the chosen standard conditions (Fig. 2B).
Transcriptome analysis of ΔCGP3 strain.
For the E. coli phage λ, it has been shown that the transcription of several genes is different in the lysogenic strain from that in the uninfected host in tryptone broth. In this case, it was shown that the pckA gene, encoding phosphoenolpyruvate carboxykinase, was significantly decreased in lysogenic strains (11). To investigate whether CGP3 influences host gene expression, we performed DNA microarray analysis of the ΔCGP3 strain and the wild type during growth on CGXII minimal medium with glucose as a carbon source. As expected, the prophage genes are no longer expressed, confirming the deletion of the respective genomic region. No other significant changes were observed (see Table S3 in the supplemental material).
Characterization of prophage deletion strains under stressful conditions.
It is known that prophages are often induced under stress conditions (16, 39). Therefore, the performance of MB001 and the ΔCGP3 strain was tested under pH stress, oxidative stress (H2O2), phosphate limitation, iron starvation or excess, UV radiation, osmotic stress, and heat shock (see Table 3). In further experiments, cells were treated with agents affecting cell wall integrity, such as penicillin, ethambutol, Tween 40, and vancomycin. In all experiments, MB001 did not show any defect in growth or survival compared to the wild type. Recent studies by our group suggested that the SOS response induces CGP3 (A. Heyer and J. Frunzke, unpublished). Therefore, the wild type and MB001 were grown in glucose minimal medium and treated with 1 μM mitomycin C, an antibiotic agent which activates the SOS response by cross-linking DNA, causing lesions in double-stranded DNA (40). Remarkably, we observed a 15 to 20% higher growth rate for MB001 than for the wild type (Fig. 2A). This indicates that MB001 exhibits an improved fitness under conditions triggering prophage induction.
Determination of transformation efficiency.
Earlier studies have shown that the deletion of the RM system located within the CGP3 region leads to a significant increase in transconjugation efficiency (41). Consequently, the deletion of the whole prophage CGP3, including the RM system, should have a similar effect. Thus, the transformation efficiency using a replicative and an integrative plasmid was analyzed in the wild type, MB001, and, as reference, a strain lacking only the RM system (ΔcglMRR). As expected, the transformation efficiency was increased in the two deletion strains over that in the wild type, i.e., 2 to 3 times for the integrative plasmid and 5 to 8 times for the replicative plasmid (Fig. 3). Although the day-to-day variations for the integrative plasmid were rather high in these experiments (high standard deviations) (Fig. 3), MB001 and the ΔcglMRR strain performed better than the wild type in 15 and 14 out of 16 experiments, respectively, underlining the higher transformation rate of strains lacking the RM system.
Fig 3.
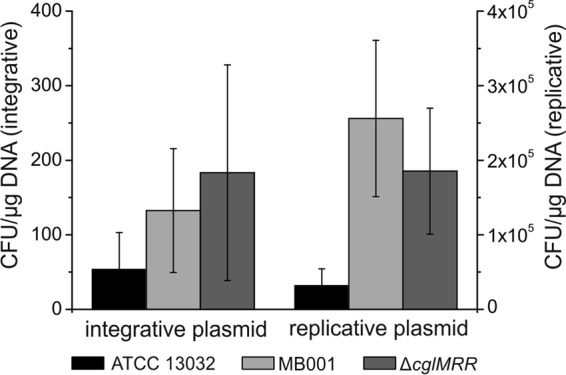
Transformation efficiency of C. glutamicum ATCC 13032, MB001, and the ΔcglMRR strain. A 150-μl cell suspension containing 109 cells was mixed on ice with 500 ng plasmid DNA. After regeneration, 10-μl and 300-μl aliquots were streaked on solid BHIS medium containing 25 mg liter−1 kanamycin. CFU were counted after 2 days incubation at 30°C, and the number of transformants was calculated as CFU per μg DNA and 109 recipient cells.
Analysis of heterologous protein production.
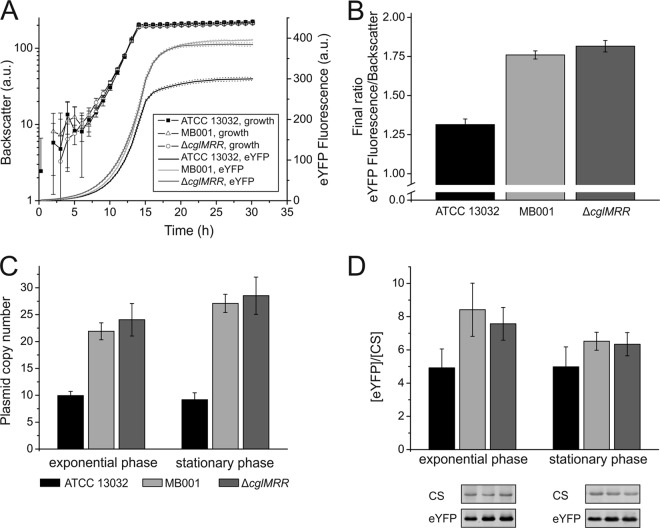
Studies of a B. subtilis strain with a 20%-reduced genome have revealed an increased production of recombinant proteins (12). Here, the performance of MB001 was tested with respect to heterologous protein production using eYFP as a model protein (pEKEx2-eyfp under the control of the tac promoter). Interestingly, we found an up to 40% higher fluorescence output in stationary phase in MB001 than in the wild type, while growth was basically unaffected (Fig. 4A and B). A similar result was observed for the ΔcglMRR strain, attributing this effect, again, to the lack of the RM system in the CGP3-deleted strain. In principle, the positive effect can result from different stages during protein production, e.g., (i) a higher copy number of the plasmid, (ii) higher promoter activity, (iii) higher mRNA stability, (iv) altered protein turnover, or (v) better protein folding conditions, leading to a higher proportion of fluorescent protein.
Fig 4.
Production of the heterologous model protein eYFP. Shown is the quantification of eYFP production and plasmid copy number in the C. glutamicum wild type, MB001, and the ΔcglMRR strain, each carrying the plasmid pEKEx2-eyfp. (A) Growth and eYFP fluorescence of the strains cultivated in CGXII minimal medium with 1% (wt vol−1) glucose and 1 mM IPTG in the Biolector at 30°C and 1,200 rpm. Cultivations were performed in seven replicates and sampled during the exponential and stationary growth phases for further analysis of plasmid copy number. Please note that the relatively large error bars of backscatter values below 10 result from technical limitations and the logarithmic presentation. (B) Biomass-specific eYFP fluorescence during the stationary growth phase of the experiment presented in panel A. (C) Plasmid copy number determined by qPCR. The cells were cultivated as described for panel A, and samples were harvested in late exponential and stationary growth phases. (D) Determination of the relative amount of eYFP. The blot was probed with primary antibodies against eYFP and citrate synthase (CS) and an anti-rabbit Cy5-coupled construct as a secondary antibody.
To better understand the observed improved protein production, we measured the plasmid copy number and the relative amount of eYFP in the exponential and stationary growth phases. In exponentially growing cells, compared to the wild type, the plasmid copy number was doubled in MB001 and the ΔcglMRR strain, and it was nearly tripled in stationary phase (Fig. 4C). In accordance with this result, the relative amount of eYFP was increased in the exponential and stationary growth phases (Fig. 4D), as determined by Western blotting. Hence, we conclude that the higher protein production can be ascribed to molecular events occurring at the beginning of the expression pathway and more specifically to an increase in the copy number of the target gene.
DISCUSSION
In this work, we have presented MB001, a prophage-free C. glutamicum strain with a genome reduced by 6%. This strain shows the same growth phenotype as the ATCC 13032 wild-type strain under standard conditions and an improved fitness under conditions triggering prophage induction. In further experiments, we could show that the strain exhibits higher transformation efficiency for replicative as well as integrative plasmids and a higher productivity of a heterologous protein.
The finding that it is possible to remove all prophages from the C. glutamicum genome without any negative impact on growth or fitness is a step toward a new platform (chassis) strain and has similarly been reported for B. subtilis, where two prophages, three prophage-like regions, and the pks operon have been deleted, leading to a genome reduction of 7% with no negative effect under laboratory conditions (10). A genome reduction of about 190 kbp has previously been reported for C. glutamicum R, where eight strand-specific islands were deleted with no effect on morphology or growth under standard laboratory conditions (15). The prophages residing in the ATCC 13032 genome encode many hypothetical proteins and proteins of unknown function (more than 80% of the predicted open reading frames [ORFs]), and hence their removal significantly reduces the complexity and thereby increases the predictability of this biological system. The higher transformation efficiency makes MB001 an ideal strain for metabolic engineering because the initial chromosomal integration of a plasmid is often the critical step. Finally, the significantly increased eYFP productivity is an interesting feature and could be relevant for all applications requiring high expression of proteins, such as protein production or the tuning of natural or synthetic metabolic pathways required for small-molecule production (42, 43).
Can we expect that removing 6% of the whole genome would reduce the energy requirement for biomass formation and thereby increase the growth rate or biomass yield of a specific strain? Strain MB001 showed no altered growth phenotype compared to the wild type under standard conditions, which seems reasonable considering the distribution of energy demand of different processes required for cell growth. Kjeldsen et al. (44) estimated that 67.8 mmol ATP is required for the formation of 1 g cell dry weight (CDW) of C. glutamicum, with maintenance, protein synthesis, and assembly of cell structures being the most energy-demanding processes. In comparison, the synthesis of nucleotides and their polymerization to DNA are rather cheap (4.44 mmol ATP g DNA−1), since only 1% (wt wt−1) of the CDW is buildup by DNA (44). Therefore, the formation of DNA molecules accounts for less than 0.1% of the cell's total ATP demand, and the reduction of this fraction by 6% in MB001 does not necessarily result in a measurable increase in growth rate or biomass yield.
The higher protein production does not result simply from the deletion of a significant fraction of the genome but could be assigned to a certain gene cluster. This is not unusual, as described for B. subtilis, where not the genome reduction by 20% in general but mainly the deletion of the rocDEF-rocR region was shown to be responsible for an elevated protein production. This region is involved in arginine degradation, and the deletion causes drastic changes in glutamate metabolism, leading to improved cell yields (45). In the case of C. glutamicum, the relevant operon codes for an RM system first described in the late 1980s (46, 47). Inactivation of these two genes led to a significant increase of fertility by several orders of magnitude in intergeneric matings and to a higher sensitivity toward infection with phage CL31 (41). The corresponding methyltransferase was discovered directly upstream of the two other genes and is likely responsible for the methylation found in C. glutamicum at the first cytidine in the sequence GGCCGC (32, 48). Genome sequencing of C. glutamicum ATCC 13032 revealed that the RM system resides within the large prophage CGP3.
The deletion of the prophage CGP3 was possible only after inactivation of the RM system, which can be explained by the “selfish” behavior of such systems (38, 49). When the genes of an RM system are deleted or inactivated, both enzymes are still present in the cells for several generations. The concentration decreases with every cell division until the amount of methyltransferase is no longer sufficient to methylate all restriction sites. At this point, even one molecule of restriction enzyme per cell can cause double strand breaks in the chromosome, which may then lead to cell death. Transferred to our deletion attempts, this explains why we could not achieve deletion of CGP3 without inactivating the RM system first. Hence, the RM system might be at least one of the reasons why CGP3 was not lost during evolution, because if CGP3 excises itself from the genome and is not replicated before the following cell division, the resulting cell without CGP3 most likely dies due to the activity of the restriction enzyme.
The copy number of the pEKEx2-eyfp plasmid was increased in both MB001 and the ΔcglMRR strain, suggesting the RM system as the main cause for this effect. The origin of replication of pEKEx2 is derived from pBL1, which replicates via the rolling-circle replication (RCR) mechanism (50), and its copy number in C. glutamicum was estimated to be in the range between 10 and 30 in earlier studies (51, 52). Replication via the RCR mechanism is initiated by a plasmid-encoded Rep protein, which introduces a site-specific nick in one of the DNA strands (53, 54). The copy number of plasmids is defined within a given host under certain conditions and is achieved by negative regulatory systems (53, 55). Our results suggest that the RM system of C. glutamicum is somehow involved in the copy number control of the pEKEx2 plasmid. An interesting hypothesis to test is that perhaps the origin of replication is modified by the methyltransferase in such a way that replication is reduced. Further investigations are required to fully understand the role of cglMRR in this context.
Altogether, MB001 represents a suitable strain for efficient metabolic engineering of C. glutamicum and will be used as basis for further genome reduction efforts, e.g., the deletion of mobile IS elements or nonessential gene clusters. These are first steps toward a flexible, predictable C. glutamicum ATCC 13032 chassis genome as a platform for the integration of synthetic pathways required for an efficient biotechnological production of small molecules or proteins.
Supplementary Material
ACKNOWLEDGMENTS
For funding, we thank the German Ministry of Education and Research (BMBF) (grants 0316017A and 0316017B), the Helmholtz Association (Young Investigator grant VH-NG-716), and the Deutsche Forschungsgemeinschaft (priority program SPP1617).
In addition, we thank Stephan Binder for providing plasmid pAN6-crimson, Cornelia Gätgens for technical assistance, and Volker Wendisch for discussions.
Footnotes
Published ahead of print 26 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01634-13.
REFERENCES
- 1.Leprince A, van Passel MWJ, Dos Santos VAPM. 2012. Streamlining genomes: toward the generation of simplified and stabilized microbial systems. Curr. Opin. Biotechnol. 23:651–658 [DOI] [PubMed] [Google Scholar]
- 2.Esvelt KM, Wang HH. 2013. Genome-scale engineering for systems and synthetic biology. Mol. Syst. Biol. 9:641. 10.1038/msb.2012.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szathmary E. 2005. Life—in search of the simplest cell. Nature 433:469–470 [DOI] [PubMed] [Google Scholar]
- 4.Kinoshita S, Udaka S, Shimono M. 1957. Studies of amino acid fermentation. I. Production of L-glutamic acid by various microorganisms. J. Gen. Appl. Microbiol. 3:193–205 [PubMed] [Google Scholar]
- 5.Becker J, Wittmann C. 2012. Systems and synthetic metabolic engineering for amino acid production—the heartbeat of industrial strain development. Curr. Opin. Biotechnol. 23:718–726 [DOI] [PubMed] [Google Scholar]
- 6.Wendisch VF. 2007. Amino acid biosynthesis—pathways, regulation and metabolic engineering. Springer-Verlag, Wiesbaden, Germany [Google Scholar]
- 7.Mizoguchi H, Sawano Y, Kato J-I, Mori H. 2008. Superpositioning of deletions promotes growth of Escherichia coli with a reduced genome. DNA Res. 15:277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ara K, Ozaki K, Nakamura K, Yamane K, Sekiguchi J, Ogasawara N. 2007. Bacillus minimum genome factory: effective utilization of microbial genome information. Biotechnol. Appl. Biochem. 46:169–178 [DOI] [PubMed] [Google Scholar]
- 9.Csörgo B, Feher T, Timar E, Blattner FR, Posfai G. 2012. Low-mutation-rate, reduced-genome Escherichia coli: an improved host for faithful maintenance of engineered genetic constructs. Microb. Cell Fact. 11:11. 10.1186/1475-2859-11-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westers H, Dorenbos R, van Dijl JM, Kabel J, Flanagan T, Devine KM, Jude F, Seror SJ, Beekman AC, Darmon E, Eschevins C, de Jong A, Bron S, Kuipers OP, Albertini AM, Antelmann H, Hecker M, Zamboni N, Sauer U, Bruand C, Ehrlich DS, Alonso JC, Salas M, Quax WJ. 2003. Genome engineering reveals large dispensable regions in Bacillus subtilis. Mol. Biol. Evol. 20:2076–2090 [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Golding I, Sawai S, Guo L, Cox EC. 2005. Population fitness and the regulation of Escherichia coli genes by bacterial viruses. PLoS Biol. 3:1276–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morimoto T, Kadoya R, Endo K, Tohata M, Sawada K, Liu S, Ozawa T, Kodama T, Kakeshita H, Kageyama Y, Manabe K, Kanaya S, Ara K, Ozaki K, Ogasawara N. 2008. Enhanced recombinant protein productivity by genome reduction in Bacillus subtilis. DNA Res. 15:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Posfai G, Plunkett G, Feher T, Frisch D, Keil GM, Umenhoffer K, Kolisnychenko V, Stahl B, Sharma SS, de Arruda M, Burland V, Harcum SW, Blattner FR. 2006. Emergent properties of reduced-genome Escherichia coli. Science 312:1044–1046 [DOI] [PubMed] [Google Scholar]
- 14.Suzuki N, Okayama S, Nonaka H, Tsuge Y, Inui M, Yukawa H. 2005. Large-scale engineering of the Corynebacterium glutamicum genome. Appl. Environ. Microbiol. 71:3369–3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki N, Nonaka H, Tsuge Y, Inui M, Yukawa H. 2005. New multiple-deletion method for the Corynebacterium glutamicum genome, using a mutant lox sequence. Appl. Environ. Microbiol. 71:8472–8480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casjens S. 2003. Prophages and bacterial genomics: what have we learned so far? Mol. Microbiol. 49:277–300 [DOI] [PubMed] [Google Scholar]
- 17.Wagner PL, Waldor MK. 2002. Bacteriophage control of bacterial virulence. Infect. Immun. 70:3985–3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canchaya C, Fournous G, Brüssow H. 2004. The impact of prophages on bacterial chromosomes. Mol. Microbiol. 53:9–18 [DOI] [PubMed] [Google Scholar]
- 19.Casjens S. 1998. The diverse and dynamic structure of bacterial genomes. Annu. Rev. Genet. 32:339–377 [DOI] [PubMed] [Google Scholar]
- 20.Kalinowski J, Bathe B, Bartels D, Bischoff N, Bott M, Burkovski A, Dusch N, Eggeling L, Eikmanns BJ, Gaigalat L, Goesmann A, Hartmann M, Huthmacher K, Krämer R, Linke B, McHardy AC, Meyer F, Möckel B, Pfefferle W, Pühler A, Rey DA, Rückert C, Rupp O, Sahm H, Wendisch VF, Wiegräbe I, Tauch A. 2003. The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J. Biotechnol. 104:5–25 [DOI] [PubMed] [Google Scholar]
- 21.Kalinowski J. 2005. The genomes of amino acid-producing corynebacteria, p 37–56 In Eggeling L, Bott M. (ed), Handbook of Corynebacterium glutamicum. CRC Press, Boca Raton, FL [Google Scholar]
- 22.Yukawa H, Omumasaba CA, Nonaka H, Kos P, Okai N, Suzuki N, Suda M, Tsuge Y, Watanabe J, Ikeda Y, Vertes AA, Inui M. 2007. Comparative analysis of the Corynebacterium glutamicum group and complete genome sequence of strain R. Microbiology 153:1042–1058 [DOI] [PubMed] [Google Scholar]
- 23.Frunzke J, Bramkamp M, Schweitzer J-E, Bott M. 2008. Population heterogeneity in Corynebacterium glutamicum ATCC 13032 caused by prophage CGP3. J. Bacteriol. 190:5111–5119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wennerhold J, Bott M. 2006. The DtxR regulon of Corynebacterium glutamicum. J. Bacteriol. 188:2907–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keilhauer C, Eggeling L, Sahm H. 1993. Isoleucine synthesis in Corynebacterium glutamicum—molecular analysis of the ilvB-ilvN-ilvC operon. J. Bacteriol. 175:5595–5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 27.Eikmanns BJ, Thumschmitz N, Eggeling L, Lüdtke KU, Sahm H. 1994. Nucleotide-sequence, expression and transcriptional analysis of the Corynebacterium glutamicum gltA gene encoding citrate synthase. Microbiology 140:1817–1828 [DOI] [PubMed] [Google Scholar]
- 28.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557–580 [DOI] [PubMed] [Google Scholar]
- 29.van der Rest ME, Lange C, Molenaar D. 1999. A heat shock following electroporation induces highly efficient transformation of Corynebacterium glutamicum with xenogeneic plasmid DNA. Appl. Microbiol. Biotechnol. 52:541–545 [DOI] [PubMed] [Google Scholar]
- 30.Grünberger A, Pyaczia N, Probst C, Schendzielorz G, Eggeling L, Noack S, Wiechert W, Kohlheyer D. 2012. A disposable picolitre bioreactor for cultivation and investigation of industrially relevant bacteria on the single cell level. Lab. Chip 12:2060–2068 [DOI] [PubMed] [Google Scholar]
- 31.Niebisch A, Bott M. 2001. Molecular analysis of the cytochrome bc1-aa3 branch of the Corynebacterium glutamicum respiratory chain containing an unusual diheme cytochrome c1. Arch. Microbiol. 175:282–294 [DOI] [PubMed] [Google Scholar]
- 32.Schäfer A, Tauch A, Droste N, Pühler A, Kalinowski J. 1997. The Corynebacterium glutamicum cglIM gene encoding a 5-cytosine methyltransferase enzyme confers a specific DNA methylation pattern in an McrBC-deficient Escherichia coli strain. Gene 203:95–101 [DOI] [PubMed] [Google Scholar]
- 33.Baumgart M, Mustafi N, Krug A, Bott M. 2011. Deletion of the aconitase gene in Corynebacterium glutamicum causes a strong selection pressure for secondary mutations inactivating citrate synthase. J. Bacteriol. 193:6864–6873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee CL, Ow DSW, Oh SKW. 2006. Quantitative real-time polymerase chain reaction for determination of plasmid copy number in bacteria. J. Microbiol. Methods 65:258–267 [DOI] [PubMed] [Google Scholar]
- 35.Tauch A, Kirchner O, Löffler B, Götker S, Pühler A, Kalinowski J. 2002. Efficient electrotransformation of Corynebacterium diphtheriae with a mini-replicon derived from the Corynebacterium glutamicum plasmid pGA1. Curr. Microbiol. 45:362–367 [DOI] [PubMed] [Google Scholar]
- 36.Dusch N, Pühler A, Kalinowski J. 1999. Expression of the Corynebacterium glutamicum panD gene encoding l-aspartate-α-decarboxylase leads to pantothenate overproduction in Escherichia coli. Appl. Environ. Microbiol. 65:1530–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rückert C, Pühler A, Kalinowski J. 2003. Genome-wide analysis of the L-methionine biosynthetic pathway in Corynebacterium glutamicum by targeted gene deletion and homologous complementation. J. Biotechnol. 104:213–228 [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi I. 2001. Behavior of restriction-modification systems as selfish mobile elements and their impact on genome evolution. Nucleic Acids Res. 29:3742–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lwoff A. 1953. Lysogeny. Bacteriol. Rev. 17:269–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomasz M, Palom Y. 1997. The mitomycin bioreductive antitumor agents: cross-linking and alkylation of DNA as the molecular basis of their activity. Pharmacol. Ther. 76:73–87 [DOI] [PubMed] [Google Scholar]
- 41.Schäfer A, Schwarzer A, Kalinowski J, Pühler A. 1994. Cloning and characterization of a DNA region encoding a stress-sensitive restriction system from Corynebacterium glutamicum ATCC 13032 and analysis of its role in intergeneric conjugation with Escherichia coli. J. Bacteriol. 176:7309–7319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rohe P, Venkanna D, Kleine B, Freudl R, Oldiges M. 2012. An automated workflow for enhancing microbial bioprocess optimization on a novel microbioreactor platform. Microb. Cell Fact. 11:144. 10.1186/1475-2859-11-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheele S, Oertel D, Bongaerts J, Evers S, Hellmuth H, Maurer K-H, Bott M, Freudl R. 2013. Secretory production of an FAD cofactor-containing cytosolic enzyme (sorbitol-xylitol oxidase from Streptomyces coelicolor) using the twin-arginine translocation (Tat) pathway of Corynebacterium glutamicum. Microb. Biotechnol. 6:202–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kjeldsen KR, Nielsen J. 2009. In silico genome-scale reconstruction and validation of the Corynebacterium glutamicum metabolic network. Biotechnol. Bioeng. 102:583–597 [DOI] [PubMed] [Google Scholar]
- 45.Manabe K, Kageyama Y, Morimoto T, Ozawa T, Sawada K, Endo K, Tohata M, Ara K, Ozaki K, Ogasawara N. 2011. Combined effect of improved cell yield and increased specific productivity enhances recombinant enzyme production in genome-reduced Bacillus subtilis strain MGB874. Appl. Environ. Microbiol. 77:8370–8381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liebl W, Bayerl A, Schein B, Stillner U, Schleifer KH. 1989. High efficiency electroporation of intact Corynebacterium glutamicum cells. FEMS Microbiol. Lett. 53:299–303 [DOI] [PubMed] [Google Scholar]
- 47.Vertes AA, Inui M, Kobayashi M, Kurusu Y, Yukawa H. 1993. Presence of mrr-like and mcr-like restriction systems in coryneform bacteria. Res. Microbiol. 144:181–185 [DOI] [PubMed] [Google Scholar]
- 48.Jang KH, Chambers PJ, Britz ML. 1996. Analysis of nucleotide methylation in DNA from Corynebacterium glutamicum and related species. FEMS Microbiol. Lett. 136:309–315 [DOI] [PubMed] [Google Scholar]
- 49.Naito T, Kusano K, Kobayashi I. 1995. Selfish behavior of restriction-modification systems. Science 267:897–899 [DOI] [PubMed] [Google Scholar]
- 50.Fernandez-Gonzalez C, Cadenas RF, Noirotgros MF, Martin JF, Gil JA. 1994. Characterization of a region of plasmid pBL1 of Bevibacterium lactofermentum involved in replication via the rolling circle model. J. Bacteriol. 176:3154–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santamaria R, Gil JA, Mesas JM, Martin JF. 1984. Characterization of an endogenous plasmid and development of cloning vectors and a transformation system in Brevibacterium lactofermentum. J. Gen. Microbiol. 130:2237–2246 [Google Scholar]
- 52.Miwa K, Matsui H, Terabe M, Nakamori S, Sano K, Momose H. 1984. Cryptic plasmids in glutamic acid-producing bacteria. Agric. Biol. Chem. 48:2901–2903 [Google Scholar]
- 53.del Solar G, Giraldo R, Ruiz-Echevarria MJ, Espinosa M, Diaz-Orejas R. 1998. Replication and control of circular bacterial plasmids. Microbiol. Mol. Biol. Rev. 62:434–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gruss A, Ehrlich SD. 1989. The family of highly interrelated single-stranded deoxyribonucleic acid plasmids. Microbiol. Rev. 53:231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.del Solar G, Espinosa M. 2000. Plasmid copy number control: an ever-growing story. Mol. Microbiol. 37:492–500 [DOI] [PubMed] [Google Scholar]
- 56.Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73 [DOI] [PubMed] [Google Scholar]
- 57.Rose RE. 1988. The nucleotide sequence of pACYC184. Nucleic Acids Res. 16:355–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hentschel E, Will C, Mustafi N, Burkovski A, Rehm N, Frunzke J. 2013. Destabilized eYFP variants for dynamic gene expression studies in Corynebacterium glutamicum. Microb. Biotechnol. 6:196–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frunzke J, Engels V, Hasenbein S, Gätgens C, Bott M. 2008. Co-ordinated regulation of gluconate catabolism and glucose uptake in Corynebacterium glutamicum by two functionally equivalent transcriptional regulators, GntR1 and GntR2. Mol. Microbiol. 67:305–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.