Abstract
Bloodstream infections due to vancomycin-resistant enterococci (VRE-BSI) result in substantial patient mortality and cost. Daptomycin and linezolid are commonly prescribed for VRE-BSI, but there are no clinical trials to determine optimal antibiotic selection. We conducted a systematic review for investigations that compared daptomycin and linezolid for VRE-BSI. We searched Medline from 1966 through 2012 for comparisons of linezolid and daptomycin for VRE-BSI. We included searches of EMBASE, clinicaltrials.gov, and national meetings. Data were extracted using a standardized instrument. Pooled odds ratios (OR) and 95% confidence intervals (95% CI) were calculated using a fixed-effects model. Our search yielded 4,243 publications, of which 482 contained data on VRE treatment. Most studies (452/482) did not present data on BSI or did not provide information on linezolid or daptomycin. Among the remaining 30 studies, 9 offered comparative data between the two agents. None were randomized clinical trials. There was no difference in microbiologic (n = 5 studies, 517 patients; OR, 1.0; 95% CI, 0.4 to 1.7; P = 0.95) and clinical (n = 3 studies, 357 patients; OR, 1.2; 95% CI, 0.7 to 2.0; P = 0.7) cures between the two antibiotics. There was a trend toward increased survival with linezolid compared to daptomycin treatment (n = 9 studies, 1,074 patients; OR, 1.3; 95% CI, 1.1 to 1.8; I2 = 0 [where I2 is a measure of inconsistency]), but this did not reach statistical significance (P = 0.054). There are limited data to inform clinicians on optimal antibiotic selection for VRE-BSI. Available studies are limited by small sample size, lack of patient-level data, and inconsistent outcome definitions. Additional research, including randomized clinical trials, is needed before conclusions can be drawn about treatment options for VRE therapy.
INTRODUCTION
Bloodstream infections due to vancomycin-resistant enterococcal species (VRE-BSI) are a rapidly growing problem in hospitals, with life-threatening consequences for patients (1–5). Despite infection prevention and control efforts, U.S. hospitalizations associated with VRE doubled between 2000 and 2006 and appear to be further increasing (1–6). National surveys of U.S. intensive care units (ICUs) indicate that VRE represented <1% of enterococcal isolates in 1990, but more recent data suggest that they now exceed 30% (1–4).
VRE-BSI primarily affect the most vulnerable patient populations, including postsurgical and trauma patients, complex internal medicine patients, and those who have undergone organ transplantation, especially liver transplantation (7–12). VRE-BSI are associated with significant mortality in cohorts of hematopoietic stem cell transplant recipients, liver transplant patients, oncology patients, and other inpatient populations (11–19). Importantly, effective antibiotic therapy and shorter duration of bacteremia are associated with lower mortality in patients with VRE-BSI (11, 19–22).
Newer antimicrobial agents with activity against VRE (daptomycin, linezolid, quinupristin-dalfopristin, and tigecycline) provide much-needed therapeutic options for VRE-BSI, but there are limited data to inform clinicians as to which among these drugs are most effective for VRE-BSI. Two phase III clinical trials for VRE-BSI were started but were subsequently aborted due to enrollment difficulties (23, 24). To our knowledge, there are no further plans to initiate phase II or phase III clinical trials for VRE-BSI.
Among currently available agents with activity against VRE, daptomycin and linezolid have been used most frequently for VRE-BSI treatment (22, 25–30). Daptomycin is a cyclic lipopeptide with a broad spectrum of activity against Gram-positive organisms, including VRE (31, 32). Higher doses of daptomycin (≥8 mg/kg) are thought to improve clinical outcomes from VRE-BSI (33, 34). Linezolid is an oxazolidinone that inhibits bacterial protein synthesis by inhibiting ribosomal complex formation. Its spectrum of activity against Gram-positive organisms includes most isolates of VRE (35). However, marrow toxicity and peripheral neuropathy from prolonged linezolid use are considered important limitations, particularly in bone marrow transplant patients with VRE-BSI (26, 36). One observational investigation suggested that linezolid was associated with a survival advantage for VRE-BSI (37); however, there have been no attempts to systematically review the literature on VRE-BSI outcomes focusing on antimicrobial therapy. We therefore conducted a systematic review and meta-analysis to quantify differences in clinical outcomes from VRE-BSI treated with daptomycin or linezolid.
MATERIALS AND METHODS
Search strategy and study selection.
We performed a literature search of Medline from 1966 to December 2012 and of EMBASE from 1980 to December 2012 to find published manuscripts evaluating linezolid and daptomycin for treatment of VRE-BSI in patients. We limited studies to those in English and using human subjects and searched for the following terms: “vancomycin-resistant,” “enterococcus,” “faecalis,” “faecium,” and “VRE.” In addition, we examined the references of all identified articles to look for additional relevant articles. We reviewed the abstracts from the annual meetings of the Infectious Disease Society of America (IDSA), the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), and the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) from 1986 to 2012.
Abstracts from each reference from our electronic search were independently reviewed for relevance by two physician investigators (D. W. Whang and J. A. McKinnell). Studies were selected for full review if they reported primary data from patients with VRE-BSI treated with either linezolid or daptomycin. Studies that did not separate data on outcomes between vancomycin-sensitive and vancomycin-resistant enterococcal infections were excluded. Studies that reported only on treatment experience with a single agent, providing no comparative data, were not included. In the final analysis, only retrospective cohort investigations comparing patients treated with linezolid against those treated with daptomycin were included. There were no exclusions for different types of patients. The intervention of interest was antibiotic selection. The comparison groups were linezolid- and daptomycin-treated patients. The outcome of interest was mortality, as defined by the study investigators. The MOOSE criteria were used to evaluate study quality (38).
Data extraction, data analysis, and statistical methods.
Each manuscript underwent independent, blinded, double-data extraction by two reviewers (D. W. Whang and N. M. Partain) using a standardized instrument. Discrepancies in data extraction underwent arbitration by a third reviewer (J. A. McKinnell), and consensus was obtained by oral discussion. Data collected from each study included year of study, number of patients, definition of infection, dose of daptomycin and linezolid used, microbiologic cure, clinical cure, and mortality. Infections, mortality, microbiologic cure rates, and clinical cure rates were defined according to descriptions provided by each study.
Additional data were collected about the patient cohorts when present, including gender, ethnicity, age, ICU versus ward-level care, comorbid conditions, source of bacteremia, vasopressor use, malignancy, organ or stem cell transplant, immunosuppression, concomitant bacteremia, and infectious disease consultation. All-cause mortality, microbiologic cure rates, and clinical cure rates were the primary outcome measures used in this meta-analysis.
Odds ratios (OR) for mortality were calculated for each study. Mantel-Haenszel statistical methods were used to calculate the pooled odds ratios, 95% confidence intervals (CI), and the associated P values of each risk factor using a fixed-effects model. We analyzed heterogeneity in publication using the I2 measure of inconsistency. We utilized a DerSimonian and Laird random-effects model to generate confidence intervals. Studies were not additionally weighted by study quality. We present a forest plot of data from each individual study (39, 40).
RESULTS
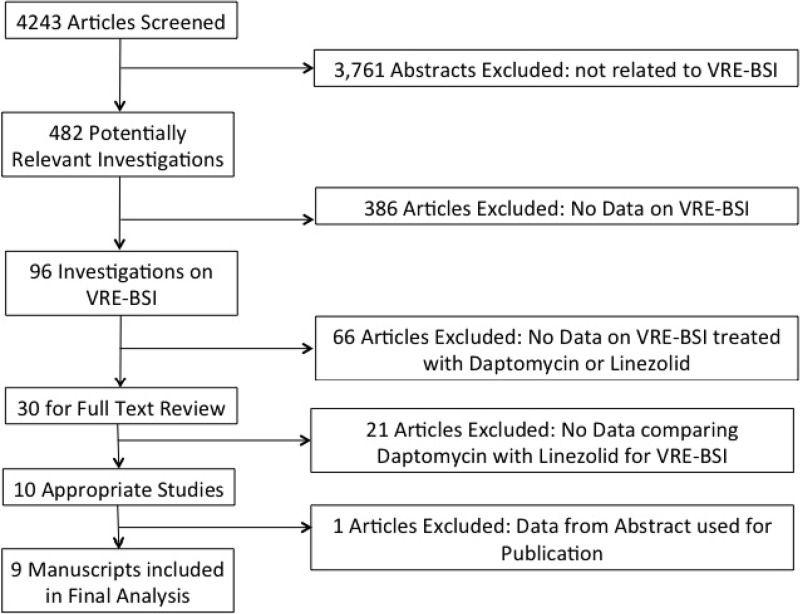
Our search yielded 4,243 publications, of which 482 contained data on treatment of VRE (Fig. 1). The majority of investigations (n = 452) did not have data on BSI as a site of infection. Among the 30 studies containing data on outcomes of VRE-BSI treated with linezolid or daptomycin, nine investigations provided comparative data on linezolid and daptomycin and were included in the final analysis (22, 26–30, 37, 41, 42). Eight of the studies were based in the United States, and one study was based in Taiwan.
Fig 1.
Flow chart representing the study selection process and reasons for exclusion.
The nine investigations that were included in our final analysis reported on 1,074 patients treated for VRE-BSI. The investigations included in our analysis differed in their cohorts and how they defined VRE-BSI (Table 1). Three investigations required at least two positive cultures for VRE to define a case of VRE-BSI (28, 30). The remaining investigations required only one positive bloodstream culture, with two studies additionally using criteria of the Centers for Disease Control and Prevention for BSI (26, 27). Studies also differed in their definition of mortality. Three investigations used 30-day all-cause mortality, one investigation reported 14-day mortality, two investigations used inpatient all-cause mortality, two investigations used all-cause mortality at end of therapy, and one manuscript used all-cause mortality 7 days after end of therapy. Linezolid was uniformly given at a dose of 600 mg every 12 h; daptomycin was usually given at 6 mg/kg, but doses ranged from 3.4 mg/kg to 10.4 mg/kg (Table 1).
Table 1.
Investigations that compared daptomycin and linezolid therapy for VRE-BSI within a single cohort
| Reference | Cohort/definition of VRE-BSI | Treatmentf | Location | Date of study (yr) | Study size (no. of patients) | Linezolid dose (mg BID)g | Mean daily daptomycin dose (mg/kg [range]) | Overall cure rate (%) |
Mortality (%) | Mortality end point | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical | Microbiologic | ||||||||||
| Bio (30) | 2 Positive culturesa | D or L (>3 days) | Philadelphia, PA | 2004–2008 | 84 | 600 | 6 (3.7–8.8)i | 52 | 88 | 36 | Within 7 days after the end of therapy |
| Crank (28) | 2 Positive culturesb,c | D or L | Chicago, Il | 2003–2007 | 101 | 600 | 5.5j | NA | NA | 41 | Inpatient all-cause mortality |
| Dubrovskaya (37) | 1 Positive culture | D or L | New York, NY | 2005–2007 | 80 | NAh | 6 (4–9) | NA | 97 | 23 | All-cause mortality at the end of therapy |
| Furuya (41) | 1 Positive culture | D or L | New York, NY | 2004–2005 | 54 | NA | 5.7 (3.9–7.9) | NA | 91 | 43 | All cause mortality at the end of therapy |
| Kraft (22) | 1 Positive cultured | D or L (>2 days) | Ann Arbor, MI | 2004–2007 | 72 | NA | NA | 76 | NA | 24 | 30-day all-cause mortality |
| Lu (42) | 2 Positive culturese | NA | Taipei, Taiwan | 2003–2010 | 149 | NA | NA | NA | NA | 48 | 14-day mortalityk |
| Mave (27) | 1 Positive culture; CDC criteria for BSI | D or L | New Orleans, LA | 2003–2007 | 98 | 600 | 6 | NA | 89 | 22 | Inpatient all-cause mortality |
| McKinnell (26) | 1 Positive culture; CDC criteria for BSI | NA | Birmingham, AL | 2005–2008 | 235 | NA | NA | NA | 78 | 35 | 30-day all-cause mortality |
| Twilla (29) | 1 Positive culture | D or L (>5 days) | Memphis, TN | 2004–2009 | 201 | 600 | 6.1 (3.4–10.40) | 74 | 94 | 20 | 30-day all-cause mortality |
Two blood cultures.
Two blood cultures or one blood culture with a second culture from another site.
Polymicrobial infections excluded.
Hematology/bone marrow transplant.
Febrile patient.
D, daptomycin; L, linezolid.
BID, twice daily.
NA, not available. The information was not stated in the article.
Value is the median (range).
Information on other doses is as follows: 4 mg/kg, n = 23; 6 mg/kg, n = 38; 8 mg/kg, n = 6.
28-Day mortality was 60%.
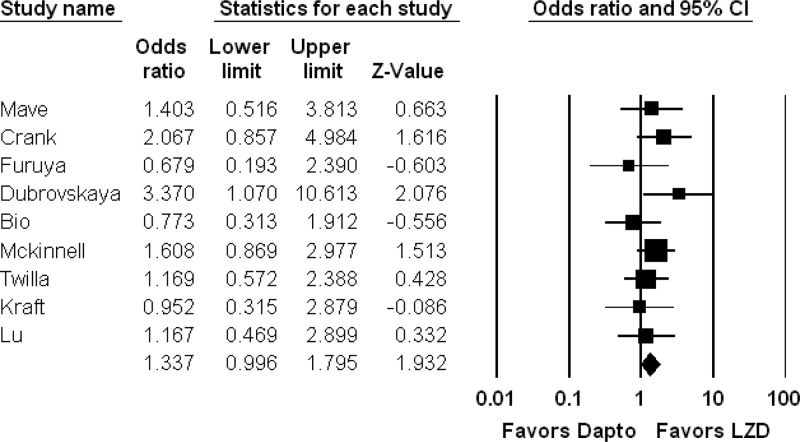
Comparisons of microbiologic cure, clinical cure, and mortality between daptomycin and linezolid are reported in Table 2. Our meta-analysis suggests that linezolid therapy is associated with increased survival for patients with VRE-BSI (n = 9 studies, 1,074 patients; OR, 1.3; 95% CI, 1.0 to 1.8; I2 = 0), but this did not reach statistical significance (P = 0.053) (Fig. 2). Only one study demonstrated a statistically significant association between daptomycin and mortality (37). Outcomes were similar between linezolid and daptomycin treatments for microbiologic cure (n = 5 studies, 517 patients; OR, 1.0; 95% CI, 0.4 to 1.7; P = 0.95) and clinical cure (n = 3 studies, 357 patients; OR, 1.2; 95% CI, 0.5 to 2.1; P = 0.68; I2 = 0) (Table 3).
Table 2.
Results from each investigations comparing linezolid and daptomycin in terms of microbiologic cure, clinical cure and mortality
| Reference | Cohort/definition of VRE-BSI | Antibioticf | Clinical cure |
Microbiologic cure |
Mortality |
|||
|---|---|---|---|---|---|---|---|---|
| No. of cured patients/no. of treated patients (%) | OR (95% CI) | No. of cured patients/no. of treated patients (%) | OR (95% CI) | No. of deaths/no. of treated patients (%) | OR (95% CI) | |||
| Bio (30) | 2 Positive culturesa | D | 22/37 (65) | 1.7 (0.7–4.0) | 32/37 (87) | 0.8 (0.2–2.8) | 12/37 (32) | 0.8 (0.3–1.9) |
| L (>3 days) | 22/47 (50) | 42/47 (90) | 18/47 (38) | |||||
| Crank (28) | 2 Positive culturesb,c | D | NAg | NA | NA | NA | 31/67 (46) | 2.1 (0.9–5.0) |
| L | NA | NA | 10/34 (29) | |||||
| Dubrovskaya (37) | 1 Positive culture | D | NA | NA | 39/40 (98) | 1.0 (0.1–16.6) | 13/40 (33) | 3.4 (1.1–10.6) |
| L | NA | 39/40 (97) | 5/40 (13) | |||||
| Furuya (41) | 1 Positive culture | D | NA | NA | 14/14 (100) | NC | 5/14 (36) | 0.7 (0.2–2.4) |
| L | NA | 35/40 (88) | 18/40 (45) | |||||
| Kraft (22) | 1 Positive cultured | D | 33/43 (77) | 1.1 (0.3–3.2) | NA | NA | 10/43 (23) | 0.9 (0.3–2.9) |
| L (>2 days) | 22/29 (76) | NA | 7/29 (24) | |||||
| Lu (42) | 2 Positive culturese | D | NA | NA | NA | NA | 11/29 (38) | 1.2 (0.5–2.9)h |
| L | NA | NA | 22/64 (34) | |||||
| Mave (27) | 1 Positive culture; CDC criteria for BSI | D | NA | NA | 27/30 (90) | 1.2 (0.3–4.9) | 8/30 (27) | 1.4 (0.5–3.8) |
| L | NA | 60/68 (88) | 14/68 (21) | |||||
| McKinnell (26) | 1 Positive culture; CDC criteria for BSI | D | NA | NA | 61/86 (71) | 0.5 (0.3–1.0) | 25/61 (29) | 3.3 (1.6–6.8) |
| L | NA | 86/104 (83) | 18/104 (18) | |||||
| Twilla (29) | 1 Positive culture | D | 47/63 (75) | 1.0 (0.5–2.1) | 59/63 (94) | 0.9 (0.3–3.1) | 15/63 (24) | 14. (0.7–2.9) |
| L (>5 days) | 102/138 (74) | 130/138 (94) | 25/138 (18) | |||||
Two blood cultures.
Two blood cultures or one blood culture with a second culture from another site.
Polymicrobial infections excluded.
Hematology/bone marrow transplant.
Febrile patient.
D, daptomycin; L, linezolid.
NA, not available. The information was not stated in the article.
For daptomycin, 28-day mortality was 17/29 (59%); for linezolid it was 33/64 (52%).
Fig 2.
Meta-analysis comparing mortality in patients treated with linezolid versus daptomycin treatment for VRE-BSI. The forest plot shows results for overall mortality in patients treated with linezolid versus daptomycin. No weighting criteria were applied to the calculations. The overall trend is for improved survival with linezolid versus daptomycin (OR, 1.3), but this is not statistically significant (P = 0.053). Dapto, daptomycin; LZD, linezolid.
Table 3.
Results of meta-analysis of studies comparing linezolid with daptomycin for the treatment of VRE-BSI
| Outcomea | No. of studies | No. of patients | ORb | 95% CI | P value |
|---|---|---|---|---|---|
| Mortality | 9 | 1,074 | 1.3 | 0.996–1.8 | 0.053 |
| Inpatient | 4 | 333 | 1.7 | 1.1–2.8 | 0.08 |
| 30-Day | 3 | 271 | 1.3 | 0.9–2.3 | 0.20 |
| Microbiologic cure | 5 | 517 | 1.0 | 0.4–1.7 | 0.95 |
| Clinical cure | 3 | 357 | 1.2 | 0.5–2.1 | 0.68 |
Outcomes of linezolid versus daptomycin treatment were as defined by the investigation.
Odds ratios greater than 1 favor linezolid treatment, and odds ratios less than 1 favor daptomycin treatment. For the paper by Lu et al. (42), the 14-day mortality numbers were used for the calculation of the mortality odds ratio. Analysis using the 28-day mortality was not significantly different.
DISCUSSION
VRE-BSI is a potentially life-threatening complication for hospitalized patients, particularly the immunocompromised. Effective antibiotic therapy has been shown to reduce mortality from VRE-BSI (13). However, the high attributable mortality associated with VRE-BSI in cohorts of hematopoietic stem cell transplant recipients, liver transplant patients, oncology patients, and inpatient populations (11–19) warrants an examination of the literature to examine which therapies may be associated with improved clinical outcomes in these vulnerable populations.
Our systematic review provides an important assessment of available literature on selection of linezolid versus daptomycin for the treatment of VRE-BSI. Although we did not identify data from clinical trials of VRE-BSI, we found a trend toward an association between linezolid therapy and patient survival in the available literature. The association between linezolid and lower patient mortality than with daptomycin treatment reached statistical significance in one analysis by Dubrovskaya et al., which examined 80 patients in an academic medical center in New York (37).
Despite the objective results from our meta-analysis showing an association between linezolid and survival, we strongly caution that our findings should not be considered conclusive. There were relatively few investigations included in our analysis, and all were retrospective cohort analyses. Differences in definitions of mortality may further have introduced bias. Moreover, there was evidence of treatment selection bias in these investigations. Four investigations demonstrated a bias toward using daptomycin in patients with hematologic abnormalities (22, 26, 29, 30). As a result, some of the observed mortality difference between treatments may be a product of the confounding by indication where “sicker” patients were given daptomycin. One method to account for differences between treatment groups would be to conduct a patient-level quantitative analysis of all studies to assess the impact of host, organism, and treatment-related factors on mortality and clinical cure. This method of analysis has proven successful in investigations of other infectious syndromes, but patient-level data were not available from the investigations included in this review (43).
Though our data trended toward an association between linezolid therapy and survival, we did not observe an association between linezolid and microbiologic cure (P = 0.95). In nearly all investigations, microbiologic cure was defined relatively late in the course of disease, typically 7 to 14 days after VRE-BSI was diagnosed. In contrast to traditional measures of microbiologic cure, duration of bacteremia and probability of repeat positive blood culture while on therapy may be more sensitive measures of antibiotic activity and are thought to be an important predictor of mortality. Diazgranados and Jernigan presented a robust analysis showing a dose-response relationship between bacteremia duration and mortality using a Cox proportional hazards models (11). Bhavnani and colleagues reported that positive follow-up cultures were associated with mortality (odds ratio, 10.1; 95% CI, 2.2 to 46.7) (21). Similarly, Kraft et al. reported that bacteremia that persisted over multiple days was associated with significantly higher mortality (22). Among the studies included in our review, one investigation found a higher likelihood of repeat cultures positive for VRE while on daptomycin (P = 0.01) and higher recurrence of VRE while patients were on daptomycin (P = 0.03) (29). Alternatively, Crank et al. and Kraft et al. found no difference in duration of bacteremia between linezolid and daptomycin treatments (22, 28).
An important consideration of this literature review is that the majority of studies described daptomycin dosing at 6 mg/kg, with relatively few patients receiving higher doses of daptomycin (≥8 mg/kg). Higher doses of daptomycin are thought to improve clinical outcomes from VRE-BSI compared to traditional doses (33, 34). Among studies included in our final analysis, there is evidence from one investigation that lower doses of daptomycin were associated with recurrently positive cultures (29). There has been limited in vitro data, and emerging clinical data suggest that combination therapy for VRE-BSI with an effective antibiotic and a β-lactam may be more effective that effective antibiotics alone (44, 45). None of the investigations in our study adjusted for β-lactam adjunctive therapy for VRE.
In summary, our results suggest that there may be a mortality difference between daptomycin and linezolid for the treatment of VRE-BSI. However, the literature on VRE-BSI is quite limited. There were no clinical trials in our review of the literature. The available manuscripts include small-cohort analyses, affected by traditional limitations of retrospective studies. There were also significant differences in study design, and importantly daptomycin may have been underdosed. With the failure of two VRE-BSI clinical trials to enroll an adequate number of subjects, the low likelihood of having a gold-standard, prospective randomized clinical trial of VRE-BSI in the near future is concerning. Until such a trial is performed, we strongly believe that further retrospective analyses of VRE-BSI that control for important clinical predictors and utilize sensitive outcomes such as duration of bacteremia, likelihood of repeat positive cultures while on therapy, time to clinical cure, and traditional endpoints such as mortality will be critical to understanding the role of antibiotic choice for this increasingly common and potentially deadly infection (46).
ACKNOWLEDGMENTS
This study and the work of J.A.M. were supported by the NIH/NCRR/NCATS (grant number KL2TR000122 to the UCLA Clinical and Translational Science Institute). J.A.M. appreciates the logistic support of the General Clinical Research Center at Harbor-UCLA Medical Center (NIH NCRR M01-RR00425).
The article's content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
We have no conflicts of interest to disclose in relation to the manuscript.
Footnotes
Published ahead of print 29 July 2013
REFERENCES
- 1.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 29:996–1011 [DOI] [PubMed] [Google Scholar]
- 2.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309–317 [DOI] [PubMed] [Google Scholar]
- 3.Deshpande LM, Fritsche TR, Moet GJ, Biedenbach DJ, Jones RN. 2007. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: a report from the SENTRY antimicrobial surveillance program. Diagn. Microbiol. Infect. Dis. 58:163–170 [DOI] [PubMed] [Google Scholar]
- 4.System NNIS. 2004. National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control 32:470–485 [DOI] [PubMed] [Google Scholar]
- 5.Reik R, Tenover FC, Klein E, McDonald LC. 2008. The burden of vancomycin-resistant enterococcal infections in US hospitals, 2003 to 2004. Diagn. Microbiol. Infect. Dis. 62:81–85 [DOI] [PubMed] [Google Scholar]
- 6.Ramsey AM, Zilberberg MD. 2009. Secular trends of hospitalization with vancomycin-resistant enterococcus infection in the United States, 2000–2006. Infect. Control Hosp. Epidemiol. 30:184–186 [DOI] [PubMed] [Google Scholar]
- 7.Orloff SL, Busch AM, Olyaei AJ, Corless CL, Benner KG, Flora KD, Rosen HR, Rabkin JM. 1999. Vancomycin-resistant Enterococcus in liver transplant patients. Am. J. Surg. 177:418–422 [DOI] [PubMed] [Google Scholar]
- 8.Kamboj M, Chung D, Seo SK, Pamer EG, Sepkowitz KA, Jakubowski AA, Papanicolaou G. 2010. The changing epidemiology of vancomycin-resistant Enterococcus (VRE) bacteremia in allogeneic hematopoietic stem cell transplant (HSCT) recipients. Biol. Blood Marrow Transplant. 16:1576–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinstock DM, Conlon M, Iovino C, Aubrey T, Gudiol C, Riedel E, Young JW, Kiehn TE, Zuccotti G. 2007. Colonization, bloodstream infection, and mortality caused by vancomycin-resistant enterococcus early after allogeneic hematopoietic stem cell transplant. Biol. Blood Marrow Transplant. 13:615–621 [DOI] [PubMed] [Google Scholar]
- 10.Ghanem G, Hachem R, Jiang Y, Chemaly RF, Raad I. 2007. Outcomes for and risk factors associated with vancomycin-resistant Enterococcus faecalis and vancomycin-resistant Enterococcus faecium bacteremia in cancer patients. Infect. Control Hosp. Epidemiol. 28:1054–1059 [DOI] [PubMed] [Google Scholar]
- 11.DiazGranados CA, Jernigan JA. 2005. Impact of vancomycin resistance on mortality among patients with neutropenia and enterococcal bloodstream infection. J. Infect. Dis. 191:588–595 [DOI] [PubMed] [Google Scholar]
- 12.Gearhart M, Martin J, Rudich S, Thomas M, Wetzel D, Solomkin J, Hanaway MJ, Aranda-Michel J, Weber F, Trumball L, Bass M, Zavala E, Steve Woodle E, Buell JF. 2005. Consequences of vancomycin-resistant Enterococcus in liver transplant recipients: a matched control study. Clin. Transplant. 19:711–716 [DOI] [PubMed] [Google Scholar]
- 13.Vergis EN, Hayden MK, Chow JW, Snydman DR, Zervos MJ, Linden PK, Wagener MM, Schmitt B, Muder RR. 2001. Determinants of vancomycin resistance and mortality rates in enterococcal bacteremia: a prospective multicenter study. Ann. Intern. Med. 135:484–492 [DOI] [PubMed] [Google Scholar]
- 14.Erlandson KM, Sun J, Iwen PC, Rupp ME. 2008. Impact of the more-potent antibiotics quinupristin-dalfopristin and linezolid on outcome measure of patients with vancomycin-resistant Enterococcus bacteremia. Clin. Infect. Dis. 46:30–36 [DOI] [PubMed] [Google Scholar]
- 15.Garbutt JM, Ventrapragada M, Littenberg B, Mundy LM. 2000. Association between resistance to vancomycin and death in cases of Enterococcus faecium bacteremia. Clin. Infect. Dis. 30:466–472 [DOI] [PubMed] [Google Scholar]
- 16.Song JH, Ko KS, Suh JY, Oh WS, Kang CI, Chung DR, Peck KR, Lee NY, Lee WG. 2008. Clinical implications of vancomycin-resistant Enterococcus faecium (VRE) with VanD phenotype and vanA genotype. J. Antimicrob. Chemother. 61:838–844 [DOI] [PubMed] [Google Scholar]
- 17.Song X, Srinivasan A, Plaut D, Perl TM. 2003. Effect of nosocomial vancomycin-resistant enterococcal bacteremia on mortality, length of stay, and costs. Infect. Control Hosp. Epidemiol. 24:251–256 [DOI] [PubMed] [Google Scholar]
- 18.Papanicolaou GA, Meyers BR, Meyers J, Mendelson MH, Lou W, Emre S, Sheiner P, Miller C. 1996. Nosocomial infections with vancomycin-resistant Enterococcus faecium in liver transplant recipients: risk factors for acquisition and mortality. Clin. Infect. Dis. 23:760–766 [DOI] [PubMed] [Google Scholar]
- 19.Camins BC, Farley MM, Jernigan JJ, Ray SM, Steinberg JP, Blumberg HM. 2007. A population-based investigation of invasive vancomycin-resistant Enterococcus infection in metropolitan Atlanta, Georgia, and predictors of mortality. Infect. Control Hosp. Epidemiol. 28:983–991 [DOI] [PubMed] [Google Scholar]
- 20. Reference deleted.
- 21.Bhavnani SM, Drake JA, Forrest A, Deinhart JA, Jones RN, Biedenbach DJ, Ballow CH. 2000. A nationwide, multicenter, case-control study comparing risk factors, treatment, and outcome for vancomycin-resistant and -susceptible enterococcal bacteremia. Diagn. Microbiol. Infect. Dis. 36:145–158 [DOI] [PubMed] [Google Scholar]
- 22.Kraft S, Mackler E, Schlickman P, Welch K, DePestel DD. 2011. Outcomes of therapy: vancomycin-resistant enterococcal bacteremia in hematology and bone marrow transplant patients. Support Care Cancer 19:1969–1974 [DOI] [PubMed] [Google Scholar]
- 23.Florescu I, Beuran M, Dimov R, Razbadauskas A, Bochan M, Fichev G, Dukart G, Babinchak T, Cooper CA, Ellis-Grosse EJ, Dartois N, Gandjini H. 2008. Efficacy and safety of tigecycline compared with vancomycin or linezolid for treatment of serious infections with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci: a phase 3, multicentre, double-blind, randomized study. J. Antimicrob. Chemother. 62(Suppl. 1):i17–i28 [DOI] [PubMed] [Google Scholar]
- 24.Carpenter CF, Chambers HF. 2004. Daptomycin: another novel agent for treating infections due to drug-resistant gram-positive pathogens. Clin. Infect. Dis. 38:994–1000 [DOI] [PubMed] [Google Scholar]
- 25.Arias CA, Contreras GA, Murray BE. 2010. Management of multidrug-resistant enterococcal infections. Clin. Microbiol. Infect. 16:555–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKinnell JA, Patel M, Shirley RM, Kunz DF, Moser SA, Baddley JW. 2011. Observational study of the epidemiology and outcomes of vancomycin-resistant Enterococcus bacteraemia treated with newer antimicrobial agents. Epidemiol. Infect. 139:1342–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mave V, Garcia-Diaz J, Islam T, Hasbun R. 2009. Vancomycin-resistant enterococcal bacteraemia: is daptomycin as effective as linezolid? J. Antimicrob. Chemother. 64:175–180 [DOI] [PubMed] [Google Scholar]
- 28.Crank CW, Scheetz MH, Brielmaier B, Rose WE, Patel GP, Ritchie DJ, Segreti J. 2010. Comparison of outcomes from daptomycin or linezolid treatment for vancomycin-resistant enterococcal bloodstream infection: A retrospective, multicenter, cohort study. Clin. Ther. 32:1713–1719 [DOI] [PubMed] [Google Scholar]
- 29.Twilla JD, Finch CK, Usery JB, Gelfand MS, Hudson JQ, Broyles JE. 2012. Vancomycin-resistant Enterococcus bacteremia: an evaluation of treatment with linezolid or daptomycin. J. Hosp. Med. 7:243–248 [DOI] [PubMed] [Google Scholar]
- 30.Bio LL, Perez ME, MacDougall C, Gallagher JC. 2011. Comparison of linezolid and daptomycin in the treatment of vancomycin-resistant enterococcal bacteremia. Infect. Dis. Clin. Pract. 19:343–347 [Google Scholar]
- 31.Eliopoulos GM, Willey S, Reiszner E, Spitzer PG, Caputo G, Moellering RC., Jr 1986. In vitro and in vivo activity of LY 146032, a new cyclic lipopeptide antibiotic. Antimicrob. Agents Chemother. 30:532–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sader HS, Streit JM, Fritsche TR, Jones RN. 2004. Antimicrobial activity of daptomycin against multidrug-resistant Gram-positive strains collected worldwide. Diagn. Microbiol. Infect. Dis. 50:201–204 [DOI] [PubMed] [Google Scholar]
- 33.Moise PA, Hershberger E, Amodio-Groton MI, Lamp KC. 2009. Safety and clinical outcomes when utilizing high-dose (≥8 mg/kg) daptomycin therapy. Ann. Pharmacother. 43:1211–1219 [DOI] [PubMed] [Google Scholar]
- 34.Kullar R, Davis SL, Levine DP, Zhao JJ, Crank CW, Segreti J, Sakoulas G, Cosgrove SE, Rybak MJ. 2011. High-dose daptomycin for treatment of complicated gram-positive infections: a large, multicenter, retrospective study. Pharmacotherapy 31:527–536 [DOI] [PubMed] [Google Scholar]
- 35.Moellering RC. 2003. Linezolid: the first oxazolidinone antimicrobial. Ann. Intern. Med. 138:135–142 [DOI] [PubMed] [Google Scholar]
- 36.Birmingham MC, Rayner CR, Meagher AK, Flavin SM, Batts DH, Schentag JJ. 2003. Linezolid for the treatment of multidrug-resistant, gram-positive infections: experience from a compassionate-use program. Clin. Infect. Dis. 36:159–168 [DOI] [PubMed] [Google Scholar]
- 37.Dubrovskaya Y, Kubin C, Furuya E. 2008. Daptomycin compared to linezolid for primary treatment of vancomycin-resistant enterococcal bacteremia (VREB), abstr K-3443, p539. Abstr. 48th Intersci. Conf. Antimcrob. Agents Chemother Washington, DC [Google Scholar]
- 38.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. 2000. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012 [DOI] [PubMed] [Google Scholar]
- 39.Bickel PJ, Hammel EA, O'Connell JW. 1975. Sex bias in graduate admissions: data from Berkeley. Science 187:398–404 [DOI] [PubMed] [Google Scholar]
- 40.Pearl J. 2000. Causality: models, reasoning, and inference. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 41.Furuya EY, Kubin C, Yin M, et al. 2005. Daptomycin experience and comparison with linezolid for the treatment of vancomycin-resistant enterococcal bacteremia, abstr K-2116, p379. Abstr. 45th Intersci. Conf. Antimcrob. Agents Chemother Washington, DC [Google Scholar]
- 42.Lu CL, Chuang YC, Chang HC, Chen YC, Wang JT, Chang SC. 2012. Microbiological and clinical characteristics of vancomycin-resistant Enterococcus faecium bacteraemia in Taiwan: implication of sequence type for prognosis. J. Antimicrob. Chemother. 67:2243–2249 [DOI] [PubMed] [Google Scholar]
- 43.Andes DR, Safdar N, Baddley JW, Playford G, Reboli AC, Rex JH, Sobel JD, Pappas PG, Kullberg BJ, Mycoses Study Group 2012. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin. Infect. Dis. 54:1110–1122 [DOI] [PubMed] [Google Scholar]
- 44.Sakoulas G, Bayer AS, Pogliano J, Tsuji BT, Yang SJ, Mishra NN, Nizet V, Yeaman MR, Moise PA. 2012. Ampicillin enhances daptomycin- and cationic host defense peptide-mediated killing of ampicillin- and vancomycin-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 56:838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moise PA, Lamp KC, DePestel DD, Yoon MJ, Zervos MJ. 2012. Daptomycin (D) with or without concomitant beta-lactams (BL) for vancomycin-resistant enterococcus (VRE) bacteremia, abstr K-276. Abstr. 52nd Intersci. Conf. Antimcrob. Agents Chemother San Francisco, CA [Google Scholar]
- 46.Talbot GH, Powers JH, Fleming TR, Siuciak JA, Bradley J, Boucher H. 2012. Progress on developing endpoints for registrational clinical trials of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections: update from the Biomarkers Consortium of the Foundation for the National Institutes of Health. Clin. Infect. Dis. 55:1114–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]