Abstract
The effect of antichagasic benznidazole (BZL; 100 mg/kg body weight/day, 3 consecutive days, intraperitoneally) on biotransformation systems and ABC transporters was evaluated in rats. Expression of cytochrome P-450 (CYP3A), UDP-glucuronosyltransferase (UGT1A), glutathione S-transferases (alpha glutathione S-transferase [GST-α], GST-μ, and GST-π), multidrug-resistance-associated protein 2 (Mrp2), and P glycoprotein (P-gp) in liver, small intestine, and kidney was estimated by Western blotting. Increases in hepatic CYP3A (30%) and GST-μ (40%) and in intestinal GST-α (72% in jejunum and 136% in ileum) were detected. Significant increases in Mrp2 (300%) and P-gp (500%) proteins in liver from BZL-treated rats were observed without changes in kidney. P-gp and Mrp2 were also increased by BZL in jejunum (170% and 120%, respectively). In ileum, only P-gp was increased by BZL (50%). The activities of GST, P-gp, and Mrp2 correlated well with the upregulation of proteins in liver and jejunum. Plasma decay of a test dose of BZL (5 mg/kg body weight) administered intraduodenally was faster (295%) and the area under the concentration-time curve (AUC) was lower (41%) for BZL-pretreated rats than for controls. The biliary excretion of BZL was higher (60%) in the BZL group, and urinary excretion of BZL did not show differences between groups. The amount of absorbed BZL in intestinal sacs was lower (25%) in pretreated rats than in controls. In conclusion, induction of biotransformation enzymes and/or transporters by BZL could increase the clearance and/or decrease the intestinal absorption of coadministered drugs that are substrates of these systems, including BZL itself.
INTRODUCTION
Chagas disease, or American trypanosomiasis, is a neglected illness that affects at least 8 million people in areas of disease endemicity in Latin America, and another 100 million people in the world are at risk of infection. The etiological agent of Chagas disease is the intracellular obligatory parasite Trypanosoma cruzi, a hemoflagellate protozoan that is transmitted to humans by hematophagous insect vectors. However, other means of transmission, such as blood transfusion, organ transplantation, congenital transmission, and laboratory accidents, have been reported. Once the parasite enters the body, all types of nucleated cells in the human host are potential targets for infection (1, 2). The occurrence of this zoonosis in areas where the disease is not endemic, such as the United States and Europe, was reported to be mainly due to the migration of infected people (3).
Benznidazole (BZL) is the only available drug for treatment of Chagas disease in most countries where it is endemic. It was reported that BZL is metabolized by a trypanosomal NADH-dependent type I nitroreductase that renders the cytotoxic and mutagenic agent glyoxal (4). In mammalians, the nitro group is reduced to an amino group by a type II nitroreductase, with formation of free-radical intermediaries and reactive oxygen species (ROS) (4, 5).
Organs such as liver, intestine, and kidney play a key role in the metabolism and elimination of endogenous and exogenous compounds. Biotransformation involves phase I reactions catalyzed by isoforms of cytochrome P-450 (CYP) and phase II reactions catalyzed by glutathione S-transferases (GST) and UDP-glucuronosyltransferases (UGT), among others. Metabolite excretion is mediated mainly by members of the ATP-binding cassette (ABC) family of transporters, such as P glycoprotein (P-gp/ABCB1/MDR1) and multidrug-resistance-associated protein 2 (Mrp2/ABCC2). P-gp transports a broad diversity of lipophilic and cationic compounds, including environmental pollutants and therapeutic agents such as cyclosporine, ritonavir, digoxin, and erythromycin. Mrp2 extrudes organic anions like bilirubin, bile salts, carcinogens, and therapeutic drugs (acetaminophen, ethynylestradiol, diclofenac, etc.) in the form of conjugates with glutathione (GSH), glucuronic acid, or sulfate (6).
The relationship between biotransformation systems and transporters and its significance in drug disposition is well-recognized (7). Changes in these proteins by endo- and xenobiotics may have a clinical impact on the normal functions of tissues with pharmacological and toxicological relevance, as mentioned above. Under these circumstances, the efficacy or toxicity of a very broad range of xenobiotics, including therapeutic agents, could be modified (6, 8).
Antiparasitic drugs have been shown to modulate biotransformation and transporter genes with an important impact on drug disposition. Bapiro et al. (9) demonstrated that quinine and albendazole induced CYP isoforms in HepG2 cells at concentrations equivalent to those achieved in therapeutic protocols, warning about the risk of combining quinine or albendazole with other drugs that are metabolized by these systems. During antimalarial treatment with artemisinin, disease reactivation was associated with decreased artemisinin plasma levels. The authors of this work postulate that artemisinin induces its own elimination, probably by increasing first-pass metabolism (10). Consistent with this postulate, Burk et al. (11) have demonstrated that LS174T cells and primary human hepatocytes treated with artemisinin show selective induction of some isoforms of CYP and MDR1 mRNA expression.
Recently we observed that BZL induces the expression and activities of CYP3A4, GST-π, P-gp, and MRP2 in a concentration-dependent manner in HepG2 cells, used as model of human hepatocytes (12). However, at present there is no information concerning the expression and activities of biotransformation systems and transporters in an in vivo model and the potential consequences in pharmacokinetics of BZL or coadministered drugs. A study carried out with patients that received BZL for 30 days (7 mg/kg body weight [b.w.]day, twice a day) showed that the maximal plasma concentration after the first dose in the morning tends to decrease with treatment time (∼20% on average after 25 days of treatment) (13). This finding suggests an increase in BZL metabolism and/or excretion that needs to be experimentally demonstrated.
Here we evaluate the expression and activities of biotransformation enzymes and transporter proteins in liver, intestine, and kidney from BZL-treated rats and how these changes modify the disposition of BZL.
MATERIALS AND METHODS
Chemicals.
Benznidazole (N-benzyl-2-nitroimidazol acetamide), 1-chloro-2,4-dinitrobenzene (CDNB), reduced glutathione (GSH), rhodamine 123 (Rh123), phenylmethylsulfonyl fluoride, pepstatin A, sucrose, and leupeptin were from Sigma-Aldrich (St. Louis, MO). Dimethyl sulfoxide (DMSO) was purchased from Merck (Darmstadt, Germany). All other chemicals were of analytical grade purity.
Animals and treatment.
Adult male Wistar rats (320 to 380 g) were used throughout. They were maintained ad libitum on a standard laboratory pellet diet and were allowed free access to water during treatment. Rats were randomly divided into two experimental groups. BZL dissolved in DMSO:propylene glycol (2:13 vehicle) was administered intraperitoneally (i.p.) in daily doses of 100 mg/kg b.w./day (14) for 3 consecutive days (BZL group). Control rats (C group) were injected with vehicle according to the same schedule. Preliminary experiments using other sets of rats that received daily doses of 25 or 50 mg/kg b.w./ day for 3 consecutive days (14) did not show statistical differences in the expression of P-gp and Mrp2. All procedures were conducted in accordance with NIH guidelines for the care and use of laboratory animals (15). Studies were performed 24 h after the last injection of BZL or vehicle.
Specimen collection.
Animals were anesthetized with pentobarbital (50 mg/kg b.w., i.p.) and sacrificed by exsanguination. Liver, kidney, proximal jejunum, and distal ileum were used in the studies. Liver-mixed plasma membranes (MPM) were prepared by differential centrifugation as described previously (16). Renal cortex was isolated and brush border membranes (BBM) were obtained by Mg-EGTA precipitation as described previously (17), with some modifications (18). For collection of the proximal jejunum, the first 10 cm starting from the pylorus valve and corresponding to the duodenum were excluded, and the following 30 cm were considered the proximal jejunum. The last 30 cm of small intestine, proximal to the ileocecal valve, were considered the distal ileum. These segments were carefully rinsed with ice-cold saline. For Western blot analysis, they were immediately opened lengthwise, the mucus layer was carefully removed, and mucosa was obtained by scraping, weighed, and used for BBM preparation (19). MPM and BBM were used for P-gp and Mrp2 immunoquantifications.
Microsomal fractions and cytosols were obtained from aliquots of the four tissues as described previously (20) and were used for UGT and GST protein detection, respectively, and for GST activity.
The protein concentrations in all these preparations were measured using bovine serum albumin as a standard (21).
Western blot analysis.
Western blotting and band quantification were performed as previously described (20, 22). The primary antibodies used were GST-α and GST-μ (GS9 and GS23, respectively; Oxford Biomedical Research, Rochester Hills, MI), GST-π (Immunotech, Marseille, France), Mrp2 (M2III-6; Enzo Life Sciences, Farmingdale, NY), and CYP3A and P-gp (H-300 and H-241, respectively; Santa Cruz Biotechnology, Santa Cruz, CA). UGT1A was detected using a rabbit polyclonal anti-rat antibody (23). Equal loading and transfer of protein were checked by detection of β-actin using a monoclonal antibody (A-2228; Sigma-Aldrich) and by Ponceau S staining of the membranes.
GST activity.
GST activity was measured according to the method of Habig et al. (24), based on the enzymatic conjugation of CDNB with GSH, thus generating dinitrophenyl-glutathione (DNP-SG). The reaction mixture contained cytosol from liver, jejunum, or ileum, phosphate-buffered saline (PBS; pH 6.50), and 1 mM CDNB. The reaction was initiated by the addition of GSH (1 mM). Formation of DNP-SG was determined spectrophotometrically at 340 nm.
P-gp and Mrp2 in vivo activities.
The activities of P-gp and Mrp2 were evaluated through assessment of the excretion rates of model substrates. To estimate P-gp activity, we used Rh123 as the substrate because it diffuses freely into the cells and then is actively extruded by this transporter (25). Mrp2 activity was evaluated through determination of the secretion rates of DNP-SG, a model substrate for Mrp2 generated endogenously after systemic administration of CDNB, and its major derivative, dinitrophenyl-cysteinylglycine (DNP-CG), resulting in gamma-glutamyl transferase action on DNP-SG at the luminal side of the secretory epithelia (26, 27).
Both studies were performed with the animals under pentobarbital anesthesia, using the in situ single-pass intestinal-perfusion technique (28) with simultaneous collections of bile and urine. After a 30-min stabilization period, a single bolus of Rh123 (0.52 μmol/kg b.w. in 1:19 dimethyl sulfoxide-saline) was administered intravenously (i.v.) for evaluation of P-gp activity. Another set of rats received CDNB (30 μmol/kg b.w. in 1:19 dimethyl sulfoxide-saline, i.v.) for estimation of Mrp2 activity.
Bile, urine, and intestinal perfusate were collected for 90 min at 10-, 30-, and 15-min intervals, respectively. Saline was administered intravenously throughout the experiment to replenish body fluids. At the end of the experiment, livers, kidneys, and perfused intestinal segments were removed and weighed. Bile, urine, and perfusate volumes were determined gravimetrically. Samples of bile, urine, and perfusate were used for assessment of Rh123 by spectrofluorometry (excitation wavelength, 485 nm; emission wavelength, 528 nm) (25) or of DNP-SG and its derivative by high-performance liquid chromatography (HPLC) (29).
Mrp2 transport activity in isolated hepatocytes.
Hepatocytes were isolated by collagenase perfusion and mechanical dissociation (30). The cells, suspended in Krebs-Henseleit Ringer-HEPES (N-2-hydroxyethylpiperazine-NÍ-2-ethanesulfonic acid) buffer (pH 7.40), were used for the determination of DNP-SG content and excretion rate. Protein concentration was determined using bovine serum albumin as the standard (21). Cell viability was determined by trypan blue exclusion and was always greater than 90%. Preloading of the hepatocytes with DNP-SG was performed by incubating the cells with CDNB (100 mM in Krebs-Henseleit Ringer-HEPES buffer [pH 7.40]) at 10°C to avoid enzymatic conjugation and transport, as described previously (31). Aliquots of cell suspensions (70,000 cells) were taken, loaded in test tubes (Beckman-type 0.4-ml polyethylene tubes) containing a lysis solution (3 M NaCl–0.1% Triton X-100) and a silicone layer (Wacker-Chemie GmbH, Munich, Germany), and incubated at 37°C for 0, 30, 60, and 90 s in CDNB-free buffer. At the end of the incubation period, the suspensions were centrifuged at 9,000 × g for 20 s. DNP-SG concentrations in the supernatants were determined by HPLC. The initial excretion rate was estimated as the slope of the regression curve of the amount of DNP-SG present in the supernatants per milligram of hepatocyte protein over time (22).
BZL pharmacokinetics.
Another set of control and BZL-treated (100 mg/kg b.w./day; 3 consecutive days) rats were used to estimate the elimination rate of BZL. For this purpose, 24 h after the last injection rats were anesthetized and bile ducts, bladders, and femoral veins were cannulated. After a 15-min stabilization period, a single bolus of BZL (5 mg/kg b.w. in saline) was intraduodenally administered. Then bile and urine were collected at designated intervals over 90 min and volumes were determined gravimetrically. Samples of venous blood were taken from the tails at selected periods of time during the experiment. Saline was administered intravenously to replenish body fluids. Bile, urine, and serum samples were subjected to solvent extraction with acetonitrile-DMSO (1:1) and deproteinized with 10% trichloroacetic acid. BZL was measured by HPLC (Waters 600; Waters, Milford, MA). Isocratic elution was performed with a C18 column (Luna 5 μ; Phenomenex) with a mobile phase of acetonitrile and water (2:3, vol/vol) at a flow rate of 1.0 ml/min as described by Morilla et al. (32). BZL was detected at 324 nm and quantified by the external standard method by the height of the peak. A one-compartment pharmacokinetic analysis was applied to the plasma concentration-versus-time data using the computer program GraphPad Prism. The area under the plasma concentration-versus-time curve (AUC) after intraduodenal administration of BZL was calculated using the linear trapezoidal rule up to the last measured plasma concentration (90 min). The first-order rate constant of elimination (k) was calculated through regression analysis of the straight tail of the curve from 30 to 90 min.
An additional group of pretreated animals (BZL, 100 mg/kg b.w./day, 3 consecutive days) was also used as a control to evaluate the plasma decay and biliary and urinary excretion rates of remnant BZL 24 h after the last injection. The data obtained with this last group were deducted from data for the BZL-pretreated group that received the BZL test dose.
BZL intestinal absorption.
In a different set of rats, the jejunum was removed, gently rinsed with ice-cold saline, and immediately used to measure the mucosa-to-serosa transport of BZL. Three-centimeter segments were filled with 750 μl of Krebs-Henseleit buffer (40 mM glucose [pH 7.40]) previously gassed with carbogen (O2CO2; 95:5) containing 50 μM BZL (mucosal compartment). They were incubated in 7 ml of the same buffer (serosal compartment) (29). The serosal medium was continuously gassed with O2CO2 (95:5), and aliquots of 100 μl were sampled for a 20-min period. Then the sacs were gently dried with filter paper and weighed. Serosal BZL concentration was determined by HPLC (32) as described above in “BZL pharmacokinetics.”
Statistical analysis.
Data are presented as means ± standard deviations (SDs). Statistical analysis was performed using the Student t test. Significance was set at P < 0.05. Studies were performed using GraphPad Prism 3.0 software (GraphPad Software, La Jolla, CA).
RESULTS
Effect of BZL on expression and activity of biotransformation systems and ABC transporters.
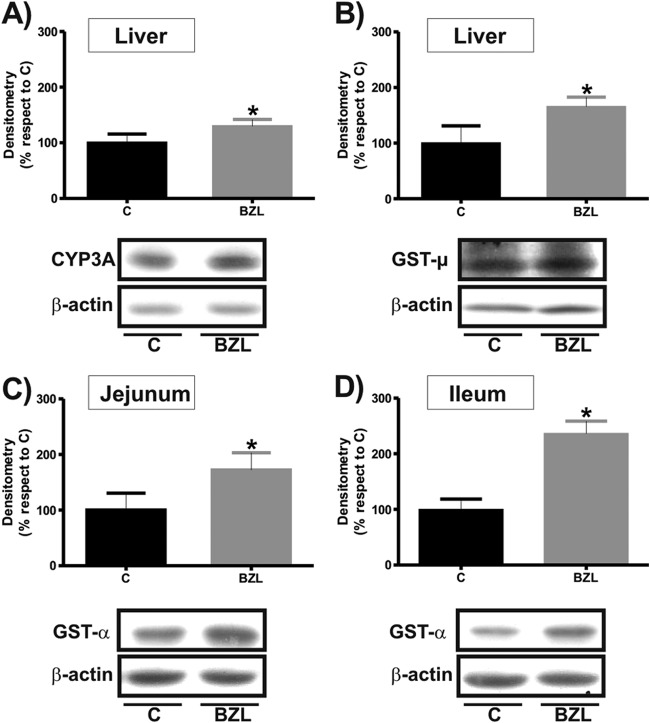
We first evaluated the effect of BZL treatment (100 mg/kg b.w./day, 3 consecutive days) on the biotransformation systems CYP450, UGT, and GST, whose participation usually precedes the transport process. As shown in Fig. 1A, BZL slightly increased CYP3A content only in liver (30%), as detected using an antibody which recognizes a common region of the C termini of CYP3A members.
Fig 1.
Effect of BZL on CYP3A expression and GST expression and activity in hepatic and intestinal tissues. (A) Microsomal CYP3A was detected by Western blotting in liver from rats treated with BZL (100 mg/kg b.w./day, 3 consecutive days) or vehicle (control [C]). (B) Cytosolic GST-μ was detected by Western blotting in liver from rats treated with BZL (100 mg/kg b.w./day, 3 consecutive days) or vehicle. (C and D) Cytosolic GST-α was detected by Western blotting in jejunum and ileum, respectively, from rats treated with BZL (100 mg/kg b.w./day, 3 consecutive days) or vehicle. Equal amounts (15 μg) of total protein were loaded in the gels. The optical densities (ODs) of CYP3A and GST were related to the OD of β-actin. Uniformity of loading and transfer from gel to polyvinylidene difluoride (PVDF) membrane were controlled with Ponceau S. Data on densitometric analysis are presented as percentages relative to the value for the control, considered 100%, and were expressed as means ± SDs (n = 3). Typical Western blot detections from each group are shown at the bottom. *, significantly different from control; (P < 0.05).
The expression of UGT1A was detected using an antibody developed against a C terminus peptide common to all isoforms of this group (23). We found that expression of UGT1A was not modified by BZL in the studied tissues (data not shown).
For GST, a modest increase in hepatic μ class GST was detected after BZL treatment (40%) (Fig. 1B). Alpha class GST was the only one increased by BZL treatment in the jejunum (72%) and ileum (136%) (Fig. 1C and D, respectively). No changes were detected in kidney (data not shown). When total GST activity toward CDNB in the liver, jejunum, and ileum was measured, an increase that correlated well with the higher levels of protein was observed for BZL-treated rats in comparison with that in the controls (2,630 ± 85 versus 1,898 ± 93, 510 ± 10 versus 257 ± 81, and 260 ± 20 versus 183 ± 35 nmol/min/mg protein, respectively; n = 3; P < 0.05).
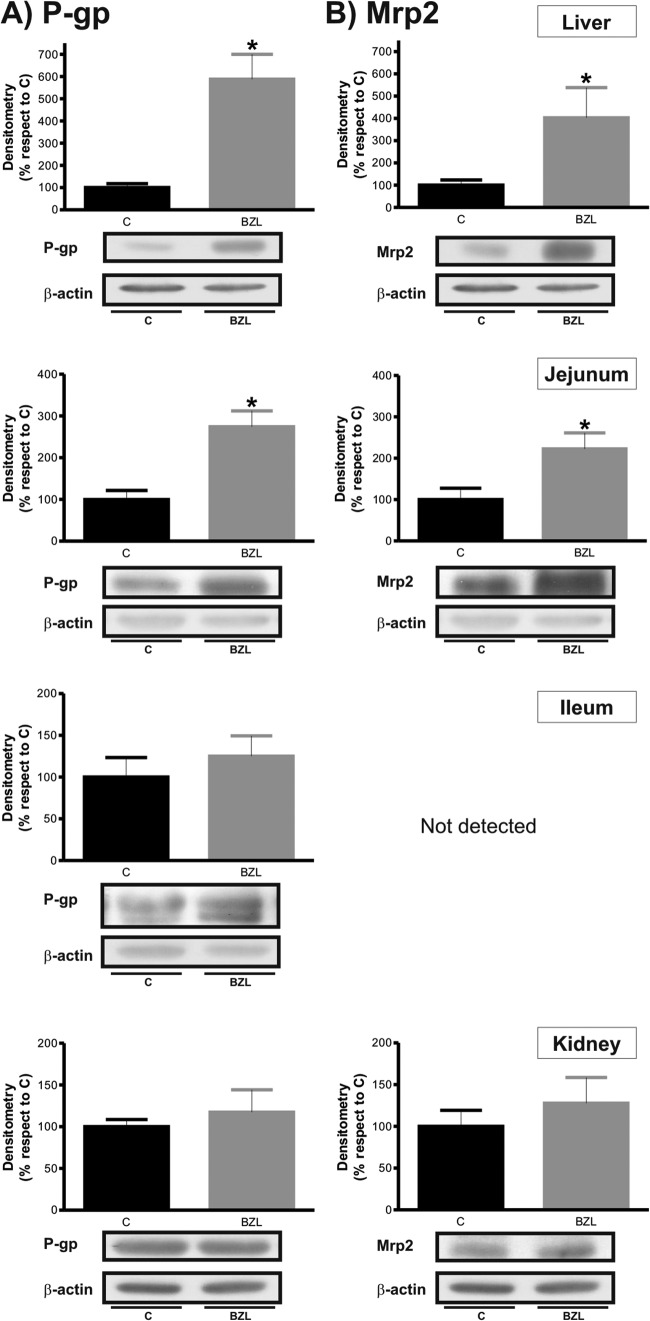
Figure 2 shows that P-gp and Mrp2 protein levels were significantly increased (about 500% and 300%, respectively) in liver from BZL-treated rats. The figure also shows an increment in P-gp and Mrp2 levels (about 170% and 120%, respectively) in jejunum from the BZL group. In ileum, BZL treatment was able to increase only P-gp levels (50%). In kidney, no change was detected (data not shown).
Fig 2.
Effect of BZL on apical transporter expression in hepatic and extrahepatic tissues. P-gp (A) and Mrp2 (B) were detected by Western blotting in liver, small intestine, and kidney from rats treated with BZL (100 mg/kg b.w./day, 3 consecutive days) or vehicle (control [C]). Equal amounts of total protein (30 μg) were loaded in the gels. The ODs of P-gp and Mrp2 were related to the OD for β-actin. Uniformity of loading and transfer from gel to PVDF membrane were controlled with Ponceau S. Data on densitometric analysis are presented as percentages relative to the value for the control, considered 100%, and were expressed as means ± SDs (n = 4). Typical Western blot detections from each group are shown at the bottom. *, significantly different from control; (P < 0.05).
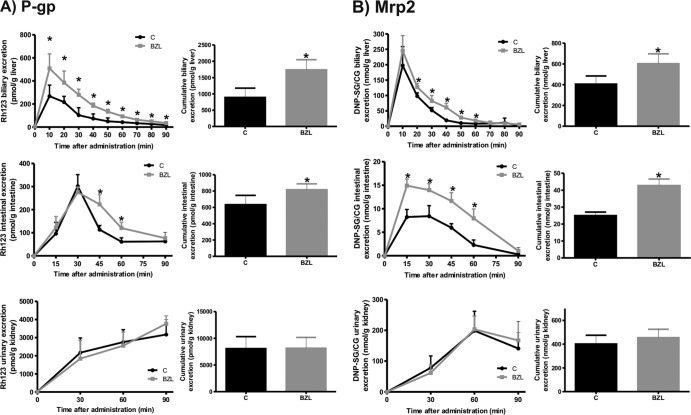
To evaluate whether the upregulation of P-gp or Mrp2 in liver and intestine could have functional consequences, we measured the efflux rate of a model substrate for each transporter in BZL-treated and control rats in an in situ single-pass intestinal perfusion model with simultaneous bile and urine collection. Figure 3A shows that biliary and intestinal excretions of the P-gp substrate Rh123 were increased by BZL (95% and 85%, respectively), which agrees well with the augmented levels of its transporter in liver and jejunum. No difference in the renal excretion rate of the dye in BZL-treated rats in comparison with that in controls was found. Furthermore, excretion of Rh123 was estimated as a percentage of the administered dose and was significantly higher in bile (11.0% versus 6.0%; P < 0.05; n = 4) and in intestinal perfusate (3.8% versus 2.7%; P < 0.05; n = 4) from BZL-treated rats than in those from controls, whereas in urine it remained unchanged (11%). These results indicate higher rates of biliary and intestinal elimination of P-gp substrates in BZL-treated rats.
Fig 3.
Effect of BZL on P-gp and Mrp2 activities. (A) Excretion rate of Rh123, a prototypical substrate for P-gp, was measured in bile, intestinal perfusate, and urine at 10-, 15- and 30-min intervals, respectively, for 90 min. Insets depict cumulative excretion of Rh123 at 90 min. The data represent means ± SDs for 4 rats per group. *, significantly different from control (C); P < 0.05. (B) Excretion rate of DNP-SG, a classical substrate for Mrp2, and its derivative, DNP-CG, was assessed in bile, intestinal perfusate, and urine at 10-, 15- and 30-min intervals, respectively, for 90 min. Insets depict cumulative excretion of DNP-SG and DNP-CG at 90 min. The data represent means ± SDs for 4 rats per group. *, significantly different from control; P < 0.05.
Figure 3B shows that the biliary excretion of DNP-SG and its major derivative, DNP-CG, as an estimation of Mrp2 activity was slightly increased (49%) in BZL-treated rats in comparison with that in controls in spite of the important increase in hepatic Mrp2 content in the BZL group. In proximal jejunum, the excretion rate of DNP-SG/CG in BZL-treated rats correlated with the augmented levels of Mrp2. No difference was found in renal excretion of Mrp2 substrate. Excretion of DNP-SG/CG was also calculated as a percentage of the dose of CDNB. The contribution of the renal route in BZL excretion was similar in BZL-treated rats and controls (10.4% and 9.7%, respectively), whereas excretion through the biliary and intestinal routes was increased by BZL (68.6% versus 46.1% and 3.04% versus 1.68%, respectively; P < 0.05; n = 4). These data suggest higher biliary and intestinal excretion rates for Mrp2 substrates in rats under BZL treatment.
Effect of BZL on DNP-SG transport in isolated hepatocytes.
Because in in vivo experiments we detected a modest change in hepatic Mrp2 activity, not correlated with the significant increase in Mrp2 expression, we decided to evaluate the transporter intrinsic activity in isolated hepatocytes. We analyzed the secretion rate of DNP-SG, an exogenous model substrate of Mrp2. DNP-SG transport into the medium was significantly increased by BZL (377 ± 116 versus 721 ± 258 pmol/min/mg protein for control and BZL groups, respectively; P < 0.05; n = 4), agreeing well with the higher Mrp2 protein content detected by Western blotting.
Effects of BZL pretreatment on its own disposition.
Plasma concentrations over time of a test dose of BZL after intraduodenal administration to control and BZL-treated rats are shown in Table 1. The decay rate of BZL concentration in plasma (k) was higher (295%) in pretreated rats than in controls. In addition, a lower AUC was seen in the BZL-pretreated group than in the controls (41%).
Table 1.
Pharmacokinetic parameters for BZL after intraduodenal administration to ratsa
| Body fluid | Parameter | Value for group |
|
|---|---|---|---|
| Control | BZL-treated | ||
| Plasma | Cmax (μM) | 18.8 ± 2.3 | 14.7 ± 1.6* |
| AUC (nmol/min/ml) | 80.0 ± 11.0 | 47.5 ± 1.2* | |
| k (min−1) | −0.0042 ± 0.0020 | −0.0166 ± 0.0045* | |
| Bile | Accumulative excretion (pmol/g liver) | 152 ± 18 | 244 ± 23* |
| Dose (%) | 0.64 ± 0.17 | 1.16 ± 0.10* | |
| Urine | Accumulative excretion (pmol/g kidney) | 1178 ± 299 | 1016 ± 184 |
| Dose (%) | 1.01 ± 0.67 | 1.55 ± 0.19 | |
BZL was administered at a dosage of 5 mg/kg b.w. Cmax, plasmatic maximal concentration; AUC, area under the concentration-time curve; k, elimination rate; *, significantly different from the control group (P < 0.05). Each value represents the mean ± SD (n = 4 to 5).
Table 1 also shows data for biliary and urinary elimination of BZL in pretreated rats over 90 min. The excreted amount of BZL was significantly increased (60%) in bile from BZL-pretreated rats in comparison with that from control rats. The amount of BZL in urine did not differ between groups.
Effects of BZL pretreatment on its intestinal absorption.
To evaluate if the induction of transporters at the intestinal level could affect the absorption of BZL, we used an in vitro model. We determined the rate of BZL absorption in sacs from jejunum, where the induction of Mrp2 and P-gp was quantitatively more significant. After 20 min of incubation, the mucosa-to-serosa transport of BZL was lower in jejunum from pretreated rats than in jejunum from controls (61 ± 15 versus 82 ± 11 pmol of BZL/g of intestine, respectively; n = 4 to 6; P < 0.05), implying that reduced absorption could occur in vivo.
DISCUSSION
The expression and activities of enzymatic systems and transporters can be altered by many factors, including diet, hormones, diseases, aging, and inducers. Thus, the effectiveness and/or toxicity of a broad range of xenobiotics, including therapeutic agents, could be modified.
Drugs with antiparasitic properties regulate biotransformation and transporter genes with an important impact on drug disposition (9–11). BZL is the only drug available for the treatment of Chagas disease in most countries where it is endemic. Although BZL can be coadministered with other drugs (diuretics, antibiotics, antiretrovirals, immunosuppressants, etc,), no study has been conducted to evaluate its effects on the systems involved in drug disposition. Recently we observed an increase in P-gp, MRP2, CYP3A4, and GST-π protein levels with a concomitant enhancement in their activities in cells from a hepatoma cell line, HepG2, treated with BZL (200 μM, 48 h) (12). Here we studied the effects of BZL treatment on phase I and phase II biotransformation enzymes and ABC efflux transporters in rat liver, small intestine, and kidney and the consequences in BZL disposition.
At present little is known about the effects of BZL on biotransformation systems. In phase I, the CYP3A subfamily as a whole significantly influences drug bioavailability in humans, since 40 to 50% of all drug metabolism involves some degree of CYP3A-mediated oxidation (33). It was reported that pentobarbital-induced sleep time is extended after acute administration of BZL to rats (30 mg/kg b.w., i.p.). This effect was associated with inhibition of the hepatic microsomal biotransformation systems aminopyrine and ethylmorphine N-demethylase by covalent interactions with BZL electrophilic metabolites (noncompetitive inhibition) (34). In addition, Workman et al. (35) observed that pharmacological concentrations of BZL were able to inhibit lomustine hydroxylation by CYP in mouse liver. They postulate that this BZL effect could explain modification of lomustine pharmacokinetics and enhanced response of mouse tumor to this drug. Our present study demonstrates a modest increase in the overall expression of CYP3A members only in liver of BZL-treated rats after 3 days of treatment (100 mg/kg b.w./day). Thus, despite the increase in CYP3A protein expression, the predominant effect of BZL seems to be inhibition of enzyme activities that can lead to drug-drug interactions.
In phase II metabolism, the expression of UGT1A was not modified by BZL in any tested tissues, indicating that glucuronidation is not essentially affected, at least for the isoforms recognized by our antibody, which represent the most relevant isoenzymes involved in the glucuronidation of phenol derivatives such as acetaminophen and endogenous compounds such as bilirubin (23).
It is known that reactive electrophilic metabolites are metabolized and excreted by a GSH-dependent process that reduces their toxicity. The conjugation reaction is generally catalyzed by GSTs, a family of detoxification enzymes that protects cellular macromolecules from attack by reactive electrophiles (36). The 2-amino reduction of BZL is catalyzed by isoforms of cytochrome P-450 nitroreductases present in host cells, rendering electrophilic metabolites (5). In the therapeutic protocol, BZL is orally administered. It is possible that increased global GST activity and expression of GST-μ in liver and GST-α in jejunum and ileum by BZL represents a presystemic compensatory mechanism to cope with overproduction of electrophilic metabolites.
Apart from overexpression of biotransformation systems, increased clearance of endo- and xenobiotics is also frequently associated with higher levels of efflux transporter proteins, such as P-gp and/or MRPs (8, 37). In this study, we observed upregulation of P-gp and Mrp2 proteins by BZL mainly in liver and jejunum. A global approach was used to further evaluate whether induction of these transporters had functional consequences. With an in vivo model, we demonstrated increased transport activity for P-gp in liver using a typical substrate, Rh123, in agreement with transporter upregulation. It is known that Rh123 is also a substrate for breast cancer resistance protein (Bcrp) (38). However, its influence in increased Rh123 transport is unlikely, since we did not observe changes in Bcrp levels (unpublished results). The induction of hepatic Mrp2 is usually followed by an increase in the excretion rate of its substrates. The slight increment in the biliary excretion of DNP-SG and DNP-CG observed in BZL-treated rats did not correlate with the significant increase in Mrp2 protein expression. This could be due to synthesis of a nonfunctional protein (e.g., Mrp2 not localized to canalicular membrane), the presence of other compounds competing with Mrp2-mediated excretion, or a nonsaturating concentration of CDNB. To further clarify this issue, we estimated the transport activity of Mrp2 in isolated hepatocytes. In the isolation process, hepatocytes are washed out of the intracellular compounds, including derivatives from BZL metabolism that could compete with DNP-SG for secretion via Mrp2. In this model, we found a direct correlation between activity and increased protein levels, suggesting that the in vivo results could be, at least in part, a consequence of competition among DNP-SG and other potential Mrp2 substrates, such as BZL-thiol or glucuronic conjugates. Thus, overexpression of these transporters in liver may result in faster biliary excretion of drugs that are their substrates, depending on their relative affinities diminishing their effectiveness and/or toxicity.
It is known that P-gp and Mrp2 expression levels vary inversely along the small intestine (29, 39). Mrp2 is the most highly expressed in the proximal intestine, whereas P-gp expression is higher distally (40). In our study, the major induction of both transporters occurred at the proximal segment of the small intestine. Interestingly, the increase in P-gp levels was extended beyond the ileum, its normal site of expression. When the intestinal excretion rate of Rh123 was tested in the in vivo model, we observed a significant increase in BZL-treated rats in comparison with controls, in agreement with P-gp upregulation. Again, Bcrp levels did not vary between groups (unpublished results). For intestinal Mrp2, when in vivo activity was measured, a higher DNP-SG/CG elimination rate was detected in BZL-treated rats than in controls, consistent with the upregulation of this protein by the drug. Consequently, the substantial increases in P-gp and Mrp2 expression rates and activities in proximal intestine can lead to an increased secretion of substances that are present in blood or a reduced absorption of drugs that are orally administered, including BZL itself.
Whether the current findings on the induction of biotransformation and transport systems also occur in patients receiving BZL is not known. The usual doses used for the treatment of Chagas disease vary between 5 and 10 mg/kg b.w. administered for 30 to 60 days, or even up to 5 months in the case of disease reactivation (41). In our study, 100 mg/kg b.w./day for 3 consecutive days was the dose of BZL that showed inductive effect. The BZL plasma concentration measured 24 h after the last injection in rats was 15 μM on average, similar to that found in patients (13 to 26 μM) 24 h after the last dose of a 30-day treatment (13). In general, rodents need a higher dose of a given compound to reproduce the same effects as in humans. Thus, an inducer effect of BZL in patients cannot be ruled out because the time of treatment is more extended than in our experimental approach. Drug-drug interactions could be particularly important in chagasic patients under immunosuppressant treatment with cyclosporine, corticosteroids, and azathioprine for heart or kidney transplantation (42, 43) or in HIV patients infected with T. cruzi and receiving antiretrovirals (44, 45).
In addition, changes in BZL pharmacokinetics would also be expected. In support of this hypothesis, Raaflaub (13) observed that the maximal plasma concentration in male patients that received BZL (7 mg/kg b.w./day for 30 days, twice a day) tends to decrease with the course of treatment (−20% on average after 25 days), suggesting an increase in BZL metabolism (autoinduction) and/or excretion and/or limited absorption. Here, the calculated BZL pharmacokinetic parameters show a higher elimination rate from plasma in the treated group than in controls. The lower AUC observed in plasma from BZL pretreated rats in comparison with controls can result from a higher amount of BZL excreted in bile 90 min post-BZL administration. In addition, as a lower maximum concentration in plasma (Cmax) was achieved in the BZL group, reduced BZL absorption is suggested. The decreased mucosa to serosa transport of BZL in intestinal sacs confirms this assumption. The participation of P-gp in BZL efflux was observed in P-gp knockdown HepG2 cells (12). Further experiments are needed to corroborate the contribution of this transporter or any other in BZL transport in an in vivo model. The data from the current study suggest the possibility of a progressive decrease in BZL absorption and/or increase in BZL metabolism/elimination after therapeutic administration. Unfortunately, we found no studies in the literature evidencing this possibility or a link with decreased therapeutic efficacy.
Although the reason CYP3A, GST, P-gp, and Mrp2 in kidney are not induced by BZL is unknown, it seems to be organ specific. The pregnane X-receptor (PXR) is a nuclear receptor that controls the expression of phase I and phase II biotransformation enzymes as well as xenobiotics transporters (6, 46). PXR is highly expressed in liver and to a lesser extent in small intestine in humans, rats, mice, and rabbits. Interestingly, these are the same tissues where biotransformation and transporter systems are most highly expressed and induced by BZL. Lower levels of PXR have also been detected in kidney (46). The knockdown of PXR in HepG2 cells was able to abolish the induction of CYP3A4, GST-π, P-gp, and MRP2 by BZL, suggesting that this nuclear receptor is involved in BZL effects (12). We postulate that the differential induction of studied systems in liver and intestine versus those in kidney could be related to tissue-specific differences in PXR expression and/or other transcription factors. Further studies are required to elucidate the mechanisms underlying the effects of BZL on these systems (i.e., transcriptional versus nontranscriptional, nuclear receptor participation, etc.).
In conclusion, BZL increases the expression and activities of P-gp and Mrp2 mainly in liver and proximal intestine along with upregulation of hepatic CYP3A and hepatic and intestinal GST. These findings suggest that under BZL treatment, drug-drug interactions could appear, especially at the excretion level, the limiting pathway in the depuration of endogenous and exogenous compounds.
ACKNOWLEDGMENTS
This work was supported by research grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET; PIP-00029), from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT-PICT; 2011-0360), and from the Universidad Nacional de Rosario (BIO 214), Argentina.
We express our gratitude to J. Elena Ochoa for her invaluable technical assistance in hepatocyte isolation and to Aldo D. Mottino for a critical review of the manuscript. We also thank S. Ikushiro (Department of Biotechnology, Faculty of Engineering, Toyama Prefectural University, Japan) for kindly providing UGT1A antibody.
Footnotes
Published ahead of print 22 July 2013
This work is dedicated to the memory of our beloved colleague and friend, Claudia G. Echenique.
REFERENCES
- 1.World Health Organization 2007. Global plan to combat neglected tropical diseases 2008–2015 World Health Organization, Geneva, Switzerland [Google Scholar]
- 2.Rassi A, Jr, Rassi A, Marín-Neto JA. 2010. Chagas disease. Lancet 375:1388–1402 [DOI] [PubMed] [Google Scholar]
- 3.Gascon J, Bern C, Pinazzo MJ. 2010. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 115:22–27 [DOI] [PubMed] [Google Scholar]
- 4.Hall BS, Wilkinson SR. 2012. Activation of benznidazole by trypanosomal type I nitroreductases results in glyoxal formation. Antimicrob. Agents Chemother. 56:115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maya JD, Cassels BK, Iturriaga-Vásquez P, Ferreira J, Faundez M, Galanti N, Ferreira A, Morello A. 2007. Mode of action of natural and synthetic drugs against Trypanosoma cruzi and their interaction with the mammalian host. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 146:601–620 [DOI] [PubMed] [Google Scholar]
- 6.Klaassen CD, Aleksunes LM. 2010. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol. Rev. 62:1–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benet LZ. 2009. The drug transporter-metabolism alliance: uncovering and defining the interplay. Mol. Pharm. 6:1631–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu C, Li CY, Kong AN. 2005. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 28:249–268 [DOI] [PubMed] [Google Scholar]
- 9.Bapiro TE, Andersson TB, Otter C, Hasler JA, Masimirembwa CM. 2002. Cytochrome P450 1A1/2 induction by antiparasitic drugs: dose-dependent increase in ethoxyresorufin O-deethylase activity and mRNA caused by quinine, primaquina and albendazole in HepG2 cells. Eur. J. Clin. Pharmacol. 58:537–542 [DOI] [PubMed] [Google Scholar]
- 10.Ashton M, Hai TN, Sy ND, Huong DX, Huong NV, Niêu NT, Công LD. 1998. Artemisinin pharmacokinetics is time-dependent during repeated oral administration in healthy male adults. Drug Metab. Dispos. 26:25–27 [PubMed] [Google Scholar]
- 11.Burk O, Arnold KA, Nussler AK, Schaeffeler E, Efimova E, Avery BA, Avery MA, Fromm MF, Eichelbaum M. 2005. Antimalarial artemisin drugs induce cytochrome P450 and MDR1 expression by activation of xenosensors pregnane X receptor and constitutive androstane receptor. Mol. Pharmacol. 67:1954–1965 [DOI] [PubMed] [Google Scholar]
- 12.Rigalli JP, Perdomo VG, Luquita MG, Villanueva SSM, Arias A, Theile D, Weiss J, Mottino AD, Ruiz ML, Catania VA. 2012. Regulation of biotransformation systems and ABC transporters by benznidazole in HepG2 cells: involvement of pregnane X-receptor. PLoS Negl. Trop. Dis. 6:e1951. 10.1371/journal.pntd.0001951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raaflaub J. 1980. Multiple-dose kinetics of the trypanosomicide benznidazole in man. Arzneimittelforschung 30:2192–2194 [PubMed] [Google Scholar]
- 14.Araújo MSS, Martins-Filho OA, Pereira MES, Brener Z. 2000. A combination of benznidazole and ketoconazole enhances efficacy of chemotherapy of Chagas' disease. J. Antimicrob. Chemother. 45:819–824 [DOI] [PubMed] [Google Scholar]
- 15.Institute of Laboratory Animal Resources 2007. Guide for the care and use of laboratory animals. Institute of Laboratory Animal Resources, Washington, DC [Google Scholar]
- 16.Meier PJ, Sztul ES, Reuben A, Boyer JL. 1984. Structural and functional polarity of canalicular and basolateral plasma membrane vesicles isolated in high yield from rat liver. J. Cell Biol. 98:991–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohoka K, Takano M, Okano T. 1993. p-Aminohippurate transport in rat renal brush-border membranes: a potential-sensitive transport system and an anion exchanger. Biol. Pharm. Bull. 16:395–401 [DOI] [PubMed] [Google Scholar]
- 18.Torres AM, MacLaughlin M, Quaglia N, Stremmel W. 2003. Role of BSP/bilirubin binding protein on p-aminohippurate transport in rat kidney. Mol. Cell. Biochem. 245:149–156 [DOI] [PubMed] [Google Scholar]
- 19.Mottino AD, Hoffman T, Jennes L, Vore M. 2000. Expression and localization of multidrug resistant protein Mrp2 in rat small intestine. J. Pharmacol. Exp. Ther. 293:717–723 [PubMed] [Google Scholar]
- 20.Catania VA, Luquita MG, Sánchez-Pozzi EJ, Mottino AD. 2000. Quantitative and qualitative gender-related differences in jejunal glutathione S-transferase in the rat: effect of testosterone administration. Life Sci. 68:467–474 [DOI] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275 [PubMed] [Google Scholar]
- 22.Ruiz ML, Villanueva SS, Luquita MG, Sánchez-Pozzi EJ, Crocenzi FA, Pellegrino JM, Ochoa JE, Vore M, Mottino AD, Catania VA. 2005. Mechanisms involved in spironolactone-induced choleresis in the rat: role of multidrug resistance-associated protein 2. Biochem. Pharmacol. 69:531–539 [DOI] [PubMed] [Google Scholar]
- 23.Ikushiro S, Emi Y, Iyanagi T. 1995. Identification and analysis of drug-responsive expression of UDP-glucuronosyltransferase family 1 (UGT1) isozyme in rat hepatic microsomes using anti-peptide antibodies. Arch. Biochem. Biophys. 324:267–272 [DOI] [PubMed] [Google Scholar]
- 24.Habig WH, Pabst MJ, Jakoby WB. 1974. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249:7130–7139 [PubMed] [Google Scholar]
- 25.Kageyama M, Fukushima K, Togawa T, Fujimoto K, Taki M, Nishimura A, Ito Y, Sugioka N, Shibata N, Takada K. 2006. Relationship between excretion clearance of rhodamine 123 and P-glycoprotein (Pgp) expression induced by representative Pgp inducers. Biol. Pharm. Bull. 29:779–784 [DOI] [PubMed] [Google Scholar]
- 26.Hinchman CA, Matsumoto H, Simmons TW, Ballatori N. 1991. Intrahepatic conversion of a glutathione conjugate to its mercapturic acid: metabolism of 1-chloro-2,4-dinitrobenzene in isolated perfused rat and guinea pig livers. J. Biol. Chem. 266:22179–22185 [PubMed] [Google Scholar]
- 27.Villanueva SS, Perdomo VG, Ruiz ML, Rigalli JP, Arias A, Luquita MG, Vore M, Catania VA, Mottino AD. 2012. Effect of glucagon-like peptide 2 on hepatic, renal, and intestinal disposition of 1-chloro-2,4-dinitrobenzene. Drug Metab. Dispos. 40:1252–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gotoh Y, Suzuki H, Kinoshita S, Hiroachi T, Kato Y, Sugiyama Y. 2000. Involvement of an organic anion transporter (canalicular multispecific organic anion transporter/multidrug resistance-associated protein 2) in gastrointestinal secretion of glutathione conjugates in rats. J. Pharmacol. Exp. Ther. 292:433–439 [PubMed] [Google Scholar]
- 29.Mottino AD, Hoffman T, Jennes L, Cao J, Vore M. 2001. Expression of multidrug resistance-associated protein 2 in small intestine from pregnant and postpartum rats. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G1261–G1273 [DOI] [PubMed] [Google Scholar]
- 30.Seglen PO. 1973. Preparation of rat liver cells. III. Enzymatic requirements for tissue dispersion. Exp. Cell Res. 82:391–398 [DOI] [PubMed] [Google Scholar]
- 31.Elferink RP, Ottenhoff R, Liefting W, de Hann J, Jansen PL. 1989. Hepatobiliary transport of glutathione and glutathione conjugate in rats with hereditary hyperbilirubinemia. J. Clin. Invest. 84:476–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morilla MJ, Benavídez PE, López MO, Romero EL. 2003. Liposomal benznidazole: a high-performance liquid chromatographic determination for biodistribution studies. J. Chromatogr. Sci. 41:405–409 [DOI] [PubMed] [Google Scholar]
- 33.Guengerich FP. 1999. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu. Rev. Pharmacol. Toxicol. 39:1–17 [DOI] [PubMed] [Google Scholar]
- 34.Masana M, de Toranzo EG, Rubio M, Castro JA. 1985. Effect of benznidazole on the mixed-function oxygenase system from rat liver microsomes. Arch. Int. Pharmacodyn. Ther. 276:4–11 [PubMed] [Google Scholar]
- 35.Workman P, Walton MI, Lee FYF. 1986. Benznidazole: nitroreduction and inhibition of cytochrome P-450 in chemosensitization of tumour response to cytotoxic drugs. Biochem. Pharmacol. 35:117–119 [DOI] [PubMed] [Google Scholar]
- 36.Hayes JD, Pulford DJ. 1995. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 30:445–600 [DOI] [PubMed] [Google Scholar]
- 37.Gottesman MM, Fojo T, Bates SE. 2002. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer 2:48–58 [DOI] [PubMed] [Google Scholar]
- 38.Alqawi O, Bates S, Georges E. 2004. Arginine 482 to threonine mutation in the breast cancer resistance protein ABCG2 inhibits rhodamine 123 transport while increasing binding. Biochem. J. 382:711–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doherty MM, Charman WN. 2002. The mucosa of the small intestine: how clinically relevant as an organ of drug metabolism? Clin. Pharmacokinet. 41:235–253 [DOI] [PubMed] [Google Scholar]
- 40.Ayrton A, Morgan P. 2001. Role of transport proteins in drug absorption, distribution and excretion. Xenobiotica 31:469–497 [DOI] [PubMed] [Google Scholar]
- 41.Coura JR, de Castro SL. 2002. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz 97:3–24 [DOI] [PubMed] [Google Scholar]
- 42.Bacal F, Silva CP, Pires PV, Mangini S, Fiorelli AI, Stolf NG, Bocchi EA. 2010. Transplantation for Chagas' disease: an overview of immunosuppression and reactivation in the last two decades. Clin. Transplant. 24:E29–E34 [DOI] [PubMed] [Google Scholar]
- 43.Silva AE, Silva AC, Faleiros AC, Guimarães CS, Corrêa RR, Oliveira FA, Correia D, Teixeira AC, Ramirez LE, de Paula Antunes Teixeira P, dos Reis MA. 2010. Acute Chagas' disease in postrenal transplant and treatment with benzonidazole. Ann. Diagn. Pathol. 14:199–203 [DOI] [PubMed] [Google Scholar]
- 44.DiazGranados CA, Saavedra-Trujillo CH, Mantilla M, Valderrama SL, Alquichire C, Franco-Paredes C. 2009. Chagasic encephalitis in HIV patients: common presentation of an evolving epidemiological and clinical association. Lancet Infect. Dis. 9:324–330 [DOI] [PubMed] [Google Scholar]
- 45.Almeida EA, Ramos Júnior AN, Correia D, Shikanai-Yasuda MA. 2011. Co-infection Trypanosoma cruzi/HIV: systematic review (1980-2010). Rev. Soc. Bras. Med. Trop. 44:762–770 [DOI] [PubMed] [Google Scholar]
- 46.Kliewer SA, Goodwin B, Willson TM. 2002. The nuclear pregnane X receptor: a key regulator of xenobiotics metabolism. Endocr. Rev. 23:687–702 [DOI] [PubMed] [Google Scholar]