Abstract
Staphylococcus epidermidis is one of the most frequent causes of device-associated infections, because it is known to cause biofilms that grow on catheters or other surgical implants. The persistent increasing resistance of S. epidermidis and other coagulase-negative staphylococci (CoNS) has driven the need for newer antibacterial agents with innovative therapeutic strategies. Thanatin is reported to display potent antibiotic activities, especially against extended-spectrum-beta-lactamase-producing Escherichia coli. The present study aimed to investigate whether a shorter derivative peptide (R-thanatin) could be used as a novel antibacterial agent. We found that R-thanatin was highly potent in vitro against coagulase-negative staphylococci, such as S. epidermidis, S. haemolyticus, and S. hominis, and inhibited biofilm formation at subinhibitory concentrations. Properties of little toxicity to human red blood cells (hRBCs) and human umbilical vein endothelial cells, a low incidence of resistance, and relatively high stability in plasma were confirmed. Excellent in vivo protective effects were also observed using a methicillin-resistant S. epidermidis (MRSE)-induced urinary tract infection rat model. Electron microscopy and confocal laser-scanning microscopy analyses suggested that R-thanatin disturbed cell division of MRSE severely, which might be the reason for inhibition of MRSE growth. These findings indicate that R-thanatin is active against the growth and biofilm formation of MRSE in vitro and in vivo. R-thanatin might be considered as a specific drug candidate for treating CoNS infections.
INTRODUCTION
Methicillin-resistant Staphylococcus epidermidis (MRSE) possesses a strong ability to exchange drug resistance genes, leading to the extensive dissemination of multidrug-resistant (MDR) strains (1–5). A study of antibiotic resistance in S. epidermidis strains found that more than 80% of the 342 clinical isolates tested were resistant to penicillin, ampicillin, cefazolin, and cefamandole (6). MRSE often causes long-lasting and recurrent infections due to its characteristic pattern of multidrug resistance (2, 3) and intrinsic genetic flexibility (7) to withstand hostile external environments. The increasing incidence of infections caused by multidrug-resistant S. epidermidis has driven the need for new antibacterial agents in innovative therapeutic strategies.
Antimicrobial peptides are cationic amphiphilic polypeptides produced by almost all species as an important component of their defense systems, and their advantages include a broad antibiotic spectrum and strong bactericidal activity. Unfortunately, the clinical use of some antimicrobial peptides with excellent bactericidal activity has been limited and some antimicrobial peptides are even abandoned during the drug development process due to their toxicities (8, 9). Mechanistically, antimicrobial peptide action centers on membrane interaction, which will inevitably produce hemolytic toxicity and other types of cytotoxicity. Therefore, low selectivity or high toxicity is the main factor limiting systemic applications of antimicrobial peptides (10). Thanatin is an inducible 21-residue insect peptide with two cysteine residues that form a disulfide bridge. Previous studies found that thanatin displayed potent antibiotic activities, especially against β-lactamase-producing Escherichia coli in vitro and in vivo, little hemolytic toxicity, and a satisfactory stability in plasma (11, 12).
It has recently been found that the γ-core motif existed widely in cysteine-stabilized antimicrobial peptides, such as thanatin, as a genetic evolution signature from primitive organisms to vertebrate animals (13, 14). Found primarily in the γ-core motif, a β-bulge is defined as a region between two consecutive β-type hydrogen bonds, which includes two or more residues on the bulged strand opposite to a single residue on the adjacent strand (15, 16). The residues on the bulged structure provide a strong local twist by impacting the directionality of β-strands. For these reasons, β-bulges conserved among antimicrobial peptides containing the γ-core motif may be associated with bactericidal activity.
In order to reduce the cost of peptide synthesis and change thanatin's antimicrobial spectrum, we made some modification of the thanatin structure. Briefly, the sequence of thanatin was truncated, only amino acids in the γ-core motif were retained, and an amino acid without positive charges in the β-bulge was replaced by an arginine with a positive charge (Table 1). In our study, the derivative of thanatin, R-thanatin, was synthesized and evaluated for efficacy. We demonstrated that R-thanatin has narrow-spectrum antimicrobial activities against coagulase-negative staphylococci (CoNS), excellent therapeutic efficacy for inhibiting biofilm formation in MRSE-caused urinary tract infection, good stability in plasma, and low cytotoxicity. In addition, R-thanatin does not induce bacterial resistance. Our findings show that R-thanatin may be considered as a drug candidate for treating CoNS specifically. The γ-core substitution design may provide a feasible strategy to develop innovative, anti-infective peptides with different antibacterial spectra.
Table 1.
Amino acid sequences and designations of the peptides investigated
| Peptide | Amino acid sequence | Designation |
|---|---|---|
| Thanatina | GSKKPVPIIYCNRRTGKCQRMCONH2 | Thanatin |
| Derivativeb | IYNCRRRFCKQRCONH2 | R-thanatin |
| Melittin | GIGAVLKVLTTGLPALISWIKRKRQQCONH2 | Melittin |
| K4-S4(1–16) | ALWKTLLKKVLKAAAKCONH2 | K4-S4(1–16)a |
Thanatin has a β-hairpin structure that is stabilized by one disulfide bond linking Cys-11 and Cys-18.
The derivative has a β-hairpin structure that is stabilized by one disulfide bond linking Cys-4 and Cys-9.
MATERIALS AND METHODS
Chemicals.
All antibiotics used were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). All other chemicals and solvents were of analytical grade.
Organisms.
The MDR clinical isolates of MRSE (XJ31106, XJ31204, XJ31276, XJ31318, and XJ75284), methicillin-resistant Staphylococcus aureus (MRSA) (XJ75302), S. haemolyticus (XJ31196 and XJ31245), and S. hominis (XJ31287 and XJ31303) were obtained from the clinical laboratory of Xijing Hospital (Xi'an, China). MRSE (SX70535, SX70582, SX70810, SX70892, and SX70893), S. haemolyticus (SX92421 and SX92464), and S. hominis (SX92357 and SX92433) isolates were collected from the clinical laboratory of Shaanxi Provincial People's Hospital (Xi'an, China). All above clinical staphylococci tested were isolated from blood cultures or urine tracts of patients. S. aureus (ATCC 29213), E. coli (ATCC 25922), and extended-spectrum-beta-lactamase-producing E. coli (ESBL-EC) (ATCC 35218) were used as references from the Chinese National Center for Surveillance of Antimicrobial Resistance.
Peptide synthesis.
Peptides (Table 1) were synthesized by the solid-phase method, applying Fmoc (9-fluorenylmethyloxycarbonyl) active ester chemistry (12). The crude compounds were purified to more than 95% chromatographic homogeneity by reverse-phase high-performance liquid chromatography (HPLC), and the purified compounds were identified by using a mass spectrometer (MS). HPLC runs were performed on a C18 column with a linear gradient of acetonitrile in water (1% per min), and both solvents contained 0.1% trifluoroacetic acid. The purified peptide in reduced form was taken up in oxidation buffer (1 mg per 10 ml) (12) and was purified by semipreparative reverse-phase HPLC. The purified compounds were confirmed by MS analysis (see Fig. S1 and Table S1 in the supplemental material).
Bacterial susceptibility testing and growth assay.
MICs were determined by a microdilution method with broth microdilution guidelines published by the Clinical and Laboratory Standards Institute (CLSI) (17). The time-kill curve for MRSE was determined by using the drop plate method (15) according to the basic microbiological techniques protocol (18). R-thanatin and ampicillin were added to MRSE cultures to a final concentration of 24 μg/ml, with the addition of an equal volume of diluent as a control. Aliquots of each culture were collected at 0.5, 1, 2, 4, and 6 h, diluted, and inoculated on solid agar. The number of CFU was calculated from the number of colonies growing on plates. Compounds were added to cell cultures containing MRSE to a final concentration of 4, 12, or 24 μg/ml R-thanatin or 24 μg/ml ampicillin. Aliquots of each culture were collected after 1 h, diluted, and inoculated on solid agar, and the plates were incubated for 24 h at 37°C.
Biofilm formation assays.
MRSE cells were seeded in 96-well microtiter plates with 100 μl tryptic soy broth (TSB) containing 0.5% glucose. R-thanatin was added to each well at a final concentration of 2 μg/ml at 8, 12, 16, and 20 h after seeding. The amounts of biofilms formed in the wells were determined by using a crystal violet staining method (19, 20). After incubation for 24 h at 37°C, supernatants were removed and the plates were gently washed with 0.1 mol/liter phosphate-buffered saline (PBS). The remaining attached bacteria were fixed with 200 μl of 99% methanol per well for 15 min. After plates were emptied, each well was incubated with 200 μl 1% crystal violet solution for 10 min. Then, plates were washed under running tap water and were air dried; 160 μl of 33% acetic acid was added. The optical density (OD) of each well was measured at 600 nm by using an automated Bio-Tek ELX800 universal microplate reader. Cells treated with diluent were used as a control. Each experiment was repeated three times, with the samples in each experiment prepared in at least eight wells.
Hemolysis assay.
The hemolytic toxicity of R-thanatin was determined using 2% and 10% suspensions of fresh human erythrocytes (21, 22). The 100-μl aliquots of suspensions of human red blood cells (hRBCs) were added to a 96-well microtiter plate containing R-thanatin or melittin at 8, 16, 32, 64, 128, 256, or 512 μg/ml for 1 or 6 h at 37°C. Samples were centrifuged, and the hemolytic toxicity was assessed as a function of hemoglobin leakage by measuring the absorbance of 200 μl of supernatant at 405 nm. Hemolytic values of 0% and 100% were determined with a spectrophotometer in PBS solution and 1% Triton X-100, respectively.
MTT assay.
The cytotoxicity of R-thanatin to the human umbilical vein endothelial cells (HUVECs) was determined by a standard MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) assay (23, 24). Briefly, cells (5 × 103 cells/well) were seeded in a 96-well plate with 100 μl Dulbecco's modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) in every well for 12 h and then exposed with or without R-thanatin at various concentrations (0.512 to 1024 μg/ml) for 48 h. After drug treatment, MTT solution (final concentration, 0.5%) was added, and cells were incubated for another 4 h at 37°C. Dimethyl sulfoxide (DMSO) (150 μl), was added to each well after removal of the supernatant, and the absorbance at 490 nm was measured with a microplate reader.
Induction of resistance.
The MIC of R-thanatin was determined against MRSE as described above. The MIC experiment was repeated for 15 days, as follows (23, 25): one-half of the MIC well from the MIC result was diluted to a 0.5 McFarland standard in Mueller-Hinton broth (MHB) and then was diluted at 1:100 to a concentration of 5 × 105 CFU/ml in MHB and used again for MIC determination in the subsequent generation. The relative MIC value was calculated from the ratio of the MIC obtained for the 15th subculture to that obtained for the first-time exposure.
Stability in 50% plasma.
Sensitivity of R-thanatin or K4-S4(1–16)a (26) to enzymatic degradation was assessed by determining the antibacterial activity after exposure to human plasma (25, 27). R-thanatin at an initial concentration of 64 times the MIC value was preincubated with 50% of human plasma in the same volume at 37°C. After incubation periods of 0, 3, and 6 h, the peptide solutions were subjected to serial 2-fold dilution in MHB medium in a 96-well plate. Inhibition of MRSE growth was determined as described for the antibacterial assay.
Protective effects of R-thanatin on urinary tract-infected rats.
The rats were anesthetized by an intraperitoneal injection of 1% pentobarbital sodium (40 mg/kg of body weight). The lower abdomen was squeezed to empty residual urine, and then the bladder was exposed and opened aseptically through a suprapubic incision at the dome (28). The 8-mm sterile stents were inserted into the bladder, and the bladder was sutured with surgical silk. After the surgical intervention, 0.1 ml nutrient broth containing 5.6 ×107 CFU/ml MRSE XJ75284 was inoculated into the bladder using a syringe. Rats received a 2.5-, 5-, or 10-mg/kg dose of R-thanatin at 16 and 28 h after bacterial challenge. The animals were returned to individual cages and were thoroughly examined daily. At day 4 after infection, the right kidney and bladder were removed and then homogenized. The implanted stents were removed and washed with PBS to remove planktonic cells. Then, these stents were prepared for scanning electron microscopy (SEM) examination or sonicated three times for 10 s each time to obtain detached staphylococci (29). Viable bacteria were enumerated by culturing serial 10-fold dilutions of the tissue homogenate and sonicated solution on Mueller-Hinton agar plates. All plates were incubated at 35°C for 24 h. CFU in blood samples were enumerated on Mueller-Hinton agar plates.
Preparation of MRSE samples for scanning and transmission electron microscopy.
In the urinary tract-infected rat model, stents with growth of S. epidermidis biofilm were analyzed using a Hitachi S-3400N scanning electron microscope. MRSE (5 × 108 CFU/ml) was exposed to 24 μg/ml R-thanatin at 120 rpm for 30, 60, and 90 min. A Hitachi S-3400N scanning electron microscope or a Joel JEM-2000EX transmission electron microscope was used to view the thin sections under standard operating conditions.
Cell membrane permeability assay.
MRSE (1 × 108 CFU/ml) was cultured with 4 or 24 μg/ml R-thanatin at 120 rpm at 37°C for 90 min. Bacteria were harvested, washed, stained using Syto 9 and propidium iodide (BacLight Bacterial Viability kit; Molecular Probes), and then examined by confocal laser-scanning microscopy using a FluoView FVlOi system (Olympus) and Epics XL flow cytometer (Beckman Coulter, USA).
Statistical analysis.
Results are expressed as means ± standard deviations (SD). Analysis of variance (ANOVA) was used for statistical evaluations, with a probability (P) value of <0.05 considered indicative of statistical significance.
RESULTS
Bacterial susceptibility testing and growth assay.
R-thanatin exerted potent bactericidal effects against all strains of S. epidermidis tested, including clinically prevalent and challenging strains, with MIC values ranging from 2 to 8 μg/ml (Table 2). The killing curves indicated that R-thanatin reduced the bacterial population from nearly 106 to 104 CFU/ml within 6 h. Ampicillin and ceftazidime at the same concentration exerted no or weaker bactericidal effects against MRSE relative to results for the control group (Fig. 1A). R-thanatin at 4, 12, and 24 μg/ml reduced the CFU of MRSE by approximately 102-, 103-, and 104-fold, respectively (Fig. 1B). While the growth of MRSE was clearly inhibited by R-thanatin in a concentration-dependent manner, it was not influenced by treatment with 24 μg/ml ampicillin.
Table 2.
MIC of R-thanatin in Mueller-Hinton broth culture
| Strain | MIC of drug (μg/ml)a |
||||||
|---|---|---|---|---|---|---|---|
| R-THA | THA | OXA | AMP | CAZ | LVX | CIP | |
| S. aureus ATCC 29213 | 64 | 256 | ≤0.25 | 1 | 8 | ≤0.25 | ≤0.25 |
| MRSA XJ75302 | 64 | >256 | >256 | 256 | 256 | 8 | 16 |
| MRSE XJ31106 | 2 | 4 | >256 | >256 | >256 | 16 | 128 |
| MRSE XJ31204 | 4 | 32 | 0.25 | >256 | 8 | 4 | 8 |
| MRSE XJ31276 | 4 | 32 | 2 | >256 | 32 | 2 | 8 |
| MRSE XJ31318 | 8 | 32 | 2 | >256 | 32 | 2 | 16 |
| MRSE XJ75284 | 4 | 16 | >256 | 256 | 256 | 8 | 8 |
| MRSE SX70535 | 2 | 32 | >256 | 128 | 32 | 16 | 8 |
| MRSE SX70582 | 4 | 16 | >256 | 64 | 64 | 4 | 2 |
| MRSE SX70810 | 4 | 16 | >256 | >256 | 128 | 4 | 8 |
| MRSE SX70892 | 8 | 32 | >256 | >256 | 32 | 16 | 4 |
| MRSE SX70893 | 4 | 16 | >256 | 128 | >256 | 8 | 4 |
| S. haemolyticus XJ31196 | 4 | 8 | >256 | >256 | >256 | 64 | 128 |
| S. haemolyticus XJ31245 | 2 | 16 | 256 | 128 | 64 | 32 | >256 |
| S. haemolyticus SX92421 | 8 | 32 | 128 | 64 | 128 | 64 | 128 |
| S. haemolyticus SX92464 | 4 | 16 | >256 | 256 | >256 | 128 | 256 |
| S. hominis XJ31287 | 2 | 8 | 0.5 | 256 | 16 | ≤0.25 | ≤0.25 |
| S. hominis XJ31303 | 8 | 16 | 1 | 128 | 32 | ≤0.25 | ≤0.25 |
| S. hominis SX92357 | 4 | 32 | 2 | >256 | 64 | ≤0.25 | ≤0.25 |
| S. hominis SX92433 | 2 | 16 | 4 | 64 | 64 | ≤0.25 | ≤0.25 |
R-THA, THA, OXA, AMP, CAZ, LVX, and CIP indicate R-thanatin, thanatin, oxacillin, ampicillin, ceftazidime, levofloxacin, and ciprofloxacin, respectively.
Fig 1.
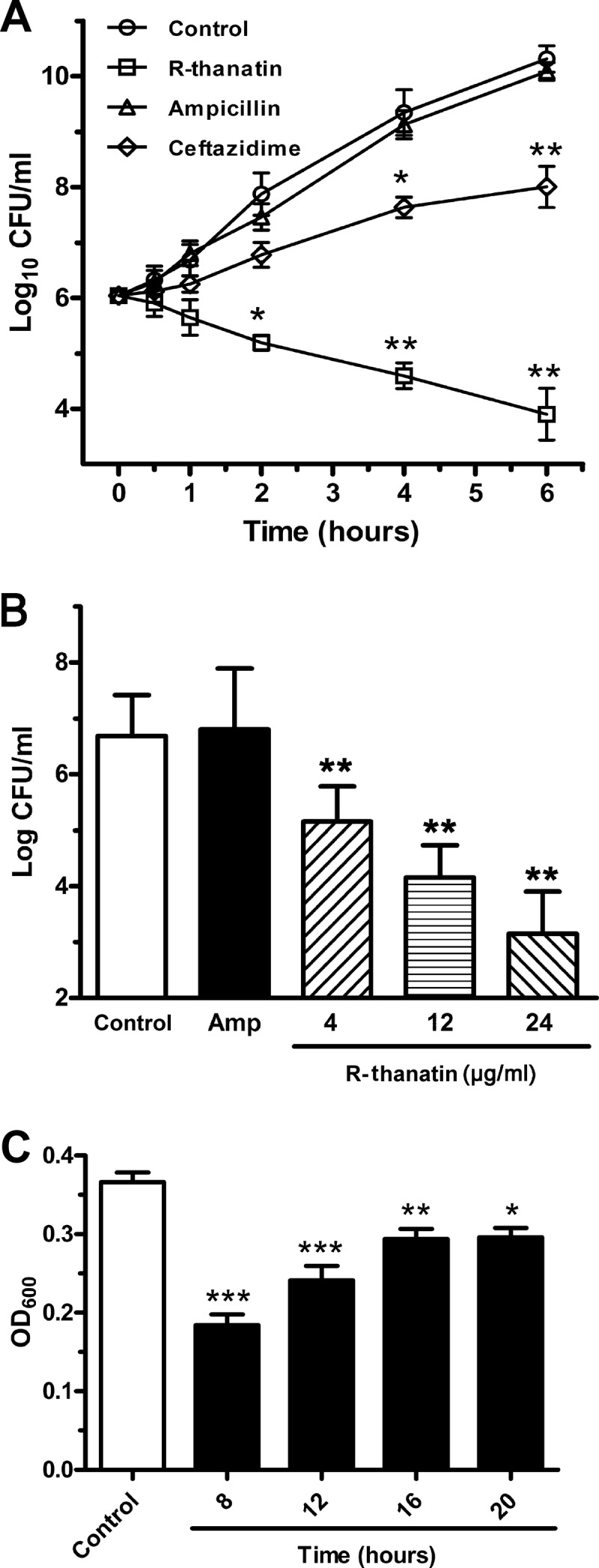
In vitro antibacterial activities of R-thanatin. (A) Time-kill curves of R-thanatin. R-thanatin, ceftazidime, and ampicillin were added to cell cultures containing MRSE to a final concentration of 24 μg/ml, with addition of an equal volume of diluent as a control. Aliquots of each culture were collected at 0.5, 1, 2, 4, and 6 h and were then diluted and inoculated on solid agar. The number of CFU was calculated from the number of colonies growing on plates. The data are shown as mean ± SD values for 3 samples: ∗, P < 0.05; ∗∗, P < 0.01 (versus results for the control). (B) Effects of R-thanatin on growth of bacteria colonies. Compounds were added to cell cultures containing MRSE to a final concentration of 4, 12, or 24 μg/ml R-thanatin or 24 μg/ml ampicillin, with the addition of an equal volume of diluent as a control. Aliquots of each culture were collected at 1 h, diluted, and inoculated on solid agar. The number of CFU was calculated from the number of colonies growing on plates. The data are shown as mean ± SD values for 8 samples: ∗∗, P < 0.01 versus results for the control. (C) Crystal violet staining of biofilms from MRSE grown for 24 h in 96-well plates with 2 μg/ml R-thanatin. R-thanatin at a subinhibitory concentration was added after MRSE cells were cultured for 0, 8, 12, 16, or 20 h. The data are shown as mean ± SD values for 16 samples: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 (versus results for the control).
Effect of R-thanatin on MRSE biofilm.
In the current study, the effect of R-thanatin on biofilm production of MRSE was investigated (Fig. 1C). After MRSE cells were seeded in 96-well microtiter plates for 8 h, and R-thanatin were added to each well every 4 h. As expected, R-thanatin at one-half the MIC significantly suppressed biofilm formation by MRSE. Furthermore, the stronger effect of R-thanatin on biofilm inhibition was achieved, if R-thanatin was applied in an early culture stage (Fig. 1C). These results indicate that R-thanatin was a biofilm inhibitor at a subinhibitory concentration.
In vitro toxicity.
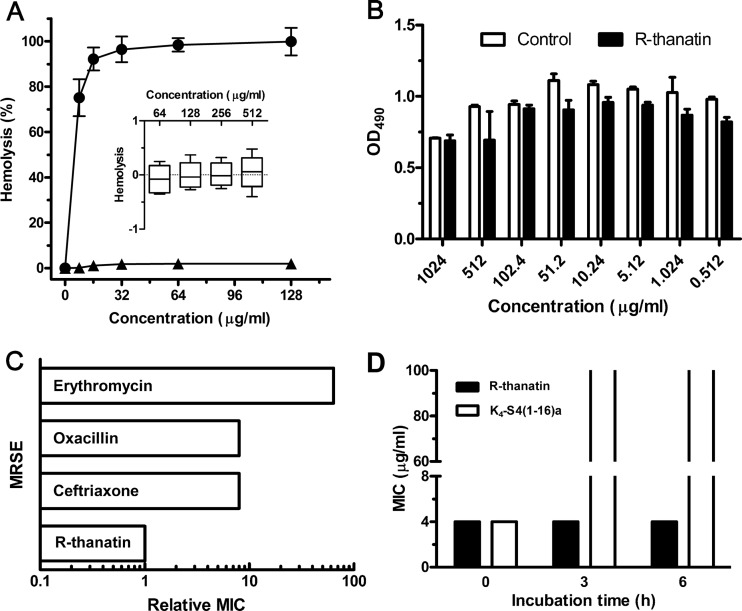
Compared to melittin, an antimicrobial peptide that exhibits powerful lytic activity against bacterial and eukaryotic cells, R-thanatin did not show hemolytic toxicity in 10% hRBC suspensions at a concentration as high as 512 μg/ml, which was 100 times higher than the MIC value for MRSE (Fig. 2A).
Fig 2.
Selectivity, stability, and antibiotic resistance induction of R-thanatin in vitro. (A) Hemolytic toxicities of R-thanatin (triangles) and melittin (circles) determined with human red blood cells (2% hematocrit) after 1 h of incubation. The inset shows toxicity of R-thanatin after 6 h of incubation with 10% hematocrit. The data are shown as mean ± SD values for 3 samples. (B) The absorbance at 490 nm of HUVE cells after incubation without or with various concentrations of R-thanatin for 48 h. Each plot was obtained from a representative experiment, and the data points are the means for four replicates ± standard deviations. (C) Emergence of resistance in MRSE after 15 serial passages in the presence of antimicrobials. “Relative MIC” is the normalized ratio of the MIC obtained for the 15th subculture to the MIC obtained upon first exposure. Data are from a representative experiment that was repeated twice with the same results. (D) Antibacterial activity before and after preincubation of R-thanatin (black) and K4-S4 (1–16)a (white) in 50% human plasma. Data are from a representative experiment that was repeated twice with the same results.
To further explore the selectivity of R-thanatin, we investigated its cytotoxicity to the human umbilical vein endothelial cells in vitro. As shown in Fig. 2B, there was no significant difference in cell viability between the control group and the R-thanatin-treated group at a concentration of 1,024 μg/ml (P > 0.05). These results imply that R-thanatin has little toxicity to mammalian cells. Given that R-thanatin has antibacterial activity at concentrations from 2 to 64 μg/ml, it has a relatively wide safety range for potential antimicrobial applications.
Induction of resistance.
In order to investigate whether R-thanatin has the potential capability to induce antibacterial resistance, MRSE was exposed to subinhibitory concentrations of R-thanatin and several antibiotics during 15 successive subcultures. The relative MIC values of classical antibiotics, including erythromycin, ceftriaxone, and oxacillin, increased by 8- to 64-fold, reflecting the emergence of resistant strains. However, the relative MIC values of R-thanatin remained unchanged, indicating no induction of antibiotic resistance (Fig. 2C).
Stability in plasma.
As shown in Fig. 2D, MIC values of R-thanatin remained consistent after incubation in 50% plasma for 3 and 6 h. However, the control peptide K4-S4(1–16)a (26), a dermaseptin S4 derivative, lost antibacterial effect after incubation with plasma. The results indicated that R-thanatin was resistant to enzymatic degradation in plasma.
In vivo antibacterial activity.
Substantial bacterial dissemination in the MRSE urinary tract infection model was inhibited after intraperitoneal administration of R-thanatin at dosages of 2.5, 5, and 10 mg/kg in a dose-dependent manner. The bacterial cell numbers in the bladder and kidney or on stents were decreased dose dependently (Fig. 3). The biofilm formation in implanted stents was also inhibited in the R-thanatin-treated group (Fig. 4).
Fig 3.
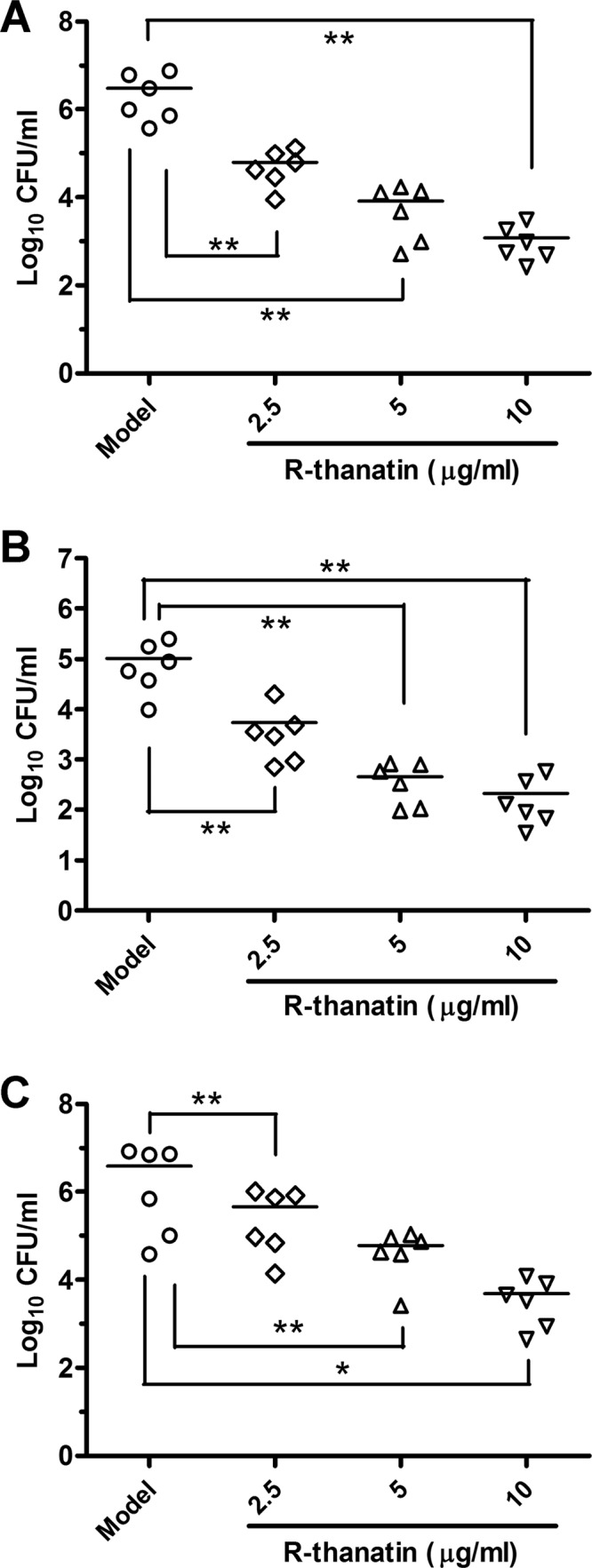
Activity of R-thanatin against the MRSE strain XJ75284 in a rat urinary tract infection model. MRSE in bladder (A), kidney (B), or stent (C) cultures of R-thanatin-treated BALB/c mice is analyzed. The data are shown as mean ± SD values for 6 samples: ∗, P < 0.05; ∗∗, P < 0.01 (versus results in the model).
Fig 4.
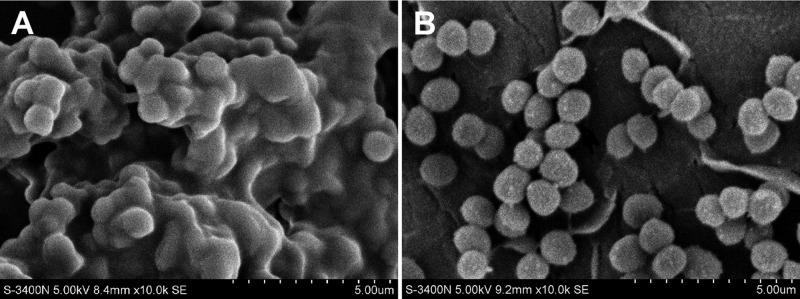
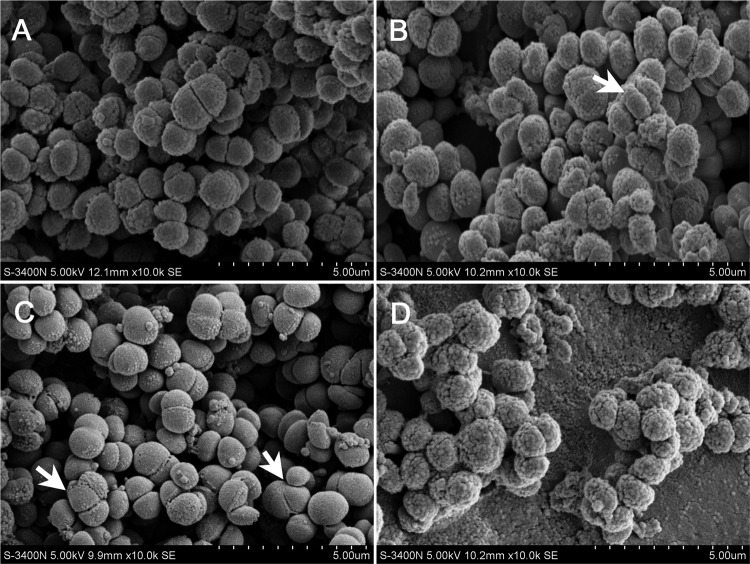
In a urinary tract-infected rat model, rats received a 10-mg/kg dose of R-thanatin at 16 and 28 h after bacterial challenge. The implanted stents were removed and washed with PBS to remove planktonic cells. At day 4 after infection, SEM images of MRSE cultured on stents in the absence (A) or presence (B) of 10 mg/kg R-thanatin were made. The images shown were taken at a magnification of ×10,000. Scale bar = 5 μm.
Bacterial morphological assay.
Scanning electron microscopy (SEM) performed during R-thanatin treatment revealed dramatic morphological changes in MRSE. The bacteria became swollen and translucent, with the number of surface microvilli decreasing (Fig. 5B and C). Several bacterial cells were observed to exhibit abnormal division (arrows in Fig. 5B and C). All of the bacteria had abnormal shapes and marked surface unevenness, and the vast majority exhibited small surface cracks. Some cells had broken into irregular pieces, and the accumulation of bacterial debris was evident (Fig. 5D).
Fig 5.
The morphology of MRSE was investigated by scanning electron microscopy at 0, 30, 60, and 90 min after treatment with 24 μg/ml R-thanatin. (A) Control; (B) MRSE treated with R-thanatin for 30 min; (C) MRSE treated with R-thanatin for 60 min; (D) MRSE treated with R-thanatin for 90 min. White arrows indicate bacterial cells with abnormal division.
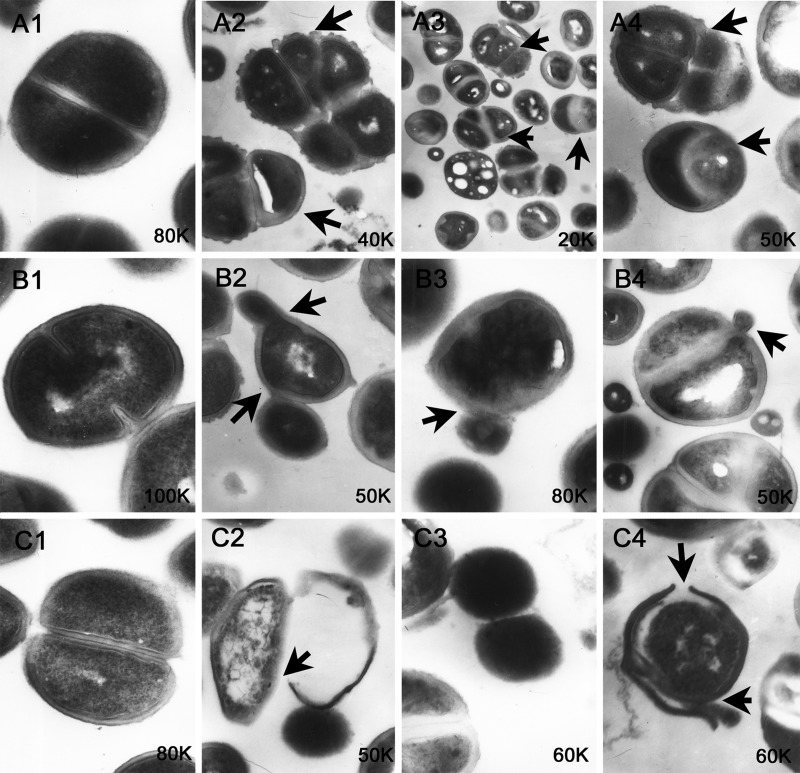
Transmission electron microscopy (TEM) revealed that few of the bacterial cells divided by binary fission (Fig. 6A1, B1, and C1), but many bacterial cells exhibited asymmetric or multiple cell divisions (Fig. 6A2 to -4) after treatment with R-thanatin. Most of the bacteria exhibited a budding division pattern (Fig. 6B2 to -4), but this phenomenon was rare in the normal cells (Fig. 6B1). The cell wall was intact in the controls (Fig. 6C1), whereas the membrane integrity was severely damaged in the R-thanatin-treated group, with the leakage of intracellular contents (Fig. 6C2 to -4).
Fig 6.
The morphology of MRSE was investigated by transmission electron microscopy after treatment with 24 μg/ml R-thanatin for 90 min. A1, B1, and C1 show the control; A2 to A4, B2 to B4, and C2 to C4 show MRSE treated with R-thanatin for 90 min. Black arrows points to the sites of bacterial cells with abnormal division, budding appearance, or bacterial membrane rupture.
Bacterial membrane permeabilization assays.
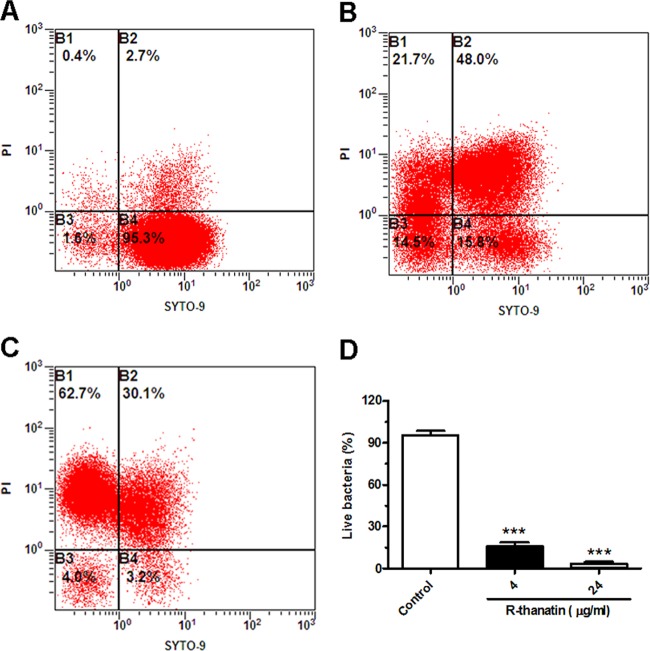
Changes in bacterial membrane permeability were quantified using the fluorescent dye pair Syto 9 and propidium iodide (PI). Bacterial membrane damage allows the non-membrane-permeating dye PI to enter the cell and displace the membrane-permeative dye Syto 9, leading to a loss of its fluorescence. In the control group, Syto 9 green fluorescence was dominantly observed and PI red fluorescence was rare (Fig. 7A, B, C, and D). After treatment with R-thanatin for 90 min, cells showed strong red fluorescence and week green fluorescence (Fig. 7E, F, G, and H), indicating cell membrane damage. A high percentage of dividing cells was found in the R-thanatin-treated group compared to that in the control group (Fig. 7D and H). Moreover, the dividing bacterial cell in the R-thanatin treatment group appeared larger than that in the control group (Fig. 7D and H). Flow cytometry assay results showed that R-thanatin at a 4-μg/ml concentration led to an approximately 80% reduction of the live MRSE compared with results for the control group. Almost no live bacteria (3.2%) were detected after MRSE cells were treated with 24 μg/ml of R-thanatin. In contrast, the percentage of dead bacteria in PI staining of MRSE was increased distinguishably in a concentration-dependent manner (Fig. 8).
Fig 7.
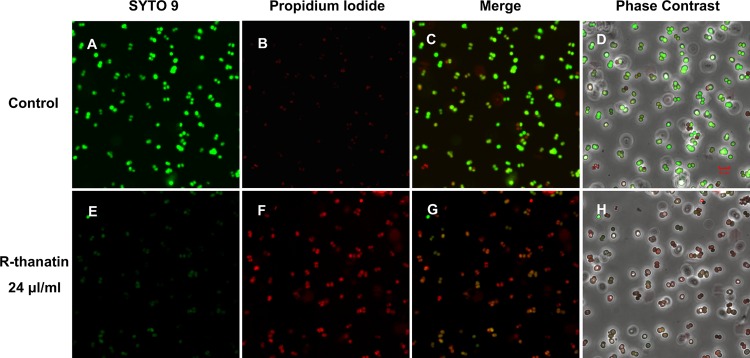
R-thanatin-mediated abnormal bacterial cell division (original magnification, ×1,800). MRSE was incubated with 24 μg/ml R-thanatin for 90 min at 37°C. Bacteria were stained with the dyes Syto 9 (which stains live bacteria) (A and E) and propidium iodide (which stains dead bacteria) (B and F) and were analyzed by confocal laser-scanning microscopy to assess viability and division (C, D, G, and H). Scale bar = 5 μm.
Fig 8.
Analysis of relative viabilities of MRSE suspensions by flow cytometry. Compounds were added to cell cultures containing MRSE to a final concentration of 4 (B) or 24 (C) μg/ml R-thanatin, with the addition of an equal volume of diluent as a control (A). Aliquots of each culture were collected at 37°C for 90 min and were stained for 15 min with the dyes Syto 9 and propidium iodide (PI). Then, bacteria were analyzed by using an Epics XL flow cytometer to assess the percentage of live or dead bacteria (A, B, and C). The numbers indicate the percentages of live bacteria within MRSE after treatment with different concentrations of R-thanatin (D). The values show the means ± SD for 3 samples. ∗∗∗, P < 0.001 compared with results for control groups.
DISCUSSION
The glycopeptide antibiotics (e.g., vancomycin) are normally reserved for use against multidrug-resistant staphylococci and are often used as drugs of last resort when battling infection caused by multidrug-resistant S. epidermidis. However, a continuously increasing resistance of S. epidermidis to glycopeptides has been reported (5, 30–33). Although S. epidermidis has a lower pathogenic potential, most staphylococcal infections are chronic and extremely challenging to cure, especially for the elderly, children, and patients with weakened immune systems. New measures to eradicate persistent staphylococcal infections are therefore urgently required.
We have previously shown that thanatin is characterized by a low inherent ability to induce microbial resistance, little hemolytic toxicity, and a high stability in plasma (11). It is gratifying that R-thanatin retains all these advantages. R-thanatin did not increase the resistance to S. epidermidis after 15 passages for sub-MIC culture; the hemolysis was not found after incubation with human red blood cells for 6 h of incubation; little toxicity to umbilical vein endothelial cells was exhibited; and also, it retained identical antibacterial activity against MRSE after 6 h of preincubation with human plasma. More importantly, R-thanatin significantly decreased bacterial loads in the bladder and kidney, and markedly inhibited biofilm formation in implanted stents. These results implied that R-thanatin might be superior to conventional antibiotics in treating drug-resistant device-associated CoNS infections.
Peptide drugs tend to be very expensive, costing about $350 per gram as an average daily dose for most systemic therapeutics or more to manufacture by the solid-phase synthesis method (8, 34), which greatly limits antimicrobial peptide research, development, and clinical application. Many endeavors to solve the issue by a variety of recombinant DNA methods using many production systems have been done. However, none of these expression systems have been confirmed to be commercially feasible and stable to date (8). The use of shorter peptides is another way to reduce the cost of goods. Compared with those of thanatin, R-thanatin's synthesis costs are reduced by almost half, which will promote its use in a clinical setting. By increasing the charges of the β-bulge, the antibacterial spectrum of R-thanatin was obviously narrowed, and Gram-negative bacteria became insensitive to it. Meanwhile, R-thanatin showed stronger antibacterial activity against Gram-positive bacteria, especially CoNS, than thanatin.
Abnormal cell divisions in MRSE after treatment with R-thanatin at a high concentration (24 μg/ml) were observed by both SEM and TEM, which indicated a major disturbance in MRSE cell division. The formation of multiple cell divisions (e.g., triploid, tetraploid, and pentaploid) would severely influence the propagation of MRSE. Treatment of MRSE by R-thanatin resulted in cell walls or membrane layers forming multiple septa that split the cells into three, four, or five compartments. Septum formation was regulated incorrectly, either spatially or temporally, resulting in morphological and genetic differences in new cells that prevented them from growing properly. In addition to producing unequal daughter cells, R-thanatin also induced considerable budding division of MRSE cells. This might be caused by abnormal division close to cell poles, which is similar to the process of spore formation or budding production. Using confocal laser-scanning microscopy, we observed that the number and volume of bacterial cells in the process of splitting were both much greater in the R-thanatin-treated group, which implied that cell division of MRSE was severely disturbed.
SEM revealed that MRSE became more swollen and translucent with the extension of R-thanatin treatment time, and a breakdown phenomenon was observed after R-thanatin treatment for 90 min. Moreover, confocal laser-scanning microscopy and flow cytometry showed that almost red fluorescence was observed in the R-thanatin-treated cells after 90 min. However, bacterial membrane rupture was observed by TEM in a small number of bacteria, the incidence of which was far less than that of abnormal or budding division. It is well known that thanatin mainly damage cell membranes (11). Compared to thanatin, R-thanatin had a weak effect in cell membrane damage and the formation of the rupture pores and obviously affected membrane permeability at high concentrations. The above-described ultrastructural changes suggest that the mechanisms underlying the effects of R-thanatin are different from those for thanatin.
In conclusion, a new antimicrobial peptide, R-thanatin, is highly effective against growth and biofilm formation of MRSE both in vivo and in vitro, exhibiting superior plasma stability and low cytotoxicity. More importantly, MRSE does not show resistance after 15 successive subcultures with R-thanatin. All of our morphological findings have shown that the main mechanism underlying the effects of R-thanatin is a severe disturbance of MRSE cell division. Further investigations are needed to reveal the fundamental molecular mechanism of how R-thanatin induces abnormal cell division.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (no. 81001460 and no. 81273555).
We thank Xiuli Xu (Xijing Hospital, Shaanxi, China) and Lixia Zhang (Shaanxi Provincial People's Hospital, Shaanxi, China) for clinical strains.
Footnotes
Published ahead of print 5 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00504-13.
REFERENCES
- 1.Ziebuhr W, Hennig S, Eckart M, Kranzler H, Batzilla C, Kozitskaya S. 2006. Nosocomial infections by Staphylococcus epidermidis: how a commensal bacterium turns into a pathogen. Int. J. Antimicrob. Agents 28(Suppl. 1):S14–S20 [DOI] [PubMed] [Google Scholar]
- 2.Bloemendaal AL, Brouwer EC, Fluit AC. 2010. Methicillin resistance transfer from Staphylocccus epidermidis to methicillin-susceptible Staphylococcus aureus in a patient during antibiotic therapy. PLoS One 5:e11841. 10.1371/journal.pone.0011841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smyth DS, Wong A, Robinson DA. 2011. Cross-species spread of SCCmec IV subtypes in staphylococci. Infect. Genet. Evol. 11:446–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews J, Ashby J, Jevons G, Marshall T, Lines N, Wise R. 2000. A comparison of antimicrobial resistance rates in Gram-positive pathogens isolated in the UK from October 1996 to January 1997 and October 1997 to January 1998. J. Antimicrob. Chemother. 45:285–293 [DOI] [PubMed] [Google Scholar]
- 5.Sendi P, Graber P, Zimmerli W. 2005. Loss of mecA gene in Staphylococcus epidermidis after prolonged therapy with vancomycin. J. Antimicrob. Chemother. 56:794–795 [DOI] [PubMed] [Google Scholar]
- 6.Arciola CR, Campoccia D, Gamberini S, Donati ME, Pirini V, Visai L, Speziale P, Montanaro L. 2005. Antibiotic resistance in exopolysaccharide-forming Staphylococcus epidermidis clinical isolates from orthopaedic implant infections. Biomaterials 26:6530–6535 [DOI] [PubMed] [Google Scholar]
- 7.Yao Y, Sturdevant DE, Villaruz A, Xu L, Gao Q, Otto M. 2005. Factors characterizing Staphylococcus epidermidis invasiveness determined by comparative genomics. Infect. Immun. 73:1856–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hancock RE, Sahl HG. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24:1551–1557 [DOI] [PubMed] [Google Scholar]
- 9.Marr AK, Gooderham WJ, Hancock RE. 2006. Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr. Opin. Pharmacol. 6:468–472 [DOI] [PubMed] [Google Scholar]
- 10.Jacobsen F, Mohammadi-Tabrisi A, Hirsch T, Mittler D, Mygind PH, Sonksen CP, Raventos D, Kristensen HH, Gatermann S, Lehnhardt M, Daigeler A, Steinau HU, Steinstraesser L. 2007. Antimicrobial activity of the recombinant designer host defence peptide P-novispirin G10 in infected full-thickness wounds of porcine skin. J. Antimicrob. Chemother. 59:493–498 [DOI] [PubMed] [Google Scholar]
- 11.Hou Z, Lu J, Fang C, Zhou Y, Bai H, Zhang X, Xue X, Chen Y, Luo X. 2011. Underlying mechanism of in vivo and in vitro activity of C-terminal-amidated thanatin against clinical isolates of extended-spectrum beta-lactamase-producing Escherichia coli. J. Infect. Dis. 203:273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fehlbaum P, Bulet P, Chernysh S, Briand JP, Roussel JP, Letellier L, Hetru C, Hoffmann JA. 1996. Structure-activity analysis of thanatin, a 21-residue inducible insect defense peptide with sequence homology to frog skin antimicrobial peptides. Proc. Natl. Acad. Sci. U. S. A. 93:1221–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeaman MR, Yount NY. 2007. Unifying themes in host defence effector polypeptides. Nat. Rev. Microbiol. 5:727–740 [DOI] [PubMed] [Google Scholar]
- 14.Yount NY, Yeaman MR. 2004. Multidimensional signatures in antimicrobial peptides. Proc. Natl. Acad. Sci. U. S. A. 101:7363–7368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie C, Prahl A, Ericksen B, Wu Z, Zeng P, Li X, Lu WY, Lubkowski J, Lu W. 2005. Reconstruction of the conserved beta-bulge in mammalian defensins using D-amino acids. J. Biol. Chem. 280:32921–32929 [DOI] [PubMed] [Google Scholar]
- 16.Chan AW, Hutchinson EG, Harris D, Thornton JM. 1993. Identification, classification, and analysis of beta-bulges in proteins. Protein Sci. 2:1574–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute 2009. Performance standards for antimicrobial susceptibility testing: 19th informational supplement, M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 18.Motyl M, Dorso K, Barrett J, Giacobbe R. 2006. Basic microbiological techniques used in antibacterial drug discovery. Curr. Protoc. Pharmacol. 31(Suppl):13A.3.1–13A.3.22. 10.1002/0471141755.ph13a03s31. [DOI] [PubMed] [Google Scholar]
- 19.Jabra-Rizk MA, Meiller TF, James CE, Shirtliff ME. 2006. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob. Agents Chemother. 50:1463–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 40:175–179 [DOI] [PubMed] [Google Scholar]
- 21.Kustanovich I, Shalev DE, Mikhlin M, Gaidukov L, Mor A. 2002. Structural requirements for potent versus selective cytotoxicity for antimicrobial dermaseptin S4 derivatives. J. Biol. Chem. 277:16941–16951 [DOI] [PubMed] [Google Scholar]
- 22.Tossi A, Scocchi M, Zanetti M, Gennaro R, Storici P, Romeo D. 1997. An approach combining rapid cDNA amplification and chemical synthesis for the identification of novel, cathelicidin-derived, antimicrobial peptides. Methods Mol. Biol. 78:133–150 [DOI] [PubMed] [Google Scholar]
- 23.Xue X, Chen X, Mao X, Hou Z, Zhou Y, Bai H, Meng J, Da F, Sang G, Wang Y, Luo X. 2013. Amino-terminated generation 2 poly(amidoamine) dendrimer as a potential broad-spectrum, nonresistance-inducing antibacterial agent. AAPS J. 15:132–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loreto ES, Mario DA, Denardi LB, Alves SH, Santurio JM. 2011. In vitro susceptibility of Pythium insidiosum to macrolides and tetracycline antibiotics. Antimicrob. Agents Chemother. 55:3588–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radzishevsky IS, Rotem S, Bourdetsky D, Navon-Venezia S, Carmeli Y, Mor A. 2007. Improved antimicrobial peptides based on acyl-lysine oligomers. Nat. Biotechnol. 25:657–659 [DOI] [PubMed] [Google Scholar]
- 26.Navon-Venezia S, Feder R, Gaidukov L, Carmeli Y, Mor A. 2002. Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob. Agents Chemother. 46:689–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radzishevsky IS, Rotem S, Zaknoon F, Gaidukov L, Dagan A, Mor A. 2005. Effects of acyl versus aminoacyl conjugation on the properties of antimicrobial peptides. Antimicrob. Agents Chemother. 49:2412–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cirioni O, Ghiselli R, Minardi D, Orlando F, Mocchegiani F, Silvestri C, Muzzonigro G, Saba V, Scalise G, Balaban N, Giacometti A. 2007. RNAIII-inhibiting peptide affects biofilm formation in a rat model of staphylococcal ureteral stent infection. Antimicrob. Agents Chemother. 51:4518–4520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saldarriaga Fernandez IC, Mei HC, Metzger S, Grainger DW, Engelsman AF, Nejadnik MR, Busscher HJ. 2010. In vitro and in vivo comparisons of staphylococcal biofilm formation on a cross-linked poly(ethylene glycol)-based polymer coating. Acta Biomater. 6:1119–1124 [DOI] [PubMed] [Google Scholar]
- 30.Sanyal D, Johnson AP, George RC, Cookson BD, Williams AJ. 1991. Peritonitis due to vancomycin-resistant Staphylococcus epidermidis. Lancet 337:54. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira Nunes AP, Martins Teixeira L, Reis Bastos CC, de Souza Fonseca L, Netto dos Santos KR. 2002. Susceptibility of Brazilian staphylococcal strains to glycopeptides evaluated by different testing methods. Curr. Microbiol. 44:385–390 [DOI] [PubMed] [Google Scholar]
- 32.Nunes AP, Schuenck RP, Bastos CC, Magnanini MM, Long JB, Iorio NL, Santos KR. 2007. Heterogeneous resistance to vancomycin and teicoplanin among Staphylococcus spp. isolated from bacteremia. Braz. J. Infect. Dis. 11:345–350 [DOI] [PubMed] [Google Scholar]
- 33.Bongiorno D, Campanile F, Mongelli G, Baldi MT, Provenzani R, Reali S, Lo Russo C, Santagati M, Stefani S. 2010. DNA methylase modifications and other linezolid resistance mutations in coagulase-negative staphylococci in Italy. J. Antimicrob. Chemother. 65:2336–2340 [DOI] [PubMed] [Google Scholar]
- 34.Fjell CD, Hiss JA, Hancock RE, Schneider G. 2012. Designing antimicrobial peptides: form follows function. Nat. Rev. Drug Discov. 11:37–51 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.