Abstract
The emergence of resistance against current antibiotics calls for the development of new compounds to treat infectious diseases. Synthetic pantothenamides are pantothenate analogs that possess broad-spectrum antibacterial activity in vitro in minimal media. Pantothenamides were shown to be substrates of the bacterial coenzyme A (CoA) biosynthetic pathway, causing cellular CoA depletion and interference with fatty acid synthesis. In spite of their potential use and selectivity for bacterial metabolic routes, these compounds have never made it to the clinic. In the present study, we show that pantothenamides are not active as antibiotics in the presence of serum, and we found that they were hydrolyzed by ubiquitous pantetheinases of the vanin family. To address this further, we synthesized a series of pantetheinase inhibitors based on a pantothenate scaffold that inhibited serum pantetheinase activity in the nanomolar range. Mass spectrometric analysis showed that addition of these pantetheinase inhibitors prevented hydrolysis of pantothenamides by serum. We found that combinations of these novel pantetheinase inhibitors and prototypic pantothenamides like N5-Pan and N7-Pan exerted antimicrobial activity in vitro, particularly against Gram-positive bacteria (Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, and Streptococcus pyogenes) even in the presence of serum. These results indicate that pantothenamides, when protected against degradation by host pantetheinases, are potentially useful antimicrobial agents.
INTRODUCTION
There is a continuous battle between humans and microorganisms, and the development of resistance against current antibiotics creates a need to develop new drug scaffolds and targets (1). There are several classes of antibiotic-resistant pathogens that are emerging as major threats. Methicillin-resistant and vancomycin-resistant Staphylococcus aureus strains (MRSA and VRSA) and multidrug-resistant Streptococcus pneumoniae are serious health problems in both hospital settings and the community. Multidrug-resistant Gram-negative bacteria (e.g., Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa) are a second group of organisms that pose a threat of untreatable infections. Finally, poverty-related conditions such as tuberculosis and parasitic diseases, which are particularly important in developing countries, are a cause of great concern, as the already-limited number of treatment modalities is decreasing because of resistance. The development of new antibiotics has been slow, and most compounds to date have been derived from a limited number of molecular scaffolds targeting a few microbial molecular pathways. Discovery of new scaffolds and new targets should clearly be a priority, although the commercial prospects of antibiotic development are unfavorable (1).
In 1970, amides derived from pantothenic acid (vitamin B5), were reported to possess antibiotic activity in vitro (2). During the last few decades, many of these pantothenamides have been synthesized and the putative modes of action have been studied in detail (3–10). Pantothenamides, of which N-pentylpantothenamide (N5-Pan) and N-heptylpantothenamide (N7-Pan) are the prototypes, are active against Gram-negative (2) and Gram-positive (11) bacteria, but they have also been shown to possess activity against fungi and malaria parasites (2, 8). Pantothenamides were found to be substrates of the key rate-controlling enzyme pantothenate kinase (CoaA), and the rate of conversion of N5-Pan was found to be more rapid than that of pantothenate itself. This led to the conclusion that the mechanism of toxicity toward E. coli is due to the formation of CoA analogs that lead to the transfer of an inactive 4′-phosphopantothenamide moiety to acyl carrier protein (ACP), which is the first step in the bacterial type II route (FASII) of fatty acid synthesis (10, 12). A recent study showed that N5-Pan causes a 10-fold reduction of cellular CoA levels in E. coli (7). The same study also showed that ethyldethia-ACP is readily hydrolyzed by ACP-phosphodiesterase (AcpH). Neither overexpression of CoA biosynthetic enzymes nor pantothenate supplementation could relieve growth inhibition by N5-Pan. These findings indicate that the mechanisms by which pantothenamides inhibit bacterial viability are not yet completely resolved.
Mammalian pantetheinases have only recently been discovered. The first vanin gene to be cloned was the mouse vanin-1 (Vnn1) gene, which encodes a GPI-anchored leukocyte cell surface protein of unknown function (13). Analysis of human vanins revealed three gene family members named VNN1, -2, and -3, clustered on chromosome 6 (14). The encoded proteins were shown to possess pantetheinase activity by the hydrolysis of pantetheine into pantothenic acid (vitamin B5) and the low-molecular-weight thiol cysteamine (15). We found vanin family members to be broadly expressed in nearly all human tissues (16). On the basis of sequence similarities, vanins were classified as members of the biotinidase branch of the nitrilase superfamily (17). The most obvious function of biotinidases and pantetheinases is that of amidases involved in vitamin recycling.
In the present paper, we address the possible application of pantothenamides as antibiotics, considering the current knowledge on human pantetheinases. Based on the fact that vanins are active on the carbon-nitrogen bond of pantetheine, we hypothesized that antibiotic pantothenamides could also be hydrolyzed by pantetheinase activity, which was indeed found to be the case. We further show that inhibition of pantetheinase activity by novel chemotypes that we recently developed (18) protects pantothenamides against hydrolysis and preserves their antibiotic activity. We hereby provide proof of concept that combination of pantetheinase inhibitors with pantothenamides might be a novel antimicrobial strategy.
MATERIALS AND METHODS
Bacterial growth assays.
Strains of Escherichia coli, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus agalactiae, Streptococcus pyogenes, Klebsiella pneumoniae and Pseudomonas aeruginosa were grown overnight in 1% Bacto tryptone medium (BD, Sparks, MD). Cultures were then diluted 1:1,000 in fresh assay medium supplemented with or without 20% fetal bovine serum (FBS; HyClone, Celbio, Logan, UT) or 20% decomplemented human serum (Sanquin Blood Bank, The Netherlands) and added to 96-well sterile enzyme-linked immunosorbent assay (ELISA) plates (100 μl per well). Moraxella catarrhalis was grown on blood agar plates, and colonies were swabbed into in 1% Bacto tryptone medium and diluted to an optical density at 600 nm (OD600) of 0.10. Subsequently, 100 μl was added to 96-well sterile ELISA plates. The compounds to be tested were diluted in the medium without serum and added to the bacteria (100 μl per well). Plates were incubated at 37°C, and bacterial growth was monitored over time by reading the optical density at 490 nm using a microplate reader (model 450; Bio-Rad Laboratories Inc., Hercules, CA). Streptococcus pneumoniae strains were grown to mid-log phase (OD620 = 0.3) and stored in 15% glycerol at −80°C till further use. Bacteria were diluted 10-fold in Mueller-Hinton medium (BD, Sparks, MD) and added to 96-well sterile ELISA plates (150 μl per well). The compounds to be tested were diluted in the medium without serum and added to the bacteria (150 μl per well). Plates were incubated overnight at 37°C in 5% CO2. The MIC was defined as the lowest concentration of pantothenamide at which no growth was visible.
Synthesis of pantothenamides and pantetheinase inhibitors.
All reactions were performed under an argon atmosphere, unless stated otherwise. Solvents were distilled from appropriate drying agents prior to use. Triethylamine was distilled and stored over KOH. Reactions were followed, Rf values were obtained using thin-layer chromatography (TLC) on silica gel-coated plates (Merck 60 F254) with the indicated eluent, and compounds were detected with UV light and/or by charring at ca. 150°C after dipping into a solution of potassium permanganate or ninhydrin. Column or flash chromatography was carried out using Acros silica gel (0.035 to 0.070 mm; pore diameter, ca. 6 mm). IR spectra were recorded on an ATI Mattson Genesis series Fourier transform infrared (FTIR) spectrometer. High-resolution mass spectra were recorded on a JEOL AccuTOF (electrospray ionization [ESI]) or a MAT900 (electric ionization [EI], chemical ionization [CI], and ESI) spectrometer. Melting points were analyzed with a Büchi B-545 melting point apparatus and are not corrected. Nuclear magnetic resonance (NMR) spectra were recorded at 298 K on a Bruker DMX 300 spectrometer (300 MHz) and a Varian 400 (400 MHz) spectrometer in the solvent indicated in front of the NMR data. Chemical shifts are given in parts per million (ppm) with respect to tetramethylsilane (0.00 ppm) or CHD2OD (3.31 ppm) as the internal standard for 1H-NMR and CDCl3 (77.16 ppm) or CD3OD (49.00 ppm) as the internal standard for 13C-NMR. Coupling constants are reported as J values in hertz (Hz). See the supplemental materials and methods for details on the synthesis of pantothenamides. Details on the synthesis of pantetheinase inhibitors were described by Jansen et al. (18).
Pantheteinase activity.
Pantetheinase activity was measured by the amount of free 7-amino-4-methylcoumarin (AMC) released by the hydrolysis of the pantetheine analogue pantothenate AMC (19). Pantothenate AMC (final concentration, 10 μM) was incubated in phosphate buffer (100 mM potassium phosphate buffer pH 8.0) in the presence of serum (1%) as the pantetheinase source with or without a potential pantetheinase inhibitor. Over time, samples were taken, and the reaction was terminated by addition of 180 μl 100 mM CaCO3 (pH 10.5). Fluorescence was measured using a luminescence spectrometer (LS55; excitation wavelength, 350 ± 2.5 nm; emission wavelength, 450 ± 2.5 nm; PerkinElmer) against samples without serum as negative controls.
For mass-spectrometric analysis, 64 μg/ml N7-Pan was incubated for 24 h at room temperature with 1% fetal bovine serum diluted in phosphate buffer (500 μM potassium phosphate buffer, pH 8.0) with and without the addition of 256 μg/ml RR6.
Mass spectrometry analysis.
Mass spectrometry was performed on a JEOL JMS-T100CS spectrometer (AccuTOF CS) connected to a Agilent 1100 series high-performance liquid chromatography (HPLC) system. Analysis was performed in infusion mode; 3 μl of sample (1% sample in methanol; HPLC grade; Fisher Scientific) was injected into a stream of methanol (HPLC grade; Fisher Scientific) containing 0.1% formic acid (puriss. p.a. for mass spectrometry; Fluka) and 50 nM PPG425 (polypropylene glycol; average molecular weight, 425; Sigma-Aldrich Chemie GmbH) for use as an internal mass drift compensator. Total analysis time with a flow rate of 100 μl/min was 2.5 min per sample. Sample information elutes between 0.3 and 1.0 min. Data between 0 and 0.3 min were used to mass drift compensate the calibration against PPG425 peaks, resulting in a mass precision better than 2 ppm.
In vitro toxicity.
Human embryonic kidney 293T cells were grown in Dulbecco's modified Eagle medium (DMEM-Glutamax; Gibco Invitrogen) containing 10% FBS (HyClone, Celbio, Logan, UT), 100 μg/ml penicillin-streptomycin, and 1% pyruvate (Gibco Invitrogen) at 37°C and 5% CO2. Pantothenamide or pantetheinase inhibitors were added to these cultures for 48 h, and the effects on growth and toxicity were determined microscopically. Cytotoxicity was detected using the LDH cytotoxicity detection kit according to the manufacturer's protocol (Roche Applied Science, Indianapolis, IN). A rate of cell death of 100% was determined after addition of 1% Triton X-100 to the cells.
Statistics.
Data were analyzed using GraphPad Prism version 5. Data are expressed as means ± standard deviations.
RESULTS
Pantothenamides are inactive as antibiotics in serum-containing medium.
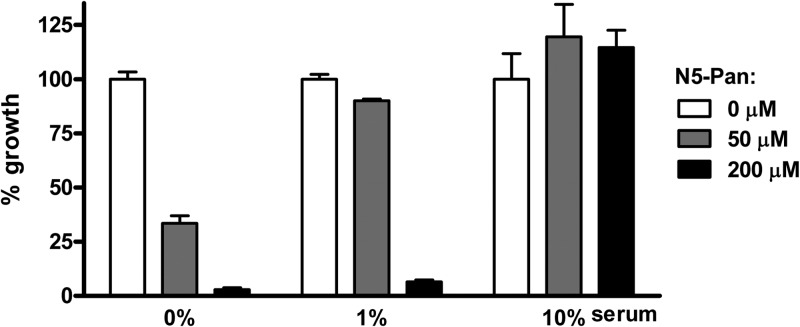
To test whether pantothenamides have antibiotic activity under physiological conditions, E. coli was cultured in minimal medium in the presence of complement-inactivated serum (in this case, fetal bovine serum, but human serum gives similar results). Addition of the prototypic pantothenamide N5-Pan to log-phase cultures of E. coli in the absence of serum shows the expected growth-inhibiting properties as described before (2) (Fig. 1). Addition of 1% and 10% serum dose-dependently abolishes the inhibitory effect of N5-Pan.
Fig 1.
Effect of serum on pantothenamides. The growth-inhibiting effect of N5-Pan on E. coli in media containing increasing amounts of serum was assessed. In serum-free medium, a low concentration of N5-Pan is sufficient to inhibit bacterial growth, while in a 1% serum medium, a high concentration N5-Pan is needed for bacterial growth inhibition. In 10% serum, a high concentration of N5-Pan is insufficient to act as an antibiotic. n = 4 for all samples.
We next investigated if the effect of serum on pantothenamides was due to a heat-labile factor. Figure 2A shows that heat-inactivated serum (5 min at 100°C) is unable to interfere with N5-Pan-mediated growth inhibition of E. coli. This made it very likely that the effect was due to one or more proteins present in serum.
Fig 2.
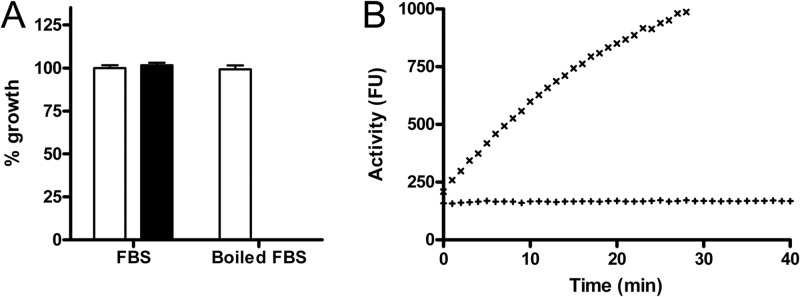
Inactivation of N5-Pan by serum is due to a heat-labile factor. (A) N5-Pan (64 μg/ml) (filled bars) does not inhibit growth of E. coli in the presence of FBS. Upon heat inactivation of serum (5 min at 100°C), N5-Pan effectively inhibits bacterial growth. Open bars, bacterial growth without N5-Pan. n = 4 for all samples. (B) A pantetheinase activity assay showed pantetheinase activity in serum (×) but none in inactivated serum (+), confirming that pantetheinases might be involved in pantothenamide inactivation.
Pantothenamides are hydrolyzed in serum.
Based on structural homology of pantothenamides with pantetheine and the knowledge that pantetheine is hydrolyzed by pantetheinases of the vanin family into pantothenic acid and cysteamine, we hypothesized that this class of enzymes could also hydrolyze pantothenamides. In a previous study, we showed that vanins are ubiquitously expressed (16). Using an aminomethylcoumarine derivative of pantothenic acid, which is a pantothenamide with a fluorescent leaving group as a substrate (19), pantetheinase activity in serum is readily detected. We found that this hydrolytic activity was completely abolished by heat inactivation of the serum (Fig. 2B).
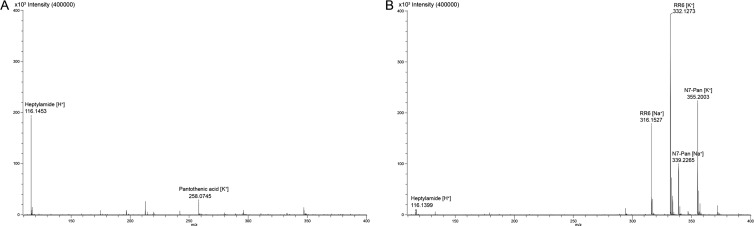
Subsequently, we investigated whether known antibiotic pantothenamides are also hydrolyzed in the presence of serum. Mass-spectrometric analysis of N7-Pan incubated in 1% serum for 24 h revealed a complete disappearance of the N7-Pan peak, whereas a clear peak of heptylamide, the expected hydrolysis product, appears (Fig. 3A). This indicates that pantothenamides are indeed hydrolyzed in the presence of serum.
Fig 3.
Hydrolysis of N7-Pan is inhibited RR6. (A) Mass spectrometric analysis of a sample containing 1% serum and N7-Pan after 24 h incubation showed a heptylamine peak but no N7-Pan-peak. Note that the produced pantothenic acid is only partially visualized with the settings used. (B) Pantetheinase inhibitor RR6 is able to protect N7-Pan against hydrolysis, as clear peaks corresponding to the N7-Pan adducts are shown on the mass spectrum. Also, the RR6 adducts and a very small peak corresponding to heptylamine were detected. The reaction was performed as described for panel A.
Pantetheinase inhibitors prevent degradation of antibiotic pantothenamides in serum.
We reasoned that inhibitors of serum pantetheinases could prevent degradation of pantothenamides in biological fluids, thereby preserving their antibiotic activity. As there were no selective high-affinity (subnanomolar) pantetheinase inhibitors available, we designed and synthesized a number of new inhibitors starting from pantetheine, the natural substrate of pantetheinases, as a scaffold. A description of the synthesis and characterization of these new chemotypes, pantetheine analogs lacking the hydrolysable amide bond, has recently been published (18). The most potent and selective inhibitor (RR6) showed a 50% inhibitory concentration (IC50) of 40 nM against pantetheinase activity present in human or bovine serum. See Table S1 in the supplemental material for the structures and IC50s of all compounds used here.
We next investigated if these pantetheinase inhibitors could protect pantothenamide antibiotics from hydrolysis by serum pantetheinase activity. Figure 3B shows the results of mass spectrometric analysis of N7-Pan incubated in 1% serum for 24 h in the presence of 256 μg/ml RR6. Whereas in Fig. 3A, a complete disappearance of the N7-Pan peaks is observed and only the hydrolysis product heptylamine can be identified, Fig. 3B shows that in the presence of RR6, the N7-Pan peaks are still present. Although we found a minor peak corresponding to heptylamide, we conclude that N7-Pan is clearly protected against hydrolysis by the addition of a pantetheinase inhibitor.
In vitro antibiotic effects of combinations of pantothenamides and pantetheinase inhibitors.
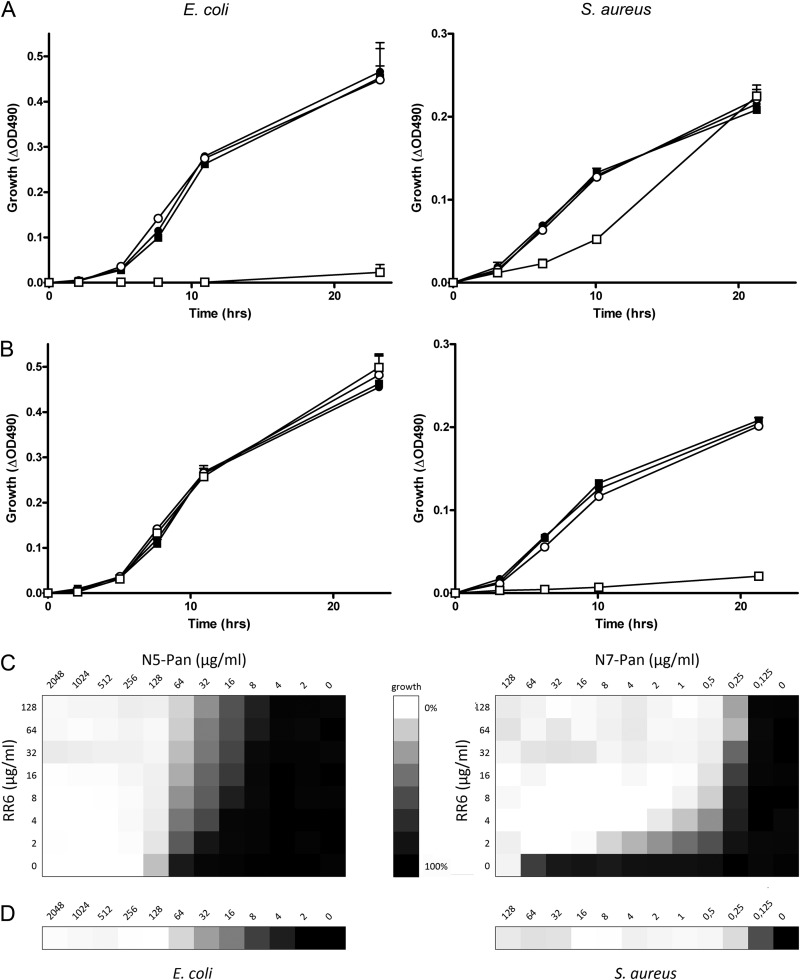
We next examined if inhibition of serum pantetheinase could preserve the antibiotic effect of the pantothenamides N5-Pan and N7-Pan, which were previously reported to be active against strains of E. coli and S. aureus, respectively. Figure 4 shows the effects of pantothenamides or a pantetheinase inhibitor (RR6) alone and combinations of these. The addition of pantothenamides had no antibacterial effect in medium with 10% FBS, as shown before for N5-Pan (Fig. 1). Similarly, the pantetheinase inhibitor RR6 on its own did not affect growth of E. coli or S. aureus. Combination of RR6 with pantothenamides, however, showed a clear antimicrobial effect for N5-Pan on E. coli (Fig. 4A) and for N7-Pan on S. aureus (Fig. 4B). We performed checkerboard titration of pantothenamides and the pantetheinase inhibitor RR6 to obtain a range of MICs for the combined compounds, as shown in Fig. 4C. The protective effect of RR6 toward pantothenamides is clearly visible for both E. coli and S. aureus. The amount of pantothenamides decreased to MIC values in cultures without serum and RR6 (Fig. 4D).
Fig 4.
Pantetheinase inhibition preserves the antibiotic effect of pantothenamides. (A) Growth curves of E. coli and S. aureus, respectively, in 1% tryptone with 10% FBS alone (■) or supplemented with 64 μg/ml N5-Pan (●), 256 μg/ml RR6 (○), or a combination of N5-Pan and RR6 (□). Only the combination of RR6 and N5-Pan showed long-term growth inhibition of E. coli. n = 3 for all samples. (B) The conditions described for panel A were used, but N7-Pan instead of N5-Pan was added. Only the combination of RR6 and N7-Pan resulted in S. aureus growth inhibition. n = 3 for all samples. (C) Growth of E. coli and S. aureus in 1% tryptone with 10% decomplemented human serum in the presence of different concentrations of RR6 and N5-Pan (E. coli) and N7-Pan (S. aureus). The checkerboard titration shows that addition of RR6 increased the potency of pantothenamides in serum-containing media to concentrations comparable to cultures without serum, as shown in panel D. n = 2. (D) Growth of E. coli and S. aureus in 1% tryptone without serum in the presence of different concentrations of N5-Pan and N7-Pan, respectively. n = 2.
Specificity and toxicity of pantothenamide antibiotics and vanin inhibitors.
The efficacy of pantothenamides has so far been studied only in vitro on a limited number of bacterial species (2, 11). To delineate the taxa that are potential targets for antibiotic therapy, we determined the MIC values for a number of clinically relevant species. Table 1 shows that only Gram-positive bacteria are sensitive to pantothenamides at pharmaceutically realistic concentrations (8 μg/ml). Sensitive species include S. aureus, S. pneumoniae, S. pyogenes, and S. epidermidis. Within one genus (Streptococcus), there is differential sensitivity, as S. agalactiae is resistant, in contrast to S. pyogenes and S. pneumoniae. We tested several strains of selected species for confirmation (see Table S2 in the supplemental material).
Table 1.
MICs of the most potent pantothenamides against various bacterial species
| Organism | Most effective pantothenamide | MIC (μg/ml) |
|---|---|---|
| S. aureus ATCC 6538 | N7-Pan | 0.5 |
| S. pneumoniae R6 | N5-Pan | 0.25 |
| S. epidermidis ATCC 12228 | N7-Pan | 1 |
| S. agalactiae RIVM 861352 | None | >256 |
| S. pyogenes SS91 | N5-Pan | 0.5 |
| E. coli ATCC 25922 | N5-Pan | 64 |
| K. pneumoniae ATCC 43816 | N5-Pan | 32 |
| M. catarrhalis RH4 | N7-Pan | 128 |
| P. aeruginosa Xen41 | None | >256 |
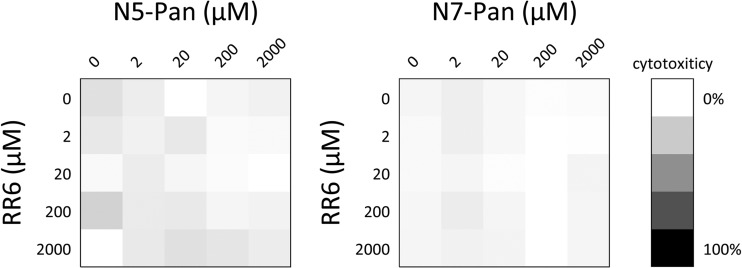
As the putative mode of action of pantothenamides is at the level of CoA-dependent biochemical pathways, this raised the question of selectivity for microbial versus host targets. We therefore investigated if pantothenamides and pantetheinase inhibitors were tolerated by mammalian cells. Using concentrations of N5-Pan, N7-Pan, and RR6 up to 2,000 μM, no effects on cell growth or viability were observed in a human cell line (293T kidney cells) (Fig. 5). Similar results were found on primary human keratinocytes (data not shown).
Fig 5.
In vitro toxicity of compounds. Cytotoxicity was measured on human embryonic kidney 293T cells treated with or without N5-Pan, N7-Pan, or RR6. None of the tested compounds or combination resulted in increased cell death up to 2,000 μM. Triton X-100 (1%) was added to cells to achieve 100% cytotoxicity.
DISCUSSION
Here we provide detailed experimental evidence for the concept that pantetheinase inhibitors can prevent the enzymatic degradation of pantothenamide antibiotics. Combination of a pantetheinase inhibitor with pantothenamides could be a successful strategy to preserve the antibiotic properties of pantothenamides in the presence of host pantetheinases. We propose that our data provide proof of concept for a novel antibiotic strategy that could be used against clinically relevant Gram-positive bacteria.
Since pantothenic acid was first described as a nutritional requirement for most if not all organisms, its potential for antibiotic drug design has been investigated. The different mechanisms by which organisms were able to obtain pantothenic acid for CoA biosynthesis and the relatively low homology between enzymes involved in pantothenic acid processing opened the field to design growth-inhibiting molecules specific for pathogens. Based on pantothenic acid, different classes of molecules were described to possess anti-bacterial, anti-fungal, and anti-parasitic activities in vitro, including N-pantoyl-substituted amines, pantoylhydrazides, pantothenones, and pantothenamides (20). Most of these analogues were expected to compete with endogenous pantothenic acid for the first enzyme of the CoA biosynthesis, CoaA. In contrast, the working mechanism of pantothenamides is expected to be different. Strauss and Begley (10) showed that N-pentylpantothenamide is a substrate for CoA rather than an inhibitor. They showed that the pantothenamide is completely processed to a CoA analogue called ethyldethia-CoA and that the conversion is over 10 times faster than pantothenic acid processing. Subsequently, Zhang et al. showed that acyl carrier protein (ACP) is the cellular target, which results in an inactive ACP analogue that lacks the sulfhydryl group for the attachment of acyl chains for fatty acid synthesis (3). Mercer et al. have shown that pantothenamide azido-pantetheine could be processed to ACP via FAS type II in bacteria, while it cannot be processed via FAS type I in the human cell line SKBR3 (21). Apparently, CoA analogues are recognized only by type II fatty acid synthase, which is found in most bacteria, while a FAS type I system is present in mammals and fungi. Because the structural arrangements of fungal and mammalian synthases differ, selective pantothenamides could theoretically also target fungal FAS type I. Assuming that pantothenamides target the bacterial FASII pathway, administration of exogenous fatty acids could potentially overcome the antibiotic effects of pantothenamides. Work by Zhang et al. showed that this is not the case for E. coli. The MIC for S. pneumoniae, however, was increased 4-fold, suggesting that pantothenamides target FASII but may also have other effects (3). A recent paper by Parsons and Rock elucidated the basis of differential susceptibility of Gram-positive bacteria to fatty acid synthesis inhibitors like cerulinin and platensimycin (22). From this work it was concluded that fatty acids cannot overcome FASII inhibition in S. aureus, in contrast to S. pneumoniae. Whether this also holds for pantothenamides remains to be investigated, as they are likely to inhibit other pathways in addition to FASII. This is supported by recent findings indicating that, at least in E. coli, both holo-ACP and ethyldethia-ACP can be hydrolyzed to Apo-ACP by ACP phosphodiesterase (AcpH) (7). Those data suggest that the antibacterial effect of N5-Pan is due to cellular depletion of CoA (7).
The first step in CoA biosynthesis is the phosphorylation of pantothenic acid by pantothenate kinase (PanK), the key regulatory enzyme that is subject to self-regulation through feedback inhibition by CoA. Structures of PanK enzymes among different species have been investigated and showed differences in binding capacity for pantothenic acid (23, 24). This might explain why N5-Pan is more active against E. coli and N7-Pan has specificity for S. aureus. Binding features of human PanK family members with pantothenic acid have not been investigated. Although structures of human PanK1α and PanK3 in complex with acetyl-CoA have been analyzed, the structure of human PanK isoforms in complex with pantothenic acid would further facilitate the knowledge of binding properties of PanK and thus would provide more insight into possible modifications of pantothenic acid to aid medicinal chemistry efforts.
We have no data on whether our pantetheinase inhibitors are PanK substrates. We have shown that pantetheinase inhibitors by themselves are not antibiotic, which suggests that they are not a substrate for the CoA biosynthetic machinery. This may be caused by the loss of the amide bond, which is likely to be a structural requirement for entering the first step of CoA synthesis leading to CoA antimetabolites, as recently suggested in a study by Mercer et al. (5).
A recent publication identified pantetheinase inhibitors derived from various chemical scaffolds in the LOPAC library, via a high-throughput approach (19). These compounds are all nonselective and have modest potency. As they are known to target various mammalian cellular pathways at concentrations that are lower than required for vanin inhibition, it is unlikely that these compounds are useful in pharmacology or biological chemistry approaches (25, 26).
The antibiotic strategy described here includes a combination of an enzyme inhibitor and a molecule that interferes with bacterial growth. Although a dual-compound formulation is not a preferred pharmaceutical approach, it is not unprecedented, as witnessed by the use of beta-lactamase inhibitors (combined with penicillin) and a dehydropeptidase inhibitor (combined with imipenem) (27). Here, we provide detailed experimental data for a concept that we previously put forward (28, 29). Recently, indirect evidence supporting this concept was found in the case of Plasmodium falciparum growth inhibition by pantothenamides (30).
Obviously, a number of steps need to be taken before combination therapy of pantothenamide antibiotics and vanin inhibitors can enter drug development. As witnessed by Fig. 4C, the potency of our current vanin inhibitors needs to be improved to achieve complete protection of pantothenamides in serum and to maintain the required MIC values (31) (e.g., around 1 μg/ml for S. aureus). We found a good bioavailability and pharmacodynamic profile of the vanin inhibitor RR6 (18), which is encouraging for further exploration of this possibility. Clearly, an increase of the potency of current pantothenamides (N5-Pan and N7-Pan) is required. If this can be achieved, their application and sensitivity may even extend beyond the Gram-positive species that we have identified here.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a Preseed grant of The Netherlands Genomics Initiative, grant 93611013.
We thank Bas Ritzen and René Aben for technical assistance and Roberta Leonardi for critical reading of the manuscript.
P.J., J.S., P.Z., P.H. and F.R. are inventors on the cited patents filed by the Radboud University Nijmegen Medical Centre.
Footnotes
Published ahead of print 22 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00603-13.
REFERENCES
- 1.Fischbach MA, Walsh CT. 2009. Antibiotics for emerging pathogens. Science 325:1089–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clifton G, Bryant SR, Skinner CG. 1970. N′-(substituted) pantothenamides, antimetabolites of pantothenic acid. Arch. Biochem. Biophys. 137:523–528 [DOI] [PubMed] [Google Scholar]
- 3.Zhang YM, Frank MW, Virga KG, Lee RE, Rock CO, Jackowski S. 2004. Acyl carrier protein is a cellular target for the antibacterial action of the pantothenamide class of pantothenate antimetabolites. J. Biol. Chem. 279:50969–50975 [DOI] [PubMed] [Google Scholar]
- 4.Virga KG, Zhang YM, Leonardi R, Ivey RA, Hevener K, Park HW, Jackowski S, Rock CO, Lee RE. 2006. Structure-activity relationships and enzyme inhibition of pantothenamide-type pantothenate kinase inhibitors. Bioorg. Med. Chem. 14:1007–1020 [DOI] [PubMed] [Google Scholar]
- 5.Mercer AC, Meier JL, Hur GH, Smith AR, Burkart MD. 2008. Antibiotic evaluation and in vivo analysis of alkynyl coenzyme A antimetabolites in Escherichia coli. Bioorg. Med. Chem. Lett. 18:5991–5994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choudhry AE, Mandichak TL, Broskey JP, Egolf RW, Kinsland C, Begley TP, Seefeld MA, Ku TW, Brown JR, Zalacain M, Ratnam K. 2003. Inhibitors of pantothenate kinase: novel antibiotics for staphylococcal infections. Antimicrob. Agents Chemother. 47:2051–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas J, Cronan JE. 2010. Antibacterial activity of N-pentylpantothenamide is due to inhibition of coenzyme a synthesis. Antimicrob. Agents Chemother. 54:1374–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spry C, Chai CL, Kirk K, Saliba KJ. 2005. A class of pantothenic acid analogs inhibits Plasmodium falciparum pantothenate kinase and represses the proliferation of malaria parasites. Antimicrob. Agents Chemother. 49:4649–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akinnusi TO, Vong K, Auclair K. 2011. Geminal dialkyl derivatives of N-substituted pantothenamides: synthesis and antibacterial activity. Bioorg. Med. Chem. 19:2696–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strauss E, Begley TP. 2002. The antibiotic activity of N-pentylpantothenamide results from its conversion to ethyldethia-coenzyme a, a coenzyme a antimetabolite. J. Biol. Chem. 277:48205–48209 [DOI] [PubMed] [Google Scholar]
- 11.Leonardi R, Chohnan S, Zhang YM, Virga KG, Lee RE, Rock CO, Jackowski S. 2005. A pantothenate kinase from Staphylococcus aureus refractory to feedback regulation by coenzyme A. J. Biol. Chem. 280:3314–3322 [DOI] [PubMed] [Google Scholar]
- 12.Zhang YM, White SW, Rock CO. 2006. Inhibiting bacterial fatty acid synthesis. J. Biol. Chem. 281:17541–17544 [DOI] [PubMed] [Google Scholar]
- 13.Aurrand-Lions M, Galland F, Bazin H, Zakharyev VM, Imhof BA, Naquet P. 1996. Vanin-1, a novel GPI-linked perivascular molecule involved in thymus homing. Immunity 5:391–405 [DOI] [PubMed] [Google Scholar]
- 14.Martin F, Malergue F, Pitari G, Philippe JM, Philips S, Chabret C, Granjeaud S, Mattei MG, Mungall AJ, Naquet P, Galland F. 2001. Vanin genes are clustered (human 6q22-24 and mouse 10A2B1) and encode isoforms of pantetheinase ectoenzymes. Immunogenetics 53:296–306 [DOI] [PubMed] [Google Scholar]
- 15.Maras B, Barra D, Dupre S, Pitari G. 1999. Is pantetheinase the actual identity of mouse and human vanin-1 proteins? FEBS Lett. 461:149–152 [DOI] [PubMed] [Google Scholar]
- 16.Jansen PA, Kamsteeg M, Rodijk-Olthuis D, van Vlijmen-Willems IM, de Jongh GJ, Bergers M, Tjabringa GS, Zeeuwen PL, Schalkwijk J. 2009. Expression of the vanin gene family in normal and inflamed human skin: induction by proinflammatory cytokines. J. Investig. Dermatol. 129:2167–2174 [DOI] [PubMed] [Google Scholar]
- 17.Brenner C. 2002. Catalysis in the nitrilase superfamily. Curr. Opin. Struct. Biol. 12:775–782 [DOI] [PubMed] [Google Scholar]
- 18.Jansen PA, van Diepen JA, Ritzen B, Zeeuwen PL, Cacciatore I, Cornacchia C, van Vlijmen-Willems IM, de Heuvel E, Botman PN, Blaauw RH, Hermkens PH, Rutjes FP, Schalkwijk J. 2013. Discovery of small molecule vanin inhibitors: new tools to study metabolism and disease. ACS Chem. Biol. 8:530–534 [DOI] [PubMed] [Google Scholar]
- 19.Ruan BH, Cole DC, Wu P, Quazi A, Page K, Wright JF, Huang N, Stock JR, Nocka K, Aulabaugh A, Krykbaev R, Fitz LJ, Wolfman NM, Fleming ML. 2010. A fluorescent assay suitable for inhibitor screening and vanin tissue quantification. Anal. Biochem. 399:284–292 [DOI] [PubMed] [Google Scholar]
- 20.Spry C, Kirk K, Saliba KJ. 2008. Coenzyme A biosynthesis: an antimicrobial drug target. FEMS Microbiol. Rev. 32:56–106 [DOI] [PubMed] [Google Scholar]
- 21.Mercer AC, Meier JL, Torpey JW, Burkart MD. 2009. In vivo modification of native carrier protein domains. Chembiochem 10:1091–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsons JB, Rock CO. 2011. Is bacterial fatty acid synthesis a valid target for antibacterial drug discovery? Curr. Opin. Microbiol. 14:544–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang K, Eyobo Y, Brand LA, Martynowski D, Tomchick D, Strauss E, Zhang H. 2006. Crystal structure of a type III pantothenate kinase: insight into the mechanism of an essential coenzyme A biosynthetic enzyme universally distributed in bacteria. J. Bacteriol. 188:5532–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong BS, Yun MK, Zhang YM, Chohnan S, Rock CO, White SW, Jackowski S, Park HW, Leonardi R. 2006. Prokaryotic type II and type III pantothenate kinases: the same monomer fold creates dimers with distinct catalytic properties. Structure 14:1251–1261 [DOI] [PubMed] [Google Scholar]
- 25.Delvaux M, Bastie MJ, Chentoufi J, Cragoe EJ, Jr, Vaysse N, Ribet A. 1990. Amiloride and analogues inhibit Na+-H+ exchange and cell proliferation in AR42J pancreatic cell line. Am. J. Physiol. 259:G842–849 [DOI] [PubMed] [Google Scholar]
- 26.Lazo JS, Nemoto K, Pestell KE, Cooley K, Southwick EC, Mitchell DA, Furey W, Gussio R, Zaharevitz DW, Joo B, Wipf P. 2002. Identification of a potent and selective pharmacophore for Cdc25 dual specificity phosphatase inhibitors. Mol. Pharmacol. 61:720–728 [DOI] [PubMed] [Google Scholar]
- 27.Drawz SM, Bonomo RA. 2010. Three decades of beta-lactamase inhibitors. Clin. Microbiol. Rev. 23:160–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansen PA, Schalkwijk J, Rutjes FP, Sauerwein R, Hermkens PH. 2011. Derivatives of pantothenic acid and their use for the treatment of malaria. Patent application EP11725212, publication WO2011152721
- 29.Jansen PA, Zeeuwen PL, Schalkwijk J, Rutjes FP, Ritzen B, Hermkens PH. 2011. Pantothenic acid derivatives and their use in the treatment of microbial infections. Patent application EP11725211, publication WO2011152720
- 30.Spry C, Macuamule C, Lin Z, Virga KG, Lee RE, Strauss E, Saliba KJ. 2013. Pantothenamides are potent, on-target inhibitors of Plasmodium falciparum growth when serum pantetheinase is inactivated. PLoS One 8:e54974. 10.1371/journal.pone.0054974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. 2005. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J. Antimicrob. Chemother. 55:601–607 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.