Abstract
A once-daily single-tablet antiretroviral regimen containing tenofovir (TFV) disoproxil fumarate, emtricitabine (FTC), elvitegravir (EVG), and cobicistat (COBI) is an approved combination for the treatment of patients infected with HIV. COBI and TFV have been reported to interact with distinct transporters in renal proximal tubules; while TFV is renally eliminated by a combination of glomerular filtration and tubular secretion via anion transporters OAT1, OAT3, and MRP4, COBI inhibits renal cation transporters, particularly MATE1, resulting in a measurable decrease in the tubular secretion of creatinine. To investigate the potential for a renal drug-drug interaction between TFV and COBI in vitro, the uptake of TFV in the presence and absence of COBI was determined in fresh human renal cortex tissue and in cells expressing the relevant renal transporters. At concentrations exceeding clinical protein-unbound plasma levels, COBI did not significantly inhibit the transport of TFV by the anion transporters OAT1, OAT3, and MRP4 (50% inhibitory concentrations [IC50s] of >15, 6.6, and 8.5 μM, respectively). Conversely, TFV had little or no effect on the cation transporters OCT2 and MATE1 (IC50 > 100 μM). Consistent with studies using individual transporters, no increase in the accumulation of TFV in freshly isolated human renal cortex tissue or renal proximal tubule cells (RPTECs) was observed in the presence of COBI. Finally, COBI alone or in combination with FTC and EVG did not affect the sensitivity to TFV of cultured primary RPTECs or cells coexpressing OAT1 and MRP4. These results illustrate that COBI and TFV interact primarily with distinct renal transporters and indicate a low potential for pharmacokinetic renal drug-drug interaction.
INTRODUCTION
STRIBILD, an antiretroviral single-tablet regimen, consists of two nucleoside/nucleotide HIV reverse transcriptase inhibitors, tenofovir (TFV) disoproxil fumarate (TDF) and emtricitabine, combined with the integrase strand transfer inhibitor elvitegravir and the pharmacokinetic enhancer cobicistat (COBI), which effectively blocks the CYP450-mediated metabolic clearance of elvitegravir (1–4). Once-daily treatment with this regimen showed noninferior and durable efficacy and safety in treatment-naive HIV-infected patients when compared head-to-head with either efavirenz (5, 6)- or atazanavir (7–9)-containing once-daily treatment regimens. A small, early, and nonprogressive increase in the levels of serum creatinine due to a COBI-mediated blockade of creatinine active tubular secretion without an effect on glomerular filtration or overall renal function has been observed during clinical trials (10).
The orally administered prodrug TDF is unstable in plasma and is rapidly hydrolyzed, releasing the parent nucleotide TFV. TFV is renally eliminated by a combination of glomerular filtration and active tubular secretion via renal transporters (11–15). TFV is taken up into proximal tubules by renal organic anion transporter 1 (OAT1) and OAT3, and its luminal efflux is mediated by ABC efflux pump multidrug resistance-related protein 4 (MRP4) (Fig. 1A) (13, 16, 30). OAT1 appears to represent the major renal uptake pathway for TFV, while OAT3 plays a secondary role (11, 17). While TFV interacts with renal organic anion transporters, recent studies have shown that COBI can inhibit cationic transporters, including the efflux pump multidrug and toxin extrusion protein 1 (MATE1) (Fig. 1B) (10, 18). The inhibition of MATE1 is the primary mechanism by which COBI affects the renal tubular secretion of creatinine, causing the serum creatinine increases observed in patients without affecting actual glomerular filtration (10, 18).
Fig 1.
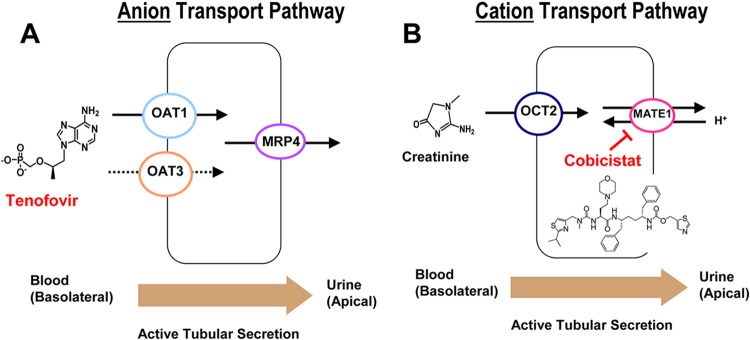
Renal tubular transporters interacting with TFV and COBI. (A) The active tubular secretion of TFV is mediated by the organic anion pathway (OATs and MRP4). (B) COBI interacts with the organic cation pathway involved in the active secretion of creatinine (MATE1) (18).
While the above findings indicate that COBI is likely to interact with a different set of renal transporters than TFV, the potential for their renal drug-drug interactions remains to be characterized. Therefore, the present studies seek to assess the potential of COBI and the boosting agent ritonavir (RTV) to affect the active tubular secretion and/or the cytotoxicity profile of TFV in various in vitro renal cell and tissue models. We observed that COBI under pharmacologically relevant conditions exhibited minimal interference with the transport pathways currently known to be involved in the renal tubular secretion of TFV. In addition, COBI did not affect the cytotoxicity of TFV in renal cell models. It should be noted, however, that the observations of this study do not affect the requirement for monitoring renal functions in patients treated with TDF-FTC-EVG-COBI in accordance with the STRIBILD prescribing information.
MATERIALS AND METHODS
Compounds.
TFV and COBI were synthesized by Gilead Sciences. RTV was purchased from Toronto Research Chemicals (North York, ON, Canada). Probenecid was purchased from Sigma (St. Louis, MO). A stock solution of TFV was prepared in water and adjusted to pH 7.0. COBI, RTV, and probenecid were dissolved in dimethyl sulfoxide (DMSO) and diluted directly into cell culture media according to individual experimental designs. [Adenine-2,8-3H]TFV (15 Ci/mmol) and [adenine-8-3H]TDF (2.6 Ci/mmol) were purchased from Moravek Biochemicals (Brea, CA). p-[Glycyl-2-3H]aminohippuric acid (PAH; 2.53 Ci/mmol) was purchased from PerkinElmer (Boston, MA).
Cells and tissues.
Human epithelial kidney 293T (HEK293T) cells were kindly provided by Warner Greene (Gladstone Institute of Virology and Immunology, San Francisco, CA). Cryopreserved and freshly isolated human renal proximal tubule cells (RPTECs) were provided by Lonza (Walkersville, MD; catalog no. C2553) and Celsis In Vitro Technologies (Halethorpe, MD), respectively. Renal cortex tissue slices were prepared by Vitron (Tucson, AZ) from freshly harvested human kidneys obtained from the Association of Human Tissue Users (Tucson, AZ) with donor consent. The donor was a healthy 36-year-old male treated with no medications who died of intracranial hemorrhaging. The kidneys were explanted from the donor under a protocol used for organ transplantation and delivered on ice in Viaspan cold preservation solution. Following decapsulation, renal cortical slices (200 μm thick) were prepared using a Brendel/Vitron tissue slicer under oxygenated, ice-cold V-7 preservation solution (Vitron). Each organ slice was placed on a titanium roller insert, blotted, and placed into glass vials containing incubation media (Waymouth's MB 752/1 tissue culture medium containing 10% fetal calf serum, 0.35 g/liter l-glutamine, 10 ml/liter Fungi-Bact, and 84 mg/liter gentamicin sulfate). This reproducible precision cut technique produces uniformly sized tissue slices, allowing for a comparison of tissue drug levels without a need for determining the relative accumulation per milligram of tissue protein.
Transient expression of OAT1, OAT3, and MRP4 transporters.
Full-length cDNA clones in pCMV-based expression vectors containing coding sequences of OAT1 variant 1 (OAT1v1; SC303532), OAT1v2 (SC123302), OAT3 (SC122673), and MRP4 (SC121947) were obtained from Origene (Rockville, MD). HEK293T cells were seeded in 6-well plates 24 h prior to transfection. Subconfluent cells were transiently transfected with the above cDNA constructs using Lipofectamine 2000 (Life Technologies, Carlsbad, CA) according to the manufacturer's recommendations. Cells were incubated at 37°C in a humidified chamber with 5% CO2 for 24 h, then trypsinized and reseeded into 12-well plates coated with poly-l-lysine (BD BioCoat; BD Sciences, San Jose, CA) at 0.3 × 106 cells per well in phenol red-free minimal essential medium (MEM) (Life Technologies, Carlsbad, CA) containing 10% fetal bovine serum (FBS). Plates were incubated another 24 h prior to use in transporter assays.
OAT1 and OAT3 uptake assays.
HEK293T cells expressing OATs were incubated with 1.2 μM [3H]TFV or 1.5 μM [3H]PAH and either DMSO, a specified concentration of the tested compounds, or 50 μM probenecid (control inhibitor of OAT-dependent uptake) for 3 to 6 min (OAT1) or 10 min (OAT3) at 37°C in Waymouth buffer (135 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 0.8 mM MgSO4, 28 mM glucose, and 13 mM HEPES, pH 7.2). At the end of the incubation, the cells were washed 3 times with ice-cold phosphate-buffered saline (PBS; 2 ml/well) and lysed directly on the plate by adding 0.4% Triton X-100 (1 ml/well) for 15 min. The intracellular level of TFV was quantified by scintillation counter after a mix with 5.0 ml Ready Safe scintillation fluid (Beckman Instruments, Fullerton, CA). The percentage of OAT-mediated uptake of [3H]TFV or [3H]PAH in the presence of each concentration of COBI or RTV was calculated according to the formula % TFV transport = 100 × (RadINH − RadPBC)/(RadCTRL − RadPBC), where RadINH, RadPBC, and RadCTRL represent the 3H radioactivity signal in the presence of tested compound, 50 μM probenecid (0% TFV transport), or DMSO (100% TFV transport), respectively. Each inhibitor concentration was tested in at least 4 independent replicates, and the resulting % TFV transport was summarized as the mean ± standard deviation (SD). Concentrations of tested compounds reducing the OAT-mediated uptake by 50% (IC50s) were calculated from individual dose response experiments using nonlinear regression analysis in XLfit software (IDBS, Guildford, United Kingdom).
MRP4 efflux assays.
HEK293T cells expressing MRP4 were incubated with 1 μM [3H]TDF or 0.02 μM dehydroepiandrosterone sulfate (DHEAS) for 90 min under ATP-depleting conditions consisting of glucose-free Dulbecco's modified Eagle medium (DMEM) supplemented with 10 mM NaN3 and 10 mM 2-deoxy-d-glucose to load the cells with TFV (13). Following incubation, the cells were washed twice with ice-cold PBS to remove extracellular [3H]TDF and supplemented with prewarmed complete DMEM containing DMSO, a specified concentration of tested compounds, or 50 μM MK-571 (control inhibitor of MRP4-dependent efflux). In some assays, 10% FBS or 100% pooled human serum (Sigma, St. Louis, MO) was added to the incubation media. Cells were incubated at 37°C for 90 min. At the end of the incubation, the cells were washed 3 times with ice-cold PBS (2 ml/well) and lysed directly on the plate by adding 0.4% Triton X-100 (1 ml/well) for 15 min. The intracellular level of 3H radioactivity was quantified by scintillation counter after a mix with 5.0 ml Ready Safe scintillation fluid (Beckman Instruments, Fullerton, CA), and the percentage of MRP4-mediated efflux of TFV in the presence of each inhibitor concentration was calculated as described above for the OAT-mediated transport.
OCT2 and MATE1 transport assays.
MATE1 and OCT2 studies were performed in stably transfected Chinese hamster ovary cell lines at SOLVO Biotechnology (Budaörs, Hungary). Twenty-four hours after the cells were plated into 96-well plates, the cultures were washed with Krebs-Henseleit buffer and incubated for 5 min (OCT2) or 20 min (MATE1) in the same buffer containing 3.6 μM [14C]tetraethylammonium ([14C]TEA) substrate and TFV at the range of tested concentrations. Following the incubation, the medium was aspirated and cells were washed twice prior to solubilization with 0.1 M NaOH. The amount of cell-associated radioactivity was determined by liquid scintillation counter. Results were compared to those for parent control CHO cells (without human OCT2 or MATE1) treated in the same manner. Verapamil (100 μM) and quinidine (100 μM) were tested in parallel as the positive-control inhibitors for OCT2 or MATE1, respectively. Fractional transport activity with the [14C]TEA substrate was calculated from the equation activity (%) = (CHO-TRANStest − CHOtest/CHO-TRANSvehicle − CHOvehicle) × 100%, where CHO-TRANS refers to transfected cells, CHO refers to the parental cell line, and subscripts test and vehicle refer to the test compound and vehicle control, respectively. IC50s were calculated from the fractional transport activity in the presence of the test compounds, with the vehicle control representing 100% transport activity. IC50s were determined by nonlinear regression using GraphPad Prism 5.0.
TFV accumulation in fresh RPTECs.
Fresh RPTEC cells were suspended at 2 × 106 cells per ml in InVitroGRO renal proximal tubule cell culture media (Celsis In Vitro Technologies, Halethorpe, MD) and prewarmed to 37°C. TFV (1.2 μM) was added into RPTEC suspensions and incubated in the presence and absence of COBI (1.5 μM and 15 μM), RTV (1.5 μM and 15 μM), or probenecid (50 μM) for 60 min at 37°C. The entire reaction mixtures were then overlaid onto preprepared microcentrifuge tubes containing 100 μl 2 N NaOH (bottom layer) and 100 μl filtration oil (top layer; 74.5:25.5 silicon oil-mineral oil mix). Samples were centrifuged immediately at 13,600 × g for 30 s to pellet the cells. The samples were then allowed to sit at room temperature for 2 h to complete cell lysis and frozen for at least 1 h at −80°C. Cell lysates were collected, neutralized with 2 N HCl solution, and extracted with acetonitrile. Following the analysis of TFV content by liquid chromatography/tandem mass spectrometry (LC/MS/MS), the percentage of TFV uptake in the presence of inhibitors relative to that for untreated controls (100%) was calculated. Mean values ± standard deviations of TFV levels were calculated from four independent measurements, and the percentage of TFV accumulation in the presence of COBI or ritonavir relative to a control in the absence of compound was determined.
TFV accumulation in fresh renal cortical tissue.
Incubation of freshly prepared renal slices with tested drugs was conducted at Vitron, Inc. (Tucson, AZ). Each individual tissue slice was incubated in 1.7 ml incubation media containing 1.2 μM [3H]TFV in the presence and absence of COBI (1.5 μM and 15 μM), RTV (1.5 μM and 15 μM), or probenecid (200 μM). Samples were gently agitated for 60 min at 37°C, washed 3 times with ice-cold phosphate-buffered saline, frozen at −80°C, and shipped to Gilead Sciences. When the samples were received, each tissue slice was lysed overnight in 0.5 ml 0.5 N NaOH at 37°C, followed by neutralization with 0.125 ml 2.0 N HCl. The amount of [3H]TFV accumulated in each tissue slice was quantified by scintillation counter after a mix with 5.0 ml Ready Safe scintillation fluid (Beckman Instruments, Fullerton, CA). Three individual renal tissue slices were analyzed for each tested condition and incubation time. Mean values ± standard deviations of TFV levels were calculated from the three independent samples, and the percentage of TFV accumulation in the presence of COBI or ritonavir relative to that for untreated controls (100%) was determined.
In vitro cytotoxicity assay in cultured RPTECs.
Cryopreserved RPTEC cells were thawed upon receipt and seeded at a density of 3.5 × 103 cells per cm2 in prewarmed REBM culture medium (Lonza, Walkersville, MD) supplemented with a growth factor cocktail (Lonza catalog no. CC 4127). Cells were utilized for cytotoxicity assays within 6 passages of thawing. Cells were seeded at a density of 7.0 × 103 cells/well in 96-well plates (Costar; Corning, Corning, NY). Twenty-four hours after seeding, medium containing TFV serially diluted 3-fold from a starting concentration of 4,000 μM was added either alone or in combination with fixed concentrations of COBI, elvitegravir, or emtricitabine. The final amount of DMSO was maintained at 1% across the plate. The concentrations used in combination with TFV corresponded to their respective peak plasma levels in treated HIV-infected patients. Cells were incubated at 37°C in a humidified chamber with 5% CO2. After 5 days, culture medium was removed and the amount of lactate dehydrogenase (LDH) released into the medium was quantified using the Cytotox-One homogeneous membrane integrity assay (Promega, Madison, WI). In addition, the cell viability was determined using CellTiter Glo (Promega, Madison, WI). Fluorescence and luminescence signals were quantified on an Envision plate reader (Perkin-Elmer, Waltham, MA).
The CC50 value was defined as the concentration inducing a 50% decrease in cell viability or an LDH release corresponding to a 50% of maximum LDH release. Data were analyzed using XLFit software (IDBS, Guildford, United Kingdom). CC50 values were calculated by nonlinear regression analysis using the sigmoidal dose-response (variable-slope) equation (four parameter logistic equation) Y = (T − B) ∗ CC50^h/(CC50^h + x^h) + B, where T is the top, B is the bottom, and h is the hill slope. The bottom and top values were fixed at 0 and 100, respectively. CC50 values were calculated as averages of four independent experiments.
In vitro cytotoxicity assay in HEK293T cells.
Subconfluent human embryonic kidney 293T cells were transfected with transporter expression plasmids using Lipofectamine 2000 (Life Technologies, Carlsbad, CA) according to the manufacturer's recommendations, incubated at 37°C in a humidified chamber with 5% CO2 for 24 h, trypsinized, and reseeded at a density of 7.0 × 103 cells/well in poly-d-lysine-coated 96-well plates (VWR, Pittsburgh, PA). After further incubation for 24 h, medium containing TFV serially diluted 3-fold from a starting concentration of 2,000 μM was added, either alone or in combination with fixed concentrations of COBI, elvitegravir, or emtricitabine. The final amount of DMSO was maintained at 1% across the plate. The concentrations used in combination with TFV corresponded to their respective peak plasma levels in treated HIV-infected patients. Cells were incubated at 37°C in a humidified chamber with 5% CO2. After 4 days, cell viability was determined by the addition of 100 μl CellTiter Glo viability reagent (Promega, Madison, WI) to each well. Luminescence signals were quantified on an EnVision luminometer (Perkin-Elmer, Waltham, MA). The CC50 values were defined as described above for RPTEC cytotoxicity assays and were calculated as averages of three independent experiments.
RESULTS
OAT-mediated uptake of TFV.
To characterize the effect of COBI, in vitro models based on the transient expression of two genetic variants of OAT1 and a consensus variant of OAT3 in HEK293T cells were established and validated. OAT1 variant 1 (OAT1v1) differs from OAT1v2 by a 13-amino-acid deletion encompassing residues 523 to 535. Transient transfection of each of the two OAT1 variants induced similar levels of TFV transport, while the transfection of OAT3 induced substantially lower levels of TFV transport than were induced by the two variants of OAT1 (see Fig. S1 in the supplemental material). The transport of TFV mediated by all three transporters was sensitive to the anion transport inhibitor probenecid. In addition, probenecid-sensitive uptake of PAH was established, indicating proper function of all three transiently expressed OATs.
COBI at concentrations ranging from 0.5 to 15 μM was tested for an effect on the active transport of TFV by OAT1 and OAT3, and its effect was compared to the effect of RTV at the same concentrations under identical conditions. To more broadly assess the effects of both pharmacokinetic boosting agents on the activity of the tested OATs, PAH was also included as a prototypic organic anion substrate. COBI showed no inhibition of TFV active transport by the two OAT1 variants up to the highest concentration tested (15 μM) (Fig. 2). Identical results were obtained with PAH as a substrate (data not shown). In comparison, RTV showed an effect on the activity of both variants of OAT1 at 15 μM, exhibiting 30 to 40% inhibition of the transport of both TFV (Fig. 2) and PAH (data not shown). The effect of RTV was dose dependent, with <10% inhibition of OAT1 detected at the concentration of 1.5 μM.
Fig 2.
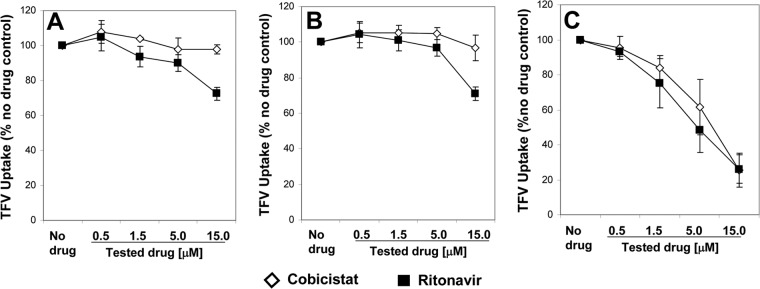
Effect of COBI and RTV on the transport of tenofovir by OAT1v1 (A), OAT1v2 (B), and OAT3 (C). Cells expressing OATs were incubated with 1.2 μM [3H]TFV and either DMSO or serially diluted tested compounds for 3 to 6 min (OAT1) or 10 min (OAT3) at 37°C. The intracellular level of [3H]TFV was quantified, and the percentage of OAT1- or OAT3-specific uptake relative to that for the untreated control was calculated after subtracting background determined in the presence of 50 μM probenecid. Results represent means ± SD from at least three independent experiments.
In comparison to that of OAT1, the activity of OAT3 was sensitive to inhibition by both COBI and RTV in a concentration-dependent manner, with similar effects observed on both tested substrates. OAT3-mediated transport of TFV was inhibited by COBI and RTV with IC50s of 6.6 and 4.8 μM, respectively. The effect of the two boosting agents on OAT3 was slightly weaker when PAH was used as a substrate. Table 1 summarizes the IC50s of COBI and RTV for the transport of TFV and PAH by the three tested OATs.
Table 1.
Effects of COBI and RTV on the activity of renal organic anion transporters
| Transporter | Function | IC50 [μM]a for transport of: |
|||
|---|---|---|---|---|---|
| TFV |
Prototype substrateb |
||||
| COBI | RTV | COBI | RTV | ||
| OAT1v1 | Uptake | >15 | >15 | >15 | >15 |
| OAT1v2 | Uptake | >15 | >15 | >15 | >15 |
| OAT3 | Uptake | 6.6 ± 1.8 | 4.8 ± 1.2 | 14.6 ± 6.8 | 8.7 ± 3.4 |
| MRP4 | Efflux | 8.5 ± 2.6 | 12.3 ± 1.3 | 14.5 ± 2.8 | >20 |
| MRP4 + 100% HSc | Efflux | >20 | >20 | NDd | ND |
IC50s are presented as means ± SD determined in at least 3 independent measurements.
PAH (1.5 μM) was used as a prototype substrate for OAT1s and OAT3; dehydroepiandrosterone sulfate (DHEAS; 0.02 μM) was used as a prototype substrate for MRP4.
MRP4 was tested in the presence of human serum (HS) to thoroughly assess the potential for in vivo drug-drug interaction under pharmacologically relevant conditions.
ND, not determined.
MRP4-mediated efflux of TFV.
The development and validation of a transient expression model system for MRP4 in HEK293T cells have been previously described (19). In this system, MRP4-expressing cells are first preloaded with TFV using its prodrug TDF under ATP-depleting conditions to minimize MRP4 efflux activity. The active efflux of TFV from the cells is then monitored after removing TDF and unblocking ATP synthesis. At the end of preloading with TDF, the intracellular concentration of TFV reached approximately 20 μM, a level substantially below the saturation of MRP4 efflux (16). In this system, both COBI and RTV inhibited the efflux of TFV by MRP4 in a dose-dependent manner (Fig. 3A), with IC50s of 8.5 and 12.3 μM, respectively (Table 1). A somewhat weaker effect of COBI and RTV on the activity of MRP4 was observed with the prototype substrate dehydroepiandrosterone sulfate (DHEAS; IC50 = 14.5 and >20 μM, respectively). Additional transport experiments were performed using MRP4-containing membrane vesicles and DHEAS as the substrate. Results similar to those in the cell-based assay were obtained, with IC50s of 20.7 and >20 μM for COBI and RTV, respectively, indicating a correlation between the two frequently used in vitro efflux models.
Fig 3.
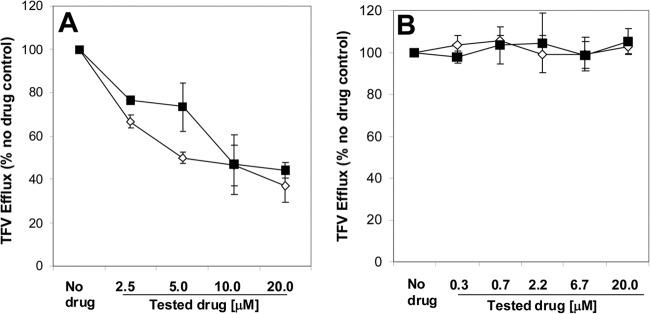
Effect of COBI and RTV on the MRP4-mediated efflux of TFV in the absence (A) or presence (B) of human serum. The results represent means ± SD from at least 3 independent measurements. MK-571 (50 μM) was used as a positive-control inhibitor of MRP4 to establish the complete inhibition of TFV transport (0% efflux).
Because the inhibition of MRP4-mediated efflux of TFV could potentially increase intracellular levels of TFV and may affect its renal toxicity profile, further evaluation under pharmacologically relevant conditions was needed to fully understand this interaction. As the free fractions of both COBI and RTV in human serum reach only 2 to 5% of the total concentration (data not shown) due to extensive binding by serum proteins, including albumin and α-acid glycoprotein, the serum proteins will decrease the availability of both drugs for interacting with MRP4. Cell-based assays in the presence of human serum are frequently used to test the interactions of drugs with intracellular targets in a more physiological environment (20, 21). To directly test the effect of protein binding, the inhibition of MRP4 by COBI and RTV was evaluated in the presence of 100% human serum. Under these conditions, neither of the two boosting agents inhibited the efflux of TFV by MRP4 up to the highest concentration tested (20 μM) (Fig. 3B).
Accumulation of TFV in fresh RPTECs and renal cortical tissue.
To confirm that the active transport of TFV is functional in the primary human renal models selected for ex vivo studies, freshly isolated RPTECs and precisely cut renal cortex slices were incubated with [3H]TFV in the absence and presence of the OAT inhibitor probenecid. TFV accumulated in both RPTECs and renal tissue slices in a time-dependent manner, with the accumulation substantially reduced in the presence of probenecid (32.0% and 36.3% uptake relative to untreated control, P = 0.013 and 0.012, respectively), confirming the presence of functional renal transport of TFV in both primary cells and fresh tissue models (Fig. 4).
Fig 4.
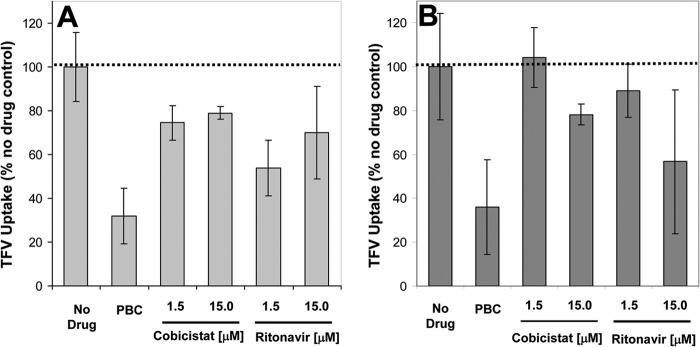
Effect of COBI and RTV on the accumulation of TFV in freshly isolated RPTECs (A) and human renal cortical slices (B). The results represent means ± SD from 4 independent measurements in RPTECs from two separate donors and 3 independent measurements in renal cortical tissue. Probenecid (PBC; 200 μM) was used as a control inhibitor of the active uptake of TFV.
The ex vivo accumulation of [3H]TFV in RPTECs and fresh cortex slices was tested in the presence of COBI and RTV at 1.5 and 15 μM concentrations, representing clinically relevant and supratherapeutic levels of the two compounds, respectively. Following a 1-hour incubation, COBI at 1.5 and 15 μM reduced the accumulation of TFV in RPTECs by approximately 25% and 21%, respectively. In the renal cortex tissue, 1.5 μM COBI showed no effect on the accumulation of TFV, while 15 μM COBI reduced the accumulation of TFV by approximately 22%, an effect similar to that observed in RPTECs. In comparison, RTV showed more-substantial effects, reducing the levels of TFV accumulation in RPTECs and renal cortex tissue by 30% and 43%, respectively, when tested at the concentration of 15 μM (Fig. 4).
Effect of TFV on OCT2- and MATE1-mediated transport.
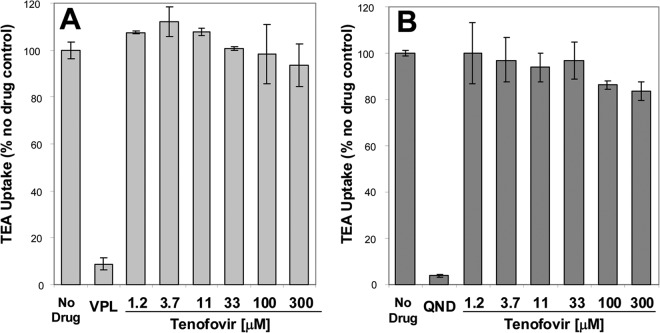
TFV was tested for its potential effect on the transport activity of OCT2 and MATE1, two renal cationic transporters that are involved in the active tubular secretion of creatinine and that have been shown to interact with COBI (18). In CHO cells stably expressing OCT2, TFV showed no effect on the OCT2-mediated transport of [3H]TEA up to the highest tested concentration of 300 μM (Fig. 5). In CHO cells stably expressing MATE1, TFV showed only a weak effect on the MATE1-mediated transport of [3H]TEA, with no inhibition up to the 33 μM concentration and a weak, approximately 20% inhibition, at the 100 to 300 μM concentrations. While the assay conditions do not allow for full determination of potential effects of TFV phosphorylated metabolites on MATE1, the likelihood that these highly negatively charged forms of TFV would interfere with MATE1 functions is very low based on the strong preference of the transporter for cationic molecules (22).
Fig 5.
Effect of TFV on the transport of tetraethylammonium (TEA) by renal organic cation transporters OCT2 (A) and MATE1 (B). The results represent means from two independent measurements. The difference in individual measurements was <13.5%. Verapamil (VPL; 100 μM) and quinidine (QND; 100 μM) were used as control inhibitors of OCT2 and MATE1, respectively.
In vitro cytotoxicity of TFV in RPTECs.
CC50 values for TFV were above the highest concentration tested (4,000 μM) in 4 individual experiments using RPTECs from 2 donors (Table 2). Emtricitabine, elvitegravir, and COBI were also tested for cytotoxicity toward RPTECs, with resulting CC50 values of >100, 13.7, and 26.2 μM, respectively. These values were consistent with the generally low cytotoxicity of TFV in RPTECs reported previously (11, 23–25). Coincubation of TFV with either COBI alone or COBI, elvitegravir, and emtricitabine in combination at pharmacologically relevant concentrations showed no measureable cytotoxicity within the range of tested concentrations of TFV (Table 2).
Table 2.
Effect of COBI, emtricitabine, and elvitegravir on the cytotoxicity of TFV in human RPTECs
| Compound | CC50 [μM]a for: |
|
|---|---|---|
| Cell viability | LDH release | |
| TFV | >4,000 | >4,000 |
| COBI | 26.2 ± 5.3 | 39.4 ± 0.8 |
| Elvitegravir | 13.7 ± 0.1 | 32.7 ± 0.1 |
| Emtricitabine | >100 | >100 |
| TFV + COBI (2 μM)b | >4,000 | >4,000 |
| TFV + COBI (2 μM) + elvitegravir (4.5 μM) + emtricitabine (8 μM)b | >4,000 | >4,000 |
The results represent means ± SD from 4 independent experiments performed in RPTECs from 2 independent donors. CC50 values were determined in parallel from cell viability readout (CellTiter Glo) and from lactate dehydrogenase (LDH) release readout.
In vitro cytotoxicity assay in cells expressing the renal anion transporters.
Functional expression of OAT1 and MRP4 following their transient transfection in HEK293T cells was confirmed by the uptake and efflux of TFV, respectively, as previously described in Materials and Methods. Both transporters showed the expected functional effect upon their expression (see Fig. S2 and S3 in the supplemental material). While TFV was not cytotoxic in control mock-transfected cells up to 2,000 μM, its cytotoxicity in cells expressing OAT1 increased substantially (CC50 = 78.7 ± 1.3 μM), an effect consistent with previously described results (24, 26) (Table 3). TFV was less toxic in cells expressing OAT1 and MRP4 (CC50 = 299.5 ± 81.3 μM) than in cells expressing OAT1 alone due to its reduced intracellular accumulation via MRP4-mediated efflux. Puromycin was also tested as a control for cytotoxicity in mock-transfected and transporter-expressing cells, with resulting CC50 values of 0.2 to 0.3 μM, respectively, irrespective of the transporter expression. Notably, in cells transiently expressing OAT1 or OAT1 and MRP4 together, the combination of TFV with either COBI or COBI, elvitegravir, and emtricitabine at pharmacologically relevant concentrations produced no significant change in cytotoxicity relative to TFV alone (Table 3). These data indicate that COBI, elvitegravir, and emtricitabine at pharmacologically relevant levels do not change the cytotoxic potential of TFV associated with its active accumulation in renal cells.
Table 3.
Effect of COBI, emtricitabine, and elvitegravir on the cytotoxicity of TFV in HEK293T cells transiently expressing renal anion transporters
| Compound | CC50 [μM]a for: |
||
|---|---|---|---|
| Control | OAT1 | OAT1 + MRP4 | |
| Puromycin | 0.35 ± 0.02 | 0.21 ± 0.10 | 0.22 ± 0.06 |
| TFV | >2,000 | 78.7 ± 1.3 | 299.5 ± 81.3 |
| TFV + COBI (2 μM)b | >2,000 | 68.3 ± 0.4 | 230.9 ± 82.6 |
| TFV + COBI (2 μM) + elvitegravir (4.5 μM) + emtricitabine (8 μM)b | >2,000 | 68.0 ± 4.1 | 228.8 ± 51.2 |
The results represent means ± SD from 3 independent experiments performed in HEK293T cells. CC50 values were determined from cell viability (CellTiter Glo) readout.
The tested concentrations of COBI, elvitegravir, and emtricitabine correspond to peak plasma levels (Cmax) in HIV-infected patients treated with a clinical dose of each compound.
DISCUSSION
Approval of the fixed-dose combination of TDF, emtricitabine, elvitegravir, and COBI (TDF-FTC-EVG-COBI; STRIBILD) in 2012 further expanded the first-line, single-tablet regimen options available to HIV-infected patients. In a 48-week head-to-head comparison with the fixed-dose combination of TDF, emtricitabine, and efavirenz (5) or TDF and emtricitabine combined with RTV-boosted atazanavir (7), a noninferior efficacy and differentiated safety profile for TDF-FTC-EVG-COBI was observed. Recent data from the 96-week analyses of both trials established the longer-term robust and durable efficacy of this regimen (6, 9). Minor elevation in serum creatinine attributed to the direct inhibitory effect of COBI on renal organic cation transporters has been noted in clinical studies with the TDF-FTC-EVG-COBI regimen (5, 7, 18). The effect of COBI on serum creatinine is similar to those observed with other drugs commonly used in this patient population such as RTV (9), dolutegravir (27), and cimetidine (28).
In the present study, we assessed the potential of COBI to directly affect the renal pharmacology of TFV. While previous findings suggested that COBI is likely to interact with a different set of renal transporters than TFV (18), the potential for renal drug-drug interaction between these two components of TDF-FTC-EVG-COBI required further characterization. Therefore, we used several complementary in vitro and ex vivo models to assess whether COBI and its structurally similar pharmacological enhancer RTV (1) exhibit a potential to affect the active renal transport and the cytotoxicity of TFV.
OAT1 and OAT3 mediate the active basolateral uptake of anionic substrates, including TFV, into proximal tubule cells during the process of tubular secretion (11, 17). COBI did not affect the transport of TFV by two variants of OAT1, even at concentrations 10-fold above its peak plasma level following the 150-mg dose used for clinical pharmacokinetic boosting. COBI affected the OAT3-mediated transport of TFV, with an IC50 of 6.7 μM, but had a minor (<20%) inhibitory effect on the transport of TFV by OAT3 at concentrations equal to its peak plasma levels. However, this effect on OAT3 transport of TFV is unlikely to be clinically relevant as OAT3 has been shown to be secondary to OAT1 in active uptake of TFV into proximal tubule cells (11, 17). In addition, reduced uptake of TFV into proximal tubules due to the interference with OAT3 would not adversely affect renal safety of TFV as it would effectively reduce the intracellular accumulation of TFV.
The ABC pump MRP4 is involved in the apical efflux of anionic substrates, including TFV, from proximal tubule cells into the nephron lumen (13). Our results showed that, while the MRP4-mediated efflux of TFV is sensitive to COBI at relatively low concentrations, the effect is eliminated in the presence of serum protein binding that effectively reduces the levels of COBI available for interacting with the transporter, indicating that COBI is unlikely to affect the renal efflux of TFV under pharmacologically relevant conditions. These results are consistent with previous observations characterizing the effect of human serum on the inhibition of MRP4 by RTV (19).
Notably, the low potential of COBI or RTV to interact with OAT3 and MRP4 was confirmed with other anion substrates such as PAH and DHEAS. It should also be noted in this context that recent studies suggested the potential involvement of MRP7 (ABCC10) in the renal efflux of tenofovir in addition to MRP4 (29). The potential for interactions between TFV and COBI at the level of MRP7 will require additional studies.
COBI has been shown in earlier studies to interact primarily with cation transporters OCT2 and MATE1, involved in the pathway of active secretion of creatinine, acting as their effective inhibitor (18). Hence, COBI showed a propensity for interacting with both cation and anion transporters. In contrast, TFV did not show any effect on OCT2 or MATE1 transporters even at extremely high concentrations, indicating its selective interaction with anion transporters.
We extended our assessment of the renal interaction between TFV and COBI to two physiologically more relevant primary cell/tissue models: human renal proximal tubule epithelial cells and fresh human renal cortical tissue slices. The presence of functional active uptake of TFV was confirmed by the effect of probenecid, a prototype inhibitor of organic anion transporters. COBI at levels mimicking clinical systemic exposure exhibited only a minor effect on the accumulation of TFV in RPTECs. Similarly, TFV accumulation in renal cortical tissue was only weakly affected in the presence of COBI at the highest tested concentration (15 μM). Consistent with the observations made in OAT1- and OAT3-transfected cells, COBI inhibited TFV uptake to an equal or lesser degree than RTV. It is important to note that the inhibition of TFV uptake, while not predicted to have any effect at pharmacologically relevant concentrations, would decrease intracellular levels of TFV in the proximal tubule.
The present study also assessed the effect of COBI and the combination of COBI, elvitegravir, and emtricitabine on the cytotoxicity of TFV in two in vitro renal cell culture models. TFV by itself exhibited minimal cytotoxicity in RPTECs, even at high concentrations, an observation consistent with prior studies (24, 25). Neither COBI alone nor the combination of COBI, elvitegravir, and emtricitabine affected the cytotoxicity of TFV in human RPTECs. However, it should be noted that the level of expression of transporters in primary RPTECs may fluctuate with the duration of primary culture. Therefore, an additional assay in which OAT1 and MRP4 transporters were coexpressed at high levels in HEK293T embryonic kidney cells was developed. Similar to the observations made in the primary culture model, the cytotoxicity of TFV in cells coexpressing OAT1 and MRP4 was not affected by COBI or the combination of COBI, elvitegravir, and emtricitabine at concentrations matching their clinical systemic peak exposures.
In summary, COBI under pharmacologically relevant conditions exhibited minimal interference with the functions of transport pathways currently known to be involved in the active renal tubular secretion of TFV. These conclusions were supported by results from primary in vitro and ex vivo renal tissue models and were corroborated by the lack of the effect of COBI on the cytotoxicity of TFV in renal cell cultures, indicating a low potential for renal drug-drug interaction between these two components of the TDF-FTC-EVG-COBI single-tablet regimen.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Javier Szwarcberg and Bernard Murray of Gilead Sciences for reviewing the manuscript and providing insightful comments. We also thank Robyn Fisher of Vitron, Inc. for supporting our fresh renal slice studies.
Footnotes
Published ahead of print 29 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00712-13.
REFERENCES
- 1.Xu L, Liu H, Murray BP, Callebaut C, Lee MS, Hong A, Strickley R, Tsai L, Stray K, Wang Y, Rhodes GR, Desai MC. 2010. Cobicistat (GS-9350): a potent and selective inhibitor of human CYP3A as a novel pharmacoenhancer. ACS Med. Chem. Lett. 1:209–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathias AA, German P, Murray BP, Wei L, Jain A, West S, Warren D, Hui J, Kearney BP. 2010. Pharmacokinetics and pharmacodynamics of GS-9350: a novel pharmacokinetic enhancer without anti-HIV activity. Clin. Pharmacol. Ther. 87:322–329 [DOI] [PubMed] [Google Scholar]
- 3.Ramanathan S, Mathias AA, German P, Kearney BP. 2011. Clinical pharmacokinetic and pharmacodynamic profile of the HIV integrase inhibitor elvitegravir. Clin. Pharmacokinet. 50:229–244 [DOI] [PubMed] [Google Scholar]
- 4.Gallant J, Koenig E, Andrade-Villanueva J, Chetchotisakd P, DeJesus E, Antunes F, Arastéh K, Moyle G, Rizzardini G, Fehr J, Liu Y, Zhong L, Callebaut C, Ramanathan S, Szwarcberg J, Rhee M, Cheng A. 2012. Cobicistat versus ritonavir as pharmacoenhancers in combination with atazanavir plus tenofovirDF/emtricitabine: phase 3 randomized, double blind, active-controlled trial, week 48 results. J. Int. AIDS Soc. 15(Suppl 3):53 [Google Scholar]
- 5.Sax PE, DeJesus E, Mills A, Zolopa A, Cohen C, Wohl D, Gallant JE, Liu HC, Zhong L, Yale K, White K, Kearney BP, Szwarcberg J, Quirk E, Cheng AK. 2012. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet 379:2439–2448 [DOI] [PubMed] [Google Scholar]
- 6.Zolopa A, Sax PE, DeJesus E, Mills A, Cohen C, Wohl D, Gallant JE, Liu HC, Plummer A, White KL, Cheng AK, Rhee MS, Szwarcberg J. 2013. A randomized double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: analysis of week 96 results. J. Acquir. Immune Defic. Syndr. 63:96–100 [DOI] [PubMed] [Google Scholar]
- 7.DeJesus E, Rockstroh JK, Henry K, Molina JM, Gathe J, Ramanathan S, Wei X, Yale K, Szwarcberg J, White K, Cheng AK, Kearney BP. 2012. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet 379:2429–2438 [DOI] [PubMed] [Google Scholar]
- 8.Lepist EI, Phan TK, Roy A, Tong L, Maclennan K, Murray B, Ray AS. 2012. Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro. Antimicrob. Agents Chemother. 56:5409–5413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rockstroh JK, DeJesus E, Henry K, Molina JM, Gathe J, Ramanathan S, Wei X, Plummer A, Abram M, Cheng AK, Fordyce MW, Szwarcberg J. 2013. A randomized, double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus coformulated emtricitabine and tenofovir DF for initial treatment of HIV-1 infection: analysis of week 96 results. J. Acquir. Immune Defic. Syndr. 62:483–486 [DOI] [PubMed] [Google Scholar]
- 10.German P, Liu HC, Szwarcberg J, Hepner M, Andrews J, Kearney BP, Mathias A. 2012. Effect of cobicistat on glomerular filtration rate in subjects with normal and impaired renal function. J. Acquir. Immune Defic. Syndr. 61:32–40 [DOI] [PubMed] [Google Scholar]
- 11.Cihlar T, Ho ES, Lin DC, Mulato AS. 2001. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids 20:641–648 [DOI] [PubMed] [Google Scholar]
- 12.Kearney BP, Flaherty JF, Shah J. 2004. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin. Pharmacokinet. 43:595–612 [DOI] [PubMed] [Google Scholar]
- 13.Ray AS, Cihlar T, Robinson KL, Tong L, Vela JE, Fuller MD, Wieman LM, Eisenberg EJ, Rhodes GR. 2006. Mechanism of active renal tubular efflux of tenofovir. Antimicrob. Agents Chemother. 50:3297–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Novoa S, Alvarez E, Labarga P, Soriano V. 2010. Renal toxicity associated with tenofovir use. Expert Opin. Drug Saf. 9:545–559 [DOI] [PubMed] [Google Scholar]
- 15.Barditch-Crovo P, Deeks SG, Collier A, Safrin S, Coakley DF, Miller M, Kearney BP, Coleman RL, Lamy PD, Kahn JO, McGowan I, Lietman PS. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in HIV-1 infected adults. Antimicrob. Agents Chemother. 45:2733–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imaoka T, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. 2007. Functional involvement of multidrug resistance associated protein 4 (MRP4/ABCC4) in the renal elimination of the anti-viral drugs, adefovir and tenofovir. Mol. Pharmacol. 71:619–627 [DOI] [PubMed] [Google Scholar]
- 17.Cihlar T, Bleasby K, Roy A, Pritchard J. 2004. Antiviral acyclic nucleotide analogs tenofovir and adefovir are substrates for human kidney organic anion, but not cation transporters: implications for potential renal drug interactions, poster A-443. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]
- 18.Lepist E-I, Murray BP, Tong L, Roy A, Bannister R, Ray AS. 2011. Effect of cobicistat and ritonavir on proximal renal tubular cell uptake and efflux transporters, abstr A1-1724. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL. American Society for Microbiology, Washington, DC [Google Scholar]
- 19.Cihlar T, Ray A, Laflamme G, Vella J, Tong L, Fuller M, Roy A, Rhodes G. 2007. Molecular assessment of the potential for renal drug interactions between tenofovir and HIV protease inhibitors. Antivir Ther. 12:267–272 [PubMed] [Google Scholar]
- 20.Boffito M, Back DJ, Blaschke TF, Rowland M, Bertz RJ, Gerber JG, Miller V. 2003. Protein binding in antiretroviral therapies. AIDS Res. Hum. Retroviruses 19:825–835 [DOI] [PubMed] [Google Scholar]
- 21.Acosta EP, Limoli KL, Trinh L, Parkin NT, King JR, Weidler JM, Ofotokun I, Petropoulos CJ. 2012. Novel method to assess antiretroviral target trough concentrations using in vitro susceptibility data. Antimicrob. Agents Chemother. 56:5938–5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, Morrissey KM, Sali A, Huang Y, Giacomini KM. 2013. Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J. Med. Chem. 56:781–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richman DD. 2001. Antiretroviral activity of emtricitabine, a potent nucleoside reverse transcriptase inhibitor. Antivir. Ther. 6:83–88 [PubMed] [Google Scholar]
- 24.Cihlar T, Birkus G, Greenwalt DE, Hitchcock MJM. 2002. Tenofovir exhibits low cytotoxicity in various human cell types: comparison with other nucleoside reverse transcriptase inhibitors. Antivir. Res. 54:37–45 [DOI] [PubMed] [Google Scholar]
- 25.Vidal F, Domingo JC, Guallar J, Saumoy M, Cordobilla B, Sanchez de la Rosa R, Giralt M, Alvarez ML, Lopez-Dupla M, Torres F, Villarroya F, Cihlar T, Domingo P. 2006. In vitro cytotoxicity and mitochondrial toxicity of tenofovir alone and in combination with other antiretrovirals in human renal proximal tubule cells. Antimicrob. Agents Chemother. 50:3824–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cihlar T, Laflamme G, Fisher R, Carey AC, Vela JE, Mackman R, Ray AS. 2009. Novel nucleotide human immunodeficiency virus reverse transcriptase inhibitor GS-9148 with a low nephrotoxic potential: characterization of renal transport and accumulation. Antimicrob. Agents Chemother. 53:150–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Lunzen J, Maggiolo F, Arribas JR, Rakhmanova A, Yeni P, Young B, Rockstroh JK, Almond S, Song I, Brothers C, Min S. 2012. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect. Dis. 12:111–118 [DOI] [PubMed] [Google Scholar]
- 28.van Acker BA, Koomen GC, Koopman MG, de Waart DR, Arisz L. 1992. Creatinine clearance during cimetidine administration for measurement of glomerular filtration rate. Lancet 340:1326–1329 [DOI] [PubMed] [Google Scholar]
- 29.Pushpakom SP, Liptrott NJ, Rodriguez-Novoa S, Labarga P, Soriano V, Albalater M, Hopper-Borge E, Bonora S, Di Perri G, Back DJ, Khoo S, Pirmohamed M, Owen A. 2011. Genetic variants of ABCC10, a novel tenofovir transporter, are associated with kidney tubular dysfunction. J. Infect. Dis. 204:145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohler JJ, Hosseini SH, Green E, Abuin A, Ludaway T, Russ R, Santoianni R, Lewis W. 2011. Tenofovir renal proximal tubular toxicity is regulated by OAT1 and MRP4 transporters. Lab. Invest. 91:852–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.German P, Warren D, West S, Hui J, Kearney BP. 2010. Pharmacokinetics and bioavailability of an integrase and novel pharmacoenhancer-containing single-tablet fixed-dose combination regimen for the treatment of HIV. J. Acquir. Immune Defic. Syndr. 55:323–329 [DOI] [PubMed] [Google Scholar]
- 32.Cohen C, Elion R, Ruane P, Shamblaw D, DeJesus E, Rashbaum B, Chuck SL, Yale K, Liu HC, Warren DR, Ramanathan S, Kearney BP. 2011. Randomized, phase 2 evaluation of two single-tablet regimens elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for the initial treatment of HIV infection. AIDS 25:F7–F12 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.