Abstract
Mixed populations of Saccharomyces cerevisiae yeasts and lactic acid bacteria occur in many dairy, food, and beverage fermentations, but knowledge about their interactions is incomplete. In the present study, interactions between Saccharomyces cerevisiae and Lactobacillus delbrueckii subsp. bulgaricus, two microorganisms that co-occur in kefir fermentations, were studied during anaerobic growth on lactose. By combining physiological and transcriptome analysis of the two strains in the cocultures, five mechanisms of interaction were identified. (i) Lb. delbrueckii subsp. bulgaricus hydrolyzes lactose, which cannot be metabolized by S. cerevisiae, to galactose and glucose. Subsequently, galactose, which cannot be metabolized by Lb. delbrueckii subsp. bulgaricus, is excreted and provides a carbon source for yeast. (ii) In pure cultures, Lb. delbrueckii subsp. bulgaricus grows only in the presence of increased CO2 concentrations. In anaerobic mixed cultures, the yeast provides this CO2 via alcoholic fermentation. (iii) Analysis of amino acid consumption from the defined medium indicated that S. cerevisiae supplied alanine to the bacterium. (iv) A mild but significant low-iron response in the yeast transcriptome, identified by DNA microarray analysis, was consistent with the chelation of iron by the lactate produced by Lb. delbrueckii subsp. bulgaricus. (v) Transcriptome analysis of Lb. delbrueckii subsp. bulgaricus in mixed cultures showed an overrepresentation of transcripts involved in lipid metabolism, suggesting either a competition of the two microorganisms for fatty acids or a response to the ethanol produced by S. cerevisiae. This study demonstrates that chemostat-based transcriptome analysis is a powerful tool to investigate microbial interactions in mixed populations.
INTRODUCTION
In the past decade, the intrinsic ambition to study microorganisms as integral systems has come within reach by the implementation of genome-wide analytical approaches. For example, DNA microarray-based transcriptome analysis and mass spectrometry-based proteome analysis enable accurate and inclusive measurement of microbial responses to environmental conditions (1–6). Chemostat cultivation is highly valuable for this type of research, as it offers unique options for experimental design. In particular, chemostat cultivation enables researchers to manipulate individual cultivation parameters with minimal impact on other process conditions and at a fixed specific growth rate and, thereby, to dissect responses to individual environmental conditions (7–10).
So far, chemostat-based transcriptome analysis has largely been confined to pure cultures. These, however, represent an extreme simplification of the situation in nature as well as in many food and beverage fermentations, where mixed microbial populations confer product characteristics such as flavor, aroma, nutritional value, and storage stability (11–14). While food fermentation processes have been intensively investigated, the mechanisms governing population composition, dynamics, and performance remain largely elusive. A better understanding of microbial interactions could contribute to improvement of mixed-culture fermentation processes. Moreover, genome-wide studies on cocultures may provide leads for functional analysis of genes whose function has not yet been identified in pure-culture studies.
A limited number of recent studies have indicated that genome-wide transcriptome analysis can provide a better insight into the nature and molecular basis of microbial interactions in mixed cultures of industrial organisms (15–18). The fact that all these reports involve lactic acid bacteria (LAB) reflects the importance of this group of bacteria in the mixed cultivations for the dairy industry and the fermentation of other foods, such as olives, sourdough, and coffee. Another common feature of all these studies is the utilization of a dynamic cultivation mode (batch) for the mixed population. In batch cultures, the inherent culture dynamics inevitably lead to changing interactions between microbial species in mixed populations, e.g., due to the depletion of substrates or to the accumulation of products. Investigating the Lactococcus lactis response to cocultivation with Saccharomyces cerevisiae, Maligoy and coworkers identified these culture dynamics to be “a crucial problem” in the dissection of microbial responses to cocultivation in batch cultures (16). Carefully designed chemostat experiments can lead to stable cocultures in which cultivation conditions are constant in time (19–22). When, in a mixed-population chemostat, interaction between two microbial strains or species is solely determined by competition for a single nutrient, stable cocultivation is possible only when a unique residual concentration is used for each set of experimental conditions (23). More stable binary cocultures can be established by growing cultures on mixtures of substrates of which each component can be used by only one of the microorganisms or in which one microorganism cannot grow on the single substrate that is provided to the culture but is able to grow on a metabolic product of its partner. To our knowledge, this concept has so far not been applied for chemostat-based transcriptome analysis of microbial interactions.
Furthermore, among the few published transcriptome studies, only one investigated the reciprocal response of all organisms involved in the cocultivation (in this case, the response of Streptococcus salivarius subsp. thermophilus and Lactobacillus delbrueckii subsp. bulgaricus involved in yoghurt fermentation [15]), while the others focused on the response of a single organism. Maligoy and coworkers investigated the response of L. lactis to cocultivation with S. cerevisiae (16), and Hervé-Jimenez and coauthors explored the impact of cocultivation of Streptomyces salivarius subsp. thermophilus with its yoghurt partner, Lb. delbrueckii subsp. bulgaricus, on the S. salivarius subsp. thermophilus transcriptome (24). A more recent study assessed S. cerevisiae's response to the presence of Oenococcus oeni during wine fermentation (17).
The present study aims to explore chemostat-based transcriptome analysis of mixed cultures by investigating interactions between the yeast S. cerevisiae and the lactic acid bacterium Lb. delbrueckii subsp. bulgaricus. S. cerevisiae and Lb. delbrueckii subsp. bulgaricus are both frequently encountered in kefir, a fermented dairy product (25). In the context of this study, this binary culture serves as a model for the many traditional food and beverage fermentation processes in which yeasts and lactic acid bacteria occur together (19, 26–30). The design of the cultivation conditions was based on the observation that Lb. delbrueckii subsp. bulgaricus, but not S. cerevisiae, can use lactose as a carbon source for growth and that S. cerevisiae, but not Lb. delbrueckii subsp. bulgaricus, can grow on the galactose that is released upon hydrolysis of lactose by the bacterial β-galactosidase.
MATERIALS AND METHODS
Strains and maintenance.
The prototrophic haploid yeast strain Saccharomyces cerevisiae CEN.PK113-7D (MATa MAL-8c SUC2 [31, 32]) was obtained from P. Kötter (J. W. Goethe Universität, Frankfurt, Germany). For storage, shake-flask cultures containing YPD medium (yeast peptone dextrose; 1% yeast extract, 2% Bacto peptone, and 2% dextrose; BD Biosciences, Breda, The Netherlands [33], pH set at 6.0) were inoculated with the yeast strain and grown to stationary phase at 30°C. After addition of sterile glycerol (final concentration, 20% [vol/vol]), 2-ml aliquots were stored in sterile vials at −80°C. Lactobacillus delbrueckii subsp. bulgaricus ATCC BAA-365 (34) was obtained from LGC Standards GmbH (Wesel, Germany). The strain was grown to stationary phase at 37°C without agitation in 100-ml screw-cap flasks containing 100 ml MRS medium (5.5% MRS broth; BD Biosciences). After mixing with glycerol (final concentration, 20% [vol/vol]), aliquots were stored at −80°C.
Design and composition of BC medium.
The chemically defined bulgaricus cerevisiae (BC) medium (see Table S1 in the supplemental material) was designed to cover all nutritional requirements of S. cerevisiae and Lb. delbrueckii subsp. bulgaricus by combining published compositions of chemically defined media for the growth of S. cerevisiae (chemically defined medium [CDM] [35]) and Lb. delbrueckii subsp. bulgaricus (milieu proche du lait [MPL] medium [36]). When a component was present in both media, the highest concentration was used in the BC medium. Under anaerobic conditions, S. cerevisiae has a strict requirement for unsaturated fatty acids (37), which can be met by including Tween 80, a sorbitol ester of oleic acid, to anaerobic growth medium (38). Tween 80 has also been shown to stimulate the growth of Lb. delbrueckii subsp. bulgaricus (39). Verduyn et al. (38) recommended an optimal concentration of 0.42 g · liter−1 Tween 80 for anaerobic growth of S. cerevisiae, while the Lb. delbrueckii subsp. bulgaricus MPL medium contains 1 g · liter−1 Tween 80. Also in this case, the highest concentration was used for the BC medium. Ergosterol, another anaerobic growth factor of S. cerevisiae (40), was added at a concentration of 10 mg · liter−1 (38). Tween 80 and ergosterol were prepared and added to the media as previously described (38). The concentration of sodium acetate, which supports efficient growth of Lb. delbrueckii subsp. bulgaricus (36), was reduced from 75 to 10 mM after showing that this reduction did not affect growth in flasks. The reducing agent thioglycolate was used only during flask cultivation to capture oxygen and not in bioreactors, in which anaerobicity was maintained by sparging with nitrogen gas (see below). In the bioreactors, the BC medium was supplemented with 73 mM (2.5%, wt/vol) lactose for monocultures of Lb. delbrueckii subsp. bulgaricus and for cocultures and with 73 mM (1.3%, wt/vol) galactose for monocultures of S. cerevisiae. These carbon sources were added to the medium after separate sterilization at 110°C.
Growth rate determination.
The specific growth rate of monocultures of yeast and bacteria was measured in four different media. Both the yeast and the lactic acid bacterium were grown in the BC medium described above and in YP (1% yeast extract, 2% Bacto peptone), a complex medium commonly used for yeast propagation. The growth of yeast was also tested in a chemically defined minimal medium (35), while the bacterial growth was tested in the complex medium MRS.
The cultures were incubated in an anaerobic chamber (Sheldon MFG Inc., Cornelius, OR) to mimic at best the conditions of the anaerobic chemostat setup used to explore yeast-bacterium interactions. The gas mixture in the anaerobic chamber was composed of 5% H2, 6% CO2, and 89% N2. Thioglycolate was not required in this anaerobic environment and was not added to the medium. The anaerobic growth factors Tween 80 and ergosterol were added to the YP medium (1 g · liter−1 and 10 mg · liter−1, respectively, identical to the concentrations added to the BC medium) to enable fast anaerobic growth. The incubation temperature was set to 35°C, and 100-ml shake flasks were placed in an orbital shaker at 200 rpm (Unimax1010 shaker; Heidolph, Kelheim, Germany). Lb. delbrueckii subsp. bulgaricus was cultivated with 2% lactose as the carbon source, while 2% galactose was used for S. cerevisiae. The pH of the media was set to 6.0. Precultures were performed in the same medium as the one used for growth rate determination. Bacterial precultures were incubated in the anaerobic chamber, while yeast precultures were performed in an aerobic environment.
Growth rates were measured from independent culture duplicates for each strain and each medium. Cultivation of the lactic acid bacteria in YP and MRS resulted in the rapid acidification of the medium and in a decline in the growth rate. To circumvent this problem, the growth rate was determined for both strains and in all media in the first 5 h following inoculation, a period during which the cultures displayed exponential growth.
Bioreactor batch and chemostat cultivation.
Yeast precultures were grown in 500-ml shake flasks on 100 ml of CDM (35) supplemented with 2% galactose. After inoculation with a frozen stock culture, precultures were incubated for 24 h at 30°C in an orbital shaker at 200 rpm. Subsequently, 1 ml of the preculture was transferred to another 500-ml shake flask containing 100 ml of BC medium supplemented with 2% galactose. After 24 h of cultivation at 30°C in an orbital shaker at 200 rpm, the second preculture was used to inoculate bioreactors (see below).
For Lb. delbrueckii subsp. bulgaricus, the first preculture was prepared by inoculating the frozen stock culture in 100-ml screw-cap flasks with 100 ml MRS medium. After 24 h of incubation without agitation at 37°C, 1 ml of this first preculture was used to inoculate a second preculture in a 100-ml screw-cap flask containing 100 ml of BC medium supplemented with 2.5% lactose. After 24 h of incubation, this second preculture was used to inoculate bioreactors.
Batch and chemostat cultures were carried out in 2-liter bioreactors (Applikon, Schiedam, The Netherlands) with working volumes of 1.4 liters and 1.0 liter, respectively. The pH was controlled at 6.0 by automated titration with 2 M KOH. The temperature was set at 35°C, and the stirring rate was set to 800 rpm. In batch cultures, anaerobicity was maintained by sparging with pure nitrogen gas or a gas mixture (2% CO2, 98% N2) at a flow rate of 0.5 liter · min−1 (CO2 2.7 [99.7% pure] and N2 5.0 [99.999% pure]; Linde Gas Benelux). In chemostat cultures, the medium reservoir was sparged with pure nitrogen gas and the culture vessel was sparged with a 2% CO2–98% N2 gas mixture. Cultures were equipped with Norprene tubing to prevent oxygen diffusion. In chemostats, the culture volume was controlled via an electrical level sensor and the dilution rate was set to 0.10 h−1. In bioreactors, the synthetic medium was supplemented with 0.3 g · liter−1 of antifoam A8011 (Sigma-Aldrich, St. Louis, MO). Chemostat cultures were considered to be in steady state when, after at least six volume changes, key parameters, such as culture dry weight, substrate concentration, and specific CO2 production rate, changed by less than 2% over two consecutive volume changes. For each chemostat condition (yeast only, LAB only, and mixed culture), three independent culture replicates were run.
Substrate, amino acid, and metabolite quantification.
Samples were centrifuged, and the supernatant was analyzed by high-performance liquid chromatography (Waters, Milford, MA) on a chromatograph equipped with Galaxie software (Varian Inc., Palo Alto, CA). An Aminex HPX-87H column (Bio-Rad, Hercules, CA) was eluted with sulfuric acid (5 mM, 0.6 ml/min) at 59°C. Detection was by means of a dual-wavelength absorbance detector (Waters 2487) and a refractive index detector (Waters 2410).
Residual sugars (lactose, glucose, and galactose) as well as glycerol were measured with enzymatic kits (BioControl Systems, Nieuwerkerk aan den IJssel, The Netherlands).
The free amino acids in the medium vessel and in the culture supernatant from chemostats were measured by two independent methods, one based on high-performance liquid chromatography according to a previously described method (41) and one based on gas chromatography-mass spectrometry as previously described (42). To calculate the amino acid concentrations for each culture condition (yeast only, LAB only, and mixed culture), the average and standard deviation of measurements from three independent culture replicates by the two analytical methods were used.
Exhaust gas was cooled in a condenser (2°C) and dried with a PermaPure dryer (model MD 110-48P-4; Inacom Instruments, Veenendaal, The Netherlands) prior to online analysis of carbon dioxide with a Rosemount NGA 2000 analyzer (Baar, Switzerland).
Biomass, cell number, and cell size determination.
To determine biomass dry weight, a known culture volume containing 0.01 to 0.03 g (dry weight) was filtered over dried nitrocellulose filters of known weight (pore size, 0.2 μm; Gelman Sciences). The filters were then washed with 20 ml demineralized water, dried for 20 min in a microwave oven at 360 W, and reweighed. The dry weights of duplicate samples differed by less than 1%. Yeast cell numbers in mixed cultures were counted with a hemocytometer (Bright-Line hemacytometer; Cambridge Instruments Inc., Buffalo, NY), and the biomass (dry weight) concentrations were estimated from a correlation between cell number and dry biomass established in pure cultures of S. cerevisiae and were used to determine the dry weight of S. cerevisiae in mixed cultures. The biomass dry weight of Lb. delbrueckii subsp. bulgaricus in mixed culture was calculated by subtracting the yeast dry weight from the total biomass dry weight. Pictures for cell size analysis were taken with an AxiocamMR microscope camera (Zeiss, Sliedrecht, The Netherlands) equipped with a ×40 objective (EC Plan-Neofluar 40×/0.75 Ph 2 M27; Zeiss). The cell sizes of Lb. delbrueckii subsp. bulgaricus were measured using the segmented line selections tool in ImageJ software (http://rsbweb.nih.gov/ij/) on the basis of 391 and 388 cells from pure and mixed cultures, respectively.
DNA microarrays and transcriptome analysis.
Sampling for transcriptome analysis from pure and mixed cultures was performed as previously described, using liquid nitrogen for rapid quenching of metabolism (43). Samples were stored at −80°C in a mixture of phenol-chloroform and TEA (Tris-EDTA-acetate) buffer until further processing for total RNA extraction. Yeast total RNA from pure and mixed cultures was recovered using phenol-chloroform extraction as previously described (43). Further processing of RNA into cDNA relies on reactions initiated by the poly(A) tail carried by eukaryotic mRNAs only. Separation of the reaction products using the Agilent bioanalyzer confirmed that carryover of Lb. delbrueckii subsp. bulgaricus RNA was negligible, thereby demonstrating that no additional purification of yeast mRNA was required. Processing of total RNA was performed according to Affymetrix's (Santa Clara, CA) instructions. RNA target preparation for microarray expression analysis was carried out according to instructions supplied with the GeneChip 3′ IVT Express kit (Affymetrix, Santa Clara, CA), using 200 ng of total RNA for both pure and mixed cultures. The protocol was carried out with minor modifications; i.e., the Affymetrix poly(A) RNA controls were excluded from the antisense RNA (aRNA) amplification protocol, and the IVT reaction mixtures were incubated for 16 h at 40°C. The quality of total RNA, cDNA, cRNA, and fragmented aRNA was checked using an Agilent Bioanalyzer 2100 instrument (Agilent Technologies). Hybridization, washing, and scanning of Affymetrix chips were performed following the manufacturer's instructions. Results for each growth condition were derived from three independent culture replicates. Processing of expression data (normalization, expression cutoff, etc.) was performed as described previously (44). Differentially expressed genes were identified using the Excel plug-in of the significance analysis of microarrays software (45) with a false-positive discovery rate (FDR) of 5%. Enrichment of the differentially expressed genes for functional categories was performed as described previously to (46).
Lb. delbrueckii subsp. bulgaricus total RNA from pure and mixed cultures was isolated as previously described (15). Five hundred milligrams of 0.1-mm-diameter zirconium beads (Biospec Products Inc., OK) was added to the extraction tubes to ensure efficient mechanical lysis of cells. Processing of RNA, labeling and hybridization, scanning, and DNA microarray analysis were also performed using Agilent custom-made arrays as described previously (15).
As S. cerevisiae RNA was not removed from the mixed-culture RNA, the influence of the presence of S. cerevisiae RNA/cDNA on the hybridization efficacy of Lb. delbrueckii subsp. bulgaricus cDNA was first tested by hybridizing Lb. delbrueckii subsp. bulgaricus monoculture cDNA against the same cDNA mixed in a 1:1 ratio with S. cerevisiae pure-culture cDNA (see Fig. S1 in the supplemental material). This showed no significant differential hybridization (fold change, greater than 2; FDR value, less than 0.05) for any Lb. delbrueckii subsp. bulgaricus probe, indicating that the presence of S. cerevisiae cDNA did not affect Lb. delbrueckii subsp. bulgaricus cDNA binding.
Samples from three independent Lb. delbrueckii subsp. bulgaricus and mixed chemostat cultures were hybridized to 12 arrays following the experimental design described in Fig. S1 in the supplemental material. Genes with a fold change in expression between mixed and pure cultures of greater than 2-fold and with an FDR of less than 0.05 between replicates were considered significantly differentially regulated. As for the S. cerevisiae data, enrichment of the differentially expressed genes for the functional categories was performed as described previously (46).
Carbon balances.
Calculations of carbon recovery were based on biomass carbon contents of 48% and 45% for S. cerevisiae and Lb. delbrueckii subsp. bulgaricus, respectively. Ethanol evaporation from cultures was experimentally determined by following ethanol evaporation from two sterile batch experiments that copied the actual fermentation conditions (i.e., 8 g/liter glucose, BC medium, and 35°C) but in the absence of biomass. The specific rates of ethanol evaporation were corrected with the measured ethanol evaporation constant of 0.009 h−1.
Microarray data accession numbers.
The complete data set is available at the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo) under accession number GSE45776. The custom Lb. delbrueckii subsp. bulgaricus microarray design and hybridization data are available at the Gene Expression Omnibus database under accession number GSE45623.
RESULTS
Anaerobic growth on lactose enables stable cocultivation of S. cerevisiae and Lb. delbrueckii subsp. bulgaricus.
Lactose is an abundant disaccharide in milk. Although S. cerevisiae is frequently encountered in fermented dairy products, such as kefir (12, 25), this yeast cannot metabolize lactose. Lb. delbrueckii subsp. bulgaricus, one of the dominant prokaryotes in kefir fermentation, grows well on lactose as the sole carbon source but, after hydrolysis of this disaccharide, can metabolize the resulting glucose but not galactose. Galactose is an excellent carbon source for growth and alcoholic fermentation by S. cerevisiae. Consequently, growth on lactose by a consortium of S. cerevisiae and Lb. delbrueckii subsp. bulgaricus might enable the establishment of stable cocultures and provide a platform to investigate interactions between these two microorganisms that are not related to carbon source metabolism.
To explore cocultivation of S. cerevisiae and Lb. delbrueckii subsp. bulgaricus, we first designed a synthetic medium and chose growth conditions that allow growth of both organisms. Lb. delbrueckii subsp. bulgaricus and S. cerevisiae have different temperature optima for growth (ca. 43°C and 33°C, respectively [47, 48]). As temperatures above 36°C negatively affect the growth and metabolism of S. cerevisiae, a temperature of 35°C (slightly below the critical point of S. cerevisiae CEN.PK113-7D [48]) was chosen for the experiments involving mixed cultures. The pH was set to 6.0, a standard pH for both organisms. A chemically defined medium (BC medium; see Materials and Methods and Table S1 in the supplemental material) was designed to supply all required nutrients in excess for the lactic acid bacterium and the yeast. Growth of both organisms was tested individually in anaerobic shake flasks with the chosen pH and temperature using BC medium as well as media commonly used for LAB and yeast growth (Table 1). The BC medium supported the growth of Lb. delbrueckii subsp. bulgaricus at a specific rate of 0.18 ± 0.003 h−1. As expected, the growth measured in this chemically defined medium was slower than that measured in the MRS and YP complex media. Conversely, in the BC medium, S. cerevisiae, having very modest nutritional requirements, grew as fast (0.31 ± 0.013 h−1) as in the complex YP medium (0.38 ± 0.003 h−1) and much faster than in minimal CDM (0.10 ± 0.007 h−1). The chosen conditions (BC medium, pH 6.0, 35°C) therefore satisfactorily supported the growth of both microorganisms under carbon source excess. The growth rate measurements indicated that cocultivation of S. cerevisiae and Lb. delbrueckii subsp. bulgaricus in a chemostat at a dilution rate of 0.1 h−1 should lead to steady-state growth of both microbes.
Table 1.
Growth rates of Lb. delbrueckii subsp. bulgaricus and S. cerevisiae measured from monocultures in several growth media under strict anaerobiosis at 35°Ca
| Microorganism | Medium | Growth rate (h−1) |
|---|---|---|
| Lb. delbrueckii subsp. bulgaricus | BC-lactose | 0.18 ± 0.003 |
| YP-lactose | 0.52 ± 0.005 | |
| MRS | 0.64 ± 0.002 | |
| S. cerevisiae | CDM-galactose | 0.10 ± 0.007 |
| BC-galactose | 0.31 ± 0.013 | |
| YP-galactose | 0.38 ± 0.003 |
For each condition, averages and standard deviations were obtained from duplicates of independent cultures.
Yeast provides the carbon dioxide required for growth of Lb. delbrueckii subsp. bulgaricus.
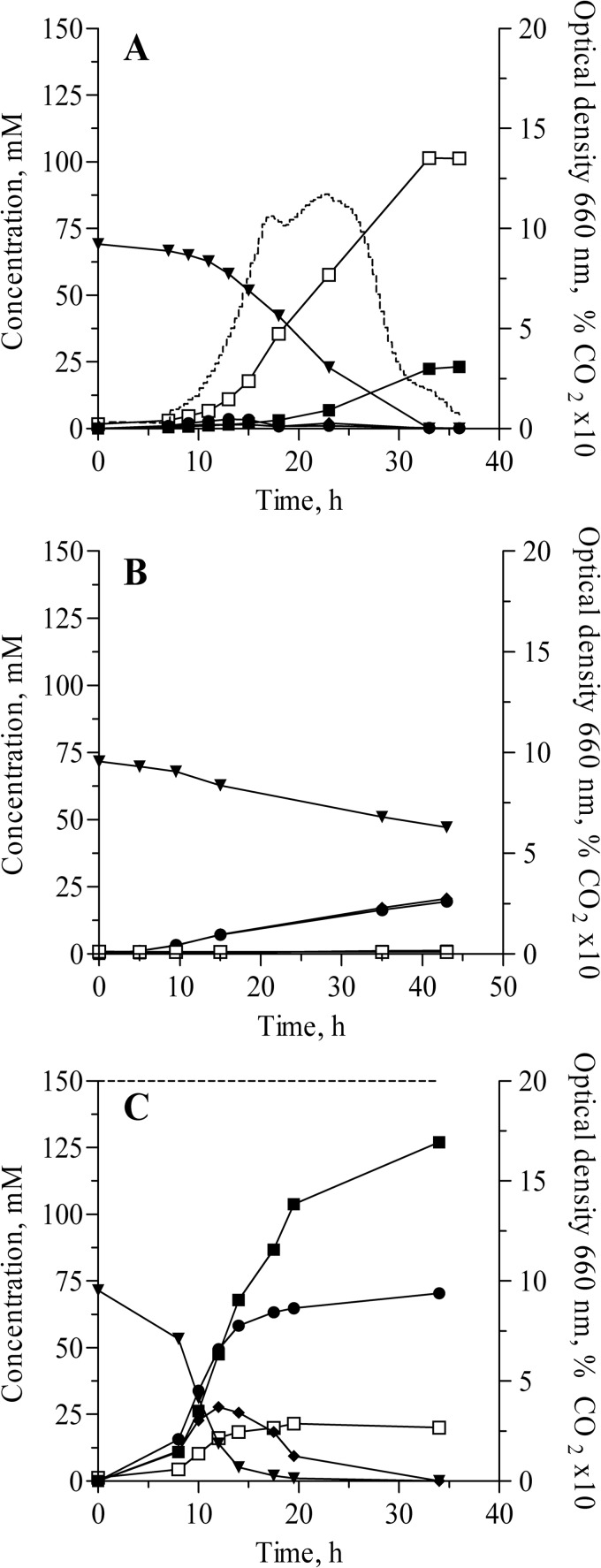
As expected from growth in anaerobic shake flasks, monocultures of S. cerevisiae grew equally well in bioreactors using the growth conditions defined above and galactose as the carbon source. Cocultures of Lb. delbrueckii subsp. bulgaricus and S. cerevisiae on lactose also grew rapidly (Fig. 1A) and consumed all sugar carbon within 35 h. However, monocultures of Lb. delbrueckii subsp. bulgaricus failed to grow on lactose in the bioreactor cultures (Fig. 1B). Although the bacteria converted the supplied lactose to glucose and galactose, neither biomass nor lactate concentrations increased. The major difference between flasks and bioreactors was the flushing of the bioreactor culture with pure nitrogen gas. It has previously been reported that the growth of Lb. delbrueckii subsp. bulgaricus is stimulated by carbon dioxide (20). While carbon dioxide was efficiently stripped from the bioreactors thanks to the continuous sparging of the vessels with pure nitrogen gas, the flasks, regularly exposed to ambient air during sampling, may contain CO2 in an amount sufficient to enable growth of the lactic acid bacteria. Furthermore, shake flasks performed for growth rate measurements were incubated in an anaerobic chamber flushed with a gas mixture containing 6% CO2. In mixed cultures, S. cerevisiae produces carbon dioxide via its alcoholic fermentation pathway, which could compensate for the CO2 stripping in the nitrogen-sparged bioreactors. The CO2 requirement of Lb. delbrueckii subsp. bulgaricus was confirmed when bioreactor batch cultures on lactose were sparged with a gas mixture containing 2% CO2 (2% CO2, 98% N2; Fig. 1C). Increasing the CO2 supply in pure Lb. delbrueckii subsp. bulgaricus culture to 4% CO2 did not increase the growth rate compared to that in 2% CO2 (data not shown).
Fig 1.
Anaerobic batch cultures of Lb. delbrueckii subsp. bulgaricus cocultivated with S. cerevisiae in the absence of added CO2 (culture sparged with 100% nitrogen gas) (A), in pure culture in the absence of added CO2 (culture sparged with 100% nitrogen gas) (B), or in pure culture with sparging of the culture vessel with a 2% CO2–98% N2 gas mixture (C). □, optical density; ▼, lactose; ◆, glucose; ●, galactose; ■, lactate. The dashed lines in panels A and C represent the percentage of CO2 in the off-gas. Cultures were run in duplicate. The duplicates differed by less than 10%, and the results for a single replicate are represented in each panel.
A slight delay in lactose utilization and lactate production was observed in the mixed cultures (Fig. 1A) compared to the Lb. delbrueckii subsp. bulgaricus monocultures sparged with 2% CO2 (Fig. 1C), probably reflecting the time required for the yeast-driven CO2 production to reach a threshold level sufficient for growth of Lb. delbrueckii subsp. bulgaricus. In further experiments, CO2 was provided to all pure and mixed cultures via sparging with a 2% CO2–98% N2 gas mixture. Although not required for growth of Lb. delbrueckii subsp. bulgaricus in mixed cultures, the additional CO2 supply prevented the dependency of Lb. delbrueckii subsp. bulgaricus on yeast for CO2 supply. The maximum specific growth rates under these experimental conditions were 0.29 ± 0.01 h−1 for S. cerevisiae and 0.20 ± 0.00 h−1 for Lb. delbrueckii subsp. bulgaricus (measured from duplicate batch cultures).
Stable chemostat cocultivation of S. cerevisiae and Lb. delbrueckii subsp. bulgaricus.
Using the experimental conditions described above (pH 6.0, 35°C, BC medium, 2% CO2–98% N2), a chemostat cultivation regime was designed for stable cocultivation. By supplying lactose as the growth-limiting nutrient, a strict dependency of the yeast on lactose hydrolysis and galactose production by the lactic acid bacterium was ensured.
At a dilution rate of 0.10 h−1, reproducible and stable cocultures of S. cerevisiae and Lb. delbrueckii subsp. bulgaricus were obtained (Table 2). Triplicate cultures showed a low residual concentration of lactose, in agreement with the reported affinities of lactose phosphotransferase transport systems in LAB (49). Additionally, low concentrations of glucose (0.34 ± 0.04 mM) were detected in the cocultures. This indicates a release of glucose by either S. cerevisiae or Lb. delbrueckii subsp. bulgaricus or, alternatively, a release of some β-galactosidase into the medium. In theory, release of glucose by Lb. delbrueckii subsp. bulgaricus, coupled to its consumption by S. cerevisiae, could result in competition for glucose between the two microorganisms. However, S. cerevisiae grew at the same biomass density and with the same biomass yield in mono- and mixed cultures (Table 2), indicating that consumption of glucose did not make a significant contribution to its growth in the mixed cultures.
Table 2.
Physiological characteristics of monocultures and cocultures of S. cerevisiae and Lb. delbrueckii subsp. bulgaricusa
| Characteristic | Value for: |
||
|---|---|---|---|
| Coculture | S. cerevisiae | Lb. delbrueckii subsp. bulgaricus | |
| Concentrations | |||
| Biomasstotal (g · liter−1) | 2.82 ± 0.08 | 1.46 ± 0.03 | 1.45 ± 0.08 |
| Biomassyeast (g · liter−1) | 1.41 ± 0.07 | 1.46 ± 0.03 | |
| BiomassLAB (g · liter−1) | 1.41 ± 0.02 | 1.45 ± 0.08 | |
| Reservoir sugar concn (mM) | 74.2 ± 1.8 | 67.8 ± 0.4b | 70.2 ± 7.1 |
| Residual lactose concn (mM) | 0.15 ± 0.07 | 2.1 ± 0.7 | |
| Residual glucose concn (mM) | 0.34 ± 0.04 | NDd | 0.54 ± 0.4 |
| Residual galactose concn (mM) | 1.57 ± 0.08 | 1.0 ± 0.1 | 63.0 ± 2.2 |
| Yields and ratesc | |||
| YLAB/glucose(gdry LAB · gglucose−1) | 0.11 ± 0.00 | 0.13 ± 0.02 | |
| YLAB/lactose (gdry LAB · glactose−1) | 0.06 ± 0.00 | 0.06 ± 0.01 | |
| Ylactate/lactose (mollactate · mollactose−1) | 1.63 ± 0.07 | 1.8 ± 0.3 | |
| qlactose (mmol · gdry LAB−1 · h−1) | 5.7 ± 0.2 | 4.7 ± 0.7 | |
| Yyeast/galactose (gdry yeast · ggalactose−1) | 0.11 ± 0.01 | 0.12 ± 0.00 | |
| Yethanol/galactose(molethanol · mollactose−1) | 1.57 ± 0.08 | 1.72 ± 0.01 | 0.00 ± 0.00 |
| Yglycerol/galactose(molglycerol · molgalactose−1) | 0.15 ± 0.03 | 0.13 ± 0.02 | 0.00 ± 0.00 |
| qgalactose (mmol · gdry yeast−1 · h−1) | NEe | 4.9 ± 0.3 | |
| qCO2 (mmol · gdry yeast−1 · h−1) | 10.2 ± 0.5 | 9.67 ± 1.05 | |
| qethanol (mmol · gdry yeast−1 · h−1) | 8.7 ± 0.2 | 8.4 ± 0.5 | |
| qglycerol (mmol · gdry yeast−1 · h−1) | 0.8 ± 0.2 | 0.7 ± 0.1 | |
| qacetate (mmol · gdry yeast−1 · h−1) | 0 | 0.01 ± 0.00 | |
| Yx/lactose (gtotal biomass · glactose−1) | 0.11 ± 0.01 | 0.06 ± 0.01 | |
| Ylactate/galactose (mollactate · molgalactose−1) | 0.02 ± 0.02 | 0 | |
| qpyruvate (mmol · gtotal biomass−1 · h−1) | 0.01 ± 0.00 | 0.02 ± 0.02 | 0.02 ± 0.01 |
| qsuccinate (mmol · gtotal biomass−1 · h−1) | 0.07 ± 0.01 | 0.01 ± 0.00 | 0.28 ± 0.09 |
| qlactate (mmol · gtotal biomass−1 · h−1) | 9.2 ± 0.4 | 0.04 ± 0.02 | 8.3 ± 0.6 |
| % carbon recovery | 98.9 ± 4.1 | 111.3 ± 2.2 | 98.6 ± 13.9 |
S. cerevisiae and Lb. delbrueckii subsp. bulgaricus were grown anaerobically in a carbon-limited chemostat at a dilution rate of 0.10 h−1. For each condition, averages and standard deviations were obtained from triplicate independent chemostat cultures.
Galactose.
Y, yield; q, biomass-specific rate.
ND, not detected.
NE, not estimated.
As anticipated, lactate and ethanol, the major fermentation products of Lb. delbrueckii subsp. bulgaricus and S. cerevisiae, respectively, were by far the major products of the cofermentation (121 ± 3 mM lactate and 130 ± 1 mM ethanol) and represented ca. 75% of the recovered carbon. A comparison of metabolic fluxes in the pure and mixed cultures showed only minor differences (Table 2) and suggested that cocultivation has a negligible impact on fluxes through central carbon metabolism in S. cerevisiae and Lb. delbrueckii subsp. bulgaricus. Carbon recovery in the galactose-grown yeast monocultures exceeded 100%, probably reflecting the coutilization of amino acids as additional carbon sources.
Cocultivation with S. cerevisiae affects the morphology of Lb. delbrueckii subsp. bulgaricus.
No changes in morphology or cell size were observed for S. cerevisiae in the presence of Lb. delbrueckii subsp. bulgaricus compared to its morphology and cell size in monocultures of this yeast. In contrast, the morphology of Lb. delbrueckii subsp. bulgaricus cells was different in pure and cocultures. Cells grown in coculture were significantly shorter (average size, 9 ± 6 μm) than those grown in pure culture (average size, 18 ± 8 μm) (Fig. 2). Bacterial filamentation is often associated with stress responses resulting from unfavorable environmental circumstances, such as the presence of antibiotics, toxic fermentation product accumulation, and depletion of growth medium components (50). This surprising observation suggested that consumption of a medium component or production of certain compounds by yeast triggers the observed morphological alteration of Lb. delbrueckii subsp. bulgaricus in the cocultures.
Fig 2.
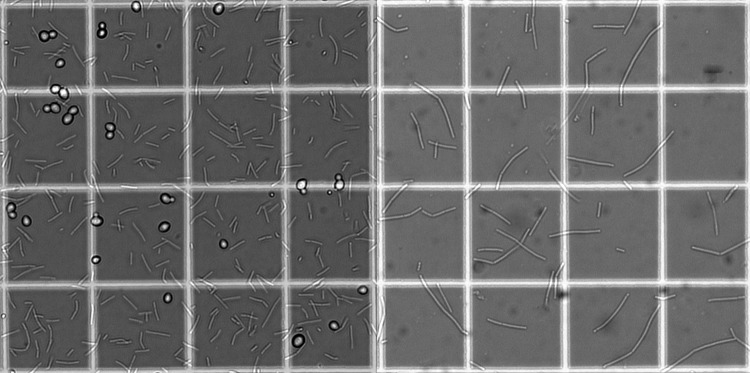
Phase-contrast pictures of Lb. delbrueckii subsp. bulgaricus cultivated in a chemostat in coculture with S. cerevisiae (left) and in pure culture (right). Small squares are 50 μm by 50 μm.
Cocultivation with Lb. delbrueckii subsp. bulgaricus elicits a transcriptional response to lactic acid in yeast.
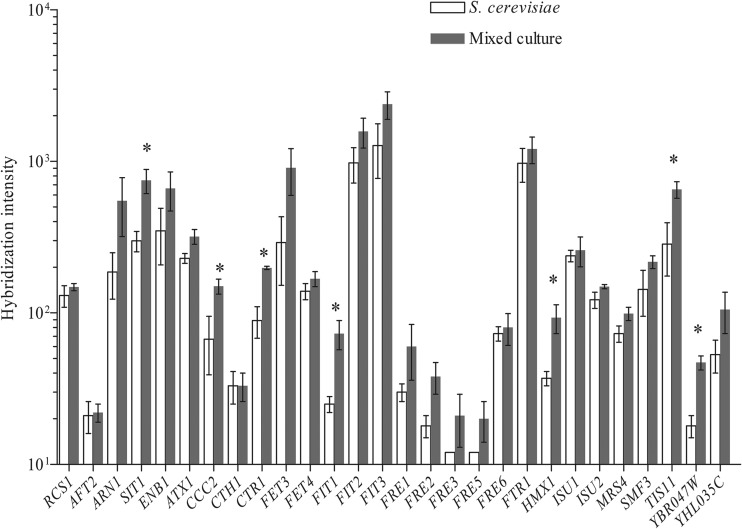
To further investigate the impact of cocultivation with Lb. delbrueckii subsp. bulgaricus, the transcriptomes of S. cerevisiae in triplicate galactose-grown monocultures and triplicate lactose-grown cocultures were compared, revealing that 17 genes (0.2% of the S. cerevisiae genome) showed a significantly different transcript level under these two cultivation regimes (Table 3). The 11 genes whose transcript levels were higher in the cocultures showed a clear overrepresentation for genes involved in iron and copper transport and homeostasis (gene ontology categories copper ion transmembrane transport and iron ion homeostasis were significantly enriched, with P values of 3.2E−07 and 4.7E−07, respectively; see Materials and Methods). Abbott and coworkers reported the upregulation of S. cerevisiae genes belonging to these functional categories in response to high lactate concentrations (51). By varying the concentration of lactic acid (500 and 900 mM), they showed the dose dependency of this effect, which was attributed to chelation of cations, and especially iron, by lactate. In cocultures, the lactate concentration produced by Lb. delbrueckii subsp. bulgaricus remained substantially lower (ca. 120 mM) than the concentrations studied by Abbott and coworkers (51). Still, out of 28 genes involved in iron and copper homeostasis that responded to high lactate concentrations, 8 showed elevated transcript levels in the cocultures relative to S. cerevisiae monocultures (Table 3). When statistical criteria were slightly relaxed, expression of most of these 28 genes (ca. 20) was found to be higher in the cocultures (Fig. 3).
Table 3.
Genes significantly differentially expressed in S. cerevisiae in response to cocultivation with Lb. delbrueckii subsp. bulgaricusa
| Type of regulation and geneb | Description | Fold change |
|
|---|---|---|---|
| In cocultures vs monoculturesc | From Abbott et al.d | ||
| Upregulated genes | |||
| CTR3e | High-affinity copper transporter of the plasma membrane, acts as a trimer | 4.6 | −1.8 |
| STL1 | Glycerol importer | 4.3 | 111 |
| FIT1e | Cell wall mannoprotein, involved in the retention of siderophore iron in the cell wall | 2.9 | 8 |
| EEB1 | Alcohol acyltransferase involved in ethyl ester biosynthesis | 2.7 | −5.2 |
| FMP23e | Mitochondrial protein of unknown function, contains Rcs1p and Aft2p binding domains | 2.7 | 19 |
| CIN5 | Transcription factor of the YAP-1 family, mediates pleiotropic drug resistance and salt tolerance | 2.5 | 12.1 |
| HMX1e | Endoplasmic reticulum localized, heme-binding peroxidase involved in the degradation of heme, expression regulated by Aft1p | 2.5 | 6 |
| SIT1e | Transporters that specifically recognize siderophore iron chelates, transcription is induced during iron deprivation and diauxic shift | 2.5 | 11 |
| TIS11e | mRNA-binding protein expressed during iron starvation and involved in mRNA degradation | 2.3 | 45 |
| CCC2e | Plasma membrane Cu2+-transporting P-type ATPase, required for export of copper from the cytosol | 2.2 | 7 |
| CTR1e | High-affinity copper transporter of the plasma membrane, mediates nearly all copper uptake under low-copper conditions | 2.2 | 2.7 |
| Downregulated genes | |||
| SUL1 | High-affinity sulfate permease | −3 | −12.4 |
| CAR2 | l-Ornithine transaminase, catalyzes the second step of arginine degradation | −2.2 | −5.1 |
| ATO3 | Plasma membrane protein, possible role in export of ammonia from the cell | −2 | −4.7 |
| ADH2 | Glucose-repressible alcohol dehydrogenase II, catalyzes the conversion of ethanol to acetaldehyde, involved in the production of certain carboxylate esters, regulated by Adr1p | −2.5 | 1.0 |
| PHM8 | Lysophosphatidic acid phosphatase involved in lysophosphatidic acid hydrolysis in response to phosphate starvation | −2.4 | 1.0 |
| XBP1 | Transcriptional repressor that binds to promoter sequences of the cyclin genes CYS3 and SMF2 | −2.5 | −1.1 |
Differential expression was identified by use of a false discovery rate of <5% (see Materials and Methods).
Genes responding to lactic acid stress in a previous study by Abbott et al. (51) are indicated in bold.
The fold change represents the increase or decrease in expression in lactose-grown chemostat cocultures compared to that in galactose-grown chemostat monocultures.
The fold change represents the increase or decrease in expression in anaerobic glucose-limited chemostat cultures grown at pH 5.0 in the presence versus absence of 900 mM lactic acid, as described by Abbott et al. (51).
Genes encoding proteins involved in iron and copper homeostasis (51).
Fig 3.
Expression in mixed and pure cultures of S. cerevisiae genes involved in iron and copper homeostasis responding to lactic acid stress according to Abbott et al. (51). ∗, genes for which the expression is significantly changed in mixed versus pure culture (see the statistical criteria in Materials and Methods).
Of the 17 yeast genes that were differentially expressed between mixed and pure cultures, 12 (i.e., 70%) were previously identified as lactate responsive (Table 3). The changes in the expression levels of these genes were remarkably lower (average, 2.7-fold) than the changes in expression levels previously measured by Abbott and coworkers (51) (Table 3), consistent with the proposed dose dependency of the lactate response. Of the remaining 5 genes, CTR3 encodes a high-affinity copper transporter that was not found to be upregulated by Abbott et al. (51). The remaining 4 genes (EEB1, ADH2, PHM8, and XBP1) did not show a clear overrepresentation of a functional category.
The presence of S. cerevisiae triggers a transcriptional response in Lb. delbrueckii subsp. bulgaricus in long-chain fatty acid (LCFA) and amino acid metabolism.
After confirming that the presence of yeast mRNA did not interfere with the bacterial mRNA during hybridization on the microarrays, RNA from three independent chemostats of lactose-grown mono- and cocultures of Lb. delbrueckii subsp. bulgaricus was hybridized to dedicated Lb. delbrueckii subsp. bulgaricus DNA microarrays (see Materials and Methods). Eighty-three Lb. delbrueckii subsp. bulgaricus genes (4% of the Lb. delbrueckii subsp. bulgaricus genome) showed a significantly different transcript level during cocultivation with S. cerevisiae. Of these 83 genes, 27 encode hypothetical proteins or do not have predicted functions. Among the 56 differentially expressed genes that are functionally annotated, 22 and 34 displayed higher and lower transcript levels in the cocultures, respectively. Two functional categories involved in biosynthesis were overrepresented among these differentially expressed genes (Table 4). The first is amino acid transport and metabolism, notably, cysteine and methionine metabolism (P value for enrichment, 8.3E−4) and, related to that, glycine, serine, and threonine (P = 7.3E−4) for conversion to cysteine and methionine, and the second is fatty acid metabolism (P = 1.5E−5).
Table 4.
Genes significantly differentially expressed in Lb. delbrueckii subsp. bulgaricus in response to cocultivation with S. cerevisiae for the enriched functional categories amino acid transport and metabolism and lipid metabolisma
| Functional category and locus tag | Description | Fold change in expression for cocultures vs monocultures |
|---|---|---|
| Amino acid transport and metabolism (14 genes)b | ||
| LBUL_0215 | ABC-type amino acid transport system permease component | −2.7 |
| LBUL_0216 | ABC-type polar amino acid transport system ATPase component | −3.6 |
| LBUL_0737 | Transcriptional regulators containing a DNA-binding helix-turn-helix domain and an aminotransferase domain (MocR family) and their eukaryotic orthologs | −15.3 |
| LBUL_0915 | Dipeptidyl aminopeptidases/acylaminoacyl peptidases | 12.1 |
| LBUL_1181 | Diaminopimelate decarboxylase | 2.2 |
| LBUL_1235 | Cysteine synthase | −2.3 |
| LBUL_1236 | Cystathionine beta-lyases/cystathionine gamma-synthases | −2.4 |
| LBUL_1257 | Aspartate semialdehyde dehydrogenase | −4.0 |
| LBUL_1353 | Homoserine trans-succinylase | −2.3 |
| LBUL_1619 | Acetylornithine deacetylase/succinyl-diaminopimelate desuccinylase and related deacylases/dipeptidase PepV | −2.5 |
| LBUL_1630 | Homoserine kinase | −2.3 |
| LBUL_1631 | Threonine synthase | −2.5 |
| LBUL_1646 | Amino acid transporters | −4.0 |
| LBUL_1668 | Gamma-aminobutyrate permease and related permeases | −9.0 |
| Lipid metabolism (10 genes)c | ||
| LBUL_0784 | Predicted acyltransferases | 5.1 |
| LBUL_0806 | 3-Hydroxy-3-methylglutaryl coenzyme A synthase | −3.0 |
| LBUL_0818d | 3-Oxoacyl-(acyl carrier protein) | 3.0 |
| LBUL_0819d | Acyl carrier protein | 3.8 |
| LBUL_0821d | 3-Oxoacyl-(acyl carrier protein) reductase/dehydrogenases with different specificities (related to short-chain alcohol dehydrogenases) | 2.2 |
| LBUL_0822d | 3-Oxoacyl-(acyl carrier protein) synthase | 2.8 |
| LBUL_0823d | Biotin carboxyl carrier protein | 3.3 |
| LBUL_0824d | 3-Hydroxymyristoyl/3-hydroxydecanoyl-(acyl carrier protein) dehydratases | 3.3 |
| LBUL_0825d | Acetyl/propionyl coenzyme A carboxylase alpha subunit | 2.6 |
| LBUL_1607d | 3-Hydroxymyristoyl/3-hydroxydecanoyl-(acyl carrier protein) dehydratases | −2.5 |
Differential expression was identified by use of a false discovery rate of <5% (see Materials and Methods). The complete list of enriched categories is available in Table S2 in the supplemental material.
The genome contained 109 genes in this category.
The genome contained 39 genes in this category.
Genes in the long-chain fatty acid production pathway.
Lb. delbrueckii subsp. bulgaricus genes involved in the biosynthesis and uptake of the amino acids cysteine, methionine, and threonine were downregulated in mixed culture, suggesting increased amino acid availability in the presence of yeast. However, analysis of the culture supernatants did not indicate differences in cysteine, methionine, or threonine concentrations between the mono- and cocultures that could explain the observed modifications in gene expression. The lowest amino acid concentration was found in monocultures of Lb. delbrueckii subsp. bulgaricus (Fig. 4), in which the residual alanine concentration was 170 ± 46 μM (out of 1,194 ± 162 μM supplied), which is in agreement with the reported affinity for amino acid transport in lactic acid bacteria (49). Under the experimental conditions, pure cultures of S. cerevisiae produced alanine (52), leading to a high residual concentration of 2,081 ± 286 μM. The absence of additional consumption of alanine and the lack of a specific transcriptional response in mixed culture compared to pure cultures of Lb. delbrueckii subsp. bulgaricus indicated that alanine availability in the pure culture was sufficient to support the requirement of Lb. delbrueckii subsp. bulgaricus.
Fig 4.
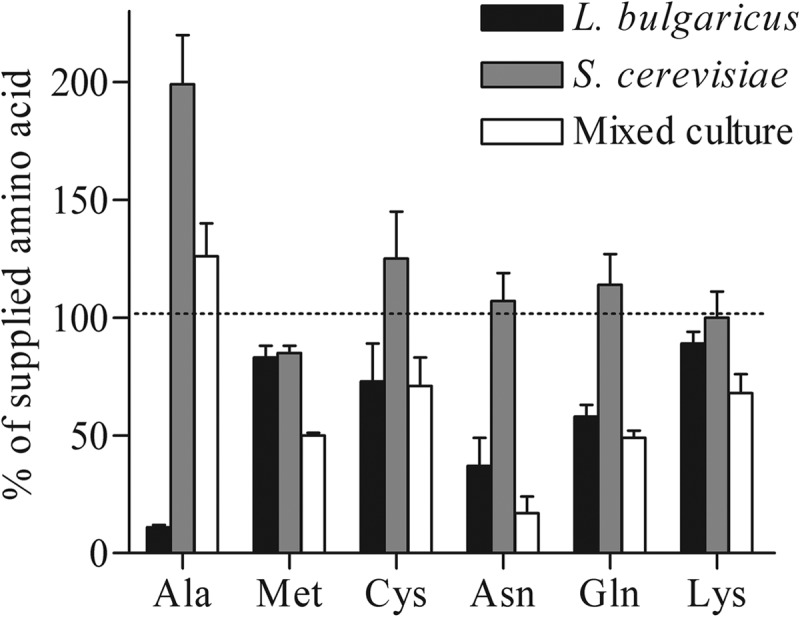
Amino acid consumption and production during anaerobic chemostat cultivation of Lb. delbrueckii subsp. bulgaricus, S. cerevisiae, and mixed populations at a dilution rate of 0.1 h−1. Data only for the amino acids with statistically significant changes between the medium and effluent concentrations (t test, P value threshold, 0.01) are represented.
The other major functional category enriched among the differentially expressed genes covered lipid metabolism and, more particularly, the biosynthesis of LCFA. Of the 10 annotated Lb. delbrueckii subsp. bulgaricus genes involved in LCFA biosynthesis, 7 were significantly upregulated in mixed culture compared to pure cultures, with fold changes ranging from 2.2 to 3.8 (Table 4).
The expression of several genes related to extracellular polysaccharide synthesis was downregulated when Lb. delbrueckii subsp. bulgaricus was cultivated in the presence of yeast (see Table S2 in the supplemental material). Genes encoding proteins involved in saccharide monomer uptake (e.g., a putative mannose/fructose phosphotransferase system), processing (e.g., mannose and glucose phosphomutase), and biosynthesis of capsular polysaccharides (CPSs; e.g., UDP-glucose 4-epimerase and one of the genes in the CPS synthase complex) were expressed at a lower level in mixed culture. Extracellular polysaccharides are known to protect cells against a variety of stresses (53) and may, like the morphological changes, indicate a decreased degree of stress when Lb. delbrueckii subsp. bulgaricus is cocultivated with yeast.
DISCUSSION
The present study shows how the design of cultivation conditions can enable stable, essentially noncompetitive cocultivation of two microorganisms under stable environmental conditions, thus facilitating the application of genome-wide expression analysis to the resulting mixed cultures and interpretation of the findings. The combination of S. cerevisiae and Lb. delbrueckii subsp. bulgaricus was primarily chosen as a laboratory model to investigate the applicability of chemostat cultures, and the carbon-limited chemostat conditions used in this study are very different from the sugar-excess conditions in real-life food and beverage fermentation processes. Nevertheless, some of the interactions between the yeast and the lactic acid bacterium demonstrated in this study are likely to be of key importance for their coexistence in mixed-culture fermentation processes.
In the late 1960s and early 1970s, a few studies had already explored the possibility of whether steady cultivation of mixed microbial populations could be reached in a chemostat by applying tailored feeding strategies. A stable cocultivation of S. cerevisiae and Lactobacillus casei was reached in rich medium, in which the growth of the yeast was limited by the carbon source (glucose), while the growth of the bacterium was limited by the supply of riboflavin (a vitamin for which S. cerevisiae is prototroph) (21). In this system, growth of the bacterium and of the yeast could be tuned independently by adjusting the glucose and riboflavin supplies, but both organisms competed for the same carbon source. In another study, a dependency of Proteus vulgaris on S. cerevisiae was created by choosing a medium leading to good yeast growth but poor growth of the bacterium (19). In this way, both organisms competed for the carbon source; while the growth of S. cerevisiae was directly limited by the carbon source (glucose), the growth of P. vulgaris was limited by one or more essential nutrients supplied by the yeast, presumably a vitamin. In an approach very similar to the one used in the present study, Acetobacter suboxydans was cultivated with Saccharomyces carlsbergensis in mannitol-limited chemostats (22). While A. suboxydans can metabolize mannitol, the yeast is incapable of using this sugar as a carbon source. A. suboxydans converts mannitol to d-fructose, a suitable carbon source for S. carlsbergensis, and d-fructose is subsequently slowly oxidized to d-ketofructose. In this system, growth of S. carlsbergensis is dependent on the d-fructose excreted in the culture medium by the bacteria. However, when mannitol is the limiting nutrient, both organisms will eventually compete for d-fructose. The present study introduces an original strategy to reach stable cocultivation by limiting both organisms involved by the carbon source while preventing competition for this carbon source.
Carbon metabolism in lactose-grown mixed cultures of S. cerevisiae and Lb. delbrueckii subsp. bulgaricus can be considered a classical example of mutualism (54). Lactose hydrolysis by Lb. delbrueckii subsp. bulgaricus and release of galactose provide S. cerevisiae with a utilizable carbon source, while generation of carbon dioxide by the yeast alcoholic fermentation pathway is required to support the growth of the lactic acid bacterium. The absence of a Leloir pathway for galactose metabolism and of β-galactosidase in Lb. delbrueckii subsp. bulgaricus and S. cerevisiae, respectively (31, 55), provides a clear biochemical explanation for the complementarity of these two species in lactose metabolism. In contrast, there is as yet no published biochemical explanation for the capnophily (requirement for high carbon dioxide concentrations) of Lb. delbrueckii subsp. bulgaricus, a phenomenon that occurs in representatives of many microbial taxa (56). In several microorganisms, including S. cerevisiae, loss-of-function mutations in the gene encoding carbonic anhydrase result in a requirement for high carbon dioxide concentrations for growth (57, 58). Since the genome of Lb. delbrueckii subsp. bulgaricus harbors an open reading frame (LBUL_0423) with very strong sequence identity with prokaryotic carbonic anhydrase genes, this does not seem to be a plausible explanation. A similar phenomenon was previously observed in Lactobacillus plantarum cultivated aerobically. Intense sparging of Lb. plantarum cultures with air resulted in a decreased growth rate and in the upregulation of CO2-producing reactions (malic enzyme, pyruvate dehydrogenase, pyruvate oxidase). As in the present study, fast growth was recovered upon sparging the culture with CO2-enriched air (air with 1% CO2 [59]). In the present study, differential expression of genes encoding CO2-producing or -consuming reactions was not observed and was not expected, as both mono- and mixed cultures were supplied with CO2. The genome of Lb. plantarum also harbors a carbonic anhydrase-like gene. The functionality of carbonic anhydrase in these two Lactobacillus species has, however, not been documented, and further research is required to understand this important aspect of the ecophysiology of lactobacilli.
Despite the carbon-limited cultivation conditions used in the present study, analysis of residual amino acid concentrations in the cultures suggested possible interactions of S. cerevisiae and Lb. delbrueckii subsp. bulgaricus at the level of nitrogen metabolism. In particular, the data suggest that alanine excreted by the yeast may be taken up by the bacterium. The physiological impact of such amino acid cross-feeding may be of much greater significance under nitrogen-limited conditions and may be experimentally addressed by performing additional experiments with different concentrations and compositions of the complex nitrogen source used in this study. Another possible interaction, unexplored in the present study, is the cross-feeding of the bacteria by peptides released by S. cerevisiae. Although very little is known about peptide transport in S. cerevisiae, this yeast harbors one di- and tripeptide transporter (Ptr2) and two transporters for bigger oligopeptides (Opt1 and Opt2 [60]). Transcripts for OPT1 were not detected, but OPT2 and PTR2 were expressed at similar levels during mono- and cocultivation of yeast and bacteria. Although mostly described for peptide uptake, Opt2 and Ptr2 could contribute to the export of yeast peptides to the culture medium.
Systematic functional analysis of microbial genomes is, almost without exception, performed in pure cultures, and even in extensively studied microorganisms, such as S. cerevisiae, a significant fraction of the genes still has no assigned biochemical function (in the case of S. cerevisiae, 12.7% [Saccharomyces Genome Database]; in the case of Lb. delbrueckii subsp. bulgaricus, 30% [NCBI database]). Our anticipation that large numbers of these genes might be activated in response to cocultivation was not confirmed by the transcriptome data. In particular, for S. cerevisiae, we have rarely seen fewer responsive genes in a chemostat-based transcriptome analysis than in the comparison of monocultures and mixed cultures with Lb. delbrueckii subsp. bulgaricus (9, 44, 57, 61, 62). Conversely, when cocultivated with the malolactic bacterium Oenococcus oeni, the wine yeast S. cerevisiae VIN13 responded by a larger change in gene expression (272 genes differentially expressed after 7 days in a setup mimicking wine fermentation), and 25% of the differentially expressed genes were classified as proteins with unknown function (17). The change in expression of these poorly annotated genes specifically responding to cocultivation may contribute to their functional annotation. In that study and in contrast to the findings of the present study, the wine yeast did not respond to the presence of lactic acid by an upregulation of genes involved in copper and iron metabolism. This difference could readily be explained by a lower lactate concentration (48 mM against 120 mM in the present study) and by the different experimental conditions that may lead to differences in iron and copper bioavailability (differences in metal concentration and form, different biomass concentrations, etc.). On the other hand, the expression of DLD1 and DLD2, encoding lactate dehydrogenases, was increased in the wine strain in response to cocultivation with O. oeni, presumably as a consequence of the lactate produced by the lactic acid bacterium. Despite the alleviation of glucose repression in the carbon-limited cultures, the expression of these glucose-repressed and lactate-induced genes was not affected in the laboratory strain in the present study. Other responses, such as a downregulation of genes involved in sterol metabolism, were identified in the wine strain in response to the presence of the lactic acid bacterium. This response, which is larger than the one observed in the present study, may be partly explained by the choice of two microorganisms isolated from wine. The frequent simultaneous occurrence of S. cerevisiae and O. oeni in wine for centuries has undoubtedly shaped their genomes toward specific interactions different from those occurring between strains isolated from other environments. Unfortunately, that study did not report the response of O. oeni to cocultivation with yeast (17).
In the present study, the number of genes that showed a significant transcriptional response to cocultivation was higher in Lb. delbrueckii subsp. bulgaricus than in S. cerevisiae and revealed an overrepresentation of genes involved in amino acid and lipid, notably, LCFA, metabolism. Since downregulation of genes involved in cysteine, methionine, and threonine metabolism in the mixed cultures did not coincide with different concentrations of these amino acids relative to those of the pure cultures, it is unlikely to reflect a direct cross-feeding of these amino acids from the yeast to the bacterium. Alternatively, the downregulation of genes involved in synthesis of sulfur-containing amino acids might, for example, reflect a release of other reduced sulfur compounds (e.g., sulfide or sulfite [63]) by the yeast. The differential regulation of these genes in Lb. delbrueckii subsp. bulgaricus may also indicate changes in the redox status of the cultures between single and mixed populations; however, if this were the case, a response from the yeast would also be expected. Regarding the upregulation of genes involved in lipid biosynthesis, a similar response was observed in Lactobacillus plantarum when this bacterium was exposed to ethanol. In Lb. plantarum, exposure to 8% ethanol resulted in the upregulation of the fab locus involved in fatty acid biosynthesis and the modification of the fatty acid composition of the cell membrane (64). Although these observations were obtained in another species with substantially higher ethanol concentrations (ca. 0.6% ethanol was produced in mixed and yeast monocultures in the present work), they suggest that the upregulation of genes involved in LCFA synthesis in Lb. delbrueckii subsp. bulgaricus could be a response to the yeast-produced ethanol. However, the upregulation of genes involved in fatty acid metabolism in Lb. delbrueckii subsp. bulgaricus may also be caused by a competition for Tween 80, which was added as an essential source of unsaturated fatty acids for both microorganisms. Optimization of Tween 80 concentrations is notoriously difficult. This source of oleic acid impairs growth (results in lower biomass yields) of both S. cerevisiae and Lb. delbrueckii subsp. bulgaricus at suboptimal concentrations but is inhibitory when present in excess, resulting in a rather narrow range of optimal concentrations in chemostat cultures at the biomass concentrations used in the present study (37–39). However, mixed and pure cultures revealed no difference in Lb. delbrueckii subsp. bulgaricus biomass yields (Table 1). Furthermore, physiological and transcriptome analysis of S. cerevisiae gave no indication of Tween 80 limitation in mixed culture. Maligoy and coauthors compared the transcriptome data for L. lactis in pure, well-controlled batch cultures with transcriptome data from cocultures with Saccharomyces cerevisiae (16). The most prominent response of the lactic acid bacterium to cocultivation with S. cerevisiae was an upregulation of genes involved in pyrimidine synthesis. While this response could not be explained by modification of the nutrient supply between mono- and mixed cultures, the authors identified ethanol as a potential factor for this response. Upregulation of genes involved in pyrimidine de novo synthesis was induced by ethanol for concentrations as low as 30 mM. Even though ethanol concentrations reached 130 mM, the corresponding genes of Lb. delbrueckii subsp. bulgaricus were not affected by cocultivation with yeast, indicating a genus dependency of the ethanol response among lactic acid bacteria. Unfortunately, Maligoy and coauthors did not investigate the reciprocal yeast response (16).
Cocultivation with S. cerevisiae led to a remarkable change in the morphology of Lb. delbrueckii subsp. bulgaricus, but we did not observe the strong physical interactions between yeast cells and lactic acid bacteria that have been reported in kefir cultures (25, 29, 65). In this respect, it may be relevant to note that S. cerevisiae CEN.PK113-7D is a laboratory strain whose ability to interact with prokaryotes may have been reduced during its construction and prolonged cultivation in the laboratory (32). Similarly, the Lb. delbrueckii subsp. bulgaricus strain used in this study was isolated from a dairy where it is fully adapted to an association with another lactic acid bacterium, Streptomyces salivarius subsp. thermophilus (55). It will be interesting to investigate whether strains isolated from environments where they naturally coexist reveal additional interactions.
This study demonstrates that chemostat-based transcriptome analysis can identify biologically relevant responses in mixed cultures. This approach can be extended to investigate interactions of other microorganisms that occur together in food fermentation (66), either synergistically or as contaminants. Furthermore, this technique seems to be excellently suited to study the cocultivation of antibiotic-producing microorganisms and relevant target organisms and the degradation of complex substrates by defined or natural consortia and evaluate metabolic engineering strategies for achieving stable cocultivation of industrially relevant microorganisms.
Supplementary Material
ACKNOWLEDGMENTS
F.M. and S.S. were supported by grants from the Kluyver Center for the Genomics of Industrial Fermentations.
Footnotes
Published ahead of print 19 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01115-13.
REFERENCES
- 1.Koskenniemi K, Laakso K, Koponen J, Kankainen M, Greco D, Auvinen P, Savijoki K, Nyman TA, Surakka A, Salusjarvi T, de Vos WM, Tynkkynen S, Kalkkinen N, Varmanen P. 2011. Proteomics and transcriptomics characterization of bile stress response in probiotic Lactobacillus rhamnosus GG. Mol. Cell. Proteomics 10:M110.002741. 10.1074/mcp.M110.002741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JY, Pajarillo EA, Kim MJ, Chae JP, Kang DK. 2013. Proteomic and transcriptional analysis of Lactobacillus johnsonii PF01 during bile salt exposure by iTRAQ shotgun proteomics and quantitative RT-PCR. J. Proteome Res. 12:432–443 [DOI] [PubMed] [Google Scholar]
- 3.Bron PA, Wels M, Bongers RS, van Bokhorst-van de Veen Wiersma A, Overmars L, Marco ML, Kleerebezem M. 2012. Transcriptomes reveal genetic signatures underlying physiological variations imposed by different fermentation conditions in Lactobacillus plantarum. PLoS One 7:e38720. 10.1371/journal.pone.0038720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11:4241–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salusjärvi L, Poutanen M, Pitkänen JP, Koivistoinen H, Aristidou A, Kalkkinen N, Ruohonen NL, Penttilä M. 2003. Proteome analysis of recombinant xylose-fermenting Saccharomyces cerevisiae. Yeast 20:295–314 [DOI] [PubMed] [Google Scholar]
- 6.de Groot MJL, Daran-Lapujade P, van Breukelen B, Knijnenburg TH, Pronk JT, Slijper M, Heck AJR. 2007. Quantitative proteomics and transcriptomics of anaerobic and aerobic yeast cultures reveals post-transcriptional regulation of key cellular processes. Microbiology 153:3864–3878 [DOI] [PubMed] [Google Scholar]
- 7.Daran-Lapujade P, Daran JM, van Maris AJ, de Winde JH, Pronk JT. 2009. Chemostat-based micro-array analysis in baker's yeast. Adv. Microb. Physiol. 54:257–311 [DOI] [PubMed] [Google Scholar]
- 8.Tai SL, Boer VM, Daran-Lapujade P, Walsh MC, de Winde JH, Daran JM, Pronk JT. 2005. Two-dimensional transcriptome analysis in chemostat cultures. Combinatorial effects of oxygen availability and macronutrient limitation in Saccharomyces cerevisiae. J. Biol. Chem. 280:437–447 [DOI] [PubMed] [Google Scholar]
- 9.Tai SL, Daran-Lapujade P, Walsh MC, Pronk JT, Daran JM. 2007. Acclimation of Saccharomyces cerevisiae to low temperature: a chemostat-based transcriptome analysis. Mol. Biol. Cell 18:5100–5112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wegkamp A, Mars AE, Faijes M, Molenaar D, de Vos RC, Klaus SM, Hanson AD, de Vos WM, Smid EJ. 2010. Physiological responses to folate overproduction in Lactobacillus plantarum WCFS1. Microb. Cell Fact. 9:100. 10.1186/1475-2859-9-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botta C, Cocolin L. 2012. Microbial dynamics and biodiversity in table olive fermentation: culture-dependent and -independent approaches. Front. Microbiol. 3:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopitz-Otsoa F, Rementeria A, Elguezabal N, Garaizar J. 2006. Kefir: a symbiotic yeasts-bacteria community with alleged healthy capabilities. Rev. Iberoam. Micol. 23:67–74 [DOI] [PubMed] [Google Scholar]
- 13.Alexandre H, Costello PJ, Remize F, Guzzo J, Guilloux-Benatier M. 2004. Saccharomyces cerevisiae-Oenococcus oeni interactions in wine: current knowledge and perspectives. Int. J. Food Microbiol. 93:141–154 [DOI] [PubMed] [Google Scholar]
- 14.Schwan RF, Wheals AE. 2004. The microbiology of cocoa fermentation and its role in chocolate quality. Crit. Rev. Food Sci. Nutr. 44:205–221 [DOI] [PubMed] [Google Scholar]
- 15.Sieuwerts S, Molenaar D, van Hijum SA, Beerthuyzen M, Stevens MJ, Janssen PW, Ingham CJ, de Bok FA, De Vos WM, van Hylckama Vlieg JE. 2010. Mixed-culture transcriptome analysis reveals the molecular basis of mixed-culture growth in Streptococcus thermophilus and Lactobacillus bulgaricus. Appl. Environ. Microbiol. 76:7775–7784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maligoy M, Mercade M, Cocaign-Bousquet M, Loubiere P. 2008. Transcriptome analysis of Lactococcus lactis in coculture with Saccharomyces cerevisiae. Appl. Environ. Microbiol. 74:485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossouw D, Du TM, Bauer FF. 2012. The impact of co-inoculation with Oenococcus oeni on the transcriptome of Saccharomyces cerevisiae and on the flavour-active metabolite profiles during fermentation in synthetic must. Food Microbiol. 29:121–131 [DOI] [PubMed] [Google Scholar]
- 18.Hervé-Jimenez L, Guillouard I, Guedon E, Boudebbouze S, Hols P, Monnet V, Maguin E, Rul F. 2009. Postgenomic analysis of Streptococcus thermophilus cocultivated in milk with Lactobacillus delbrueckii subsp. bulgaricus: involvement of nitrogen, purine, and iron metabolism. Appl. Environ. Microbiol. 75:2062–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shindala A, Bungay HR, III, Krieg NR, Culbert K. 1965. Mixed-culture interactions. I. Commensalism of Proteus vulgaris with Saccharomyces cerevisiae in continuous culture. J. Bacteriol. 89:693–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Driessen FM, Kingma F, Stadhouders J. 1982. Evidence that Lactobacillus bulgaricus in yoghurt is stimulated by carbon dioxide produced by Streptococcus thermophilus. Neth. Milk Dairy J. 36:135–144 [Google Scholar]
- 21.Megee RD, Drake JF, Fredrick AG, Tsuchiya HM. 1972. Studies in intermicrobial symbiosis. Saccharomyces cerevisiae and Lactobacillus casei. Can. J. Microbiol. 18:1733–1742 [DOI] [PubMed] [Google Scholar]
- 22.Chao C, Reilly PJ. 1972. Symbiotic growth of Acetobacter suboxydans and Saccharomyces carlsbergensis in a chemostat. Biotechnol. Bioeng. 14:75–92 [Google Scholar]
- 23.Harder W, Kuenen JG, Matin A. 1977. A review. Microbial selection in continuous culture. J. Appl. Bacteriol. 43:1–24 [DOI] [PubMed] [Google Scholar]
- 24.Hervé-Jimenez L, Guillouard I, Guedon E, Gautier C, Boudebbouze S, Hols P, Monnet V, Rul F, Maguin E. 2008. Physiology of Streptococcus thermophilus during the late stage of milk fermentation with special regard to sulfur amino-acid metabolism. Proteomics 8:4273–4286 [DOI] [PubMed] [Google Scholar]
- 25.Simova E, Beshkova D, Angelov A, Hristozova T, Frengova G, Spasov Z. 2002. Lactic acid bacteria and yeasts in kefir grains and kefir made from them. J. Ind. Microbiol. Biotechnol. 28:1–6 [DOI] [PubMed] [Google Scholar]
- 26.Basson NJ, van Wyk CW. 1996. The establishment of a community of oral bacteria that controls the growth of Candida albicans in a chemostat. Oral Microbiol. Immunol. 11:199–202 [DOI] [PubMed] [Google Scholar]
- 27.Hobson PN, Wallace RJ. 1982. Microbial ecology and activities in the rumen: part 1. Crit. Rev. Microbiol. 9:165–225 [DOI] [PubMed] [Google Scholar]
- 28.Bokulich NA, Bamforth CW, Mills DA. 2012. Brewhouse-resident microbiota are responsible for multi-stage fermentation of American coolship ale. PLoS One 7:e35507. 10.1371/journal.pone.0035507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheirsilp B, Shoji H, Shimizu H, Shioya S. 2003. Interactions between Lactobacillus kefiranofaciens and Saccharomyces cerevisiae in mixed culture for kefiran production. J. Biosci. Bioeng. 96:279–284 [DOI] [PubMed] [Google Scholar]
- 30.Jakobsen M, Narvhus J. 1996. Yeasts and their possible beneficial and negative effects on the quality of dairy products. Int. Dairy J. 6:755–768 [Google Scholar]
- 31.Nijkamp JF, van den Broek M, Datema E, de Kok S, Bosman L, Luttik MA, Daran-Lapujade P, Vongsangnak W, Nielsen J, Heijne WH, Klaassen P, Paddon CJ, Platt D, Kotter P, van Ham RC, Reinders MJ, Pronk JT, de Ridder D, Daran JM. 2012. De novo sequencing, assembly and analysis of the genome of the laboratory strain Saccharomyces cerevisiae CEN.PK113-7D, a model for modern industrial biotechnology. Microb. Cell Fact. 11:36. 10.1186/1475-2859-11-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Entian KD, Kötter P. 2007. Yeast genetic strain and plasmid collections, p 629–666 In Stansfield I, Stark MJR. (ed), Yeast gene analysis, 2nd ed, vol 36 Academic Press, Elsevier, Amsterdam, The Netherlands [Google Scholar]
- 33.Sherman F. 1991. Getting started with yeast. Methods Enzymol. 194:3–21 [DOI] [PubMed] [Google Scholar]
- 34.Makarova K, Slesarev A, Wolf Y, Sorokin A, Mirkin B, Koonin E, Pavlov A, Pavlova N, Karamychev V, Polouchine N, Shakhova V, Grigoriev I, Lou Y, Rohksar D, Lucas S, Huang K, Goodstein DM, Hawkins T, Plengvidhya V, Welker D, Hughes J, Goh Y, Benson A, Baldwin K, Lee JH, Diaz-Muniz I, Dosti B, Smeianov V, Wechter W, Barabote R, Lorca G, Altermann E, Barrangou R, Ganesan B, Xie Y, Rawsthorne H, Tamir D, Parker C, Breidt F, Broadbent J, Hutkins R, O'Sullivan D, Steele J, Unlu G, Saier M, Klaenhammer T, Richardson P, Kozyavkin S, Weimer B, Mills D. 2006. Comparative genomics of the lactic acid bacteria. Proc. Natl. Acad. Sci. U. S. A. 103:15611–15616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verduyn C, Postma E, Scheffers WA, van Dijken JP. 1992. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 8:501–517 [DOI] [PubMed] [Google Scholar]
- 36.Chervaux C, Ehrlich SD, Maguin E. 2000. Physiological study of Lactobacillus delbrueckii subsp. bulgaricus strains in a novel chemically defined medium. Appl. Environ. Microbiol. 66:5306–5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andreasen AA, Stier TJB. 1954. Anaerobic nutrition of Saccharomyces cerevisiae. 2. Unsaturated fatty acid requirement for growth in a defined medium. J. Cell. Comp. Physiol. 43:271–281 [DOI] [PubMed] [Google Scholar]
- 38.Verduyn C, Postma E, Scheffers WA, van Dijken JP. 1990. Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J. Gen. Microbiol. 136:395–403 [DOI] [PubMed] [Google Scholar]
- 39.Partanen L, Marttinen N, Alatossava T. 2001. Fats and fatty acids as growth factors for Lactobacillus delbrueckii. Syst. Appl. Microbiol. 24:500–506 [DOI] [PubMed] [Google Scholar]
- 40.Andreasen AA, Stier TJB. 1953. Anaerobic nutrition of Saccharomyces cerevisiae. 1. Ergosterol requirement for growth in a defined medium. J. Cell. Comp. Physiol. 41:23–26 [DOI] [PubMed] [Google Scholar]
- 41.Krause I, Bockhardt A, Neckerman H, Henle T, Klostermeyer H. 1995. Simultaneous determination of amino acids and biogenic amines by reversed-phase high-performance liquid chromatography of the dabsyl derivatives. J. Chromatogr. A 715:67–79 [Google Scholar]
- 42.de Jonge LP, Buijs NA, ten Pierick A, Deshmukh A, Zhao Z, Kiel JA, Heijnen JJ, van Gulik WM. 2011. Scale-down of penicillin production in Penicillium chrysogenum. Biotechnol. J. 6:944–958 [DOI] [PubMed] [Google Scholar]
- 43.Piper MDW, Daran-Lapujade P, Bro C, Regenberg B, Knudsen S, Nielsen J, Pronk JT. 2002. Reproducibility of oligonucleotide microarray transcriptome analyses: an interlaboratory comparison using chemostat cultures of Saccharomyces cerevisiae. J. Biol. Chem. 277:37001–37008 [DOI] [PubMed] [Google Scholar]
- 44.Boer VM, de Winde JH, Pronk JT, Piper MD. 2003. The genome-wide transcriptional responses of Saccharomyces cerevisiae grown on glucose in aerobic chemostat cultures limited for carbon, nitrogen, phosphorus, or sulfur. J. Biol. Chem. 278:3265–3274 [DOI] [PubMed] [Google Scholar]
- 45.Storey JD, Tibshirani R. 2003. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. U. S. A. 100:9440–9445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knijnenburg TA, de Winde JH, Daran JM, Daran-Lapujade P, Pronk JT, Reinders MJT, Wessels LFA. 2007. Exploiting combinatorial cultivation conditions to infer transcriptional regulation. BMC Genomics 8:25. 10.1186/1471-2164-8-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adamberg K, Kask S, Laht TM, Paalme T. 2003. The effect of temperature and pH on the growth of lactic acid bacteria: a pH-auxostat study. Int. J. Food Microbiol. 85:171–183 [DOI] [PubMed] [Google Scholar]
- 48.Postmus J, Canelas AB, Bouwman J, Bakker BM, van Gulik W, de Mattos MJ, Brul S, Smits GJ. 2008. Quantitative analysis of the high temperature-induced glycolytic flux increase in Saccharomyces cerevisiae reveals dominant metabolic regulation. J. Biol. Chem. 283:23524–23532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Konings WN, Poolman B, Driessen AJ. 1989. Bioenergetics and solute transport in lactococci. Crit. Rev. Microbiol. 16:419–476 [DOI] [PubMed] [Google Scholar]
- 50.Neidhart FC, Ingraham JL, Schaechter M. 1990. The cell cycle, p 389–417 In Neidhart FC, Ingraham JL, Schaechter M. (ed), Physiology of the bacterial cell. Sinauer Associates, Sunderland, MA [Google Scholar]
- 51.Abbott DA, Suir E, van Maris AJ, Pronk JT. 2008. Physiological and transcriptional responses to high concentrations of lactic acid in anaerobic chemostat cultures of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 74:5759–5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chico E, Olavarria JS, Nunez de Castro I. 1978. l-Alanine as an end product of glycolysis in Saccharomyces cerevisiae growing under different hypoxic conditions. Antonie Van Leeuwenhoek 44:193–201 [DOI] [PubMed] [Google Scholar]
- 53.Jolly L, Vincent SJ, Duboc P, Neeser JR. 2002. Exploiting expolysaccharides from lactic acid bacteria. Antonie Van Leeuwenhoek 82:367–374 [DOI] [PubMed] [Google Scholar]
- 54.Fredrickson AG. 1977. Behavior of mixed cultures of microorganisms. Annu. Rev. Microbiol. 31:63–87 [DOI] [PubMed] [Google Scholar]
- 55.van de Guchte M, Penaud S, Grimaldi C, Barbe V, Bryson K, Nicolas P, Robert C, Oztas S, Mangenot S, Couloux A, Loux V, Dervyn R, Bossy R, Bolotin A, Batto JM, Walunas T, Gibrat JF, Bessieres P, Weissenbach J, Ehrlich SD, Maguin E. 2006. The complete genome sequence of Lactobacillus bulgaricus reveals extensive and ongoing reductive evolution. Proc. Natl. Acad. Sci. U. S. A. 103:9274–9279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ueda K, Tagami Y, Kamihara Y, Shiratori H, Takano H, Beppu T. 2008. Isolation of bacteria whose growth is dependent on high levels of CO2 and implications of their potential diversity. Appl. Environ. Microbiol. 74:4535–4538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aguilera J, van Dijken JP, de Winde JH, Pronk JT. 2005. Carbonic anhydrase (Nce103p): an essential biosynthetic enzyme for growth of Saccharomyces cerevisiae at atmospheric carbon dioxide pressure. Biochem. J. 391:311–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bahn YS, Muhlschlegel FA. 2006. CO2 sensing in fungi and beyond. Curr. Opin. Microbiol. 9:572–578 [DOI] [PubMed] [Google Scholar]
- 59.Stevens MJ, Wiersma A, de Vos WM, Kuipers OP, Smid EJ, Molenaar D, Kleerebezem M. 2008. Improvement of Lactobacillus plantarum aerobic growth as directed by comprehensive transcriptome analysis. Appl. Environ. Microbiol. 74:4776–4778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiles AM, Cai H, Naider F, Becker JM. 2006. Nutrient regulation of oligopeptide transport in Saccharomyces cerevisiae. Microbiology 152:3133–3145 [DOI] [PubMed] [Google Scholar]
- 61.Daran-Lapujade P, Jansen ML, Daran JM, van Gulik W, de Winde JH, Pronk JT. 2004. Role of transcriptional regulation in controlling fluxes in central carbon metabolism of Saccharomyces cerevisiae, a chemostat culture study. J. Biol. Chem. 279:9125–9138 [DOI] [PubMed] [Google Scholar]
- 62.de Nicola R, Hazelwood LA, Hulster EAF, Walsh MC, Knijnenburg TA, Reinders MJT, Walker GM, Daran JM, Daran-Lapujade P. 2007. Physiological and transcriptional responses of Saccharomyces cerevisiae to zinc limitation in chemostat cultures. Appl. Environ. Microbiol. 73:7680–7692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshida S, Imoto J, Minato T, Oouchi R, Kamada Y, Tomita M, Soga T, Yoshimoto H. 2011. A novel mechanism regulates H2S and SO2 production in Saccharomyces cerevisiae. Yeast 28:109–121 [DOI] [PubMed] [Google Scholar]
- 64.van Bokhorst-van de Veen H, Abee T, Tempelaars M, Bron PA, Kleerebezem M, Marco ML. 2011. Short- and long-term adaptation to ethanol stress and its cross-protective consequences in Lactobacillus plantarum. Appl. Environ. Microbiol. 77:5247–5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheirsilp B, Shimizu H, Shioya S. 2003. Enhanced kefiran production by mixed culture of Lactobacillus kefiranofaciens and Saccharomyces cerevisiae. J. Biotechnol. 100:43–53 [DOI] [PubMed] [Google Scholar]
- 66.Sieuwerts S, de Bok FA, Hugenholtz J, van Hylckama Vlieg JE. 2008. Unraveling microbial interactions in food fermentations: from classical to genomics approaches. Appl. Environ. Microbiol. 74:4997–5007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.