Abstract
We compared the fitness and lung pathogenicity of two isogenic clinical isolates of Acinetobacter baumannii, one resistant (ABCR) and the other susceptible (ABCS) to colistin. In vitro, ABCR exhibited slower growth kinetics than ABCS. In a rat model of pneumonia, ABCR was associated with less pronounced signs of infection (lung bacterial count, systemic dissemination, and lung damage) and a better outcome (ABCR and ABCS mortality rates, 20 and 50%, respectively [P = 0.03]).
TEXT
The emergence of colistin-resistant strains of Acinetobacter baumannii is perceived as a formidable threat in clinical settings. Recent contradictory clinical observations and experimental data have questioned the virulence of such strains (1, 2, 3). Experimental models of surgical infections have previously assessed the virulence of A. baumannii with in vitro-induced colistin resistance (4), but a specific model of pneumonia with parental clinical isolates is required to focus on lung pathogenicity.
We compared the fitness and virulence, in vitro and in vivo, of A. baumannii according to its sensitivity to colistin in a rat model of acute pneumonia using two isogenic clinical strains.
Two strains of A. baumannii were successively isolated from the respiratory tract of a patient with ventilator-associated pneumonia. The first was colistin susceptible (ABCS), and the second was colistin resistant (ABCR). Whole-genome sequencing confirmed that they were parental strains, with ABCR differing from ABCS by a mutation in the pmrA and rpoB genes and by the loss of a prophage (5, 6).
The ABCS strain was obtained from bronchoalveolar lavage fluid (colistin MIC = 0.064 mg/liter) (5). The ABCR strain was isolated from tracheal aspirates after the patient had received intravenous colistin (colistin MIC = 32 mg/liter) (5). Apart from the colistin and rifampin susceptibility of ABCS, both strains were resistant to all of the antibiotics tested, including cefepime and sulbactam.
The 24-h growth curves of ABCR, ABCS, and reference strain AYE (7) showed a reduced slope in the exponential phase for ABCR versus ABCS (0.14 ± 0.0038 versus 0.18 ± 0.0053 [P = 0.01]) and versus AYE (0.14 ± 0.0038 versus 0.23 ± 0.0066 [P = 0.003]), indicating slower growth of ABCR bacteria.
Both the ABCR and ABCS strains were tested for virulence in an animal model of acute pneumonia. Adult Sprague-Dawley male pathogen-free rats (weighing 300 to 350 g) were used for in vivo experiments after approval was obtained from the departmental ethics committee (study agreement 58-08112012).
Sixty animals received 250 μl of phosphate-buffered saline (PBS) containing 109 CFU/ml of ABCR (n = 30) or ABCS (n = 30) and 250 μl of porcine mucin diluted to 10% via the tracheal route. Ten controls received only 250 μl of PBS and 250 μl of porcine mucin. The follow-up period lasted 48 h with daily body weight recording. After death, blood and spleen samples were cultured to assess bacteremia and the right lung was cultured. A histological severity score (HSS; minimum, 0; maximum, 4) was calculated on the basis of the number of bronchopneumonia lesions present in the left lung (8).
The data were expressed as the mean ± standard deviation or the median and interquartile range according to the distribution of the data. The difference in bacterial lung counts was analyzed by the Mann-Whitney test. Data analysis was performed with SPSS for Windows, version 12.0 (SPSS, Chicago, IL). P ≤ 0.05 was considered statistically significant.
The results showed that 6 (20%) of the 30 rats infected with ABCR died spontaneously during the first 48 h, compared with 15 (50%) of 30 in the ABCS group (P = 0.03). None of the 10 control animals died (Fig. 1).
Fig 1.
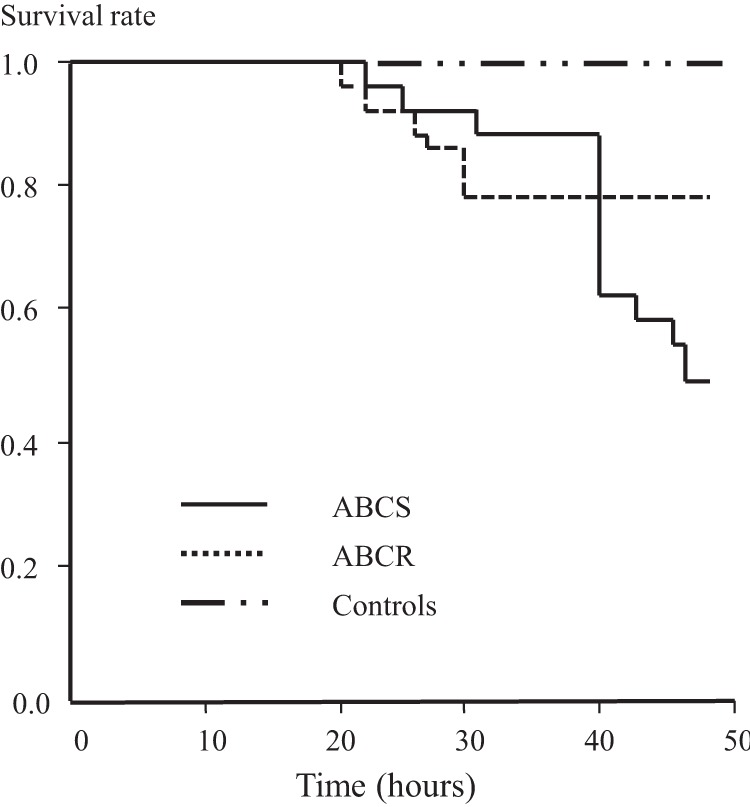
Forty-eight-hour survival curves of animals infected with the same inoculum of ABCS or ABCR compared to that of control (uninfected) animals. The log rank of the Kaplan-Meier curve was P = 0.04 between the ABCR and ABCS curves.
The decrease in body weight was less pronounced in the ABCR group than in the ABCS group, with end follow-up values of 295 ± 55 g for ABCR and 263 ± 39 g for ABCS (P = 0.029).
The weight of the right lung indexed to body weight was lower in the ABCR group than in the ABCS group (6 [5.5 to 7.2] versus 9.2 [6.5 to 13.8] g/kg, respectively [P < 0.001]), attesting to less intense edema and lung injury. The indexed lung weight in the control group was 2.5 [2 to 3.2] g/kg.
The lung bacterial count was significantly lower in the ABCR group than in the ABCS group (1.4 × 106 [5.3 × 105 to 1.1 × 107] versus 1.6 × 109 [8.5 × 106 to 2.6 × 1010] CFU/g of lung, respectively [P = 0.011]).
A lower percentage of the animals in the ABCR group showed bacteremia (15% versus 63% of those in the ABCS group [P < 0.001]).
The HSS was significantly lower in ABCR rats than in ABCS rats (2.5 ± 0.4 versus 3.8 ± 0.2, respectively [P = 0.02]). In the ABCR group, some areas of normal lung parenchyma could be observed and no abscesses were detected, in contrast to the ABCS group (Fig. 2).
Fig 2.
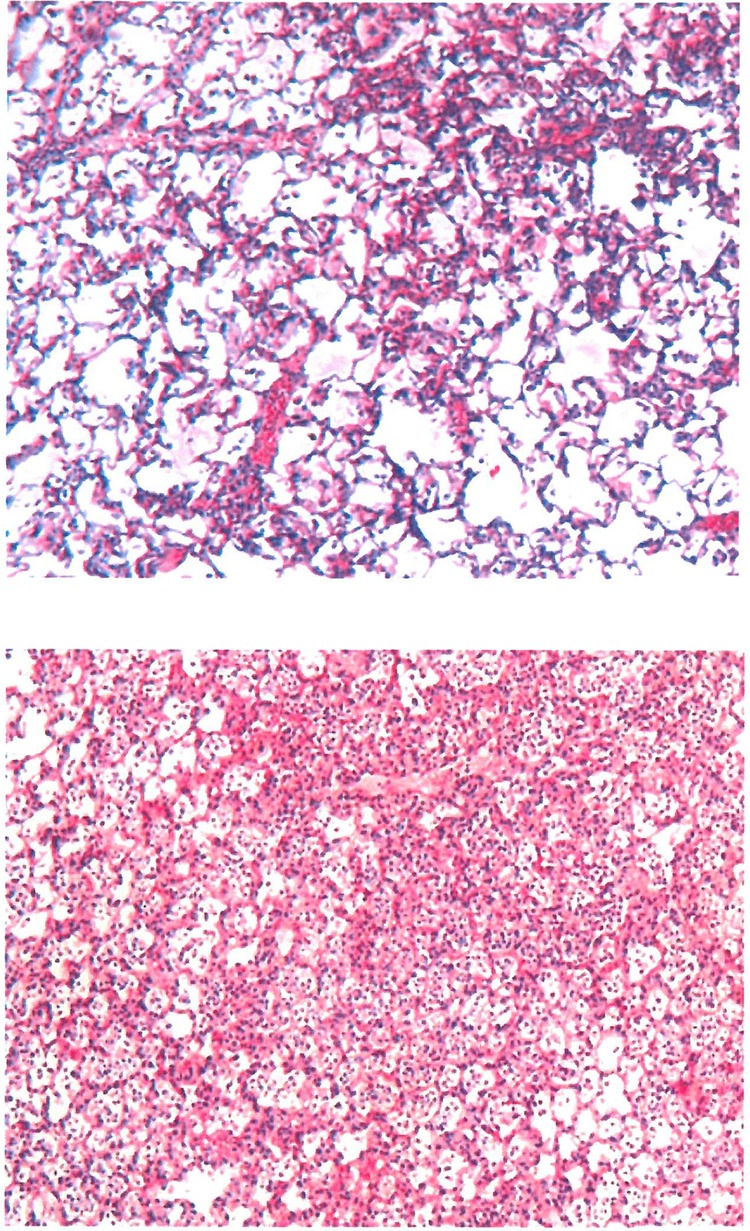
Pathological mapping of lung tissue samples representative of the ABCR and ABCS groups. Giemsa staining and a magnification of ×50 were used. ABCR lung tissue showed fewer confluent lesions of bronchopneumonia with areas of preserved lung architecture, in contrast to ABCS lung tissue.
In the present work, we developed a reproducible and reliable rat model of acute pneumonia with strains that emerged from environmental selection and showed that the colistin-resistant strain of A. baumannii had reduced growth kinetics and was less virulent than the colistin-susceptible one.
Mutation-induced changes in the expression of membrane proteins, cytoplasmic activation of signal factors, and metabolic enzymes have been suggested to be responsible for a reduction in virulence in colistin-resistant A. baumannii (9). A reduction of biofilm-forming ability related to the acquisition of colistin resistance by A. baumannii (10) has also been suggested to explain the loss of virulence.
Mechanisms of A. baumannii colistin resistance were recently reviewed in detail (1) and may involve (i) total loss of lipopolysaccharide and (ii) PmrAB two-component system-regulated lipid A modification. In our ABCR strain, resistance to colistin was associated with mutations in the PmrA (E8D)-encoding gene (5), arguing that the reduced virulence we report here was strongly associated with the colistin resistance phenotype of the ABCR strain. Differences between the two strains also included the loss of a prophage in the ABCR strain compared with the ABCS strain (5), which may have contributed to the decrease in in vivo virulence.
Our study demonstrated the reduced virulence and lung pathogenicity of the ABCR strain and is in agreement with the clinical outcome of the patient it came from (2). It would, however, be premature to extend our findings to other clinical settings or strains.
ACKNOWLEDGMENTS
We acknowledge C. Guervilly for his contributions to the figures and statistics in this report and L. Hadjaj and A. Barnau for their valuable help.
There are no conflicts of interest to declare.
Funding was provided by a grant from URMITE.
Footnotes
Published ahead of print 8 July 2013
REFERENCES
- 1.Beceiro A, Tomás M, Bou G. 2013. Antimicrobial resistance and virulence: a successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 26:185–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rolain JM, Roch A, Castanier M, Papazian L, Raoult D. 2011. Acinetobacter baumannii resistant to colistin with impaired virulence: a case report from France. J. Infect. Dis. 204:1146–1147 [DOI] [PubMed] [Google Scholar]
- 3.López-Rojas R, Jimenez-Mejias ME, Lepe JA, Pachon J. 2011. Acinetobacter baumannii resistant to colistin alters its antibiotic resistance profile: a case report from Spain. J. Infect. Dis. 204:1147–1148 [DOI] [PubMed] [Google Scholar]
- 4.López-Rojas R, Dominguez-Herrera J, McConnell MJ, Docobo-Perez F, Smani Y, Fernández-Reyes M, Rivas L, Pachon J. 2011. Impaired virulence and in vivo fitness of colistin-resistant Acinetobacter baumannii. J. Infect. Dis. 203:545–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rolain JM, Diene SM, Kempf M, Gimenez G, Robert C, Raoult D. 2013. Real-time sequencing to decipher the molecular mechanism of resistance of a clinical pan-drug-resistant Acinetobacter baumannii isolate from Marseille, France. Antimicrob. Agents Chemother. 57:592–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kempf M, Rolain JM, Azza S, Diene S, Joly-Guillou ML, Dubourg G, Colson P, Papazian L, Richet H, Fournier PE, Ribeiro A, Raoult D. 2013. Investigation of Acinetobacter baumannii resistance to carbapenems in Marseille hospitals, south of France: a transition from an epidemic to an endemic situation. APMIS 121:64–71 [DOI] [PubMed] [Google Scholar]
- 7.Fournier PE, Vallenet D, Barbe V, Audic S, Ogata H, Poirel L, Richet H, Robert C, Mangenot S, Abergel C, Nordmann P, Weissenbach J, Raoult D, Claverie JM. 2006. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2:e7. 10.1371/journal.pgen.0020007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hraiech S, Brégeon F, Brunel JM, Rolain JM, Lepidi H, Andrieu V, Raoult D, Papazian L, Roch A. 2012. Antibacterial efficacy of inhaled squalamine in a rat model of chronic Pseudomonas aeruginosa pneumonia. J. Antimicrob. Chemother. 67:2452–2458 [DOI] [PubMed] [Google Scholar]
- 9.Fernández-Reyes M, Rodriguez-Falcon M, Chiva C, Pachon J, Andreu D, Rivas L. 2009. The cost of resistance to colistin in Acinetobacter baumannii: a proteomic perspective. Proteomics 9:1632–1645 [DOI] [PubMed] [Google Scholar]
- 10.Li J, Nation RL, Owen RJ, Wong S, Spelman D, Franklin C. 2007. Antibiograms of multidrug-resistant clinical Acinetobacter baumannii: promising therapeutic options for treatment of infection with colistin-resistant strains. Clin. Infect. Dis. 45:594–598 [DOI] [PubMed] [Google Scholar]


