Abstract
Bacterial binuclear iron monooxygenases play numerous physiological roles in oxidative metabolism. Monooxygenases of this type found in actinomycetes also catalyze various useful reactions and have attracted much attention as oxidation biocatalysts. However, difficulties in expressing these multicomponent monooxygenases in heterologous hosts, particularly in Escherichia coli, have hampered the development of engineered oxidation biocatalysts. Here, we describe a strategy to functionally express the mycobacterial binuclear iron monooxygenase MimABCD in Escherichia coli. Sodium dodecyl sulfate-polyacrylamide gel electrophoretic analysis of the mimABCD gene expression in E. coli revealed that the oxygenase components MimA and MimC were insoluble. Furthermore, although the reductase MimB was expressed at a low level in the soluble fraction of E. coli cells, a band corresponding to the coupling protein MimD was not evident. This situation rendered the transformed E. coli cells inactive. We found that the following factors are important for functional expression of MimABCD in E. coli: coexpression of the specific chaperonin MimG, which caused MimA and MimC to be soluble in E. coli cells, and the optimization of the mimD nucleotide sequence, which led to efficient expression of this gene product. These two remedies enabled this multicomponent monooxygenase to be actively expressed in E. coli. The strategy described here should be generally applicable to the E. coli expression of other actinomycetous binuclear iron monooxygenases and related enzymes and will accelerate the development of engineered oxidation biocatalysts for industrial processes.
INTRODUCTION
Bacterial binuclear iron monooxygenases are a family of proteins that contain a binuclear iron center at the active site and are widely distributed throughout prokaryotes (1–3). These enzymes play numerous physiological roles in the oxidative metabolism of organic compounds, including alkanes, alkenes, and aromatics. Monooxygenases of this type found in actinomycetes constitute a new subfamily within the family of binuclear iron monooxygenases (1–3). These actinomycetous monooxygenases consist of four components, an oxygenase large subunit, an oxygenase small subunit, a reductase, and a coupling protein. Notably, the oxygenase component in these actinomycetous enzymes comprises two subunits in an αβ or an α2β2 quaternary structure, whereas this component in the enzymes of other bacteria, including methanotrophs and pseudomonads, comprises three subunits in an α2β2γ2 quaternary structure. The oxygenase component activates molecular oxygen using electrons that are transferred from NAD(P)H by the reductase component (4, 5). The coupling protein interacts with the oxygenase component and is essential for full oxidation activity (6, 7). The actinomycetous monooxygenases catalyze various interesting reactions and have attracted much attention as oxidation biocatalysts. For example, AmoABCD from Nocardia corallina (Rhodococcus rhodochrous) strain B-276 catalyzes the stereoselective epoxidation of various aliphatic alkenes (8, 9). In particular, this enzyme is able to catalyze the epoxidation of terminal alkenes, which is particularly difficult to perform by chemical methods. PmoABCD from Mycobacterium sp. strain M156 also shows epoxidation activity toward alkenes (10). In addition, propane monooxygenase (PrmABCD) (11, 12) and tetrahydrofuran monooxygenase (ThmABCD) (13) from actinomycetous strains have high catalytic potential for applications in biocatalysis and biodegradation.
The gene clusters encoding the actinomycetous monooxygenases described above have been successfully identified and cloned, while attempts to express these gene clusters in heterologous hosts have encountered difficulties (10, 14). In particular, expression of these gene clusters in Escherichia coli has been unsuccessful, although this extensively characterized and developed model microorganism is an ideal host for biochemical characterization and biotechnological applications of enzymes. For example, although functional expression of amoABCD from N. corallina B-276 in E. coli cells has been reported (9), experiments to confirm the reproducibility of the experiment were unsuccessful (14). Similarly, E. coli cells transformed with pmoABCD from Mycobacterium sp. strain M156 were not able to acquire oxidation activity (10). Chan Kwo Chion et al. suggested that the unsuccessful expression could be attributed to overlapping reading frames between pmoA and pmoB and between pmoC and pmoD (10). In addition to these actinomycetous monooxygenases, it has been reported that several binuclear iron monooxygenases of other bacteria, including methanotrophs and a Xanthobacter strain, were not functionally expressed in E. coli cells (15, 16). These studies suggest that the oxygenase components are unstable in E. coli hosts. The fact that these fascinating binuclear iron monooxygenases are difficult to express in E. coli has hampered the development of the engineered oxidation biocatalysts and prevents the practical application of these enzymes (17–19).
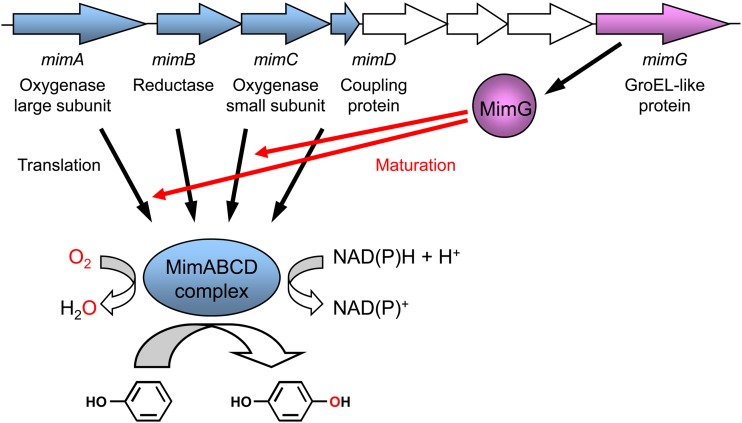
More recently, we succeeded in functionally expressing the mimABCD gene clusters from Mycobacterium smegmatis strain mc2155 and Mycobacterium goodii strain 12523 in the actinomycetous strain Rhodococcus opacus B-4 (20). The four genes mimA, mimB, mimC, and mimD encode an oxygenase large subunit, a reductase, an oxygenase small subunit, and a coupling protein, respectively (Fig. 1). The mimABCD gene cluster plays essential roles in propane and acetone metabolism in these mycobacteria (21, 22). Interestingly, MimABCD fortuitously catalyzes the regioselective oxidation of phenol to hydroquinone, which is of biotechnological importance (21). We have already found that MimABCD requires the specific chaperonin-like protein MimG, which is encoded downstream from the mimABCD gene cluster (Fig. 1), for functional expression in the Rhodococcus host; when the mimG gene was coexpressed with the mimABCD gene cluster in R. opacus strain B-4, this host successfully acquired oxidation activity toward phenol (20). Furthermore, we demonstrated that MimG was involved in the productive folding of the oxygenase large subunit MimA (20). We speculated that this chaperonin-like protein might also be an important factor in active expression in E. coli.
Fig 1.
Scheme showing genetic organization and enzymatic reaction of the mycobacterial binuclear iron monooxygenase.
In this study, we attempted to reconstitute the active MimABCD complex in E. coli. Based on the findings described above, the mimABCD gene cluster was coexpressed with the mimG gene in an E. coli host. Furthermore, the nucleotide sequence of the mimD gene was optimized for an expression system in E. coli, as we found that the expression level of the MimD protein in E. coli cells was extremely low. These attempts led to the successful expression of the mycobacterial binuclear iron monooxygenase in E. coli. The approach described here provides a robust platform applicable for the functional expression of other actinomycetous binuclear iron monooxygenases.
MATERIALS AND METHODS
Bacterial strains, plasmids, and cultivation media.
The bacterial strains and plasmids that were used or constructed in this study are listed in Table 1. Schematic maps of the plasmids are shown in Fig. S1 in the supplemental material. Bacteria were grown in Luria-Bertani (LB) medium, which contained (per liter) Bacto tryptone (10 g), Bacto yeast extract (5 g), and NaCl (10 g), pH 7.0.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristicsa | Reference(s) or source |
|---|---|---|
| Strains | ||
| E. coli JM109 | Host used for cloning | TaKaRa Bio |
| E. coli Rosetta 2(DE3)pLysS | Host used for expression | Novagen |
| Plasmids | ||
| pETmimABCDgo | pET21a containing mimABCDgo | 20, 21 |
| pTipmimGsm | pTip-RT2 containing mimGsm | 20 |
| pRSFDuet-1 | Vector used for cloning, RSF origin, Knr | Novagen |
| pCDFDuet-1 | Vector used for cloning, CDF origin, Smr | Novagen |
| pETDuet-1 | Vector used for cloning, pBR322 origin, Apr | Novagen |
| pRSFDmimA | pRSFDuet-1 containing mimA in MCS-1 under the control of the T7 promoter | This study |
| pRSFDmimAC | pRSFDuet-1 containing mimA in MCS-1 and mimC in MCS-2 under the control of the T7 promoters | This study |
| pCDFDmimG | pCDFDuet-1 containing mimG in MCS-1 under the control of the T7 promoter | This study |
| pCDFDmimG-groES | pCDFDuet-1 containing mimG in MCS-1 and groES in MCS-2 under the control of the T7 promoters | This study |
| pETDmimB | pETDuet-1 containing mimB in MCS-1 under the control of the T7 promoter | This study |
| pETDmimBD | pETDuet-1 containing mimB in MCS-1 and mimD in MCS-2 under the control of the T7 promoters | This study |
| pETDmimDop | pETDuet-1 containing mimDop in MCS-2 under the control of the T7 promoter | This study |
| pETDmimBDop | pETDuet-1 containing mimB in MCS-1 and mimDop in MCS-2 under the control of the T7 promoters | This study |
| pETDmimB5′opDop | pETDuet-1 containing mimB5′op in MCS-1 and mimDop in MCS-2 under the control of the T7 promoters | This study |
Knr, kanamycin resistance; Smr, streptomycin resistance; Apr, ampicillin resistance.
Construction of mimABCD, mimG, and groES expression plasmids.
The plasmids used for expression of the mimA and mimC genes in E. coli cells were constructed using the pRSFDuet-1 vector (Table 1). Two oligonucleotide primers, mimA-F and mimA-R (see Table S1 in the supplemental material), were designed to amplify the mimA gene. The region between the two oligonucleotide primers was amplified from the pETmimABCDgo plasmid (Table 1) by PCR. This amplified DNA fragment was digested with BspHI and EcoRI and was then inserted into multicloning site 1 (MCS-1) of pRSFDuet-1. The resulting plasmid, pRSFDmimA (Table 1), was amplified in E. coli JM109 cells (Table 1). Next, the mimC gene was amplified from pETmimABCDgo by PCR using two oligonucleotide primers, mimC-F and mimC-R (see Table S1). This amplified DNA fragment was digested with NdeI and FseI and was then inserted into MCS-2 of pRSFDmimA to construct pRSFDmimAC (Table 1).
In a similar technique, the plasmids used for expression of the mimG and groES genes in E. coli cells were constructed using the pCDFDuet-1 vector (Table 1). The mimG gene was amplified from the pTipmimGsm plasmid (Table 1) by PCR using two oligonucleotide primers, mimG-F and mimG-R (see Table S1 in the supplemental material). This amplified DNA fragment was digested with NcoI and EcoRI and was then inserted into MCS-1 of pCDFDuet-1 to construct pCDFDmimG (Table 1). Next, two oligonucleotide primers, groES-F and groES-R (see Table S1), were designed to amplify the groES gene that corresponds to the Msmeg_1582 open reading frame (ORF), based on the genome sequence of M. smegmatis strain mc2155 (GenBank accession number NC_008596). PCR was used to amplify the region between the two oligonucleotide primers from genomic DNA of M. smegmatis strain mc2155. This amplified DNA fragment was digested with NdeI and BglII and was then inserted into MCS-2 of pCDFDmimG to construct pCDFDmimG-groES (Table 1).
The plasmids used for expression of the mimB and mimD genes in E. coli cells were constructed using the pETDuet-1 vector (Table 1). The mimB gene was amplified from pETmimABCDgo by PCR using two oligonucleotide primers, mimB-F and mimB-R (see Table S1). This amplified DNA fragment was digested with NcoI and EcoRI and was then inserted into MCS-1 of pETDuet-1 to construct pETDmimB (Table 1). Next, the mimD gene was amplified from pETmimABCDgo by PCR using two oligonucleotide primers, mimD-F and mimD-R (see Table S1). This amplified DNA fragment was digested with NdeI and BglII and was then inserted into MCS-2 of pETDmimB to construct pETDmimBD (Table 1).
These plasmids were introduced into E. coli Rosetta 2(DE3)pLysS cells (Table 1) by heat shock. When three plasmids were introduced into E. coli cells, two plasmids, derived from pRSFDuet-1 and pCDFDuet-1, were first simultaneously introduced into E. coli cells. After preparation of the competent E. coli cells carrying the two plasmids, the third pETDuet-1-derived plasmid was introduced into the cells by electroporation.
Construction of mimDop and mimB5′op expression plasmids.
The overall nucleotide sequence of the mimD gene and the partial nucleotide sequence of the mimB gene in the translation initiation region were optimized for the E. coli expression system based on codon usage using software by Fasmac Co., Ltd. (Kanagawa, Japan). The nucleotide sequences of the optimized genes, mimDop and mimB5′op, are shown in Fig. S2 and S3, respectively, in the supplemental material. The mimDop gene was chemically synthesized by Fasmac Co., Ltd. The mimDop gene was amplified from the synthetic gene by PCR using two oligonucleotide primers, mimDop-F and mimDop-R (see Table S1). This amplified DNA fragment was digested with NdeI and BglII and was then inserted into MCS-2 of pETDuet-1 to construct pETDmimDop (Table 1) or into MCS-2 of pETDmimB to construct pETDmimBDop (Table 1). The mimB5′op gene was synthesized and amplified from pETmimABCDgo by PCR using two oligonucleotide primers, mimB5′op-F and mimB5′op-R (see Table S1). The optimized nucleotide sequence was included in the 5′-terminal primer mimB5′op-F. This amplified DNA fragment was digested with NcoI and BamHI and was then inserted into MCS-1 of pETDmimDop to construct pETDmimB5′opDop (Table 1).
Preparation of whole cells.
The transformed E. coli Rosetta 2(DE3)pLysS cells were cultivated at 30°C in LB medium supplemented with chloramphenicol (34 μg/ml), kanamycin (15 μg/ml), streptomycin (50 μg/ml), and/or ampicillin (50 μg/ml). When the cell growth reached an optical density at 600 nm (OD600) of 0.6 to 0.8, ferric citrate (100 μg/ml), ammonium ferric citrate (100 μg/ml), and l-cysteine (0.2 mM) were added to the medium to supply iron and sulfur for an iron protein (i.e., MimA) and an iron-sulfur protein (i.e., MimB) (23, 24). Isopropyl-β-d-thiogalactopyranoside (IPTG, 0.1 mM) was also added to the medium, and cultivation was continued at 25°C for an additional 15 h. Cells were harvested by centrifugation at 15,000 × g for 10 min at 4°C, washed with potassium phosphate buffer (50 mM, pH 7.5) containing glycerol (10%, vol/vol), and stored at −80°C until use.
SDS-PAGE analysis.
The expression levels of Mim-related proteins were examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. Frozen cells were suspended in potassium phosphate buffer (50 mM, pH 7.5) containing glycerol (10%, vol/vol) and were disrupted by sonication. Disrupted cells were used for the preparation of whole-cell samples that included both soluble and insoluble proteins. After centrifugation at 15,000 × g for 30 min at 4°C, the resulting supernatant was used for the preparation of soluble-fraction samples. Protein concentrations were measured using a Coomassie protein assay kit (Pierce, Rockford, IL) with a bovine serum albumin standard (25). Samples (2.5 to 5 μg protein) were treated with sodium dodecyl sulfate and then loaded onto a polyacrylamide gel. The acrylamide concentration was adjusted to 7.5% or 15%, depending on the molecular weights of Mim proteins.
Reaction using whole cells.
In whole-cell assays using transformed E. coli cells, the reaction mixture (250 μl) contained cells of the E. coli strain (2 g dry cell weight per liter), the substrate phenol (10 mM), ethanol (1%, vol/vol), and aqueous basal medium (20, 26) containing glucose (5 g/liter).
Product analysis.
High-performance liquid chromatography (HPLC) analysis was performed using an HPLC system (1100 series; Agilent, Palo Alto, CA) with a Wakosil-II 5C18 HG column (4.6 mm by 150 mm, 4.2- to 4.7-μm particle size; Wako pure chemicals, Osaka, Japan). Following the reaction of the transformed E. coli cells with phenol, methanol (250 μl) was added to the reaction mixture. The resulting sample (10 μl) was then injected into the HPLC system. Mobile phase A was composed of a mixture of acetonitrile-methanol-potassium phosphate buffer (10 mM, pH 2.7) at a ratio of 2.5:2.5:95, and phase B was methanol. Samples were eluted with 20% B for 30 min at a flow rate of 0.5 ml/min. The reaction product hydroquinone was detected spectrophotometrically at a wavelength of 220 nm.
Sequence analysis.
The GC contents of the nucleotide sequence were calculated using Genetyx version 10 (Genetyx Corporation, Tokyo, Japan). The minimum free energy associated with the mRNA secondary structure was also calculated using Genetyx version 10.
RESULTS
Construction of mimABCD expression system in E. coli.
We have previously attempted to express the mimABCD gene cluster in E. coli cells using pETmimABCD, in which the tandem mimABCD gene cluster was located downstream from the T7 promoter and the ribosome-binding site (RBS) on the pET21a vector (20). In this plasmid, the mimA gene utilizes the vector-derived RBS, whereas the mimB, mimC, and mimD genes have their natural RBSs. The transformed E. coli cells, however, did not oxidize the substrate phenol. SDS-PAGE analysis revealed the absence of any bands corresponding to MimB, MimC, or MimD and the presence of a band corresponding to MimA in the insoluble fraction (20).
In this study, we designed and exploited a new system for mimABCD expression using three compatible plasmids to overcome the low-level expression and insolubilization of the Mim components in E. coli cells. Here, the mimA, mimB, mimC, and mimD genes were placed under the respective T7 promoters and E. coli RBSs. The mimA and mimC genes, encoding the oxygenase large and small subunits, respectively, were cloned into the two MCSs of the pRSFDuet-1 vector (Table 1). In a similar technique, the mimB and mimD genes, encoding a reductase and a coupling protein, respectively, were cloned into the two MCSs of the pETDuet-1 vector (Table 1). Furthermore, the mimG gene, whose GroEL-like product had been required for the productive folding of MimA in R. opacus cells (20), was cloned into MCS-1 of the pCDFDuet-1 vector (Table 1). In addition, GroELs generally require a cochaperonin, GroES, to assist in the folding of target proteins. The genome sequence of M. smegmatis strain mc2155 has only one copy of the groES gene, Msmeg_1582 (20, 27). This groES gene was cloned into MCS-2 of the pCDFDuet-1 vector (Table 1).
Expression analysis of mimA and mimC in E. coli.
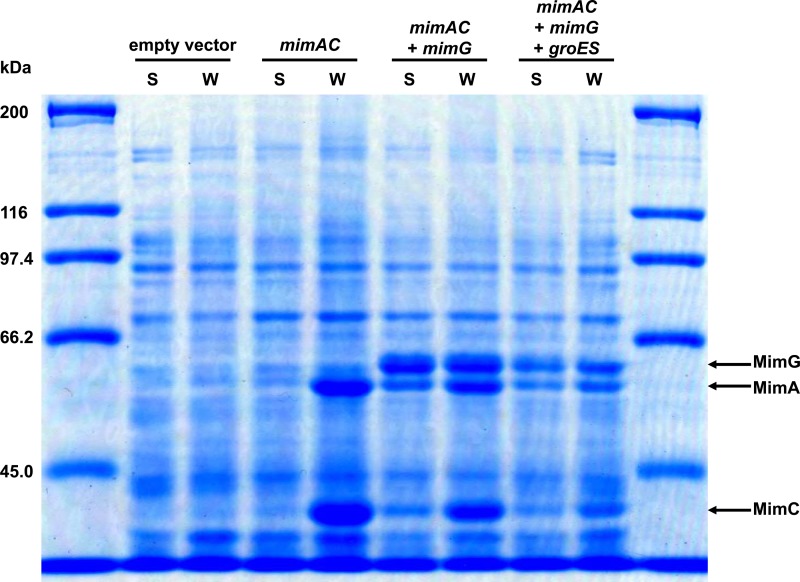
To reconstitute the active MimABCD complex in E. coli cells, we first attempted to express the oxygenase large and small subunits, MimA and MimC, in the soluble fraction of E. coli cells. The pRSFDmimAC plasmid (Table 1) was introduced into E. coli Rosetta 2(DE3)pLysS cells, and the expression of the mimA and mimC genes was induced by IPTG. As observed for the expression analysis of MimA in R. opacus cells, SDS-PAGE analysis revealed that the MimA protein was insoluble in E. coli cells (Fig. 2, mimAC). Furthermore, we found that MimC was also insoluble (Fig. 2, mimAC). In contrast, when the mimG gene was coexpressed with the mimA and mimC genes, these gene products were successfully expressed as their soluble forms (Fig. 2, mimAC + mimG). These results indicate that MimG functioned as a chaperonin for the productive folding of MimA and MimC even in E. coli cells. We also examined the effects of mycobacterial cochaperonin GroES on the expression of MimA and MimC. By SDS-PAGE analysis using 15% polyacrylamide gel, we confirmed that the GroES protein (10.8 kDa) was expressed in the soluble fraction (data not shown). However, the coexpression of the groES gene with the mimA, mimC, and mimG genes did not further enhance the proportions of the soluble forms of MimA and MimC compared to their expression without the coexpression of groES (Fig. 2, mimAC + mimG + groES).
Fig 2.
SDS-PAGE analysis of expression of mimA and mimC in E. coli. Samples prepared from E. coli cells carrying pRSFDuet-1 and pCDFDuet-1 (empty vector), pRSFDmimAC and pCDFDuet-1 (mimAC), pRSFDmimAC and pCDFDmimG (mimAC + mimG), or pRSFDmimAC and pCDFDmimG-groES (mimAC + mimG + groES) were loaded onto a polyacrylamide gel (7.5%). S, soluble-fraction sample; W, whole-cell sample. The molecular masses corresponding to MimA, MimC, and MimG are indicated by arrows.
Expression analysis of mimB and mimD in E. coli.
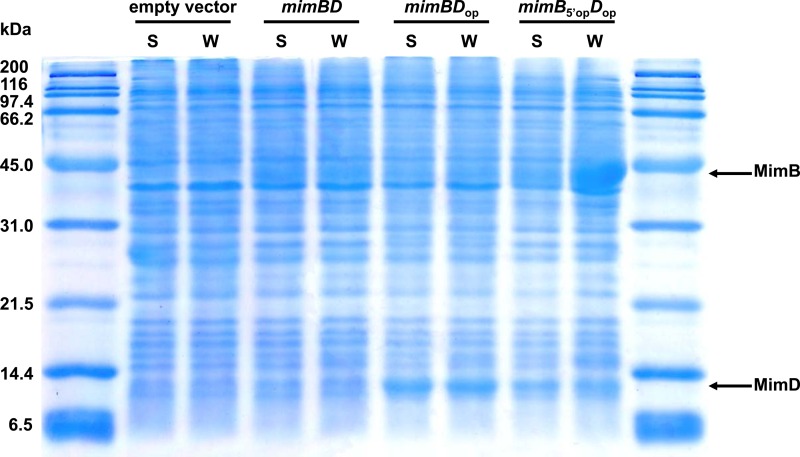
We next examined the expression of the mimB and mimD genes in E. coli cells using the pETDmimBD plasmid carrying these genes (Table 1). Although SDS-PAGE analysis revealed very low-level expression of the MimB protein in the soluble fraction of E. coli cells, a band corresponding to MimD was not evident (Fig. 3, mimBD). These results indicate the difficulties in expressing native mycobacterial genes in Gram-negative E. coli, which is phylogenetically distant from Gram-positive actinomycetous Mycobacterium species and possesses transcription and translation systems different from those of mycobacteria.
Fig 3.
SDS-PAGE analysis of expression of mimB and mimD in E. coli. Samples prepared from E. coli cells carrying pETDuet-1 (empty vector), pETDmimBD (mimBD), pETDmimBDop (mimBDop), or pETDmimB5′opDop (mimB5′opDop) were loaded onto a polyacrylamide gel (15%). S, soluble-fraction sample; W, whole-cell sample. The molecular masses corresponding to MimB and MimD are indicated by arrows.
In order to overcome low-level expression of the MimD protein in E. coli cells, the overall nucleotide sequence of the mimD gene (345 bp) was optimized for the expression system of E. coli using software (see Materials and Methods). The synthetic gene designated mimDop (see Fig. S2 in the supplemental material) shares 77% nucleotide identity with the native mimD gene, whereas the encoded protein sequences are identical for the two genes. The GC content decreased to 51% from 62% through the optimization. The mimDop gene was cloned into the pETDuet-1 vector instead of the native mimD gene, and was then introduced into the E. coli host. The induction of the expression of this gene by IPTG resulted in markedly high-level expression of MimD in E. coli; SDS-PAGE analysis revealed the presence of a major band corresponding to MimD in the soluble fraction of E. coli cells (Fig. 3, mimBDop).
We also attempted to express the MimB protein at higher levels in E. coli cells. Because MimB is encoded by a relatively long nucleotide sequence (1,047 bp), we examined the substitution of a partial nucleotide sequence. Recent studies have suggested that poor expression levels of recombinant proteins in E. coli were often caused by the formation of excessively stable secondary structures of mRNA in the translation initiation region (28, 29). Thus, we calculated the minimum free energy associated with the mRNA secondary structure of the translation initiation region for mimB using Genetyx software (29). The folding energy for the region from nucleotide −4 to +37 relative to the start site was found to be −6.6 to ∼−6.2 kcal/mol. This value was lower than the threshold predicted for inhibition of translation initiation, ca. −6 kcal/mol (29–31). When the partial nucleotide sequence of the mimB gene in the translation initiation region was optimized for the expression system of E. coli, the folding energy increased to −3.9 to ∼−3.8 kcal/mol. Therefore, we synthesized the mimB gene with an optimized nucleotide sequence from +1 to +37 (see Materials and Methods). The synthetic gene designated mimB5′op (see Fig. S3 in the supplemental material) is different in only three nucleotides from the native mimB gene, whereas the encoded protein sequences are identical for the two genes. The GC contents are almost the same (64%) for the two genes. The mimB5′op gene was cloned into the pETDuet-1 vector instead of the native mimB gene and then expressed in the E. coli host. Through this optimization, the expression level of MimB was extensively improved; SDS-PAGE analysis, however, revealed the presence of MimB in the insoluble fraction of E. coli cells (Fig. 3, mimB5′opDop). We also confirmed that induction of mimB5′op gene expression by IPTG under 15°C instead of 25°C did not affect the solubility of this gene product.
Catalytic activity of E. coli cells carrying mimABCD toward phenol.
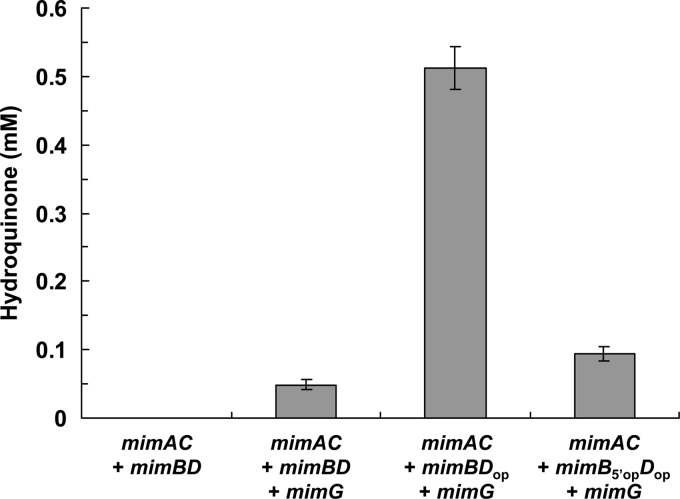
We examined the catalytic activity of E. coli cells carrying the mimABCD genes toward phenol using the three-recombinant-plasmid system. As expected, E. coli cells carrying only the mimABCD genes did not oxidize phenol. In contrast, when the mimABCD genes were coexpressed with the mimG gene, E. coli cells successfully acquired oxidation activity toward phenol, although the activity was low (0.067 μmol per gram dry cell weight per minute) (Fig. 4). When the mimDop gene was used as a substitute for mimD, however, the oxidation activity was extensively enhanced; E. coli cells carrying mimABCDop and mimG rapidly oxidized phenol to hydroquinone (Fig. 4). The initial rate of hydroquinone production by cells carrying mimABCDop and mimG was estimated to be 0.71 μmol per gram dry cell weight per minute, which was 11 times higher than that by cells carrying mimABCD and mimG. When the mimB gene was optimized in addition to the mimD gene, E. coli cells carrying mimAB5′opCDop and mimG showed lower oxidation activities than cells carrying mimABCDop and mimG (Fig. 4).
Fig 4.
Monooxygenase activities of engineered E. coli cells toward phenol. Whole cells of E. coli carrying pRSFDmimAC, pETDmimBD, and pCDFDuet-1 (mimAC + mimBD), pRSFDmimAC, pETDmimBD, and pCDFDmimG (mimAC + mimBD + mimG), pRSFDmimAC, pETDmimBDop, and pCDFDmimG (mimAC + mimBDop + mimG), or pRSFDmimAC, pETDmimB5′opDop, and pCDFDmimG (mimAC + mimB5′opDop + mimG) were reacted with phenol for 6 h, and the monooxygenation product hydroquinone was quantified using HPLC. Bars represent the means of three independent experiments, and error bars represent standard deviations of the means.
DISCUSSION
In this study, we succeeded in reconstituting the active MimABCD complex in E. coli. We found that the following factors are important for functional expression of the mycobacterial binuclear iron monooxygenase in E. coli: the coexpression of MimG, which caused MimA and MimC to be soluble in E. coli cells, and the optimization of the mimD nucleotide sequence, which led to efficient expression of this gene product (Fig. 1). To our knowledge, this is the second report regarding the active expression of an actinomycetous binuclear iron monooxygenase in an E. coli host. The first functional expression in E. coli was reported for AmoABCD of N. corallina B-276; E. coli cells carrying only the native amoABCD gene cluster were able to oxidize 3,3,3-trifluoropropene (9). However, it has also been reported that experiments to carefully confirm the reproducibility of the experiment were unsuccessful (14).
The GroEL-like protein MimG functioned as a chaperonin for the productive folding of the oxygenase large subunit MimA in E. coli cells, as well as in R. opacus cells. Notably, in the absence of MimG, the oxygenase small subunit MimC was also insoluble in E. coli cells (Fig. 2), although this protein had been soluble in R. opacus cells (20). These results suggest that the environment in E. coli cells tended to render the MimC protein unstable. Nevertheless, MimG was able to support MimC in the maturation process in E. coli cells (Fig. 2). Because the oxygenase large and small subunits are known to be a paralogous protein and show similarity in quaternary structure (2), it is unsurprising that MimG plays a role for both MimA and MimC. Coexpression of the mycobacterial cochaperonin GroES with MimG did not further enhance the proportions of the soluble forms of MimA and MimC compared to their proportions without groES coexpression (Fig. 2). It is possible that MimG did not require any cochaperonin for its function or that a proper cochaperonin was sufficiently supplied to MimG by the host E. coli strain. The genome sequence of E. coli BL21(DE3), the parent strain of Rosetta 2(DE3)pLysS (GenBank accession number NC_012971), contains one copy of the groES gene, ECD_04012, whose gene product shares ca. 45% amino acid identity with the mycobacterial GroES.
In this study, we succeeded in reconstituting the active MimABCD complex using protein components whose amino acid sequences are identical to those of the native Mim components. The optimization of the mimD nucleotide sequence for the expression system of E. coli provided efficient expression of this mimDop gene product (Fig. 3), which endowed E. coli cells with high oxidation activity (Fig. 4). We also achieved high-level expression of MimB in E. coli by increasing the folding energy associated with the mRNA secondary structure of the translation initiation region. However, this mimB5′op gene product was insoluble in E. coli cells (Fig. 3), and this situation reduced the oxidation activity of the transformed E. coli cells (Fig. 4). It was reported that the reductase components of binuclear iron monooxygenases that contain [Fe-S] centers were amenable to inactivation in E. coli cells, particularly under high-level-expression conditions (15, 24, 32). For example, the reductase component of toluene 4-monooxygenase has been functionally expressed under the control of a weak promoter (32). Similarly, it would be important to restrict the expression of MimB at low levels to escape inactivation. As a result, the engineered E. coli cells carrying mimABCDop and mimG acquired the highest oxidation activity (0.71 μmol per gram dry cell weight per minute) toward phenol. This E. coli biocatalyst has great potential for the industrial production of hydroquinone from phenol. To further enhance the oxidation activity of the engineered E. coli cells, it would be important to achieve high-level and soluble expression of MimB. It has recently been reported that fusion of the reductase component of tetrahydrofuran monooxygenase to maltose binding protein markedly increased the solubility of this component (24). Similar to this, fusion of MimB to an appropriate tag sequence might lead to its high-level soluble expression. Also, the genome sequence of M. smegmatis strain mc2155 has two other copies of the groEL gene, Msmeg_0880 and Msmeg_1583, in addition to mimG (Msmeg_1978) (27). These mycobacterial chaperonins might be helpful in the solubilization of MimB.
Finally, the strategy described here should be generally applicable to the active expression of other actinomycetous binuclear iron monooxygenases in E. coli. As we have reported previously (20), many homologous gene clusters encoding binuclear iron monooxygenase exist in cloned nucleotide sequences and published genome sequences of actinomycetes, and these are always accompanied by mimG homologs. This strongly suggests the high potential of our strategy for wider application. Furthermore, although methane monooxygenase is the archetypal and most investigated member of the binuclear iron monooxygenase family, active expression of this monooxygenase in E. coli has yet to be achieved (18, 19). Because it was found that a specific chaperonin was involved in the expression system of the methane monooxygenase (33, 34), our experimental platform may be helpful for the functional expression of this monooxygenase in E. coli. The approaches described here accelerate not only the biochemical characterization of these binuclear iron monooxygenase systems but also the development of the engineered oxidation biocatalysts and will pave the way for industrial application of these enzymes.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by Japan Society for the Promotion of Science, Grant-in-Aid for Young Scientists (B) 24780083 to T.F.
Footnotes
Published ahead of print 26 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01856-13.
REFERENCES
- 1.Coleman NV, Bui NB, Holmes AJ. 2006. Soluble di-iron monooxygenase gene diversity in soils, sediments and ethene enrichments. Environ. Microbiol. 8:1228–1239 [DOI] [PubMed] [Google Scholar]
- 2.Leahy JG, Batchelor PJ, Morcomb SM. 2003. Evolution of the soluble diiron monooxygenases. FEMS Microbiol. Rev. 27:449–479 [DOI] [PubMed] [Google Scholar]
- 3.Notomista E, Lahm A, Di Donato A, Tramontano A. 2003. Evolution of bacterial and archaeal multicomponent monooxygenases. J. Mol. Evol. 56:435–445 [DOI] [PubMed] [Google Scholar]
- 4.Fox BG, Froland WA, Dege JE, Lipscomb JD. 1989. Methane monooxygenase from Methylosinus trichosporium OB3b. Purification and properties of a three-component system with high specific activity from a type II methanotroph. J. Biol. Chem. 264:10023–10033 [PubMed] [Google Scholar]
- 5.Tinberg CE, Lippard SJ. 2011. Dioxygen activation in soluble methane monooxygenase. Acc. Chem. Res. 44:280–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandstetter H, Whittington DA, Lippard SJ, Frederick CA. 1999. Mutational and structural analyses of the regulatory protein B of soluble methane monooxygenase from Methylococcus capsulatus (Bath). Chem. Biol. 6:441–449 [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, McCormick MS, Lippard SJ, Cho US. 2013. Control of substrate access to the active site in methane monooxygenase. Nature 494:380–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furuhashi K. 1992. Biological routes to optically active epoxides, p 167–186 In Collins AN, Sheldrake GN, Crosby J. (ed), Chirality in industry, Wiley & Sons Ltd,Chichester, United Kingdom [Google Scholar]
- 9.Saeki H, Furuhashi K. 1994. Cloning and characterization of a Nocardia corallina B-276 gene cluster encoding alkene monooxygenase. J. Ferment. Bioeng. 78:399–406 [Google Scholar]
- 10.Chan Kwo Chion CK, Askew SE, Leak DJ. 2005. Cloning, expression, and site-directed mutagenesis of the propene monooxygenase genes from Mycobacterium sp. strain M156. Appl. Environ. Microbiol. 71:1909–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotani T, Yamamoto T, Yurimoto H, Sakai Y, Kato N. 2003. Propane monooxygenase and NAD+-dependent secondary alcohol dehydrogenase in propane metabolism by Gordonia sp. strain TY-5. J. Bacteriol. 185:7120–7128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharp JO, Sales CM, LeBlanc JC, Liu J, Wood TK, Eltis LD, Mohn WW, Alvarez-Cohen L. 2007. An inducible propane monooxygenase is responsible for N-nitrosodimethylamine degradation by Rhodococcus sp. strain RHA1. Appl. Environ. Microbiol. 73:6930–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiemer B, Andreesen JR, Schräder T. 2003. Cloning and characterization of a gene cluster involved in tetrahydrofuran degradation in Pseudonocardia sp. strain K1. Arch. Microbiol. 179:266–277 [DOI] [PubMed] [Google Scholar]
- 14.Smith TJ, Lloyd JS, Gallagher SC, Fosdike WL, Murrell JC, Dalton H. 1999. Heterologous expression of alkene monooxygenase from Rhodococcus rhodochrous B-276. Eur. J. Biochem. 260:446–452 [DOI] [PubMed] [Google Scholar]
- 15.Champreda V, Zhou NY, Leak DJ. 2004. Heterologous expression of alkene monooxygenase components from Xanthobacter autotrophicus Py2 and reconstitution of the active complex. FEMS Microbiol. Lett. 239:309–318 [DOI] [PubMed] [Google Scholar]
- 16.West CA, Salmond GP, Dalton H, Murrell JC. 1992. Functional expression in Escherichia coli of proteins B and C from soluble methane monooxygenase of Methylococcus capsulatus (Bath). J. Gen. Microbiol. 138:1301–1307 [DOI] [PubMed] [Google Scholar]
- 17.Leak DJ, Sheldon RA, Woodley JM, Adlercreutz P. 2009. Biocatalysts for selective introduction of oxygen. Biocatal. Biotransform. 27:1–26 [Google Scholar]
- 18.Murrell JC. 2002. Expression of soluble methane monooxygenase genes. Microbiology 148:3329–3330 [DOI] [PubMed] [Google Scholar]
- 19.Wood TK. 2002. Active expression of soluble methane monooxygenase from Methylosinus trichosporium OB3b in heterologous hosts. Microbiology 148:3328–3329 [DOI] [PubMed] [Google Scholar]
- 20.Furuya T, Hayashi M, Semba H, Kino K. 2013. The mycobacterial binuclear iron monooxygenases require a specific chaperonin-like protein for functional expression in a heterologous host. FEBS J. 280:817–826 [DOI] [PubMed] [Google Scholar]
- 21.Furuya T, Hirose S, Osanai H, Semba H, Kino K. 2011. Identification of the monooxygenase gene clusters responsible for the regioselective oxidation of phenol to hydroquinone in mycobacteria. Appl. Environ. Microbiol. 77:1214–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furuya T, Hirose S, Semba H, Kino K. 2011. Identification of the regulator gene responsible for the acetone-responsive expression of the binuclear iron monooxygenase gene cluster in mycobacteria. J. Bacteriol. 193:5817–5823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaganaman S, Pinto A, Tarasev M, Ballou DP. 2007. High levels of expression of the iron-sulfur proteins phthalate dioxygenase and phthalate dioxygenase reductase in Escherichia coli. Protein. Expr. Purif. 52:273–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oppenheimer M, Pierce BS, Crawford JA, Ray K, Helm RF, Sobrado P. 2010. Recombinant expression, purification, and characterization of ThmD, the oxidoreductase component of tetrahydrofuran monooxygenase. Arch. Biochem. Biophys. 496:123–131 [DOI] [PubMed] [Google Scholar]
- 25.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 26.Hamada T, Maeda Y, Matsuda H, Sameshima Y, Honda K, Omasa T, Kato J, Ohtake H. 2009. Effect of cell-surface hydrophobicity on bacterial conversion of water-immiscible chemicals in two-liquid-phase culture systems. J. Biosci. Bioeng. 108:116–120 [DOI] [PubMed] [Google Scholar]
- 27.Rao T, Lund PA. 2010. Differential expression of the multiple chaperonins of Mycobacterium smegmatis. FEMS Microbiol. Lett. 310:24–31 [DOI] [PubMed] [Google Scholar]
- 28.Kudla G, Murray AW, Tollervey D, Plotkin JB. 2009. Coding-sequence determinants of gene expression in Escherichia coli. Science 324:255–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szeker K, Niemitalo O, Casteleijn MG, Juffer AH, Neubauer P. 2010. High-temperature cultivation and 5′ mRNA optimization are key factors for the efficient overexpression of thermostable Deinococcus geothermalis purine nucleoside phosphorylase in Escherichia coli. J. Biotechnol. 156:268–274 [DOI] [PubMed] [Google Scholar]
- 30.de Smit MH, van Duin J. 1990. Secondary structure of the ribosome binding site determines translational efficiency: a quantitative analysis. Proc. Natl. Acad. Sci. U. S. A. 87:7668–7672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Smit MH, van Duin J. 1994. Control of translation by mRNA secondary structure in Escherichia coli. A quantitative analysis of literature data. J. Mol. Biol. 244:144–150 [DOI] [PubMed] [Google Scholar]
- 32.Bailey LJ, Elsen NL, Pierce BS, Fox BG. 2008. Soluble expression and purification of the oxidoreductase component of toluene 4-monooxygenase. Protein. Expr. Purif. 57:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scanlan J, Dumont MG, Murrell JC. 2009. Involvement of MmoR and MmoG in the transcriptional activation of soluble methane monooxygenase genes in Methylosinus trichosporium OB3b. FEMS Microbiol. Lett. 301:181–187 [DOI] [PubMed] [Google Scholar]
- 34.Stafford GP, Scanlan J, McDonald IR, Murrell JC. 2003. rpoN, mmoR and mmoG, genes involved in regulating the expression of soluble methane monooxygenase in Methylosinus trichosporium OB3b. Microbiology 149:1771–1784 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.