TEXT
In May 2012, a number of us listened spellbound as Janet Woodcock of the FDA announced at a meeting at the Brookings Institution that the agency would “reboot” their entire approach to antibiotic development (J. Woodcock, presented at an Expert Workshop on Facilitating Antibacterial Drug Development, 9 May 2012). She recognized that there is a public health crisis of antibiotic resistance that continues to grow worse and that development of new antibacterial agents to deal with the threat is inadequate. Finally, she acknowledged that the approach taken by the Office of Antimicrobials at FDA to change clinical trial designs for antibacterial agents over the past decade had contributed to this crisis. Dr. Woodcock went on to emphasize the need for a new pathway for development focusing on patients with unmet medical needs—that is those with infections due to pathogens that are pan-drug resistant (PDR) or that are extremely drug resistant (XDR) (1). Further discussion showed that the agency also understands that development for traditional indications such as pneumonia also needs “rebooting.” Their thoughts on the reboot follow the recommendations of a working group from the pharmaceutical industry (2). How and why did we get here?
The first warning of the exodus of industry from antibacterial development was published as far back as 2002 (3). The change in industry interest in this area was due to a convergence of unattractive economics around antibacterial development (4–6) combined with a fundamental rethink regarding statistical principles of non-inferiority trial designs that began in the late 1990s (7–9). Although this rethink was not specific to antibacterial agents, it disproportionately affected antibacterial development (8). A dangerous inflection point occurred in 2006, after the public spectacle surrounding telithromycin, which was discovered to cause very rare but life-threatening hepatotoxicity only after the drug was approved (8–10). Ironically, this statistical rethink focusing entirely on proving efficacy (and doubting that antibiotics were effective) had been triggered by a safety problem with telithromycin (9).
In the aftermath of telithromycin, FDA rules governing trial conduct became increasingly stringent to the point of making antibacterial trials infeasible, nonsensical, or both. For example, changes to trial design meant to increase the scientific purity of the trials (such as excluding any patients who receive even 1 dose of prestudy antibiotic from enrollment in a clinical trial) made it virtually impossible to enroll patients into the trials in the United States. Worries that antibiotics were no more effective than placebo were finally put to rest by the FDA's own analysis of preantibiotic era data showing treatment effects consistently higher than 20% and frequently over 50% depending on the infection (11, 12). In spite of these large treatment effects, some of which would justify noninferiority margins greater than 20%, the FDA always “discounted” the treatment effect by a sufficient amount such that the resulting noninferiority margins were always 10% (lower margins = higher sample sizes, more patients, and greater costs) (13).
A new endpoint for skin infections was developed in which patients whose infections have not improved at all after 3 days of therapy are declared treatment successes merely because they stop getting worse (14, 15). This endpoint was convenient for statisticians because they could comfortably calculate a treatment effect of oral sulfonamide antibiotics versus placebo equivalent (UV lamp therapy) based on unverifiable data from two studies published in 1937 (in which the background therapy for skin infections consisted of a liquid diet and a mandatory hot-liquid paraffin soap-and-water enema) (16, 17). The endpoint used in these 80-year-old studies was cessation of spread of the skin lesion. Although this did provide a feasible way forward for companies desiring to develop antibiotics for skin infection, this endpoint, in our view, is invalid and has little clinical relevance (B. Spellberg, presented at an Expert Workshop on Facilitating Antibacterial Drug Development at the Brookings Institution, 9 May 2012 [http://www.brookings.edu/~/media/events/2012/5/09%20antibacterial%20drug%20development/panel%201%20brad%20spellberg%20presentation]).
Clearly, the FDA process of determining how antibacterial trials should be conducted has badly lost its way. (For a general overview on this opinion, see the Spellberg presentation cited above.) There have been three results. (i) Many companies do not invest in the trials. (ii) Those that do invest in trials enroll patients in countries where it is possible to withhold therapy while the patients are enrolled in trials, with resulting ethical concerns. (iii) The results become less meaningful and relevant to patients in the United States, because U.S. patients are not enrolled. Pharmaceutical companies have voted with their feet. Twenty years ago, more than 20 large companies had active discovery and development programs for antibacterial agents; in 2013, only four have active discovery programs (18). Our approval rate for new antibiotics has fallen to dismally low levels (Fig. 1).
Fig 1.
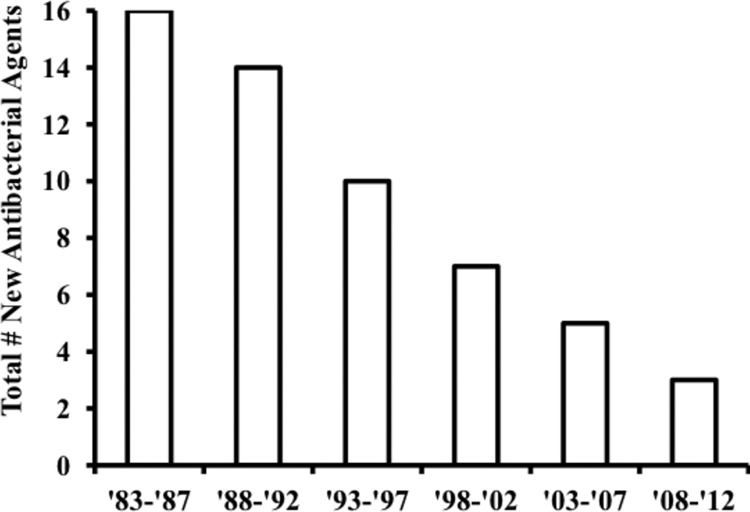
Antimicrobial agents approved by FDA. The number of new antibacterial agents is shown from 1983 to 2012.
The combination of the above has logically contributed to the hesitancy of companies to invest in the area and has led to a lack of both antibacterial drugs in the pipeline and to an absence of ongoing trials in indications where trial designs required by FDA are infeasible such as pneumonia. The FDA now recognizes that not having a robust antibiotic pipeline is a risk to patient health and safety, and this recognition has, in part, led to their decision to “reboot.”
At the same time, antibiotic resistance has continued its inexorable climb globally. The United States is no exception to rising resistance, as recent data published by the Centers for Disease Control and Prevention have dramatically shown (19, 20). This clearly represents a major risk to patient health and safety, and it is this medical need that drives the urgency of the FDA reboot.
We have carried out our own analysis of the situation in the United States (Table 1). The data are derived from hospitals distributed throughout the United States, and we believe that they provide a fair picture of the United States as a whole (see Table S1 in the supplemental material). In fact, this database was, in part, used by the CDC for their study (19).
Table 1.
Resistance among key Gram-negative pathogens in U.S. hospitals from 2009 to 2012
| Gram-negative pathogen | % Resistance (na) in nonurinary isolates |
% Resistance (n) in urinary isolates |
||||||
|---|---|---|---|---|---|---|---|---|
| ICU |
Non-ICU |
ICU |
Non-ICU |
|||||
| Ceftazidime-resistant | Imipenem-resistant | Ceftazidime-resistant | Imipenem-resistant | Ceftazidime-resistant | Imipenem-resistant | Ceftazidime-resistant | Imipenem-resistant | |
| Escherichia coli | 11.0 (3,084) | 0.3 (3,287) | 6.9 (43,445) | 0.1 (47,559) | 8.0 (10,258) | 0.1 (11,537) | 3.7 (744,532) | 0.0 (794,072) |
| Klebsiella pneumoniae | 26.8 (1,780) | 11.5 (1,907) | 14.5 (16,475) | 5.8 (17,228) | 19.3 (3,583) | 7.1 (3,834) | 8.0 (130,088) | 2.4 (131,464) |
| Acinetobacter baumannii | 60.1 (550) | 52.0 (535) | 35.4 (5,532) | 28.0 (4,37) | 71.7 (247) | 56.7 (230) | 37.1 (3,436) | 23.9 (2,758) |
| Pseudomonas aeruginosa | 18.6 (2,615) | 23.2 (2,869) | 7.3 (35,210) | 8.4 (35,810) | 13.8 (3,056) | 17.5 (3,285) | 5.8 (53,835) | 8.8 (52,758) |
n is the number of isolates tested.
As background for our view of the FDA and its implications for public health, it is worthwhile considering the rules governing new antibiotic development in terms of risk and benefit. The risk is that of a highly resistant infection. The benefit is that of having an approved, efficacious antibiotic prescribed early. Toward this end, we carried out an analysis of data from Eurofins' The Surveillance Network (TSN) surveillance of resistance in the United States. The methods for obtaining these data are provided in the supplemental material, as are a list of geographic locations of sites providing the data. We analyzed data for the years 2009 to 2012. Because there was little variation for these years among the strains and resistance monitored, we present average data here. In the report from CDC in 2008 (20), using isolates taken from intensive care units (ICUs), the resistance among Escherichia coli to third-generation cephalosporins was 5%, while in our analysis (using different methods), it stands at 8 to 11%. Klebsiella pneumoniae resistance to third-generation cephalosporins was 15% in the CDC study, while in our updated analysis, it ranges from 20 to 27%. Resistance to carbapenems among these isolates is now between 7 and 11%. For Acinetobacter baumannii, the resistance is even more drastic. In the CDC report, 11% were carbapenem resistant, while our data show that number to be over 50%.
These data indicate that for Acinetobacter baumannii infections, the carbapenems are already obsolete. This holds true for both intensive care and non-intensive care unit patients and for urinary and nonurinary infections. The same can be said for our third-generation cephalosporins (here indicated by ceftazidime) in the treatment of K. pneumoniae infections. For these organisms, the carbapenems are also rapidly losing efficacy. Even among E. coli isolates, our third-generation cephalosporins are no longer completely reliable, although the carbapenems remain a solid backup.
Our late-stage pipeline does hold out some hope for treating these infections, but none of the pipeline antibiotics by themselves can address all these resistance problems. We will therefore continue to confront serious infections caused by pathogens for which the treatment options are either limited or nonexistent (21).
With this picture in mind, we need to examine the role of our regulatory system in bringing needed new antibiotics to the patients and physicians who need them. As has been documented repeatedly by the Infectious Diseases Society of America and others, the approval rate of the FDA for new antibiotics is dismal and getting worse (Fig. 1).
It has now been over a year since the FDA's announcement of the “reboot” of their approach to antibacterial drugs. Just 2 days after the submission of this commentary, the FDA released their guidance on antibacterial therapies for patients with unmet medical needs (22). There are a number of positive aspects to this guidance, and much of it follows prior discussions that have occurred in the context of the Brookings Institution. To us, the two most promising aspects of the guidance are (i) the open attitude of the FDA to discussion of novel trial designs with sponsors and (ii) their willingness to consider externally or historically controlled studies. The latter could include pharmacometric approaches to establishing control levels of response as has been suggested previously (1). The FDA suggests that safety databases as small as 300 patients might be acceptable in the context of unmet needs. For other details, readers are referred to the guidance document itself (22).
It is also clear from discussions with the agency that their approach to the development of antibacterial agents in traditional indications such as pneumonia and urinary tract infection has been mixed. In a clear sign of progress, they now allow approval for two indications (e.g., complicated urinary tract infection [cUTI] and complicated intra-abdominal infection [cIAI]) following a single trial in each. However, the continued existence of now outdated guidance (e.g., community-acquired bacterial pneumonia [CABP], hospital-acquired bacterial pneumonia [HABP], and ventilator-associated bacterial pneumonia [VABP]) remains confusing to industry. The FDA has not indicated whether they will rescind their current guidance requiring what they now recognize are infeasible trial designs and, sometimes, irrelevant endpoints.
We must recognize that regulatory reform may not be enough to entice large pharmaceutical companies to restart or even continue their efforts in antibacterial research and development. For example, Astra-Zeneca has announced that they will “reduce” their investment in antibacterial research in favor of other therapeutic areas. While they recognize that progress is occurring at FDA, they remain concerned about the potential return on their investment in the antibacterial space. Without the participation of industry, especially that of the large companies, our pipeline will continue to lie fallow for years to come.
Antibiotic resistance is already at crisis levels in U.S. hospitals, especially in intensive care units. Our current late-stage pipeline will address some of these resistant pathogens, but not all. That said, the complete FDA reboot cannot come too soon. It is becoming clear, though, that even this will not be enough. Industry must also clearly see that there is a path for a return on their investment in antibiotics. This will probably require, at the very least, one company to bring one of the late-stage drugs with activity against resistant pathogens all the way to the marketplace such that pricing negotiations can occur. These negotiations will become as critical as the FDA reboot. We hope that value-based pricing and a rebooted FDA process will both come to pass. The alternative is too terrible to contemplate.
Supplementary Material
Footnotes
Published ahead of print 29 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01277-13.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.Spellberg B, Brass EP, Bradley JS, Lewis R, Shlaes D, Ambrose P, Das A, Boucher HW, Doi Y, Bartlett JG, Bonomo RA, Larosa SP, Talbot GH, Benjamin D, Guidos RJ, Jezek A, Gilbert DN, on behalf of the Infectious Diseases Society of America 2012. White paper: recommendations on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. Clin. Infect. Dis. 55:1031–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rex JH, Eisenstein BI, Alder J, Goldberger M, Meyer R, Dane A, Friedland I, Knirsch C, Sanhai WR, Tomayko J, Lancaster C, Jackson J. 2013. A comprehensive regulatory framework to address the unmet need for new antibacterial treatments. Lancet Infect. Dis. 13:269–275 [DOI] [PubMed] [Google Scholar]
- 3.Shlaes DM, Moellering RC., Jr 2002. The United States Food and Drug Administration and the end of antibiotics. Clin. Infect. Dis. 34:420–422 [DOI] [PubMed] [Google Scholar]
- 4.Projan SJ, Shlaes DM. 2004. Antibacterial drug discovery: is it all downhill from here? Clin. Microbiol. Infect. 10(Suppl 4):18–22 [DOI] [PubMed] [Google Scholar]
- 5.Sharma P, Towse A. 2011. New drugs to tackle antimicrobial resistance: analysis of EU policy options. Office of Health Economics, London, United Kingdom: http://www.ohe.org/publications/article/new-drugs-to-tackle-antimicrobial-resistance-5.cfm [Google Scholar]
- 6.Spellberg B, Sharma P, Rex JH. 2012. The critical impact of time discounting on economic incentives to overcome the antibiotic market failure. Nat. Rev. Drug Discov. 11:168. 10.1038/nrd3560-c1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Temple R, Ellenberg SS. 2000. Placebo-controlled trials and active-control trials in the evaluation of new treatments. Part 1: ethical and scientific issues. Ann. Intern. Med. 133:455–463 [DOI] [PubMed] [Google Scholar]
- 8.Spellberg B. 2010. The antibacterial pipeline: why is it drying up and what must be done about it?, p 299–332 In Choffnes ER, Relman DA, Mack A. (ed), Antibiotic resistance: implications for global health and novel intervention strategies. Workshop Summary. Institute of Medicine of the National Academies. National Academies Press, Washington, DC: [PubMed] [Google Scholar]
- 9.Echols R. 2012. A long and winding road; evolution of antimicrobial drug development - crisis management. Expert Rev. Anti Infect. Ther. 10:1311–1318 [DOI] [PubMed] [Google Scholar]
- 10.Shlaes DM, Moellering RC., Jr 2008. Telithromycin and the FDA: implications for the future. Lancet Infect. Dis. 8:83–85 [DOI] [PubMed] [Google Scholar]
- 11.Food and Drug Administration 2009. Guidance for industry. Community-acquired bacterial pneumonia: developing drugs for treatment. Center for Drug Evaluation and Research, Food and Drug Administration, US Department of Health and Human Services, Silver Spring, Maryland: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM123686.pdf [Google Scholar]
- 12.Food and Drug Administration 2010. Guidance for industry. Hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia: developing drugs for treatment. Center for Drug Evaluation and Research, Food and Drug Administration, US Department of Health and Human Services, Silver Spring, Maryland: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM234907.pdf [Google Scholar]
- 13.Shlaes DM. 2010. Antibiotics: the perfect storm, p 29–50 Springer-Verlag, New York, NY [Google Scholar]
- 14.Food and Drug Administration 2010. Guidance for industry. Acute bacterial skin and skin structure infections: developing drugs for treatment. Center for Drug Evaluation and Research, Food and Drug Administration, US Department of Health and Human Services, Silver Spring, Maryland: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071185.pdf [Google Scholar]
- 15.Spellberg B. 2011. Acute bacterial skin and skin structure trials: the bad is the enemy of the good. Clin. Infect. Dis. 53:1308–1309 [DOI] [PubMed] [Google Scholar]
- 16.Snodgrass WR, Anderson T. 1937. Prontosil in the treatment of erysipelas. A controlled series of 312 cases. BMJ 2:101–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snodgrass WR, Anderson T. 1937. Sulphanilamide in the treatment of erysipelas: a controlled series of 270 cases. BMJ 2:1156–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper M, Shlaes DM. 2011. Fix the antibiotic pipeline. Nature 472:32. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention 2013. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb. Mortal. Wkly. Rep. 62:165–170 [PMC free article] [PubMed] [Google Scholar]
- 20.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK; National Healthcare Safety Network Team 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect. Control Hosp. Epidemiol. 29:996–1011 [DOI] [PubMed] [Google Scholar]
- 21.Spellberg B, Bartlett JG, Gilbert DN. 2013. The future of antibiotics and resistance. N. Engl. J. Med. 368:299–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Food and Drug Administration 2013. Guidance for industry. Antibacterial therapies for patients with unmet medical need for the treatment of serious bacterial diseases. Center for Drug Evaluation and Research, Food and Drug Administration, US Department of Health and Human Services, Silver Spring, Maryland: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM359184.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


