Abstract
Treatment of disseminated Trichosporon infections still remains difficult. Amphotericin B frequently displays inadequate fungicidal activity and echinocandins have no meaningful antifungal effect against this genus. Triazoles are currently the drugs of choice for the treatment of Trichosporon infections. This study evaluates the inhibitory and fungicidal activities of five triazoles against 90 clinical isolates of Trichosporon asahii. MICs (μg/ml) were determined according to Clinical and Laboratory Standards Institute microdilution method M27-A3 at 24 and 48 h using two endpoints, MIC-2 and MIC-0 (the lowest concentrations that inhibited ∼50 and 100% of growth, respectively). Minimum fungicidal concentrations (MFCs; μg/ml) were determined by seeding 100 μl of all clear MIC wells (using an inoculum of 104 CFU/ml) onto Sabouraud dextrose agar. Time-kill curves were assayed against four clinical T. asahii isolates and the T. asahii ATCC 201110 strain. The MIC-2 (∼50% reduction in turbidity compared to the growth control well)/MIC-0 (complete inhibition of growth)/MFC values that inhibited 90% of isolates at 48 h were, respectively, 8/32/64 μg/ml for fluconazole, 1/2/8 μg/ml for itraconazole, 0.12/0.5/2 μg/ml for voriconazole, 0.5/2/4 μg/ml for posaconazole, and 0.25/1/4 μg/ml for isavuconazole. The MIC-0 endpoints yielded more consistent MIC results, which remained mostly unchanged when extending the incubation to 48 h (98 to 100% agreement with 24-h values) and are easier to interpret. Based on the time-kill experiments, none of the drugs reached the fungicidal endpoint (99.9% killing), killing activity being shown but at concentrations not reached in serum. Statistical analysis revealed that killing rates are dose and antifungal dependent. The lowest concentration at which killing activity begins was for voriconazole, and the highest was for fluconazole. These results suggest that azoles display fungistatic activity and lack fungicidal effect against T. asahii. By rank order, the most active triazole is voriconazole, followed by itraconazole ∼ posaconazole ∼ isavuconazole > fluconazole.
INTRODUCTION
Fungal genera and species that are less susceptible or resistant to antifungal drugs in use have now emerged as rare causes of invasive infections in immunocompromised patients. One of the genera which remains relatively resistant to antifungal therapy is Trichosporon (1–8). Trichosporon spp. are basidiomycetous yeast-like anamorphic organisms (classified in Basidiomycota, Hymenomycetes, Tremelloidae, and Trichosporonales) (3). Thus far, 38 species of Trichosporon have been identified, 8 of which (Trichosporon cutaneum, Trichosporon asahii, Trichosporon asteroides, Trichosporon mucoides, Trichosporon inkin, Trichosporon ovoides, Trichosporon mycotoxinivorans, and Trichosporon insectorum) have been detected as causative agents in human infections and allergic disorders (4, 5, 9–11). T. mucoides and T. asahii mostly cause invasive trichosporonosis, while T. asteroides and T. cutaneum have been identified in superficial infections, and T. ovoides and T. inkin in white piedra (12). The reports published prior to this more recently accepted taxonomy of Trichosporon spp. refer only to Trichosporon beigelli. It remains partly difficult to determine the definitive species distribution of Trichosporon strains isolated in various clinical settings. With the contribution of more recently published data, T. asahii is now considered to be the predominant species encountered in invasive Trichosporon infections (13, 14).
Amphotericin B has long remained as a commonly used drug in treatment of trichosporonosis. However, its in vitro activity and particularly its fungicidal effect may remain inadequate against some strains of Trichosporon (15). The activity of echinocandins, on the other hand, is inherently limited against this genus (16, 17). While triazoles are among the most commonly studied antifungal drugs against Trichosporon (7, 18), there are still uncertainties regarding the optimal drug to be chosen for treatment of trichosporonosis and data to compare the in vitro activities of available triazoles and to rationally determine the optimal one for clinical use are limited (6).
Although any possible benefit of fungicidal activity remains at least partly unclear particularly in a patient setting of immunosuppression with multiple comorbidities, fungicidal activity has proven to be significant in a number of patients with trichosporonosis. One major example for this is the report on the lack of clinical response to amphotericin B in cases of disseminated trichosporonosis where amphotericin B was inhibitory but not fungicidal in vitro against the infecting Trichosporon strains. Specifically, the correlation of the lack of fungicidal activity of amphotericin B with refractory, disseminated trichosporonosis in granulocytopenic patients was emphasized (15). On the other hand, studies on comparative killing activities and pharmacodynamic properties of various triazoles against Trichosporon spp. are lacking (3, 14).
This study was undertaken (i) to determine and compare the MIC and minimum fungicidal concentration (MFC) values of five triazoles—fluconazole, itraconazole, voriconazole, posaconazole, and isavuconazole—against clinical isolates of T. asahii, (ii) to further analyze the comparative killing activities of these triazoles by time-kill assay for a limited number of selected Trichosporon strains, and finally (iii) to rank the in vitro activities of these agents so as to constitute the essential knowledge for optimal clinical choices.
(This study was presented in part at the 4th Trends in Medical Mycology Meeting, 18 to 21 October 2009, Athens, Greece, abstr. P-018.)
MATERIALS AND METHODS
Clinical isolates.
A total of 90 clinical isolates were tested. These strains were isolated from clinical samples of patients treated at Hacettepe University Medical School Hospital, Ankara, Turkey. The clinical samples were urine (n = 71, including one urolithiasis sample), blood (n = 6), sterile body fluids (n = 4), respiratory tract samples (n = 4, sputum samples/deep tracheal aspirates), pus (n = 3), vascular catheter culture (n = 1), and fine-needle aspiration biopsy sample (n = 1).
The strains were classified to the species level by using conventional methods, including the colony morphology, assimilation profiles detected by ID 32C (bioMérieux, France), microscopic properties on cornmeal Tween 80 agar, and urease enzyme activity (19). The isolates were stored at −80°C in brain heart infusion broth supplemented with 10% glycerol. Before tests were performed, the isolates were examined for purity and viability by subculturing at least twice and grown for 24 to 48 h at 35°C on Sabouraud dextrose agar (SDA).
Antifungal drugs.
Fluconazole (Pfizer Ireland Pharmaceuticals, Ireland), itraconazole (Janssen Pharmaceutica N.V., Belgium), voriconazole (Pfizer, Inc., USA), posaconazole (Schering-Plough Research Institute, USA), and isavuconazole (Basilea Pharmaceutica International, Ltd., Switzerland) were provided from their respective manufacturers as standard powders. Antifungal stock solutions were prepared by dissolving fluconazole in water, and other triazoles in dimethyl sulfoxide, as recommended by the Clinical and Laboratory Standards Institute (CLSI) (20). Stock solutions were stored at −80°C until used (maximum of 3 months).
Antifungal susceptibility testing.
The CLSI M27-A3 broth microdilution method was used to determine MIC (μg/ml) values (20). Candida krusei ATCC 6258 was included in each run of the susceptibility experiments as a quality control. Final drug concentrations in the microdilution plates ranged from 64 to 0.125 μg/ml for fluconazole and from 8 to 0.015 μg/ml for itraconazole, voriconazole, posaconazole, and isavuconazole. Broth microdilution method was performed by using two different inocula. A lower inoculum of ∼103 CFU/ml was used for MIC determinations, as recommended in the CLSI M27-A3 document (20). A higher inoculum of ∼104 CFU/ml was also studied to be used for MFC determinations (21).
Determination of MICs.
Since no definitive MIC reading endpoint and time have yet been determined for susceptibility testing of azoles against Trichosporon strains, the MICs were recorded by using both MIC-0 (complete inhibition of growth) and MIC-2 (∼50% reduction in turbidity compared to the growth control well) endpoints and at 24 and 48 h of incubation.
Determination of MFCs.
MFCs were determined by the method reported by Cantón et al. (21). Briefly, content of each visually clear MIC well with the highest inoculum of ∼104 CFU/ml was homogenized with a micropipette, and 100 μl of the content of each was subcultured onto SDA. All plates were incubated at 35°C for 24 and 48 h. The MFC was defined as the lowest drug concentration that killed ≥99.9% of the final inocula (detection of ≤1 colony on subculture agar plate). MFCs were determined at 24 as well as at 48 h.
Time-kill assay.
Time-kill studies were performed for four clinical isolates and T. asahii ATCC 201110 strain. The clinical isolates were selected depending on their relatively distinctive MIC value for any of the triazoles tested and compared to the other strains. Specifically, clinical isolate 1 presented with a relatively low fluconazole MIC-2, and clinical isolate 2 presented with a relatively high fluconazole MIC-2 value. Clinical isolates 3 and 4, on the other hand, had relatively high voriconazole and isavuconazole MIC-2 values, respectively.
The MIC values obtained for the isolates tested in time-kill experiments are shown in Table 1. Antifungal carryover effect and time-kill studies were performed as described previously (22, 23). Time-kill studies were carried out in RPMI 1640 medium by using an inoculum size of 1 × 103 to 5 × 103 CFU/ml with a 10-ml volume and concentrations of 32, 16, 8, 4, 2, 1, and 0.5× the MICs for each drug and test isolate. At predetermined time points (0, 6, 12, 24, 36, and 48 h), aliquots of 100 μl were removed from each control (drug-free) and test solution tube and then serially diluted in sterile water. A volume of 100 μl from serially diluted aliquots was placed on SDA plates to determine the number of CFU/ml after incubation at 35°C for 24 h. All experiments were performed twice for each isolate and triazole tested.
Table 1.
MIC and MFC values of azole agents for T. asahii isolates tested in time-kill experiments (n = 5)
| Agent | MIC or MFCa | MIC (μg/ml) for the indicated isolate at the indicated time |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ATCC 201110 |
Clinical isolate 1 |
Clinical isolate 2 |
Clinical isolate 3 |
Clinical isolate 4 |
|||||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | ||
| Fluconazole | MIC-2 | 0.5 | 4 | 0.5 | 0.5 | 4 | 16 | 0.5 | 2 | 4 | 4 |
| MIC-0 | 4 | 4 | 4 | 4 | 16 | 32 | 16 | 16 | 32 | 32 | |
| MFC | 8 | 32 | 16 | 32 | 32 | 64 | 16 | 16 | 64 | 64 | |
| Itraconazole | MIC-2 | 0.25 | 0.5 | 0.12 | 0.12 | 0.06 | 0.25 | 1 | 1 | 0.12 | 0.12 |
| MIC-0 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | |
| MFC | 2 | 2 | 8 | >8 | 2 | 2 | 2 | 2 | 4 | 4 | |
| Voriconazole | MIC-2 | ≤0.015 | 0.06 | ≤0.015 | 0.03 | 0.06 | 0.25 | 0.12 | 0.25 | 0.03 | 0.03 |
| MIC-0 | 0.12 | 0.12 | 0.06 | 0.12 | 0.25 | 0.5 | 0.25 | 0.5 | 0.5 | 0.5 | |
| MFC | 0.25 | 0.25 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | |
| Posaconazole | MIC-2 | 0.12 | 0.12 | 0.06 | 0.06 | ≤0.015 | 0.5 | 0.06 | 0.12 | 0.25 | 0.5 |
| MIC-0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | |
| MFC | 2 | 2 | 2 | 8 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Isavuconazole | MIC-2 | ≤0.015 | ≤0.015 | ≤0.015 | 0.03 | ≤0.015 | 0.12 | 0.12 | 0.12 | 0.25 | 0.5 |
| MIC-0 | 0.5 | 0.5 | 0.25 | 0.5 | 0.25 | 0.5 | 1 | 1 | 1 | 1 | |
| MFC | 8 | 8 | 2 | 4 | 2 | 2 | 2 | 2 | 2 | 2 | |
MIC-2, reading endpoint which provides ∼50% reduction in turbidity compared to the growth control well; MIC-0, reading endpoint at concentration which provides complete inhibition of growth. MFC, minimum fungicidal concentration.
Data analysis.
The MICs and MFCs that inhibited 50 and 90% of isolates (MIC50, MIC90, MFC50, and MFC90), the geometric mean (GM) MIC and GM MFC, and also the MIC and MFC range values were determined for each antifungal drug for both MIC and time reading endpoints. Time-kill data were fitted to an exponential equation, Nt = N0 × ekt (Nt, viable cells at time t; N0, starting inoculum; k, killing rate [or lethality]; t, incubation time). The exponential equation was transformed into a line by applying natural logarithms (log Nt = log N0 + kt). The slope of this line gives k values or killing rates. Killing activities of the drugs for each isolate were compared by use of the k values, positive values of which indicate growth, and negative values indicate killing. The generation time for each isolate/azole/concentration combination was calculated by the k value (0.30103/k). The goodness of fit for each isolate-drug combination was assessed by the R2 value (>0.8) (24, 25). A multivariate mixed-effects analysis (26) was performed to determine significant differences in killing rates among azoles and concentrations. In order to know the effect of these specific drugs on the whole isolate population, the variable isolates were considered as a random factor. The effect of the concentration was analyzed both as μg/ml and as multiples of MIC. A P value of ≤0.05 was considered significant.
RESULTS
MICs and MFCs of the tested triazoles against the 90 clinical T. asahii isolates included in the study are shown in Table 2. The highest MICs and MFCs were obtained for fluconazole, followed by itraconazole, posaconazole, and isavuconazole with similar degrees of activities compared to each other. The MICs and MFCs of itraconazole, posaconazole, and isavuconazole vary by no more than three 2-fold dilutions compared to one another. Voriconazole generated the lowest MICs and MFCs of all. The influence of the incubation time on MIC values was minor for MIC-0, where the agreement between 24 and 48 h results ranged from 98 to 100%, while for the MIC-2 it varied from 64 to 79% (Table 2).
Table 2.
In vitro activity of azole agents against 90 clinical isolates of T. asahiia
| Agent | MIC or MFC | MIC (μg/ml) at 24 h |
MIC (μg/ml) at 48 h |
% Agreementb (±1 dilution) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | MIC50 or MFC50 | MIC90 or MFC90 | GM | Range | MIC50 or MFC50 | MIC90 or MFC90 | GM | |||
| Fluconazole | MIC-2 | ≤0.12–8 | 1 | 4 | 1.54 | 0.5–16 | 4 | 8 | 3.24 | 64.4 |
| MIC-0 | 2–32 | 8 | 32 | 10.66 | 4–32 | 8 | 32 | 11.31 | 98.9 | |
| MFC | 2–64 | 16 | 64 | 20.78 | 4–64 | 32 | 64 | 23.69 | ||
| Itraconazole | MIC-2 | 0.03–1 | 0.25 | 0.5 | 0.23 | 0.12–1 | 0.25 | 1 | 0.37 | 78.9 |
| MIC-0 | 0.5–2 | 1 | 2 | 1.06 | 0.5–4 | 2 | 2 | 1.44 | 98.9 | |
| MFC | 1–>8 | 2 | 8 | 2.43 | 1–>8 | 2 | 8 | 3.1 | ||
| Voriconazole | MIC-2 | ≤0.015–0.12 | 0.03 | 0.06 | 0.04 | ≤0.015–0.25 | 0.06 | 0.12 | 0.06 | 75.6 |
| MIC-0 | 0.06–0.5 | 0.25 | 0.5 | 0.21 | 0.12–0.5 | 0.25 | 0.5 | 0.25 | 98.9 | |
| MFC | 0.25–4 | 0.5 | 2 | 0.71 | 0.25–8 | 1 | 2 | 0.83 | ||
| Posaconazole | MIC-2 | ≤0.015–1 | 0.12 | 0.5 | 0.16 | 0.06–1 | 0.25 | 0.5 | 0.25 | 76.7 |
| MIC-0 | 0.5–2 | 1 | 1 | 1.06 | 1–2 | 1 | 2 | 1.12 | 100 | |
| MFC | 0.5–8 | 2 | 2 | 1.68 | 0.5–8 | 2 | 4 | 1.92 | ||
| Isavuconazole | MIC-2 | ≤0.015–0.5 | 0.03 | 0.25 | 0.07 | ≤0.015–0.5 | 0.12 | 0.25 | 0.1 | 67.8 |
| MIC-0 | 0.12–2 | 0.5 | 1 | 0.53 | 0.25–2 | 1 | 1 | 0.75 | 97.8 | |
| MFC | 0.5–8 | 2 | 4 | 1.79 | 0.5–8 | 2 | 4 | 2.14 | ||
MIC-2 and MIC-0, MICs that inhibit 50 and 100% of growth, respectively; MIC50 and MIC90, concentrations that inhibit 50 and 90% of isolates, respectively; MFC, minimum fungicidal concentration; GM, geometric mean.
That is, agreement between results obtained at 24 and 48 h.
As an important observational note, MIC-2 values were difficult to evaluate particularly at 24 h due primarily to the relatively weak and nonhomogenous growth pattern of most of the isolates. On the contrary, we observed that MICs evaluated by MIC-0 endpoint could easily be determined at both reading time points.
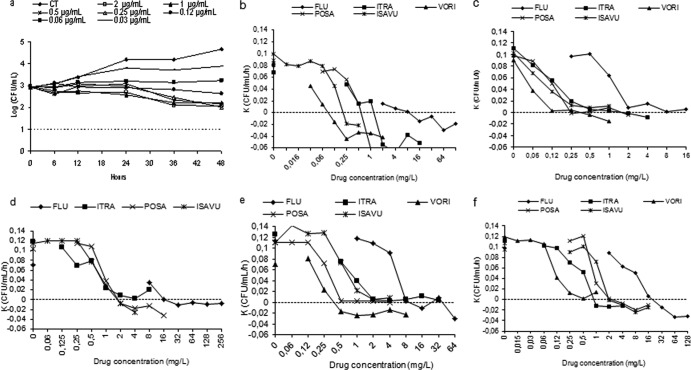
No carryover antifungal effect was detected for any of the triazoles tested. The time killing curves show that all agents were fungistatic; the activity increased as the incubation time and concentration increased, reaching the maximum log decrease (regarding the number of CFU/ml) at 48 h. Figure 1 a shows representative kill curves of voriconazole obtained for the T. asahii ATCC strain. For clinical isolates, the maximum log reduction in CFU, with respect to control at the same time point, obtained at 32× the MIC-2 varies with the isolate and agent, and ranged from 1.48 to 2.72 log for fluconazole, from 1.83 to 2.78 log for itraconazole, from 2.05 to 2.58 log for voriconazole, from 2.05 to 2.58 log for posaconazole, and from 1.87 to 2.65 log for isavuconazole. Table 3 shows the generation time for each isolate at MIC-2 and 4× the MIC-2. The assessment of the generation time for the clinical isolates and at 4× the MIC-2 led to the following conclusions. For fluconazole, it ranged from 3.3 h to 41.8 h at 4× the MIC, except for isolate 2, where killing activity was observed. For itraconazole it ranged from 5.7 to >48 h; for voriconazole from 7.2 to >48 h except for isolate 3, where killing activity was observed. For posaconazole, on the other hand, the generation time ranged from 33.1 to >48 except for isolate 1 (killing activity) and for isavuconazole from 3.9 to 8.2 h, except for isolate 4. In general, the generation time obtained at 4× the MIC was significantly greater (P < 0.05) than the corresponding generation time of each isolate in the absence of drug: 3.03 ± 0.2 h (isolate 1), 3.0 ± 0.9 h (isolate 2), 2.9 ± 0.8 h (isolate 3), and 2.9 ± 0.3 h (isolate 4). The fungicidal endpoint (99.9% killing or 3-log decreases in CFU/ml) was not reached by any azole although, in all agents, killing activity against some isolates was observed (negative k values). Figure 1b to f depict the relationship between the killing rate and concentration for the four clinical isolates tested and the T. asahii ATCC strain. The killing rate increased linearly with concentration and was not related to the MIC (e.g., for isolate 1, fluconazole killing rate at 2× the MIC [32 μg/ml] was −0.013 CFU/ml/h, whereas for the other isolates this rate was reached at 8× the MIC). Negative k values were obtained against 3 of the 4 clinical isolates tested at concentration ≥16 μg/ml (2 and 8× the MIC-2) of fluconazole and for the other agents against 2 of the 4 isolates, itraconazole at ≥1 μg/ml (8× and 32× the MIC-2), voriconazole at ≥0.5 μg/ml (2× and 32× the MIC-2), posaconazole at ≥2 μg/ml (4× and 8× the MIC-2), and isavuconazole at ≥2 μg/ml (4× and 16× the MIC-2) (Fig. 1b to f). Voriconazole was the agent that reached the greatest killing rates. Statistical analysis showed significant differences in the killing rates (k values) of isavuconazole and fluconazole (P < 0.001) when considering multiples of MIC, and when considering μg/ml, the relationship between k and concentration is significantly different for each azole (P < 0.001).
Fig 1.
Representative time-kill plots for ATCC T. asahii after exposure to voriconazole (a) and killing rates of fluconazole (FLU), itraconazole (ITRA), voriconazole (VORI), posaconazole (POSA), and isavuconazole (ISAVU) against T. asahii ATCC 201110, isolate 1, isolate 2, isolate 3, and isolate 4 (b to f). CT, drug-free control; above the dotted line, growth; dotted line, the starting point of killing activity. The voriconazole correlation coefficient value is low (R2 < 0.8) for isolate 2 and so has been omitted.
Table 3.
Time of generation obtained at the MIC and 4× the MIC for each isolate and azole
| Azole | Generation time (h) for the indicated isolate and concna |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ATCC 201110 |
Clinical isolate 1 |
Clinical isolate 2 |
Clinical isolate 3 |
Clinical isolate 4 |
||||||
| MIC | 4× MIC | MIC | 4× MIC | MIC | 4× MIC | MIC | 4× MIC | MIC | 4× MIC | |
| Fluconazole | 38.62 | Killing | 3 | 34 | >48 | Killing* | 2.6 | 3.3 | 4.8 | 41.8 |
| Itraconazole | 19.95 | Killing | 5.4 | >48 | 4.3 | 12.5 | 7.7 | >48 | 3.1 | 5.7 |
| Voriconazole | 22.31 | Killing | 6.4 | >48 | ND | ND | 13 | Killing | 2.7 | 7.2 |
| Posaconazole | 4.09 | 19.1 | 3.4 | Killing | 3.8 | 33.1 | 2.7 | >48 | 2.5 | >48 |
| Isavuconazole | 3.81 | 3.8 | 2.9 | 8.2 | 2.5 | 3.9 | 2.4 | 4.1 | 3 | Killing |
ND, not determined. *, Number of viable cells below starting inoculum.
DISCUSSION
We compared here the inhibitory and any possibly existing fungicidal activities of the triazoles against clinical isolates of T. asahii. For assessment of the inhibitory activities, we used the CLSI M27-A3 microdilution method standardized for Candida spp. and Cryptococcus neoformans. The very recently proposed test parameters for standardization of antifungal susceptibility testing for Trichosporon spp., as well as other nonfermentative yeasts (27), will hopefully and eventually provide a standard methodology and comparable data to be used in future studies for these fungal genera.
As indicated in Table 2, the analysis of our MIC results in general at both endpoint and time point readings suggests that voriconazole is the most active triazole in vitro against T. asahii. It is followed by itraconazole, posaconazole, and isavuconazole, which exhibit similar activities. Fluconazole, on the other hand, has the lowest activity among the five triazoles.
MIC values of triazoles against isolates of T. asahii have been previously reported by other authors. While some of these used the CLSI or similar broth microdilution methodology (2, 4, 6, 18, 28–30), others applied Etest or disk diffusion assays (18, 31, 32). Using the CLSI M27-A2 method, MIC-2 as the endpoint, and 72 h as the incubation period, Thompson et al. (29) reported MIC90 values of 2, 0.06, 0.25, and 0.125 μg/ml for fluconazole, voriconazole, posaconazole, and isavuconazole, respectively, for 40 isolates of T. asahii. Our corresponding values of 8, 0.125, 0.5, and 0.25 μg/ml, respectively, for the MIC-2 endpoint and at 48 h of incubation remained similar to those reported by these authors.
In vitro activities of fluconazole, itraconazole, and voriconazole against multicenter T. asahii isolates (n = 107) have also been studied by Kalkanci et al. (5), using the commercially available ASTY colorimetric microdilution panel (Kyokuto Pharmaceuticals, Japan). Similar to our results, MIC90 values of 16, 2, and 0.25 μg/ml were reported for fluconazole, itraconazole, and voriconazole, respectively, in the denoted study. Rodriguez-Tudela et al. (28) tested their T. asahii isolates (n = 15) by EUCAST broth microdilution methodology with modifications of incubation at 30°C for 48 h and agitation of the microplates during incubation. The GM MIC values were found to be 4.3, 0.57, and 0.18 for fluconazole, itraconazole, and voriconazole, respectively. Paphitou et al. (6) reported MIC50 values of 2, 0.125, 0.06, and 0.125 μg/ml for fluconazole, itraconazole, voriconazole, and posaconazole, respectively, at 48 h using the MIC-2 endpoint and CLSI M27-A3 methodology for 24 T. asahii isolates. These results are also similar to the MIC50 values we generated (4, 0.25, 0.06, and 0.25 μg/ml, respectively). In accordance with our results, these reports, as well as others by Mekha et al. (101 T. asahii isolates) (4), Chagas-Neto et al. (15 T. asahii isolates) (33), Guo et al. (36 T. asahii isolates) (34), and Xia et al. (8 T. asahii isolates) (35) all emphasize the relatively higher activity of voriconazole compared to fluconazole and itraconazole. Except for the data we present here, the only available data for the activity of isavuconazole against T. asahii were published by Thompson et al. (29). In that study, an MIC90 value of 0.125 μg/ml (at 72 h and with an MIC-2 endpoint) was reported for isavuconazole against 40 isolates of T. asahii, similar to the 0.25 μg/ml (at 48 h and with an MIC-2 endpoint) we reported for 90 isolates in the present study. In general, our data as a whole add more to these previously published reports by providing head-to-head comparison of all five triazoles (fluconazole, itraconazole, voriconazole, posaconazole, and isavuconazole) in terms of not only inhibitory but also the fungicidal activities against a relatively high number of T. asahii isolates (n = 90).
In our study, MIC values of all triazoles were determined at both 24 and 48 h, using two MIC reading endpoints (MIC-2 and MIC-0). Taking into account a difference of ±1 2-fold dilution, the similarity of 24 h MIC-0 values to those at 48 h are very high (>97% for all triazoles tested) (Table 2). However, the similarities of MICs at 24 h to those at 48 h vary between 64 to 79% when results are evaluated by the MIC-2 endpoint. In addition to the ability of MIC-0 to provide more consistent MICs at 24 and 48 h compared to MIC-2, we also observed that it is technically easier to evaluate the results by using the MIC-0 endpoint. We thus recommend the use of the MIC-0 endpoint for the evaluation of the MIC results of triazoles against T. asahii. Paphitou et al. (6) also compared MIC-0 versus MIC-2 endpoints and 24 versus 48 h MICs for testing fluconazole, itraconazole, voriconazole, posaconazole, and ravuconazole against 39 Trichosporon isolates (24 T. asahii). They similarly observed that MICs at 24 and 48 h were within 1 or 2 dilutions of each other. They also stated that MIC-0 values were found to be often similar to those evaluated by MIC-2 (with the MIC-0 being 1 to 3 2-fold higher than the MIC-2 in general). Importantly, significant trailing was noted for some isolates included in the study by Paphitou et al., suggesting the possibly more optimal utility of the MIC-0 endpoint.
The number of previous reports on MFCs of triazoles against isolates of Trichosporon is limited. To our knowledge, our report is the first that provides head-to-head comparison of the fungicidal activities of the five triazoles against T. asahii. We detected MFC90 values of 64, 8, 2, 4, and 4 μg/ml for fluconazole, itraconazole, voriconazole, posaconazole, and isavuconazole, respectively, at 48 h. Similarly, and being within a two 2-fold dilution range of our results, MFC90 values of 16, 0.5, 4, and 2 μg/ml were reported for fluconazole, voriconazole, posaconazole, and isavuconazole, respectively, by Thompson et al. for 40 isolates of T. asahii (29). On the other hand, MFC50 values of 0.5 and 1 μg/ml (compared to the 1 and 2 μg/ml generated here) were reported for voriconazole and posaconazole, respectively, by Paphitou et al. for 24 T. asahii isolates (6). These results, as well as ours, show that the fungicidal activities of the triazoles, as assessed by MFC values, remain less optimal in general. Voriconazole has the highest and fluconazole the lowest fungicidal activity and itraconazole, posaconazole, and isavuconazole lie in between with activities similar to each other.
In order to investigate the comparative killing activities in more detail, we also performed time-kill experiments against four clinical isolates and T. asahii ATCC 201110 strain. These experiments show that the killing rate depends on the agent and increases with concentration but MIC is independent and that voriconazole and posaconazole are the most active triazoles. To better demonstrate differences in time killing activity among isolates, azoles, and concentration, we calculated the generation time (or time required to double the number of viable CFU/ml) (Table 3); in general, it increases significantly at 4× the MIC. The same as occurs with Candida species, all agents show killing activity but near to the peak concentration reached in serum after standard doses (i.e., 6.7 μg/ml for fluconazole, 0.5 μg/ml for itraconazole, 2 μg/ml for voriconazole, 2 μg/ml for posaconazole, and 1 μg/ml for isavuconazole) but without reaching the fungicidal endpoint (99.9% killing) (36–40). To our knowledge, this is the first study that reported a head-to-head comparison of the killing kinetics of the five triazoles against T. asahii, and thus the lack of similar reports in the literature for this species has precluded comparisons. Thus far, time-kill curves of different azoles have been evaluated for Candida species (including Candida lusitaniae, Candida guilliermondii, Candida krusei, and Candida tropicalis) (41–45). Our results indicate that these triazoles are fungistatic against T. asahii.
In conclusion, when assessed by MIC and MFC determinations, voriconazole is the most active and fluconazole is the least active triazole tested against T. asahii. Itraconazole, posaconazole, and isavuconazole are in between and have similar and favorable in vitro activities against this fungal species. Using the MIC-0 endpoint appears to be optimal for determination of MICs of these triazoles against T. asahii, based on the ease of interpretation and generation of consistent results. In parallel with the MIC and MFC data and as assessed by time-kill experiments, the lowest concentration at which killing activity begins is for voriconazole and the highest for fluconazole. However, by time-kill experiments the fungicidal endpoint cannot be achieved with any of the triazoles tested, and all remain fungistatic against T. asahii. The MFC is not a good predictor of fungicidal activity of azoles against this species.
ACKNOWLEDGMENTS
This study was funded by Hacettepe University Scientific Research Unit as the project number 08D07101002.
Trichosporon asahii ATCC strain 201110 and travel support for presentation of the initial results at the 4th Trends in Medical Mycology Meeting (18 to 21 October 2009, Athens, Greece, abstr. P-018) by G.H. was provided by Basilea Pharmaceutica International, Ltd., Switzerland. S.A.A. does not have any potential conflict of interest related to this paper. S.A.A. has received investigator initiated research grant support from Pfizer and speaker honoraria from Merck and Pfizer.
Footnotes
Published ahead of print 22 July 2013
REFERENCES
- 1.Cornely OA. 2008. Aspergillus to Zygomycetes: causes, risk factors, prevention, and treatment of invasive fungal infections. Infection 36:296–313 [DOI] [PubMed] [Google Scholar]
- 2.Taj-Aldeen SJ, Al-Ansari N, El Shafei S, Meis JF, Curfs-Breuker I, Theelen B, Boekhout T. 2009. Molecular identification and susceptibility of Trichosporon species isolated from clinical specimens in Qatar: isolation of Trichosporon dohaense Taj-Aldeen, Meis & Boekhout sp. nov. J. Clin. Microbiol. 47:1791–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colombo AL, Padovan AC, Chaves GM. 2011. Current knowledge of Trichosporon spp. and trichosporonosis. Clin. Microbiol. Rev. 24:682–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mekha N, Sugita T, Ikeda R, Nishikawa A, Autthateinchai R, Poonwan N, Sawanpanyalert P. 2010. Genotyping and antifungal drug susceptibility of the pathogenic yeast Trichosporon asahii isolated from Thai patients. Mycopathologia 169:67–70 [DOI] [PubMed] [Google Scholar]
- 5.Kalkanci A, Sugita T, Arikan S, Yucesoy M, Ener B, Otag F, Kiraz N, Kustimur S, Sancak B, Evci C, Emektas G. 2010. Molecular identification, genotyping, and drug susceptibility of the basidiomycetous yeast pathogen Trichosporon isolated from Turkish patients. Med. Mycol. 48:141–146 [DOI] [PubMed] [Google Scholar]
- 6.Paphitou NI, Ostrosky-Zeichner L, Paetznick VL, Rodriguez JR, Chen E, Rex JH. 2002. In vitro antifungal susceptibilities of Trichosporon species. Antimicrob. Agents Chemother. 46:1144–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Lu Q, Wan Z, Zhang J. 2010. In vitro combined activity of amphotericin B, caspofungin, and voriconazole against clinical isolates of Trichosporon asahii. Int. J. Antimicrob. Agents 35:550–552 [DOI] [PubMed] [Google Scholar]
- 8.Thomas C, Chagas-Neto Guilherme Chaves M, Arnaldo Colombo L. 2008. Update on the genus Trichosporon. Mycopathologia 166:121–132 [DOI] [PubMed] [Google Scholar]
- 9.Gueho E, Smith MT, de Hoog GS, Billon-Grand G, Christen R, Batenburg-van der Vegte WH. 1992. Contributions to a revision of the genus Trichosporon. Antonie Van Leeuwenhoeck 61:289–316 [DOI] [PubMed] [Google Scholar]
- 10.Sugita T, Nishikawa A, Shinoda T. 1994. Reclassification of Trichosporon cutaneum by DNA relatedness by using the spectrophotometric method and chemiluminometric method. J. Gen. Appl. Microbiol. 40:397–408 [Google Scholar]
- 11.Sugita T, Nishikawa A, Shinoda T, Kume H. 1995. Taxonomic position of deep-seated, mucosa-associated, and superficial isolates of Trichosporon cutaneum from trichosporonosis patients. J. Clin. Microbiol. 33:1368–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li HM, Du HT, Liu W, Wan Z, Li RY. 2005. Microbiological characteristics of medically important Trichosporon species. Mycopathologia 160:217–225 [DOI] [PubMed] [Google Scholar]
- 13.Walsh TJ, Groll A, Hiemenz J, Fleming R, Roilides E, Anaissie E. 2004. Infections due to emerging and uncommon medically important fungal pathogens. Clin. Microbiol. Infect. 10:48–66 [DOI] [PubMed] [Google Scholar]
- 14.Girmenia C, Pagano L, Martino B, D'Antonio D, Fanci R, Specchia G, Melillo L, Buelli M, Pizzarelli G, Venditti M, Martino P, Infection Program GIMEMA 2005. Invasive infections caused by Trichosporon species and Geotrichum capitatum in patients with hematological malignancies: a retrospective multicenter study from Italy and review of the literature. J. Clin. Microbiol. 43:1818–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh TJ, Melcher GP, Rinaldi MG, Lecciones J, McGough DA, Kelly P, Lee J, Callender D, Rubin M, Pizzo PA. 1990. Trichosporon beigelii, an emerging pathogen resistant to amphotericin B. J. Clin. Microbiol. 28:1616–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denning DW. 2003. Echinocandin antifungal drugs. Lancet 362:1142–1151 [DOI] [PubMed] [Google Scholar]
- 17.Mattiuzzi GN, Alvarado G, Giles FJ, Ostrosky-Zeichner L, Cortes J, O'brien S, Verstovsek S, Faderl S, Zhou X, Raad II, Bekele BN, Leitz GJ, Lopez-Roman I, Estey EH. 2006. Open-label, randomized comparison of itraconazole versus caspofungin for prophylaxis in patients with hematologic malignancies. Antimicrob. Agents Chemother. 50:143–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arıkan S, Hasçelik G. 2002. Comparison of NCCLS microdilution method and Etest in antifungal susceptibility testing of clinical Trichosporon asahii isolates. Diagn. Microbiol. Infect. Dis. 43:107–111 [DOI] [PubMed] [Google Scholar]
- 19.Larone DH. 2011. Yeast and yeast-like organisms, p 151–152 In Medically important fungi: a guide to identification, 5th ed. ASM Press, Washington, DC [Google Scholar]
- 20.Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts, approved standard. CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 21.Cantón E, Pemán J, Viudes A, Quindós G, Gobernado M, Espinel-Ingroff A. 2003. Minimum fungicidal concentrations of amphotericin B for bloodstream Candida species. Diagn. Microbiol. Infect. Dis. 45:203–206 [DOI] [PubMed] [Google Scholar]
- 22.Klepser ME, Ernst EJ, Lewis RE, Ernst ME, Pfaller MA. 1998. Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrob. Agents Chemother. 42:1207–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klepser ME, Wolfe EJ, Jones RN, Nightingale CH, Pfaller MA. 1997. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrob. Agents Chemother. 41:1392–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantón E, Pemán J, Gobernado M, Viudes A, Espinel-Ingroff A. 2004. Patterns of amphotericin B killing kinetics against seven Candida species. Antimicrob. Agents Chemother. 48:2477–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cantón E, Pemán J, Gobernado M, Viudes A, Espinel-Ingroff A. 2005. Synergistic activities of fluconazole and voriconazole with terbinafine against four Candida species determined by checkerboard, time-kill, and Etest methods. Antimicrob. Agents Chemother. 49:1593–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang J. 2007. Linear and generalized linear mixed models, p 4–28 In Linear, generalized linear mixed models and their applications. Springer, Dordrecht, Netherlands [Google Scholar]
- 27.Zaragoza O, Mesa-Arango AC, Gómez-López A, Bernal-Martínez L, Rodríguez-Tudela JL, Cuenca-Estrella M. 2011. Process analysis of variables for standardization of antifungal susceptibility testing of nonfermentative yeasts. Antimicrob. Agents Chemother. 55:1563–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Tudela JL, Diaz-Guerra TM, Mellado E, Cano V, Tapia C, Perkins A, Gomez-Lopez A, Rodero L, Cuenca-Estrella M. 2005. Susceptibility patterns and molecular identification of Trichosporon species. Antimicrob. Agents Chemother. 49:4026–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson GR, III, Wiederhold NP, Sutton DA, Fothergill A, Patterson TF. 2009. In vitro activity of isavuconazole against Trichosporon, Rhodotorula, Geotrichum, Saccharomyces, and Pichia species. J. Antimicrob. Chemother. 64:79–83 [DOI] [PubMed] [Google Scholar]
- 30.Arıkan S, Sancak B, Bolat S, Hascelık G. 2005. Comparative in vitro activity of posaconazole (POS), voriconazole (VOR), itraconazole (ITR) and fluconazole (FLU) against Trichosporon asahii, abstr M-1634. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]
- 31.Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Bijie H, Dzierzanowska D, Klimko NN, Letscher-Bru V, Lisalova M, Muehlethaler K, Rennison C, Zaidi M, Global Antifungal Surveillance Group 2009. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of noncandidal yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J. Clin. Microbiol. 47:117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metin DY, Hilmioglu-Polat S, Hakim F, Inci R, Tumbay E. 2005. Evaluation of the microdilution, Etest, and disk diffusion methods for antifungal susceptibility testing of clinical strains of Trichosporon spp. J. Chemother. 17:404–408 [DOI] [PubMed] [Google Scholar]
- 33.Chagas-Neto TC, Chaves GM, Melo AS, Colombo AL. 2009. Bloodstream infections due to Trichosporon spp.: species distribution, Trichosporon asahii genotypes determined on the basis of ribosomal DNA intergenic spacer 1 sequencing and antifungal susceptibility testing. J. Clin. Microbiol. 47:1074–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo LN, Xiao M, Kong F, Chen SC, Wang H, Sorrell TC, Jiang W, Dou HT, Li RY, Xu YC. 2011. Three-locus identification, genotyping, and antifungal susceptibilities of medically important Trichosporon species from China. J. Clin. Microbiol. 49:3805–3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia Z, Yang R, Wang W, Cong L. 2012. Genotyping and antifungal drug susceptibility of Trichosporon asahii isolated from Chinese patients. Mycopathologia 173:127–133 [DOI] [PubMed] [Google Scholar]
- 36.Rex JH, Pfaller MA, Galgiani JN, Bartlett MS, Espinel-Ingroff A, Ghannoum MA, Lancaster M, Odds FC, Rinaldi MG, Walsh TJ, Barry AL, Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin. Infect. Dis. 24:235–247 [DOI] [PubMed] [Google Scholar]
- 37.Sabo JA, Abdel-Rahman SM. 2000. Voriconazole: a new triazole antifungal. Ann. Pharmacother. 34:1032–1043 [DOI] [PubMed] [Google Scholar]
- 38.Pemán J, Salavert M, Cantón E, Jarque I, Romá E, Zaragoza R, Viudes A, Gobernado M. 2006. Voriconazole in the management of nosocomial invasive fungal infections. Ther. Clin. Risk Manag. 2:129–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitt-Hoffmann A, Roos B, Maares J, Heep M, Spickerman J, Weidekamm E, Brown T, Roehrle M. 2006. Multiple-dose pharmacokinetics and safety of the new antifungal triazole BAL4815 after intravenous infusion and oral administration of its prodrug, BAL8557, in healthy volunteers. Antimicrob. Agents Chemother. 50:286–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conte JE, Jr, Golden JA, Krishna G, McIver M, Little E, Zurlinden E. 2009. Intrapulmonary pharmacokinetics and pharmacodynamics of posaconazole at steady state in healthy subjects. Antimicrob. Agents Chemother. 53:703–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roling EE, Klepser ME, Wasson A, Lewis RE, Ernst EJ, Pfaller MA. 2002. Antifungal activities of fluconazole, caspofungin (MK0991), and anidulafungin (LY 303366) alone and in combination against Candida spp. and Cryptococcus neoformans via time-kill methods. Diagn. Microbiol. Infect. Dis. 43:13–17 [DOI] [PubMed] [Google Scholar]
- 42.Oz Y, Dag I, Kiraz N. 2012. Broth microdilution and time-kill testing of caspofungin, voriconazole, amphotericin B, and their combinations against clinical isolates of Candida krusei. Mycopathologia 173:27–34 [DOI] [PubMed] [Google Scholar]
- 43.Cantón E, Pemán J, Valentín A, Bosch M, Espinel-Ingroff A, Gobernado M. 2008. Comparison of posaconazole and voriconazole in vitro killing against Candida krusei. Diagn. Microbiol. Infect. Dis. 62:177–181 [DOI] [PubMed] [Google Scholar]
- 44.Di Bonaventura G, Spedicato I, Picciani C, D'Antonio D, Piccolomini R. 2004. In vitro pharmacodynamic characteristics of amphotericin B, caspofungin, fluconazole, and voriconazole against bloodstream isolates of infrequent Candida species from patients with hematologic malignancies. Antimicrob. Agents Chemother. 48:4453–4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sóczó G, Kardos G, McNicholas PM, Balogh E, Gergely L, Varga I, Kelentey B, Majoros L. 2007. Correlation of posaconazole minimum fungicidal concentration and time kill test against nine Candida species. J. Antimicrob. Chemother. 60:1004–1009 [DOI] [PubMed] [Google Scholar]