Abstract
Rhodococcus equi, the causal agent of rhodococcosis, is a major pathogen of foals and is also responsible for severe infections in immunocompromised humans. Of great concern, strains resistant to currently used antibiotics have emerged. As the number of drugs that are efficient in vivo is limited because of the intracellular localization of the bacterium inside macrophages, new active but cell-permeant drugs will be needed in the near future. In the present study, we evaluated, by in vitro and ex vivo experiments, the ability of the alpha-helical equine antimicrobial peptide eCATH1 to kill intracellular bacterial cells. Moreover, the therapeutic potential of the peptide was assessed in experimental rhodococcosis induced in mice, while the in vivo toxicity was evaluated by behavioral and histopathological analysis. The study revealed that eCATH1 significantly reduced the number of bacteria inside macrophages. Furthermore, the bactericidal potential of the peptide was maintained in vivo at doses that appeared to have no visible deleterious effects for the mice even after 7 days of treatment. Indeed, daily subcutaneous injections of 1 mg/kg body weight of eCATH1 led to a significant reduction of the bacterial load in organs comparable to that obtained after treatment with 10 mg/kg body weight of rifampin. Interestingly, the combination of the peptide with rifampin showed a synergistic interaction in both ex vivo and in vivo experiments. These results emphasize the therapeutic potential that eCATH1 represents in the treatment of rhodococcosis.
INTRODUCTION
Rhodococcus equi, previously known as Corynebacterium equi, was initially described in 1923 by Magnusson in foals presenting pyogranulomatous pneumonia (1). Rhodococcosis is now typically reported in foals from 1 to 6 months of age and is one of the most common causes of mortality at this age (2–5). The pathogen, however, can infect a wide variety of other animal species, including humans. Since the first case was described in 1967, the number of reports on the infection have increased substantially in humans, with ∼200 cases in the last 3 decades, preferentially in persons whose immune system was compromised, either naturally or due to illness or medical treatment (6–9).
Many antimicrobials are effective against the extracellular pathogen in vitro. However, the number of antimicrobials available for treatment of rhodococcosis is limited because of the intracellular location of the bacterium. The combination of rifampin and a macrolide, such as erythromycin, or more recently of azithromycin and clarithromycin, is considered the treatment of choice today, as these antibiotics have the ability to penetrate abscesses and cells due their lipophilic nature and to accumulate in granulocytes and macrophages to kill the bacterium (10). Since the introduction of these antibiotics in rhodococcosis treatment, the survival rate of foals has increased dramatically, from less than 20% up to 97% (K. Chaffin, presented at the 49th Annual Ocala Equine Conference, Ocala, FL, 21 to 24 October 2011). Unfortunately, some observations suggest that there might be a problem in the treatment of R. equi infections in the near future due to the emergence of rhodococci resistant to these antibiotics and potentially fatal side effects of macrolides for horses (11–14). With the rise of bacterial resistance to antibiotics, there is a growing interest in anti-infective agents with mechanisms of action fundamentally different from those of conventional antibiotics (15, 16). The concept of using antimicrobial peptides (AMPs) as therapeutic tools dates back to the 1990s, and they are now emerging as particularly promising candidates for new anti-infective agents in the antimicrobial research area (17). In nature, these peptides participate in the first line of host defense against pathogens by combining antimicrobial activity with immunomodulatory properties (18).
We recently reported the in vitro activity of the alpha-helical equine AMP eCATH1 against antibiotic-resistant and antibiotic-susceptible strains of R. equi. The peptide proved to be extracellularly active at low micromolar concentrations (0.16 to 1.27 μM) against the pathogen independently of the antibiotic resistance profile (19; M. Schlusselhuber, K. Guldbech, C. Sévin, C. Laugier, M. Leippe, J. Grötzinger, R. Leclercq, S. Giguère, and J. Cauchard, submitted for publication). Aiming at extending the therapeutic potential of eCATH1 against rhodococcosis, the present study was designed to analyze the efficacy of the peptide in killing intracellular R. equi and combating the pathogen in an infection model in mice.
MATERIALS AND METHODS
Cell line, bacterial strain, and growth conditions.
The mouse macrophage cell line J774.2 (European Collection of Cell Cultures [ECACC] reference number 85011428), purchased from Sigma-Aldrich, was grown in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Rockville, MD, USA) supplemented with 10% (vol/vol) fetal bovine serum (FBS) (Pan Biotech GmbH, Aidenbach, Germany), 100 μg/ml streptomycin, 100 units/ml penicillin (complete medium) and incubated at 37°C in a humidified atmosphere containing 5% CO2. Before assays, cell numbers and the viability of cells were determined by exclusion of the vital dye trypan blue. Virulent, vapA-positive R. equi ATCC 33701 was used in this study. A pure strain, freshly thawed, was grown in brain heart infusion medium (BHI) (BD Difco, Franklin Lakes, NJ, USA) before each experiment to avoid loss of the virulence plasmid. Cultures were incubated aerobically at 37°C for 24 to 48 h. For cell line infection, bacteria were suspended with an appropriate volume of DMEM supplemented with 5% FBS. The actual number of cells was confirmed by plating serial dilutions on BHI agar plates. For mouse inoculation, bacteria were harvested from a liquid culture by centrifugation, washed, and resuspended in phosphate-buffered saline (PBS) (Gibco, Rockville, MD, USA) at the required bacterial concentration.
Antimicrobials used.
eCATH1 was chemically synthesized at a purity grade of at least 96% by GenScript USA Inc. (Piscataway, NJ, USA) and dissolved in 10 mM acetic acid at a final concentration of 1 mg/ml. Rifampin stock solution was prepared by dissolving antibiotic powder (reference R7382; Sigma-Aldrich, St. Louis, MO, USA) in methanol at a final concentration of 50 mg/kg. Stock solutions of antimicrobials were stored at −20°C until use.
Survival of R. equi in a mouse macrophage cell line.
Before the use of animals, the activity of eCATH1 against intracellular rhodococci was evaluated in vitro. J774.2 murine monocyte/macrophage-like cells were washed with PBS, detached by scraping, and resuspended in complete medium at a final concentration of 5 × 105 cells/ml. Monocytes were allowed to attach for 3 h on an 8-well Lab-Tek chamber slide (200 μl of cell suspension per well) at 37°C in a humidified atmosphere containing 5% CO2. Infection of mammalian cells was performed by washing the cells with PBS before adding 200 μl of bacterial suspension per well at a bacterium-to-monocyte ratio of 2:1. The chamber slides were incubated for 45 min at 37°C in a humidified atmosphere containing 5% CO2 to allow serum opsonization of the rhodococci. Infected monolayers were carefully washed three times with PBS to remove unbound bacteria. After the last wash, 200 μl of DMEM supplemented with either eCATH1 (final concentration, 20 μg/ml) or the same volume of peptide solvent (acetic acid; final concentration, 0.2 mM) was added to infected cells before incubation at 37°C in a humidified atmosphere containing 5% CO2. Viable intracellular bacteria were assessed after 24 h by staining with the LIVE/DEAD Baclight bacterial-viability kit (Molecular Probes Europe BV, Leiden, The Netherlands) in saponin solution (final concentration, 0.05% [wt/vol]) to permeabilize macrophage membranes, as previously described (20). The slides were examined by fluorescence microscopy (Axioskop 40 microscope; Zeiss). The bacterial-viability kit is composed of propidium iodide, which stains dead cells red, and Syto 9, which stains viable cells green. Therefore, nuclei of permeabilized macrophages appear red. Before the experiment, the effect of 20 μg/ml of eCATH1 on the plasma membrane integrity of the J774.2 cell line was assessed by the lactate dehydrogenase (LDH) release assay after 24 h of treatment. One hundred percent lysis was achieved by using 1% (vol/vol) Triton X-100. The LDH release assay was carried out according to the manufacturer's instructions using a commercially available kit (Tox-7; Sigma-Aldrich, Saint Louis, MO, USA).
Animal studies.
Pathogen-free BALB/c mice (Harlan Italy S.r.l., San Pietro al Natisone, Udine, Italy) were housed with free access to water and standard mouse food at animal facilities of the Sacred Heart University (Rome, Italy). All animals used for experiments were females 10 weeks of age weighing approximately 20 to 25 g. For the experiments, animals were sacrificed by cervical dislocation. The animal experiments were performed under a protocol approved by the Institutional Animal Use and Care Committee at Università Cattolica del S. Cuore, Rome, Italy (permit number N21, 5 December 2010) and authorized by the Italian Ministry of Health, according to Legislative Decree 116/92, which implemented the European Directive 86/609/EEC on laboratory animal protection in Italy. Animal welfare was routinely checked by veterinarians of the Service for Animal Welfare.
Survival of R. equi in mouse peritoneal macrophages.
The survival of R. equi in mouse peritoneal macrophages after treatment with eCATH1, rifampin, or eCATH1 combined with rifampin was assessed by using an ex vivo infection model as described previously (21, 22). Briefly, mice were infected with 107 to 108 CFU of R. equi by intraperitoneal injection. After an 8-h infection period, the peritoneal macrophages were collected by peritoneal lavage, centrifuged, and suspended in DMEM containing 10 mM HEPES, 2 mM glutamine, 10% (vol/vol) FBS, and 1× nonessential amino acids supplemented with vancomycin (10 μg/ml) and gentamicin (150 μg/ml). The cell suspension was dispensed into 24-well tissue culture plates and incubated at 37°C under 5% CO2 for 2 h. After exposure to antibiotics to kill extracellular bacteria (i.e., at 10 h postinfection), the infected macrophages were washed, and triplicate wells of macrophages were lysed with detergent. After dilution with BHI broth, the lysates were plated on BHI agar to quantify the viable intracellular bacteria. The remaining wells containing infected macrophages were maintained in DMEM and treated with eCATH1 (20 μg/ml), rifampin (0.5, 5, and 10 μg/ml), or eCATH1 (20 μg/ml) combined with rifampin (5 μg/ml) for the duration of the experiment. At 24 h postinfection, supernatant fluid was removed from each well, and intracellular bacteria were quantified by counting on BHI agar plates. Before the experiment, the toxicity of eCATH1 and rifampin on mouse peritoneal macrophages was assessed with a range of concentrations up to 80 μg/ml in order to determine the concentration of antimicrobials used in the study. To assess cell viability, macrophages treated for 24 h were analyzed using the alamarBlue test (Invitrogen, Milan, Italy) following the manufacturer's instructions.
Activity of eCATH1 against R. equi-infected mice.
The activity of eCATH1 alone and in combination with rifampin was assessed on rhodococcosis induced in mice. Before therapeutic trials, comparison of the natural clearance of R. equi CFU from organs was established under our experimental conditions to determine time points of treatment and the sublethal dose. Briefly, 10 mice per group were inoculated intravenously with 107, 108, and 109 CFU of R. equi, and two animals from each group were sacrificed at days 1, 2, 6, 8, and 10 postinfection for bacterial counts in liver, spleen, and lung. The intravenous route was chosen based on the experience of Takai et al., who reported higher virulence of the R. equi ATCC 33701 strain by this route than by the intraperitoneal route (23). All the mice were monitored for survival throughout the experimental period. Based on these preliminary results, mice were intravenously challenged with 200 μl of a sublethal dose (108 CFU) of R. equi. Therapeutic trials with BALB/c mice were initiated 1 day after bacterial inoculation to let the infection develop and symptoms appear and before natural clearance of rhodococci from mouse organs. Stock solutions of antimicrobials were appropriately freshly diluted in isotonic sodium chloride before subcutaneous injection of 200 μl into the animals. Infected mice were treated once a day with eCATH1 (1 mg/kg body weight; group I), rifampin (10 mg/kg body weight; group II), a combination of both in the same concentrations as mentioned above (group III), or the same volume of isotonic sodium chloride (control group) for 7 days. The doses, periodicities, and route of injections were chosen based on previous reports (24–26). Five mice per group were sacrificed 1, 4, and 8 days postinfection (i.e., at the start, middle, and end of treatment), and their spleens and livers were aseptically removed. The organs were weighed and separately homogenized in PBS. The R. equi concentrations in the organs (CFU/g organ) were determined by plating 10-fold serial dilutions of the tissue homogenates on BHI agar. The plates were incubated at 37°C for 24 to 48 h before bacterial enumeration.
In vivo toxicity of eCATH1.
Noninfected mice were used to assess the in vivo toxicity of eCATH1 by behavioral and histopathological analysis. Five mice were treated once a day with eCATH1 for 7 days (1 mg/kg body weight) by subcutaneous injection, while a second group of five nontreated mice was used as a control group. All the mice were monitored for survival and the presence of any drug-related adverse effect (local signs of inflammation, weight loss, diarrhea, and behavioral alterations) throughout the experimental period. The mice were sacrificed after 7 days of treatment, and organs were aseptically removed (intestine, spleen, liver, lung, kidney, and stomach). Tissue samples were fixed in 3.7% formaldehyde solution, processed for paraffin embedding, and stained with hematoxylin and eosin before analysis at the Veterinary Pathological Anatomy Laboratory (Amboise, France) by pathologists.
Statistics.
Ex vivo and in vivo experiments were performed in triplicate and duplicate, respectively, and the results were subjected to statistical analysis by using one-way analysis of variance (ANOVA) with a Bonferroni correction posttest using GraphPad Prism version 5.04 for Windows (GraphPad Software, San Diego, CA).
RESULTS
Survival of R. equi in a mouse macrophage cell line after addition of eCATH1.
Before the assessment of the bactericidal activity of eCATH1 on R. equi inside J774.2 mouse macrophages, the effects of the peptide on the plasma membrane integrity of the host cells were evaluated by the LDH release assay. At a concentration of 20 μg/ml, eCATH1 exerted no detectable cytotoxic effect on J774.2 cells (data not shown).
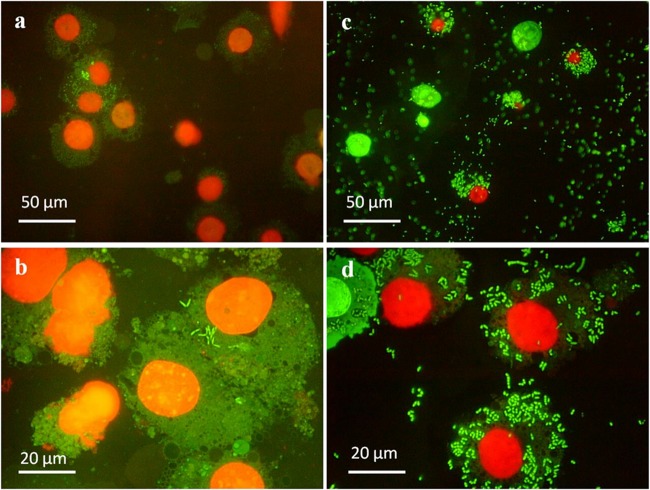
The activity of eCATH1 against the intracellular pathogen was first assessed by fluorescence microscopy. J774.2 macrophages were infected with R. equi for 45 min, and cells were subsequently incubated for 24 h with either 20 μg/ml of eCATH1 or the peptide solvent (negative control). Fluorescent staining of intracellular R. equi with the LIVE/DEAD Baclight bacterial-viability kit revealed clear differences in rhodococcal survival inside macrophages between the two conditions. Indeed, very few macrophages were found to be infected in the presence of peptide, and those cells contained substantially lower numbers of viable bacteria (stained in green) than observed in the negative control (Fig. 1). Moreover, the overall number of macrophages was higher in the presence of eCATH1 than in the nontreated sample. In the negative control, no dead bacteria (stained red) were observed, indicating that the virulent strain used in this study is able to successfully survive inside macrophages (Fig. 1c and d). Dead rhodococci were also not observed in the presence of eCATH1, but this was most likely the result of rapid lysis of bacteria by the peptide. Extracellular bacteria were observed in the negative control, but not in the treated sample. Before adding the peptide or its solvent, infected cells were carefully washed several times to remove any noninternalized bacteria. Therefore, the presence of extracellular bacteria might be due either to the necrotic death of macrophages after bacterial multiplication for 24 h; to the chemical action of saponin on the macrophage membrane, which may release bacteria; or to a mechanical rupture of eukaryotic membranes by the positioning of the coverslip.
Fig 1.
Activity of eCATH1 against R. equi residing in mouse macrophages as observed by fluorescence microscopy. Mouse macrophages of the cell line J774.2 were infected with a virulent strain of R. equi for 45 min and then treated with 20 μg/ml of eCATH1 (left) or the peptide solvent as a negative control (right) for 24 h. Following permeabilization of eukaryotic membranes by saponin, the viability of the rhodococci was assessed by staining with a bacterial-viability kit (viable cells are green, and dead cells are red). Nuclei of permeabilized macrophages appear red. Representative micrographs were taken at ×400 (a and c) and ×1,000 (b and d) magnification with a fluorescence microscope.
Survival of R. equi in mouse peritoneal macrophages.
To confirm the in vitro study, complementary ex vivo experiments were performed. For this, mouse macrophages were naturally infected in vivo and further collected for in vitro treatment with eCATH1 and rifampin before CFU enumeration. As the two antimicrobials exhibited a synergistic interaction in vitro against extracellular R. equi (19), the combination eCATH1 and rifampin in the present ex vivo model was also assessed. Before the ex vivo experiment, cytotoxicity of eCATH1 and rifampin on mouse peritoneal macrophages revealed that, similarly to the J774.2 cell line, eCATH1 did not exhibit significant toxicity at 20 μg/ml, whereas rifampin was found to exert significant toxicity at 20 μg/ml (data not shown) (ANOVA test; P < 0.05). The concentrations of antimicrobials chosen for use in the ex vivo experiment were therefore 20 μg/ml for eCATH1 and 0.5, 5, and 10 μg/ml for rifampin. Supporting the in vitro data, it was observed that eCATH1 produced significant killing (P < 0.001) of intracellular R. equi at a concentration of 20 μg/ml after 14 h of treatment, i.e., ∼60% decrease of R. equi in macrophages compared to the control (Fig. 2). The same killing rate was already found at 5 μg/ml of rifampin. Interestingly, the combination of both antimicrobials led to an even more significant decrease of the overall number of R. equi CFU residing in macrophages (∼90%; P < 0.00001) (Fig. 2). However, from these data, it was not possible to conclude whether this was synergy or only an additive effect.
Fig 2.
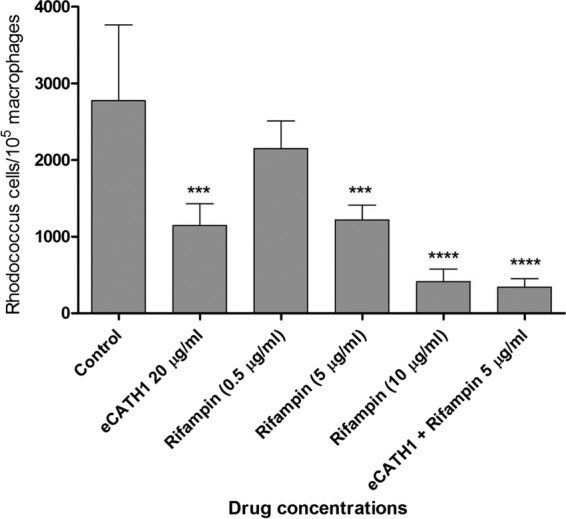
Bactericidal activity of eCATH1 against R. equi residing inside murine peritoneal macrophages. Following mouse infection with a virulent strain of R. equi, peritoneal macrophages were collected. The infected macrophages were further treated with eCATH1 (20 μg/ml), rifampin at various concentrations, or a combination of both drugs (20 μg/ml eCATH1 plus 5 μg/ml rifampin). Survival of R. equi inside macrophages was assessed 24 h postinfection by CFU enumeration. Experiments were done in triplicate, and the results are expressed as means and standard deviations (SD); statistical significance in comparison to the control is shown as follows: ***P < 0.001; **** P < 0.00001.
Activity of eCATH1 against R. equi-infected mice.
Before the therapeutic trials, comparison of the natural clearance of R. equi CFU from mouse organs for various bacterial inocula (107 to 109 CFU) was assessed (data not shown). Intravenous injection of 109 CFU of R. equi led to the death of mice after 2 to 4 days. In contrast, an inoculum of 108 CFU led to the onset of symptoms from days 1 to 6 postinfection, the in vivo multiplication of the pathogen, and progressive clearance of bacteria from spleen, liver, and lungs after 4 days. Therefore, this sublethal dose was selected for the therapeutic trials. Moreover, the lung was no longer used for bacterial counting in the therapeutic trials in our study, as a lower concentration of bacteria was found in that organ than in the spleen and liver.
For therapeutic trials, mice were infected with the sublethal dose of R. equi. One day later, therapy started, with daily injections of antimicrobials for 7 days. The results of CFU enumeration in spleen and liver are presented in Fig. 3. After 3 days of treatment, bacterial loads were significantly decreased in comparison to the control in a similar manner in spleen and liver. Indeed, 1 mg/kg of body weight of eCATH1, as well as 10 mg/kg of body weight of rifampin, led to a decrease of ∼2 log10 CFU in both organs (i.e., >99%) compared to the control. eCATH1 combined with rifampin (1 mg/kg and 10 mg/kg body weight, respectively) showed the highest antimicrobial activity in mice, with a decrease of ∼3 log10 CFU in organs (i.e., >99.9%).
Fig 3.
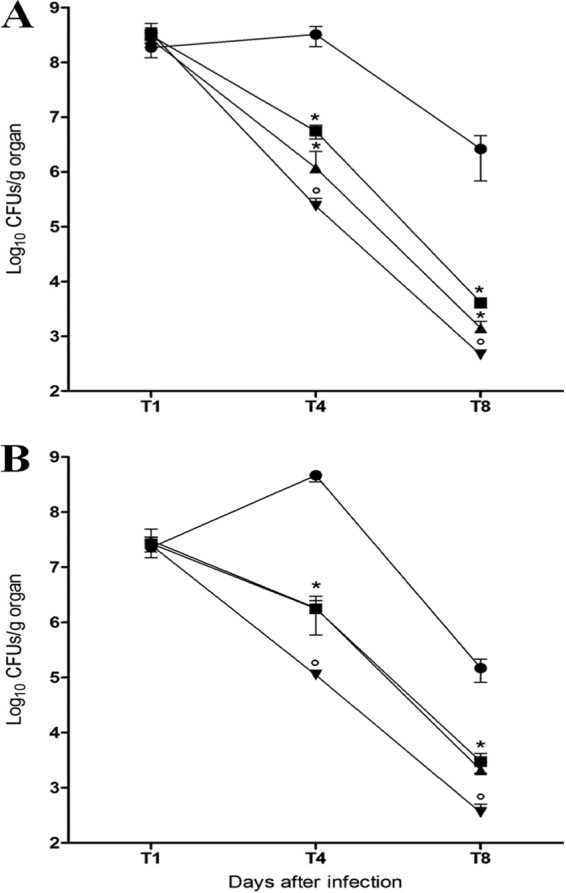
CFU enumeration in the spleens and livers of infected mice treated with eCATH1, rifampin, or a combination of both drugs. Mice were infected with 108 CFU of virulent R. equi via the tail vein. One day postinfection, drugs were subcutaneously injected daily for 7 days. After completion of the therapy, CFU were enumerated in preparations of spleens (A) and livers (B). The results are expressed as log10 CFU per gram of organ. Values are means ± SD for 5 animals in each group at each time point from two independent experiments. Significance: *, P < 0.05 (eCATH1 or rifampin versus no drug), and °, P < 0.05 (eCATH1 plus rifampin versus eCATH1 or rifampin). ●, no drug; ■, eCATH1, 1 mg/kg; ▲, rifampin, 10 mg/kg; ▼, eCATH1 plus rifampin.
In vivo toxicity of eCATH1.
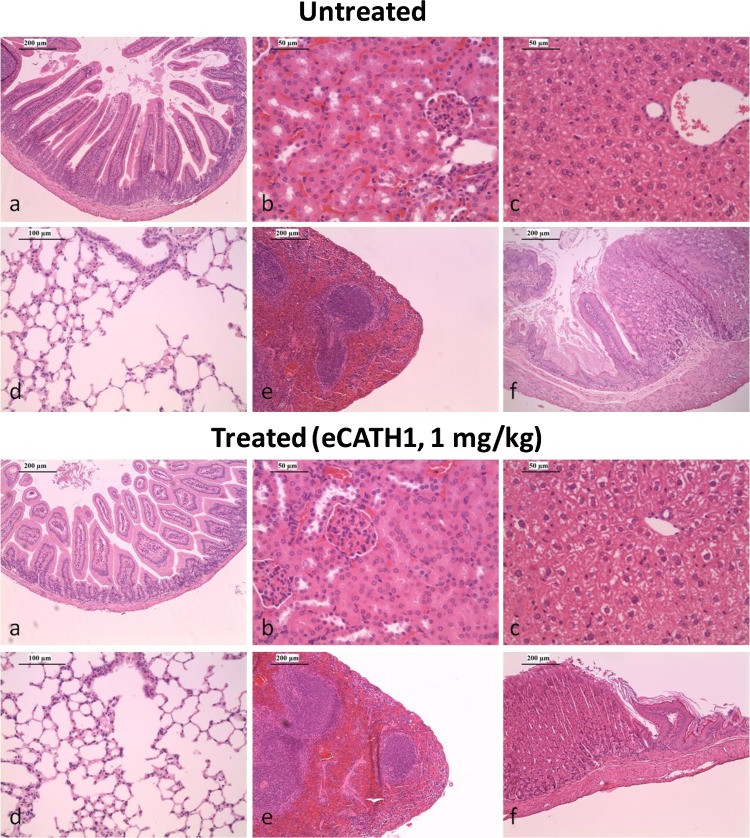
Daily injection of 1 mg/kg body weight of eCATH1 for 7 days did not result in any behavioral changes, differences in body weight, or clinical evidence of antimicrobial-related adverse effects (data not shown). Moreover, the histopathology analysis performed on the intestines, spleens, livers, lungs, kidneys, and stomachs of treated and nontreated mice did not reveal any histological changes between the groups (Fig. 4).
Fig 4.
Histopathology of organs from BALB/c mice upon eCATH1 treatment. The organs of untreated mice and mice receiving a daily subcutaneous dose of 1 mg/kg body weight of eCATH1 for 7 days were stained with hematoxylin and eosin. Representative examples are shown of small intestine (a), kidney (b), liver (c), lung (d), spleen (e), and stomach (f) sections. The histopathology analysis did not reveal any histological changes between treated and nontreated mice.
DISCUSSION
The data presented in this study represent the first demonstration of the intracellular antirhodoccoccal properties of an antimicrobial peptide by application of in vitro, ex vivo, and in vivo methods. Indeed, the present in vitro and ex vivo studies proved that eCATH1 remained active against bacteria inside the host cell, although a higher concentration was needed to reach the intracellular pathogen. These results support similar work in Mycobacterium species. Sharma et al. demonstrated in vitro the direct bactericidal activity of the human neutrophil peptide 1 (HNP-1) against intracellular Mycobacterium tuberculosis. The number of cells per macrophage was reduced by 1 log10 with 20 μg/ml and by 2 log10 with 40 μg/ml of peptide after 3 days of treatment (27). More recently, Jena et al., reported the activity of NK-2, a shortened peptide derived from the porcine NK-lysin, against intracellular Mycobacterium smegmatis. This study revealed that 10 μM peptide (∼30 μg/ml) decreased the number of bacteria per macrophage by ∼40% after 8 h of treatment (28).
From the mouse rhodococcosis model, the main conclusion is that eCATH1 remained effective against the facultative intracellular pathogen. Interestingly, the peptide appeared to be more active in vivo than in vitro in comparison to rifampin activity. A 4-fold-lower concentration of rifampin led to a decrease of rhodococci per macrophage similar to that with eCATH1 in vitro, while in vivo, a 10-fold-higher concentration of rifampin than of the peptide is required to equally decrease the bacterial load in organs. Previously, similar data were reported by Sharma et al. (26). As little as 1 to 5 μg of HNP-1 per mouse was found to decrease tuberculosis infection, while 40 μg/ml of peptide was needed to kill intramacrophage mycobacteria in ex vivo experiments (26, 27). These observations might indicate that eCATH1 has a more complex effect in vivo than in vitro. As previously proposed by Sharma et al., we hypothesize that the in vivo activity of eCATH1 is a combination of (i) immunomodulatory properties leading to indirect killing of bacteria and (ii) a direct killing effect on intra- and extracellular bacteria. Some reports in the literature about immunomodulatory properties of human and bovine cathelicidins could support our hypothesis. The human cathelicidin LL-37 exerts a chemoattractive activity on immune cells through direct interaction with granulocytes and mononuclear cell receptors and through induction of the production of chemokines, which hypothetically increase the numbers of neutrophils and monocytes at sites of infection (29–33). Interestingly, some bovine cathelicidins were also found to be chemoattractants for neutrophils and had the property of enhancing the phagocytic and degranulation activities of these cells (B. W. Paget, J. L. Harper, and B. J. Haigh, presented at the 3rd Antimicrobial Peptide Symposium, Lille, France, 13 to 15 June 2012). Moreover, macrophages have the ability to take up granules of apoptotic neutrophils to acquire AMPs that traffic via early endosomes to intracellular-pathogen-containing vacuoles, where they exert their bactericidal activity (34). Chemoattraction of immune cells, such as neutrophils, by eCATH1 might limit the spread of R. equi infection in the host in a similar way. Furthermore, this hypothesis would be in accordance with the observation that neutrophils are critical for the control of rhodococcosis (35). Nevertheless, the immunomodulatory properties of eCATH1 remain to be elucidated for a better understanding of the in vivo activities of the peptide. The mechanism by which exogenously applied AMPs might gain access to intracellular bacteria is not fully understood. Some clues, however, were first provided by Sharma et al. in 2000 (27). The studied peptide was initially found to localize at the macrophage membrane and then to be taken up inside the cell (27). In a pathway similar to that of neutrophil AMP uptake by macrophages discussed above (34), eCATH1 might be internalized by macrophages in early endosomes that would further fuse with R. equi-containing vacuoles, where the peptide could exert its bactericidal activity.
Interestingly, the slight positive interaction between eCATH1 and rifampin against extracellular rhodococci described previously was observed against the facultative intracellular bacterium in the in vivo setting. as well (19). Our observations are consistent with the work of Cirioni et al., who similarly reported a synergistic effect between rifampin and α-helical AMPs (magainin II and cecropin A) in Pseudomonas aeruginosa infection models in rats (25). It was hypothesized that AMPs allow rifampin, an RNA polymerase inhibitor, to access its intracellular target by permeabilization of the bacterial membrane (19, 36).
In vivo degradation by proteases, inactivation by a physiological salt concentration, rapid clearance by kidneys, and toxicity are often described in the literature as major limiting factors that hamper the development of AMPs as therapeutic options. In the present study, we proved that the bactericidal activity of eCATH1 was conserved in vivo at doses that might be compatible with clinical use without detectable deleterious effects for the host even after 7 days of daily subcutaneous injections. The results of the in vivo toxicity and activity studies presented here agree with our previous investigations of eCATH1. Indeed, we recently reported that the in vitro activity of eCATH1 was not hampered by a physiological salt concentration and that the peptide was not toxic for various mammalian cell types in vitro (19).
Taken together, our data suggest that eCATH1 might be a valuable template for a therapeutic molecule to treat rhodococcosis in both equids and humans in addition to antibiotics.
ACKNOWLEDGMENTS
This work was supported by grants from the European Regional Development Fund, the Regional Council of Low Normandy, the Institut Français du Cheval et de l'Equitation, and the French Agency for Food, Environmental and Occupational Health Safety.
We thank Jean-Loïc Le Net from the Veterinary Pathological Anatomy Laboratory (Amboise, France) for the excellent histopathology analysis performed.
Footnotes
Published ahead of print 1 July 2013
REFERENCES
- 1.Magnusson H. 1923. Spezifische infektiose pneumonie biem fohlen. Ein neurer eiterreger biem pferd. Arch. Wiss. Prakt. Tierheilk. 50:22–38 [Google Scholar]
- 2.Mauger C. 2009. Retrospective study of equine rhodococcosis observed at autopsy on 1617 foals at the “LERPE” (AFSSA, Dozulé) from 1986 to 2006. D.V.M. thesis. Université Paul-Sabatier de Toulouse, Toulouse, France: (In French.) [Google Scholar]
- 3.Laugier C. 2004. Rhodococcose: bilan partiel des cas enregistrés à l'autopsie en 2004 (du 1er janvier au 26 août). Bull. Réseau Epidémiosurveill. Pathol. Equine 13:3 [Google Scholar]
- 4.Takai S, Sasaki Y, Tsubaki S. 1995. Rhodococcus equi infection in foals: current concepts and implication for future research. J. Equine Sci. 6:105–119 [Google Scholar]
- 5.Yager JA. 1987. The pathogenesis of Rhodococcus equi pneumonia in foals. Vet. Microbiol. 14:225–232 [DOI] [PubMed] [Google Scholar]
- 6.Weinstock DM, Brown AE. 2002. Rhodococcus equi: an emerging pathogen. Clin. Infect. Dis. 34:1379–1385 [DOI] [PubMed] [Google Scholar]
- 7.Golub B, Falk G, Spink W. 1967. Lung abscess due to Corynebacterium equi. Report of first human infection. Ann. Intern. Med. 66:1174–1177 [DOI] [PubMed] [Google Scholar]
- 8.Topino S, Galati V, Grilli E, Petrosillo N. 2010. Rhodococcus equi infection in HIV-infected individuals: case reports and review of the literature. AIDS Patient Care STDS 24:211–222 [DOI] [PubMed] [Google Scholar]
- 9.Yamshchikov AV, Schuetz A, Lyon GM. 2010. Rhodococcus equi infection. Lancet Infect. Dis. 10:350–359 [DOI] [PubMed] [Google Scholar]
- 10.Heidmann P, Madigan JE, watson JL. 2006. Rhodococcus equi pneumonia: clinical findings, diagnosis, treatment and prevention. Clin. Tech. Equine Pract. 5:203–2010 [Google Scholar]
- 11.Asoh N, Watanabe H, Fines-Guyon M, Watanabe K, Oishi K, Kositsakulchai W, Sanchai T, Kunsuikmengrai K, Kahintapong S, Khantawa B, Tharavichitkul P, Sirisanthana T, Nagatake T. 2003. Emergence of rifampin-resistant Rhodococcus equi with several types of mutations in the rpoB gene among AIDS patients in northern Thailand. J. Clin. Microbiol. 41:2337–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyen F, Pasmans F, Haesebrouck F. 2011. Acquired antimicrobial resistance in equine Rhodococcus equi isolates. Vet. Rec. 168:101. [DOI] [PubMed] [Google Scholar]
- 13.Buckley TM, Stanbridge ES. 2007. Resistance studies of erythromycine and rifampin for Rhodococcus equi over a 10-year period. Irish Vet. J. 60:728–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenney DG, Robbins SC, Prescott JF, Kaushik A, Baird JD. 1994. Development of reactive arthritis and resistance to erythromycin and rifampin in a foal during treatment for Rhodococcus equi pneumonia. Equine Vet. J. 26:246–248 [DOI] [PubMed] [Google Scholar]
- 15.Hancock RE, Sahl HG. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24:1551–1557 [DOI] [PubMed] [Google Scholar]
- 16.Levy SB, Marshall B. 2004. Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 10:S122–S129 [DOI] [PubMed] [Google Scholar]
- 17.Zucca M, Savoia D. 2010. The post-antibiotic era: promising developments in the therapy of infectious diseases. Int. J. Biomed. Sci. 6:77–85 [PMC free article] [PubMed] [Google Scholar]
- 18.Steinstraesser L, Kraneburg UM, Hirsch T, Kesting M, Steinau HU, Jacobsen F, Al-Benna S. 2009. Host defense peptides as effector molecules of the innate immune response: a sledgehammer for drug resistance? Int. J. Mol. Sci. 10:3951–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlusselhuber M, Jung S, Bruhn O, Goux D, Leippe M, Leclercq R, Laugier C, Grötzinger J, Cauchard J. 2012. In vitro potential of equine DEFA1 and eCATH1 as alternative antimicrobial drugs in rhodococcosis treatment. Antimicrob. Agents Chemother. 56:1749–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thulin P, Johansson L, Low DE, Gan BS, Kotb M, McGeer A, Norrby-Teglund A. 2006. Viable group A streptococci in macrophages during acute soft tissue infection. PLoS Med. 3:e53. 10.1371/journal.pmed.0030053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verneuil N, Sanguinetti M, Le Breton Y, Posteraro B, Fadda G, Auffray Y, Hartke A, Giard JC. 2004. Effects of the Enterococcus faecalis hypR gene encoding a new transcriptional regulator on oxidative stress response and intracellular survival within macrophages. Infect. Immun. 72:4424–4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gentry-Weeks CR, Karkhoff-Schweizer R, Pikis A, Estay M, Keith JM. 1999. Survival of Enterococcus faecalis in mouse peritoneal macrophages. Infect. Immun. 67:2160–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takai S, Sasaki Y, Tsubaki S. 1992. Influence of inoculation route on virulence of Rhodococcus equi in mice. Microbiol. Immunol. 36:895–898 [DOI] [PubMed] [Google Scholar]
- 24.Nordmann P, Kerestedjian JJ, Ronco E. 1992. Therapy of Rhodococcus equi disseminated infections in nude mice. Antimicrob. Agents Chemother. 36:1244–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cirioni O, Silvestri C, Ghiselli R, Orlando F, Riva A, Mocchegiani F, Chiodi L, Castelletti S, Gabrielli E, Saba V, Scalise G, Giacometti A. 2008. Protective effects of the combination of alpha-helical antimicrobial peptides and rifampicin in three rat models of Pseudomonas aeruginosa infection. J. Antimicrob. Chemother. 62:1332–1338 [DOI] [PubMed] [Google Scholar]
- 26.Sharma S, Verma I, Khuller GK. 2001. Therapeutic potential of human neutrophil peptide 1 against experimental tuberculosis. Antimicrob. Agents Chemother. 45:639–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma S, Verma I, Khuller GK. 2000. Antibacterial activity of human neutrophil peptide-1 against Mycobacterium tuberculosis H37Rv: in vitro and ex vivo study. Eur. Respir. J. 16:112–117 [DOI] [PubMed] [Google Scholar]
- 28.Jena P, Mishra B, Leippe M, Hasilik A, Griffiths G, Sonawane A. 2011. Membrane-active antimicrobial peptides and human placental lysosomal extracts are highly active against mycobacteria. Peptides 32:881–887 [DOI] [PubMed] [Google Scholar]
- 29.Yang BD, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. 2000. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192:1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, Nagaoka I. 2002. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology 106:20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowdish DM, Davidson DJ, Speert DP, Hancock RE. 2004. The human cationic peptide LL-37 induces activation of the extracellular signal-regulated kinase and p38 kinase pathways in primary human monocytes. J. Immunol. 172:3758–3765 [DOI] [PubMed] [Google Scholar]
- 32.Bowdish DM, Davidson DJ, Lau YE, Lee K, Scott MG, Hancock RE. 2005. Impact of LL-37 on anti-infective immunity. J. Leukoc. Biol. 77:451–459 [DOI] [PubMed] [Google Scholar]
- 33.Mookherjee N, Brown KL, Bowdish DM, Doria S, Falsafi R, Hokamp K, Roche FM, Mu R, Doho GH, Pistolic J, Powers JP, Bryan J, Brinkman FS, Hancock RE. 2006. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 176:2455–2464 [DOI] [PubMed] [Google Scholar]
- 34.Tan BH, Meinken C, Bastian M, Bruns H, Legaspi A, Ochoa MT, Krutzik SR, Bloom BR, Ganz T, Modlin RL, Stenger S. 2006. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J. Immunol. 177:1864–1871 [DOI] [PubMed] [Google Scholar]
- 35.Martens RJ, Cohen ND, Jones SL, Moore TA, Edwards JF. 2005. Protective role of neutrophils in mice experimentally infected with Rhodococcus equi. Infect. Immun. 73:7040–7042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cassone M, Otvos L., Jr 2010. Synergy among antibacterial peptides and between peptides and small-molecule antibiotics. Expert Rev. Anti Infect. Ther. 8:703–716 [DOI] [PubMed] [Google Scholar]