Abstract
Linezolid has emerged as an important therapeutic option for the treatment of Staphylococcus aureus in patients with cystic fibrosis. We report the rapid emergence, upon treatment with linezolid, of linezolid-resistant S. aureus clinical isolates through the accumulation of resistance-associated 23S rRNA mutations, together with acquisition of an altered mutator phenotype.
TEXT
Staphylococcus aureus is a major pathogenic microorganism in the respiratory tracts of pediatric and adult patients with cystic fibrosis (CF). In 2011, 25.9% of CF patients in the United States were infected with methicillin-resistant S. aureus (MRSA) (1). Amid this rising trend, linezolid (LZD) has emerged as a therapeutic option for the treatment of MRSA in CF patients. LZD is effective through inhibition of protein synthesis after binding to domain V of the 23S rRNA (2). A few LZD-resistant S. aureus (LRSA) clinical isolates have been reported since 2001 (3), from both CF and non-CF patients (4). Mostly mutations in the 23S rRNA subunit were incriminated, and these involved at least one of the five or six chromosomal copies of the 23S rRNA gene (3, 5). Alterations in ribosomal proteins L3 and L4 of the peptidyltransferase center were also reported (6), as were alterations in the plasmid-mediated ribosomal methyltransferase cfr gene (7). Here we report the rapid emergence of resistance to LZD in MRSA in the course of LZD treatment in an adult CF patient.
A 24-year-old woman (with a homozygous ΔF508 genotype) had been diagnosed with CF at the age of 14 months and had been presenting chronic bronchial colonization with MRSA and Pseudomonas aeruginosa since the age of 10. She had been treated with inhaled antibiotics (tobramycin and colistin), azithromycin, two intravenous antibiotic courses per year, and oral antibiotics (60 to 180 days per year). Her pulmonary function was stable, with a forced expiratory volume in 1 s at 75% of the predicted value.
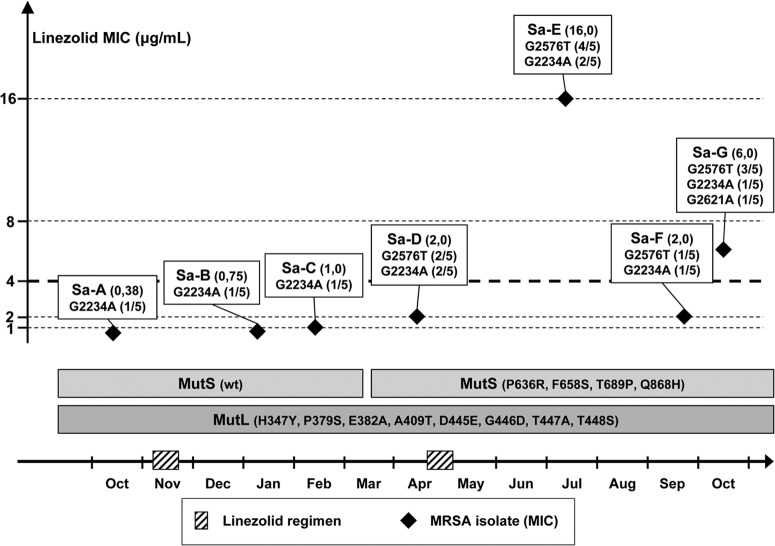
Poor clinical response to fusidic acid and minocycline treatment in an episode of pulmonary exacerbation led to the initial administration of LZD (600 mg orally twice daily, for 14 days) (Fig. 1). LZD was readministered 5 months later, as an alternative to a monthly 14-day course of fusidic acid or minocycline. Two months after the second administration of LZD, routine sputum microbiological monitoring showed LZD-resistant MRSA (isolate Sa-E in Fig. 1 [MIC = 16 μg/ml]), using the disk diffusion method and confirmation by Etest (breakpoint of 4.0 μg/ml per CLSI and EUCAST recommendations). All MRSA isolates obtained before the second administration of LZD (Sa-A to Sa-D) were LZD susceptible. The MRSA isolates obtained after Sa-E were susceptible to LZD, except for Sa-G, which was obtained 3 months after Sa-E. LZD treatment was reintroduced 2 years after discontinuation, having been replaced by one intravenous (i.v.) course of vancomycin and three annual courses of minocycline in the meantime, with good clinical outcome. No LRSA has been isolated since LZD was reintroduced, and no other patient attending the clinic was infected by LRSA. Taken together, the emergence of LRSA had limited clinical impact.
Fig 1.
Characteristics of the MRSA strains isolated over the period of treatment with LZD. LZD MICs (listed in parentheses after the isolate names) were determined by Etest; a thick dotted line shows the 4-μg/ml cutoff value. Mutations in domain V of the 23S rRNA gene are indicated for each MRSA isolate (Sa-A to Sa-G), as are the relative numbers of mutated copies (in parentheses). MutS and MutL genotypes are indicated for all isolates. LZD total therapy from first use to isolation of LRSA was 28 days. wt, wild type.
None of the isolates (Sa-A to Sa-G) were distinguishable from each other in pulsed-field gel electrophoresis analysis (8), suggesting that they were phylogenetically related. No cfr-associated resistance was detected, and all chromosomal copies of the 23S rRNA gene were sequenced (5). Among the five copies of the 23S rRNA gene, a G2234A mutation was identified on 1 copy in Sa-A, Sa-B, Sa-C, Sa-F, and Sa-G and on 2 copies in Sa-D and Sa-E. A G2576T mutation was identified on 1 copy in Sa-F, 2 copies in Sa-D, 3 copies in Sa-G, and 4 copies in Sa-E. A G2621A mutation was also found on one copy in Sa-G. Attempts to obtain LZD-resistant clones in vitro from LZD-susceptible isolates through daily iterative exposure to a sub-MIC LZD concentration (9), for up to 20 days, remained unsuccessful. However, two isolates showing LZD-susceptible and -resistant phenotypes (Sa-C and Sa-E, respectively) were investigated for their mutator phenotype by measuring the emergence rate of resistance to streptomycin (10). Whereas no peculiar mutator phenotype was observed for the LZD-susceptible Sa-C isolate, compared to S. aureus ATCC 25923, which was used as a control (mutation frequencies of 1.6 × 10−6 and 2.3 × 10−6, respectively [P = 0.03, Student t test]), the LZD-resistant Sa-E isolate showed a mutation rate (8.2 × 10−5) that was increased, although moderately, compared to that of Sa-C (ca. 50-fold [P = 0.0002]) and that of the control (ca. 35-fold [P = 0.0002]). Genotypic results supported these data, since identical MutS mutations not previously described (P636R, F658S, and T689P) and one MutS mutation previously reported (Q868H) (10) were found in Sa-D to Sa-G, whereas no MutS mutation was observed in Sa-A to Sa-C compared to a reference (AAW38145.1). All isolates displayed identical mutations in MutL (H347Y, P379S, E382A, A409T, D445E, G446D, T447A, and T448S) compared to the reference (AAW38146.1).
These data highlight the rapidness with which resistance-associated mutations can arise in MRSA upon LZD treatment, since only 14 days were sufficient for the emergence of a G2576T mutation in the 23S rRNA gene and 28 days of total therapy were sufficient for the emergence of LRSA. The rapid emergence of resistance described in this case is in contrast with previous reports that strengthened the impact of long-lasting use of LZD in CF patients on the selection of LRSA (11, 12).
The G2576T mutation in the 23S rRNA gene, observed in isolates Sa-D to Sa-G, was previously reported to mediate resistance to LZD (3), with MICs increasing together with the number of mutated copies (9, 13). Neither G2234A nor G2621A mutations have been reported so far. G2234A existed prior to the administration of LZD, and its having a role in decreased susceptibility to LZD is therefore unlikely. The G2621A mutation was observed in Sa-G in a single copy, in association with three copies of the G2576T mutation, thus rendering difficult the assessment of its direct role in resistance to LZD.
The hypermutator phenotype was not associated so far with the emergence of LRSA in CF, but the altered mutator phenotype observed for Sa-E, compared to Sa-C, is consistent with the previously reported high rate of mutable strains in CF patients (10, 14). Although one cannot exclude the possibility that, in this case, other mechanisms are also involved in the emergence of the resistance phenotype (15), it is striking that mutator phenotype and genotype have evolved concomitantly with the increasing number of mutations in 23S rRNA, MutS sequences being different for isolates Sa-A to Sa-C and Sa-D to Sa-G. The use of iterative exposure to LZD subinhibitory concentrations did not allow us to obtain in vitro LZD-resistant S. aureus mutants. Difficulty to obtain LZD-resistant clones was also reported in a different setting, with genetically engineered hypermutable S. aureus strains in an in vitro pharmacokinetic-pharmacodynamic model (16). These in vitro observations are in contrast with the rapid emergence of resistance that was observed in this patient. Conditions for the emergence of resistance to LZD in CF patients thus probably involve additional factors, such as pharmacokinetics. Despite similar LZD levels in sputum and serum (17), the bioavailability of LZD was reported to be reduced in CF patients (18), and a twice-daily 600-mg regimen was suggested to be insufficient to reach the target pharmacodynamic exposure for strains presenting with MICs above the 1- to 2-μg/ml range (18, 19). In this report, the isolates preceeding the emergence of LZD resistance did not show MICs exceeding 2.0 μg/ml, but wide interindividual pharmacodynamic variations have been reported (17) and further work in this population is needed to evaluate the benefit in resistance prevention and the potential adverse effects associated with the administration of a third daily dose (20).
Finally, the persistence of strains harboring 23S rRNA gene mutations, as for Sa-D after 5 months without LZD antibiotic pressure, is in contrast with the reduced bacterial fitness of hypermutable S. aureus that was reported in a model of chronic bone infection (21). This persistence rather supports the controversial hypothesis stemming from in vitro experiments, that LZD resistance-associated mutations would have a minimal impact on the fitness of S. aureus (5, 9) and that mutations in the 23S RNA gene may arise in the absence of exposure to LZD (22). The chronology for the isolation of strains Sa-E to Sa-G shows an alternation of LZD-susceptible and -resistant isolates, as well as different patterns for the mutated copies of the 23S RNA gene. One cannot formally exclude the possibility that Sa-G is a derivative of Sa-E through successive reversions of G2576T (23) and G2234A mutations and with the acquisition of the G2621A mutation. Alternatively, we favor the hypothesis that several isogenic mutants with different fitness behaviors emerged upon LZD treatment and that some isolates that appear predominant at different time points are successively isolated in routine sputum analysis.
In conclusion, this case illustrates a rapid in vivo selection of LRSA upon treatment with LZD and highlights the need for efficient and reliable monitoring of susceptibility to LZD in MRSA strains isolated from CF patients.
ACKNOWLEDGMENTS
C. Poyart has received reimbursement for attending meetings from bioMérieux, BioRad, Cepheid, and Novartis and has received research funding from bioMérieux, Wyeth, Siemens, and Oxoid.
Footnotes
Published ahead of print 5 August 2013
REFERENCES
- 1.Cystic Fibrosis Foundation 2012. Patient registry 2011 annual data report. Cystic Fibrosis Foundation, Bethesda, MD [Google Scholar]
- 2.Aoki H, Ke L, Poppe SM, Poel TJ, Weaver EA, Gadwood RC, Thomas RC, Shinabarger DL, Ganoza MC. 2002. Oxazolidinone antibiotics target the P site on Escherichia coli ribosomes. Antimicrob. Agents Chemother. 46:1080–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsiodras S, Gold HS, Sakoulas G, Eliopoulos GM, Wennersten C, Venkataraman L, Moellering RC, Ferraro MJ. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207–208 [DOI] [PubMed] [Google Scholar]
- 4.Gu B, Kelesidis T, Tsiodras S, Hindler J, Humphries RM. 2013. The emerging problem of linezolid-resistant Staphylococcus. J. Antimicrob. Chemother. 68:4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pillai SK, Sakoulas G, Wennersten C, Eliopoulos GM, Moellering RC, Jr, Ferraro MJ, Gold HS. 2002. Linezolid resistance in Staphylococcus aureus: characterization and stability of resistant phenotype. J. Infect. Dis. 186:1603–1607 [DOI] [PubMed] [Google Scholar]
- 6.Locke JB, Hilgers M, Shaw KJ. 2009. Novel ribosomal mutations in Staphylococcus aureus strains identified through selection with the oxazolidinones linezolid and torezolid (TR-700). Antimicrob. Agents Chemother. 53:5265–5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toh SM, Xiong L, Arias CA, Villegas MV, Lolans K, Quinn J, Mankin AS. 2007. Acquisition of a natural resistance gene renders a clinical strain of methicillin-resistant Staphylococcus aureus resistant to the synthetic antibiotic linezolid. Mol. Microbiol. 64:1506–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannerman TL, Hancock GA, Tenover FC, Miller JM. 1995. Pulsed-field gel electrophoresis as a replacement for bacteriophage typing of Staphylococcus aureus. J. Clin. Microbiol. 33:551–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besier S, Ludwig A, Zander J, Brade V, Wichelhaus TA. 2008. Linezolid resistance in Staphylococcus aureus: gene dosage effect, stability, fitness costs, and cross-resistances. Antimicrob. Agents Chemother. 52:1570–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prunier AL, Malbruny B, Laurans M, Brouard J, Duhamel JF, Leclercq R. 2003. High rate of macrolide resistance in Staphylococcus aureus strains from patients with cystic fibrosis reveals high proportions of hypermutable strains. J. Infect. Dis. 187:1709–1716 [DOI] [PubMed] [Google Scholar]
- 11.Endimiani A, Blackford M, Dasenbrook EC, Reed MD, Bajaksouszian S, Hujer AM, Rudin SD, Hujer KM, Perreten V, Rice LB, Jacobs MR, Konstan MW, Bonomo RA. 2011. Emergence of linezolid-resistant Staphylococcus aureus after prolonged treatment of cystic fibrosis patients in Cleveland, Ohio. Antimicrob. Agents Chemother. 55:1684–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill RL, Kearns AM, Nash J, North SE, Pike R, Newson T, Woodford N, Calver R, Livermore DM. 2010. Linezolid-resistant ST36 methicillin-resistant Staphylococcus aureus associated with prolonged linezolid treatment in two paediatric cystic fibrosis patients. J. Antimicrob. Chemother. 65:442–445 [DOI] [PubMed] [Google Scholar]
- 13.Wilson P, Andrews JA, Charlesworth R, Walesby R, Singer M, Farrell DJ, Robbins M. 2003. Linezolid resistance in clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 51:186–188 [DOI] [PubMed] [Google Scholar]
- 14.Oliver A, Mena A. 2010. Bacterial hypermutation in cystic fibrosis, not only for antibiotic resistance. Clin. Microbiol. Infect. 16:798–808 [DOI] [PubMed] [Google Scholar]
- 15.Prunier AL, Leclercq R. 2005. Role of mutS and mutL genes in hypermutability and recombination in Staphylococcus aureus. J. Bacteriol. 187:3455–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ba BB, Arpin C, Bikie Bi Nso B, Dubois V, Saux MC, Quentin C. 2010. Activity of linezolid in an in vitro pharmacokinetic-pharmacodynamic model using different dosages and Staphylococcus aureus and Enterococcus faecalis strains with and without a hypermutator phenotype. Antimicrob. Agents Chemother. 54:1443–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saralaya D, Peckham DG, Hulme B, Tobin CM, Denton M, Conway S, Etherington C. 2004. Serum and sputum concentrations following the oral administration of linezolid in adult patients with cystic fibrosis. J. Antimicrob. Chemother. 53:325–328 [DOI] [PubMed] [Google Scholar]
- 18.Keel RA, Schaeftlein A, Kloft C, Pope JS, Knauft RF, Muhlebach M, Nicolau DP, Kuti JL. 2011. Pharmacokinetics of intravenous and oral linezolid in adults with cystic fibrosis. Antimicrob. Agents Chemother. 55:3393–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosso JA, Flume PA, Gray SL. 2004. Linezolid pharmacokinetics in adult patients with cystic fibrosis. Antimicrob. Agents Chemother. 48:281–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Paolo A, Malacarne P, Guidotti E, Danesi R, Del Tacca M. 2010. Pharmacological issues of linezolid: an updated critical review. Clin. Pharmacokinet. 49:439–447 [DOI] [PubMed] [Google Scholar]
- 21.Daurel C, Prunier AL, Chau F, Garry L, Leclercq R, Fantin B. 2007. Role of hypermutability on bacterial fitness and emergence of resistance in experimental osteomyelitis due to Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 51:344–349 [DOI] [PubMed] [Google Scholar]
- 22.Quiles-Melero I, Garcia-Perea A, de Pablos M, Gomez-Gil R, Mingorance J. 2012. Resistance to linezolid in a methicillin-susceptible Staphylococcus aureus clinical isolate without previous exposure to oxazolidinones. Int. J. Med. Microbiol. 302:145–147 [DOI] [PubMed] [Google Scholar]
- 23.Meka VG, Gold HS, Cooke A, Venkataraman L, Eliopoulos GM, Moellering RC, Jr, Jenkins SG. 2004. Reversion to susceptibility in a linezolid-resistant clinical isolate of Staphylococcus aureus. J. Antimicrob. Chemother. 54:818–820 [DOI] [PubMed] [Google Scholar]