Abstract
The aims of this study were to describe the pharmacokinetics of zidovudine (ZDV) and its biotransformation to its metabolite, 3*-azido-3*-deoxy-5*-glucuronylthymidine (G-ZDV), in HIV-infected children, to identify factors that influence the pharmacokinetics of ZDV, and to compare and evaluate the doses recommended by the World Health Organization (WHO) and the Food and Drug Administration (FDA). ZDV concentrations in 782 samples and G-ZDV concentrations in 554 samples from 247 children ranging in age from 0.5 to 18 years were retrospectively measured. A population pharmacokinetic model was developed with NONMEM software (version 6.2), and the pharmacokinetics of ZDV were best described by a one-compartment model with first-order absorption and elimination. The effect of body weight on the apparent elimination clearance and volume of distribution was significant. The mean population parameter estimates were as follows: absorption rate, 2.86 h−1; apparent elimination clearance, 89.7 liters · h−1 (between-subject variability, 0.701 liters · h−1); apparent volume of distribution, 229 liters (between-subject variability, 0.807 liters); metabolic formation rate constant, 12.6 h−1 (between-subject variability, 0.352 h−1); and elimination rate constant of G-ZDV, 2.27 h−1. On the basis of simulations with FDA and WHO dosing recommendations, the probabilities of observing efficient exposures (doses resulting in exposures of between 3 and 5 mg/liter · h) with less adverse events (doses resulting in exposures below 8.4 mg/liter · h) were higher when the FDA recommendations than when the WHO recommendations were followed. In order to improve the FDA recommendations, ZDV doses should be reconsidered for the weight band (WB) of 20 to 40 kg. The most appropriate doses should be decreased from 9 to 8 mg/kg of body weight twice a day (BID) for the WB from 20 to 29.9 kg and from 300 to 250 mg BID for the WB from 30 to 39.9 kg. The highest dose, 300 mg BID, should be started from body weights of 40 kg.
INTRODUCTION
Zidovudine (ZDV) was the first antiretroviral drug allowed by the Food and Drug Administration (FDA) and approved for the treatment of children with HIV infection (1). It was the most prescribed monotherapy in children and adults, and currently, ZDV-containing regimens are still recommended as first-line combination regimens for infants and children by the World Health Organization (WHO) (2). However, limited pharmacokinetic (PK) studies have been conducted in children (small size, narrow range of ages and/or body weight, single-dose regimen, and the doses administered were different from the last doses recommended) (3–11).
Recommended dosing regimens have changed in recent years. Before 2009, the ZDV dosage was based on the body surface area (BSA). The recommended oral dose was 480 mg/m2/day given as 240 mg/m2 twice daily (BID) or 160 mg/m2 three times daily (TID) (12). The equivalent weight-based dose of 480 mg/m2/day for an average child with a BSA of 1 m2 and a weight of 32 kg is approximately 15 mg/kg of body weight (9). Currently, the FDA and WHO recommend higher doses (~19 mg/kg). The doses recommended by WHO are expressed as mg, whereas the FDA-approved doses are expressed as mg/kg BID for the youngest children. Furthermore, the weight bands (WB) defined are not the same. Current recommendations are detailed in Table 1.
Table 1.
ZDV dosages recommended by FDA and WHO
| Recommending authority and body wt range (kg) | Dosage form | Dose or dosage regimena | Total daily dose (mg/kg/day) |
|---|---|---|---|
| WHO | |||
| 3 to <6 | Syrup | 60 mg | 20–40 |
| ≥6 to <10 | Syrup | 90 mg | 18–30 |
| ≥10 to <14 | Syrup | 120 mg | 17–24 |
| ≥14 to <20 | Tablet | 150 mg | 15–21 |
| ≥20 to <25 | Tablet | 225 mgb | 18–23 |
| ≥25 | Tablet | 300 mg | 4c-24 |
| FDA | |||
| 4 to <9 | Syrup | 12 mg/kg | 24 |
| ≥9 to <30 | Syrup | 9 mg/kg | 18 |
| ≥30 | Tablet | 300 mg | 4c-20 |
All doses or dosage regimens are BID.
Patients received an oral dose of 300 mg every morning and 150 mg every evening.
For an adult weighing 150 kg.
For children older than age 6 weeks, the doses recommended are based on only the body weight (2, 12). Nevertheless, a previous study suggested an effect of age on the apparent ZDV clearance: a decrease was observed in children less than 2 years old (13). Moreover, another study (14) demonstrated that the neonatal doses recommended by the WHO produced very high ZDV exposure.
Many adverse effects of treatment with ZDV have been observed. Anemia, neutropenia, and mitochondrial toxicity have been described in an important number of infants that were exposed to ZDV during the first weeks or months of life (1, 15–18).
The physiopathological mechanism of anemia is not well understood (17), but mitochondrial dysfunction can be clearly attributed to ZDV (19). The adverse effects of ZDV are concentration dependent. Two previous studies have reported exposure-toxicity relationships. A mean ZDV area under the curve (AUCZDV) of 8.4 mg/liter · h was found to increase the risk of anemia by 32% (from 7.6% to 23.4%) (13), while a mean AUCZDV of greater than 19.2 mg/liter · h was found to significantly increase the risk of neutropenia (P = 0.01) (4).
ZDV is mainly metabolized by uridine diphosphate glucuronosyltransferase (UGT) to an inactive metabolite, 3*-azido-3*-deoxy-5*-glucuronylthymidine (G-ZDV) (20, 21). Seventy percent of ZDV is eliminated via metabolism, whereas 30% is eliminated via the renal route. Modeling of the pharmacokinetics of ZDV plus G-ZDV allows estimation of the rate of formation of the metabolite and then identification of covariates, for example, age, that can influence this parameter.
Plasma ZDV and G-ZDV data were collected for a large population of infants, children, and adolescents in order to characterize the pharmacokinetics of ZDV and its biotransformation to G-ZDV by using a population approach and to identify significant covariates (age, body weight, and combined antiretroviral drugs) that can explain some of the between-subject variability. Thanks to the final population model, it was possible to compare and evaluate the doses recommended by FDA and WHO and then to refine the dose regimens in children.
MATERIALS AND METHODS
Patients.
The population included 247 HIV-infected infants, children, and adolescents in the greater Paris, France, area who had received ZDV-containing antiretroviral regimens. Plasma concentrations were monitored on a routine basis, and the concentrations in the samples were measured in the pharmacology unit of the Hospital Cochin. They were collected from 1998 to 2012 during medical visits with therapeutic drug monitoring. For each patient, age, body weight (BW), cotreatments (CoTs), gender, time of administration, time of sampling, and dosage form were recorded. Samples were collected for therapeutic drug monitoring during the visit; therefore, the times that had elapsed between drug administration and sampling times were variable. Ethics committee approval and patient consent are not compulsory in France in order to use therapeutic drug monitoring data, and thus they were not obtained.
Treatments.
ZDV was administered as an oral solution (100 mg/10 ml), capsules (100 mg or 250 mg), or tablets in combination with other nucleoside reverse transcriptase inhibitors (NRTIs; Combivir, which contains lamivudine [3TC] and ZDV at 300 mg; Trizivir, which contains abacavir [ABC], 3TC, and ZDV at 300 mg). ZDV was administered twice daily or three times daily. Some patients also received 3TC and/or didanosine (ddI), ABC, lopinavir (LPV), ritonavir (RTO), nelfinavir (NFV), nevirapine (NVP), or efavirenz (EFV) as cotreatments.
Analytical method.
Plasma ZDV and G-ZDV concentrations were determined by high-performance liquid chromatography, as previously described (22). The lower limit of quantification (LLOQ) for both was 0.05 mg/liter. The mean interassay precisions of the lowest concentration of the quality controls were 10 and 13.9% for ZDV and its metabolite, respectively. Overall recovery from plasma was 89% for ZDV and 82% for G-ZDV.
Population pharmacokinetic analysis and modeling.
A population approach was used to analyze simultaneously the concentrations of ZDV and its metabolite, G-ZDV, obtained by biotransformation. Plasma drug concentrations were assumed to be at steady state and were expressed in molarities in order to mix the data for ZDV and G-ZDV. Doses (in mg) and plasma drug concentrations (in mg · liter−1) were divided by the ZDV and G-ZDV molar masses (267.2 and 443.3 g · mol−1, respectively).
The data were analyzed using the nonlinear mixed-effect modeling software NONMEM (version 6.2), and the FOCE (first order conditional estimation) method was applied (23). ZDV was exclusively given orally, so clearance (CL) and volume of distribution (V) are apparent parameters CL/F and V/F, respectively, where F is the unknown bioavailability fraction.
A one-compartment model with first-order absorption and elimination was used to describe plasma ZDV concentrations. An additional compartment for G-ZDV connected to the ZDV compartment with a first-order rate constant was added. The parameters of this model were the apparent volumes of distribution of ZDV and G-ZDV (V/F and Vm/F, respectively, where Vm is the volume of distribution of the metabolite), the first-order absorption rate constant for ZDV (ka), the apparent elimination clearance for ZDV (CLp/F, where “p” stands for “parent”), and the apparent metabolic clearance (CLm/F) and the elimination rate constant (kel) for G-ZDV. The parameters estimated with the model were ka, CLp/F, V/F, CLm/Vm (metabolic formation rate constant), and kel. Plasma ZDV concentrations below the limit of quantification (LOQ) were replaced by the LOQ/2 value or by the M2 and M3 method. The M2 method consists of discarding BQL observations but adjusting the likelihood for the remaining data, whereas the M3 method involves maximizing the likelihood for the data above the limit of quantification and treating BQL data as censored (24). Between-subject variabilities (BSVs) were assumed to be exponential. An additive, proportional, or mixed error model was tested to describe the residual variability.
The influence of each patient covariate was systematically tested. Indeed, the covariates were evaluated via upward-backward model building, as previously described (25). A covariate was selected if (i) it produced a minimum decrease of 6.63 units (χ2 with 1 degree of freedom, P < 0.01) in the objective function value (OFV), (ii) it produced a reduction in the variability of the pharmacokinetic parameter(s), as assessed by the associated BSV, and (iii) its effect was physiologically plausible.
The continuous covariates, age and BW, were tested according to the following equation, using, for example, CL: , where θCL is the typical value of clearance for a patient with the median covariate value and is the estimated influential factor for the continuous covariate. The median value from all of the other patients was used if a covariate was missing.
CoTs, gender, the dosage form (galenic), and age were considered binary covariates, and their influence was tested as follows: , where CoT is equal to 1 if cotreatment is present and CoT is equal to 0 if it is not, and is the estimated influential factor for the cotreatments. CoTs were tested individually, e.g., the effect of lopinavir alone, or by pharmaceutical class, e.g., the effect of the protease inhibitor.
Evaluation and validation.
Diagnostic graphics were used for graphical evaluation of the goodness of fit. Observed versus predicted concentrations and weighted residuals (WRES) versus time and/or predicted concentrations (PRED). The stability of the model and accuracy of the parameters were assessed by a bootstrap method implemented in Wings for NONMEM (WfN; http://wfn.sourceforge.net/).
The final model was also evaluated by use of the visual predictive check (VPC) (26). ZDV concentration profiles were simulated and compared with the observed data in order to evaluate the predictive performance of the model. The final population model was used to simulate 500 vectors of pharmacokinetic parameters from all the patients. The simulations were performed using NONMEM. The 5th, 50th, and 95th percentiles of the simulated concentrations at each time were then overlaid on the observed concentrations using RfN (http://wfn.sourceforge.net/), and a visual inspection was performed.
ZDV pharmacokinetic parameters.
For each patient, the time that the maximum concentration (Cmax) was observed (Tmax) and the half-life (t1/2) were calculated from the estimated individual pharmacokinetic parameters. Doses administered by WB were highly variable, so it was not possible to compare the mean AUCZDV and Cmax with those of previous studies.
Dose evaluations and simulations.
The weight bands used by FDA are different from those used by WHO (Table 1). In order to compare the doses recommended by these organizations, patients were divided into several weight classes: from 6 to 9.9 kg, from 10 to 13.9 kg, from 14 to 19.9 kg, from 20 to 50 kg in steps of 5 kg, and from 50 kg to the maximum. The first WBs are those recommended by the WHO for the youngest children (2), and the others were created by ourselves.
For each patient, 400 simulations were performed using the FDA and WHO recommendations. The AUCZDV values from 0 to 24 h were also derived from the estimated individual pharmacokinetic parameters.
RESULTS
Demographic data.
A total of 247 patients (119 boys and 128 girls; 48% and 52%, respectively) ranging in age from 6 months to 18 years (median age, 10.6 years) were available for pharmacokinetic evaluation. Data on a total of 782 plasma ZDV concentrations and 554 plasma G-ZDV concentrations were collected (mean, 3.6 samples; range, 1 to 19 samples per child). The median values for body weight were 32.2 kg (minimum and maximum, 6.1 and 84 kg, respectively). The characteristics of the patients are summarized in Table 2.
Table 2.
Characteristics of the population
| Characteristic | Value or median | Range |
|---|---|---|
| Gender (% male/% female) | 48/52 | |
| Age (yr) | 10.8 | 0.15–18 |
| Wt (kg) | 32.2 | 6–85 |
| No. of samples/patient | 3.6 | 1–19 |
| Dose/day (mg) | 600 | 36–600 |
Among the treatments that the patients received, 51% of patients also received 3TC, 10% also received ddI, 11% also received ABC, 39% also received LPV, 41% also received RTO, 13% also received NFV, 18% also received NVP, and 11% also received EFV. ZDV was administered in syrup (33.7%) or as a tablet (66.3%).
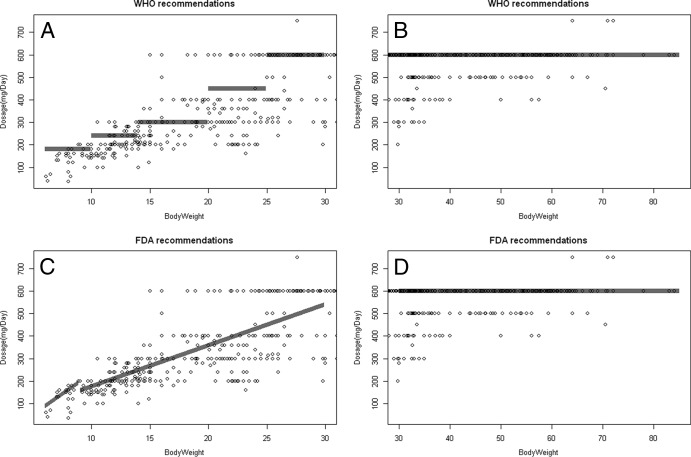
ZDV was administered every 8 h (5.6%) or every 12 h (94.4%). The median ZDV dose administered was 300 mg (range, 18 to 300 mg) twice daily. As shown in Fig. 1, the doses recommended by FDA and WHO were compared to the current doses. A majority of the doses were below the actual recommendations, mostly for the weight band of 6 to 30 kg, and the maximum doses were given to children weighing greater than 15 kg instead of 25 kg or 30 kg, depending on the recommendations.
Fig 1.
Comparison of ZDV doses administered in the study and those recommended by WHO (A, B) and FDA (C, D) for patients with body weights less than 30 kg (A, C) and patients with body weights greater than 30 kg (B, D). Gray areas represent the doses recommended according to the different weight bands. Points represent the dose of ZDV administered (mg/day).
The high variability of the doses can be explained by the long period of patient inclusion and the changes in recommendations in recent years.
Population pharmacokinetics.
All concentrations were at steady state, and the ZDV concentrations in 46 (5.9%) plasma samples and the G-ZDV concentrations in 13 (2.3%) plasma samples were below the limit of quantification.
Use of the M3 method and the built-in M2 method did not improve the results. So, we used the method of setting those concentrations to half of the LOQ (27).
The best model to describe plasma ZDV concentrations was a one-compartment model with first-order absorption and elimination and an additional compartment for G-ZDV. The two-compartment model with or without lag time or a zero-order absorption rate did not improve the fit.
We did not have enough data to correctly estimate the ZDV ka, but several studies have reported some estimates (13, 28, 29). The stability of the model was improved when the value was fixed to 2.86 h−1. This value was reported by Panhard et al. (28) and used in another study (30).
Residual variabilities were best described by a combined error model for ZDV and by a proportional error model for G-ZDV. The additive part of the combined error model was fixed at the LOQ/2 value (0.025 mg/liter, i.e., 0.1 mmol−1). No between-occasion variability could be estimated, but BSV could be estimated for CLp/F, V/F, and Clm/Vm.
The covariance between CLp/F and V/F was significant, decreasing the OFV by 51.3 units. Covariates were tested on CLp/F, Clm/Vm, and V/F in an upward procedure.
The most significant covariate effect was the effect of body weight on the apparent elimination clearance for ZDV, decreasing the OFV by 110 units, and then on the apparent volume of distribution, with an additional decrease of 15.7 units. Also, BSVs of CLp/F and V/F decreased on inclusion of the body weight effect. An allometric model was tested but did not significantly improve the results. The effect of age was tested as a continuous covariate or a categorical covariate: patients were separated into two groups, those less than 2 years old and those more than 2 years old (13), but the separation did not significantly improve the results. The final population pharmacokinetic estimates are summarized in Table 3.
Table 3.
Population pharmacokinetic parameters of ZDV and GZDV from the final modela
| Structural model |
Statistical model |
||||||
|---|---|---|---|---|---|---|---|
| Parameter | Estimate | % RSE | 95% CI | Parameter | Estimate | % RSE | 95% CI |
| ka (h−1) | 2.86b | ||||||
| CLp/F (liters · h−1) | 89.7 | 7.1 | 77–102 | 0.701 | 17.9 | 0.572–0.820 | |
| 0.858 | 11.1 | 0.67–1.1 | |||||
| V/F (liters) | 229 | 12.4 | 181–291 | ωV/F | 0.807 | 22.9 | 0.597–0.963 |
| 0.534 | 24.5 | 0.22–0.81 | |||||
| Clm/Vm (h−1) | 12.6 | 20.7 | 8.2–18 | 0.352 | 20.7 | 0.213–0.473 | |
| kel (h−1) | 2.27 | 16.2 | 1.6–3.1 | ||||
| Corr (CLp, V) | 0.733 | 0.5–0.85 | |||||
| a (mmol/liter) | 0.1b | ||||||
| σZDV | 0.56 | 8.5 | 0.514–0.614 | ||||
| σG-ZDV | 0.69 | 7.0 | 0.643–0.742 | ||||
RSE, relative standard error (standard error of the estimate divided by the estimate and multiplied by 100); ω, coefficient of variation for between-subject variability; Corr, correlation between two parameters; a, additive part of the combined error model; σ, parameters of errors model.
Fixed value.
Validation.
The parameters and their associated BSVs were accurately estimated, and the confidence intervals (CIs) derived from the bootstrap analysis were reasonably narrow and did not include the value 0.
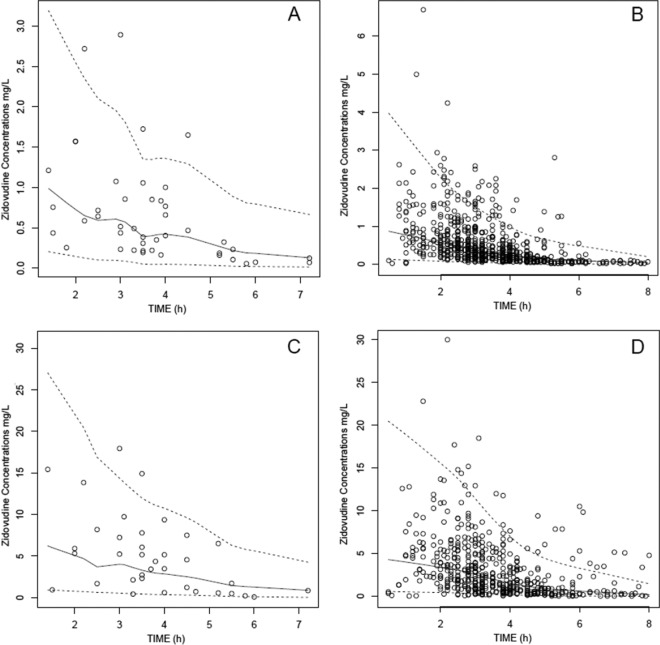
The visual predictive check (VPC) performed on the final model showed that the average prediction matched the observed concentration-time courses and that the variability was reasonably estimated for the TID and BID regimens. Since patients received different drug dosages, we normalized the observed and the predicted concentrations for a mean 300-mg dose in Fig. 2.
Fig 2.
Visual predictive check for comparison of the 5th (bottom dashed lines), 50th (full lines), and 95th (top dashed lines) percentiles obtained from 400 simulations and the observed data (points) for plasma ZDV concentration for TID administration (A), plasma ZDV concentration for BID administration (B), G-ZDV plasma concentration for TID administration (C), and G-ZDV plasma concentration for BID administration (D). The model predictions and observations have been normalized to the median ZDV dose, 300 mg.
ZDV results and simulations.
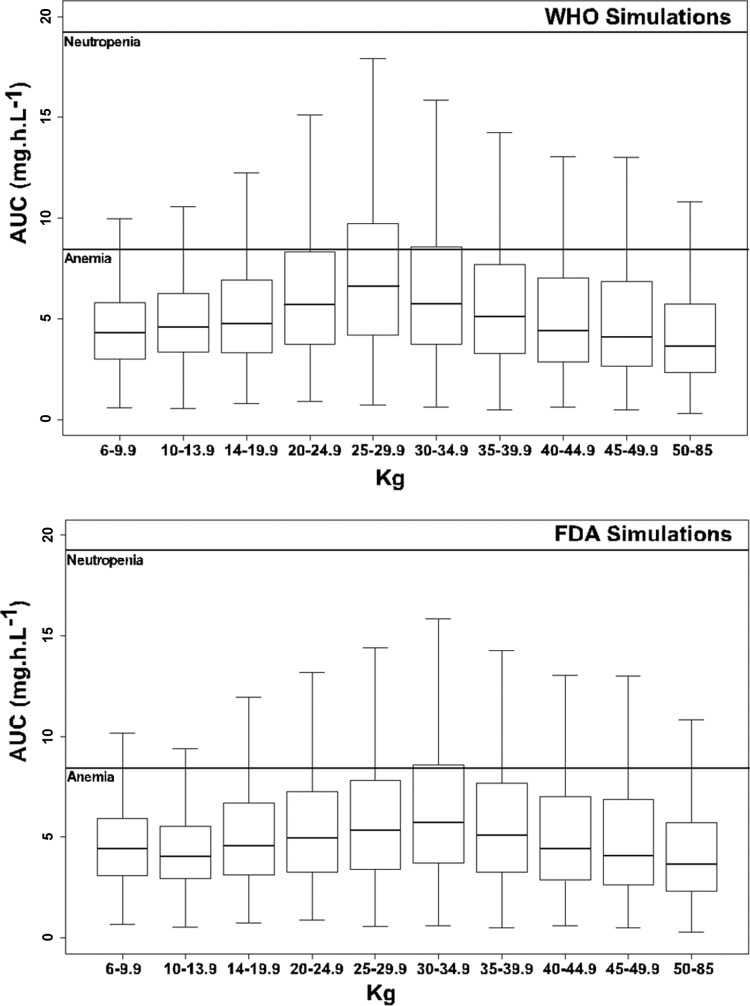
Doses recommended by FDA and by WHO were simulated 400 times for each patient, in order to evaluate and compare the FDA and WHO recommendations. Our goal was to target the median adult AUCZDV (i.e., between 3 and 5 mg/liter · h) for the lowest percentage of children with an AUCZDV of >8.4 mg/liter · h. The individual AUCZDV values were calculated for each simulation. They were split by group and are represented in Fig. 3.
Fig 3.
ZDV exposures obtained with 400 simulations of the doses recommended by FDA and WHO. Patients were grouped according to the different weight bands. Solid lines correspond to exposures of 8.4 and 19.2 mg · h · liter−1.
The median exposures are in agreement with the targets defined previously, except for the WB of 20 to 40 kg, in which the median AUCZDV was greater than 5 mg/liter · h. In this group, the percentage of children who had a high exposure and who were at risk for the development of adverse effects is more important.
For the WHO recommendations, the percentage of patients with an exposure higher than 8.4 mg/liter · h varied from 21.5% to 40.0%, whereas the percentage of patients with an exposure higher than 19.2 mg/liter · h varied from 0.90% to 2.1%. Using the FDA recommendations, the proportions are 17.9% to 26.8% and 0.35% to 1.43%, respectively. Details are shown in Table 4.
Table 4.
Results of the 400 simulations for WHO, FDA, and our recommendations
| Recommending authority and body weight (kg) | Dose or dosage regimena | Median AUCZDV (mg/liter · h)c | % subjects with AUCZDV of: |
|
|---|---|---|---|---|
| >8.4 mg/liter · h | >19.2 mg/liter · h | |||
| WHO | ||||
| ≥20 to <25 | 225 mgb | 5.8 | 25.4 | 0.9 |
| ≥25 to <30 | 300 mg | 6.6 | 40 | 2.1 |
| ≥30 to <35 | 300 mg | 5.8 | 26.7 | 1.43 |
| ≥35 to <40 | 300 mg | 5.2 | 21.5 | 0.94 |
| FDA | ||||
| ≥20 to <25 | 9 mg/kg | 5.2 | 17.9 | 0.35 |
| ≥25 to <30 | 9 mg/kg | 5.4 | 21.4 | 0.61 |
| ≥30 to <35 | 300 mg | 5.9 | 26.8 | 1.43 |
| ≥35 to <40 | 300 mg | 5.2 | 21.5 | 0.94 |
| Authors of this study | ||||
| ≥20 to <25 | 8 mg/kg | 4.5 | 12.4 | 0.1 |
| ≥25 to <30 | 8 mg/kg | 4.8 | 15.3 | 0.28 |
| ≥30 to <35 | 250 mg | 4.8 | 17 | 0.48 |
| ≥35 to <40 | 250 mg | 4.4 | 13.1 | 0.31 |
All doses or dosage regimens are BID.
Patients received an oral dose of 300 mg every morning and 150 mg every evening.
AUCZDV values are from 0 to 24 h.
We simulated other doses for this WB in order to obtain the adult AUCZDV in the range fixed above: 8 mg/kg BID for BWs from 20 to 29.9 kg, 250 mg BID for BWs from 30 to 39.9 kg, and 300 BID mg for BWs above 40 kg.
The median AUCZDV was 4.5 mg/liter · h for the WB of 20 to 25 kg, 4.8 mg/liter · h for the WB of 25 to 30 kg, 4.8 mg/liter · h for the WB of 30 to 35 kg, and 4.4 mg/liter · h for the WB of 35 to 40 kg. The percentage of patients in these WBs with an exposure higher than 8.4 mg/liter · h should decrease 31% (from 17.9% to 12.4%), 29% (from 21.4% to 15.3%), 37% (from 26.8% to 17.0%), and 39% (from 21.5% to 13.1%), respectively. Results are presented in Table 4.
DISCUSSION
A joint model was developed to simultaneously describe plasma ZDV and G-ZDV concentration-time courses in 247 children ranging in age from 0.5 to 18 years. The pharmacokinetics of ZDV was satisfactorily described by a one-compartment model with a linear absorption and elimination rate and an additional compartment for G-ZDV.
Several previous studies found a structural model with one compartment for ZDV (13, 28, 30, 31), but to our knowledge, only one study has investigated the pharmacokinetics of ZDV in children (13). Bootstrap and VPC procedures validated this model.
The following observations support the model developed: the median half-life of ZDV (1.45 h) was consistent with that previously reported from studies with children (from 1 to 2 h) (3, 4, 7, 13), as was the median Tmax of 0.76 h, which ranged from 0.5 to 1 h in previous studies (7, 32, 33). The median apparent clearance (3.02 liters/h/kg) was also consistent with previously reported values, which ranged from 2.95 to 3.89 liters/h/kg, obtained from studies with children and adults (8, 33, 34).
In our model, the effect of body weight on apparent clearance and apparent volume of distribution was the most significant. The allometric model, with fixed power exponents of 1 and 3/4 for volume and clearance terms, respectively, did not improve the results. No additional effect of age on CL/F was found using a continuous relationship or a categorical relationship with an age cutoff of 2 years, as in previous studies (13, 32). This was not unexpected, since body weight and age are highly correlated in this pediatric population.
The effects of age and body weight were tested on Clm/Vm. No effect was found, which is in agreement with the findings of the study of Hirt et al. (14), who suggested a fast maturation of this biotransformation during the first month of life, reaching adult values at age 2 months.
This model was used to test the effect of the other antiretroviral drugs on Clm/Vm. Indeed, some drugs, such as EFV and NVP, are inducers or inhibitors of many enzymes (35), but in our study no effect of the cotreatments on Clm/Vm were found. The effect of these coadministered antiretroviral drugs on CLp/F and V/F was also tested. An effect of ddI and NFV on CL/F and V/F was already found (13, 28), but these effects were not significant in our study.
The effects of dosage form and gender on Clm/Vm were also tested. Bazzoli et al. (30) found an effect of gender on CL/F, but no significant differences between administration route and gender were found in the present study.
FDA and WHO recommendation and simulations.
Four hundred simulations were performed for each patient in order to evaluate and compare FDA and WHO recommendations. Because no relationship between concentration and efficacy is available for ZDV, the target was defined as the median ZDV adult exposure. This varies from 3 mg · h · liter−1 in adults (33, 34) to 5 mg/liter · h in children (9). It had been demonstrated that the variability of ZDV is very important on both intersubject and intrasubject components (28, 30). Adverse effects of ZDV are concentration dependent, as shown by two previous studies that have reported exposure-toxicity relationships. A value of AUCZDV greater than 8.4 mg/liter · h increased the risk of anemia by 32% (from 7.6% to 23.4%) (13), and a value of AUCZDV greater than 19.2 mg/liter · h significantly increased the risk of neutropenia (P = 0.01) (4).
Therefore, AUCZDV values were compared by WB group, and the efficacy target was set to 3 to 5 mg/liter · h. The lowest percentages of children with exposures below the levels related to adverse effects were used as safety targets.
FDA recommendations seem to be more appropriate than WHO recommendations. Median AUCZDVs were over the interval of 3 to 5 mg/liter · h in at least 50% of the children, and the risk of development of adverse effects is lower than the one expected by following the WHO recommendations (Table 4). Moreover, we pointed out an effect of body weight on apparent clearance, so the recommendations should be weight dependent, similar to the FDA recommendations.
Nevertheless, simulations of the doses recommended by FDA showed that the risks of development of adverse effects are greater in the WB of 20 to 40 kg. To reduce these risks, other simulations were performed and showed that the most appropriate doses for the WB of 20 to 40 kg would be (i) 8 mg/kg BID for BWs ranging from 20 to 29.9 kg, (ii) 250 mg BID for BWs ranging from 30 to 39.9 kg, and (iii) 300 BID mg for BWs greater than 40 kg. The target exposures should maintain drug efficacy and minimize the risk of development of adverse effects.
In conclusion, this study reports the pharmacokinetics of ZDV and G-ZDV in infants, children, and adolescents weighing from 6 to 85 kg. The pharmacokinetic parameters were consistent with those from previous studies. According to simulations, to decrease the risk of anemia and neutropenia and to reach a median AUCZDV close to 3 to 5 mg/liter · h, the highest dose should be above 40 kg. The doses administered should be decreased for those ranging in weight from 20 to 39.9 kg. These conclusions should be prospectively confirmed.
Footnotes
Published ahead of print 22 July 2013
REFERENCES
- 1.Richman DD, Fischl MA, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, Leedom JM, Groopman JE, Mildvan D, Hirsch MS. 1987. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N. Engl. J. Med. 317:192–197 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization 2010. Antiretroviral therapy for HIV infection in infants and children; towards universal access: recommendations for a public health approach. World Health Organization, Geneva, Switzerland: http://www.who.int/hiv/pub/paediatric/infants2010/en/index.html [PubMed] [Google Scholar]
- 3.Balis FM, Pizzo PA, Eddy J, Wilfert C, McKinney R, Scott G, Murphy RF, Jarosinski PF, Falloon J, Poplack DG. 1989. Pharmacokinetics of zidovudine administered intravenously and orally in children with human immunodeficiency virus infection. J. Pediatr. 114:880–884 [DOI] [PubMed] [Google Scholar]
- 4.Balis FM, Pizzo PA, Murphy RF, Eddy J, Jarosinski PF, Falloon J, Broder S, Poplack DG. 1989. The pharmacokinetics of zidovudine administered by continuous infusion in children. Ann. Intern. Med. 110:279–285 [DOI] [PubMed] [Google Scholar]
- 5.Boucher FD, Modlin JF, Weller S, Ruff A, Mirochnick M, Pelton S, Wilfert C, McKinney R, Jr, Crain MJ, Elkins MM. 1993. Phase I evaluation of zidovudine administered to infants exposed at birth to the human immunodeficiency virus. J. Pediatr. 122:137–144 [DOI] [PubMed] [Google Scholar]
- 6.Gibb D, Barry M, Ormesher S, Nokes L, Seefried M, Giaquinto C, Back D. 1995. Pharmacokinetics of zidovudine and dideoxyinosine alone and in combination in children with HIV infection. Br. J. Clin. Pharmacol. 39:527–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wintergerst U, Rolinski B, Vocks-Hauck M, Wahn V, Debatin KM, Notheis G, Grosch-Wörner I, Goebel FD, Roscher AA, Belohradsky BH. 1995. Pharmacokinetics of orally administered zidovudine in HIV-infected children and adults. Infection 23:344–348 [DOI] [PubMed] [Google Scholar]
- 8.Fletcher CV, Yogev R, Nachman SA, Wiznia A, Pelton S, McIntosh K, Stanley K. 2004. Pharmacokinetic characteristics of ritonavir, zidovudine, lamivudine, and stavudine in children with human immunodeficiency virus infection. Pharmacotherapy 24:453–459 [DOI] [PubMed] [Google Scholar]
- 9.Bergshoeff AS, Fraaij PLA, Verweij C, van Rossum AMC, Verweel G, Hartwig NG, de Groot R, Burger DM. 2004. Plasma levels of zidovudine twice daily compared with three times daily in six HIV-1-infected children. J. Antimicrob. Chemother. 54:1152–1154 [DOI] [PubMed] [Google Scholar]
- 10.Chokephaibulkit K, Cressey TR, Capparelli E, Sirisanthana V, Muresan P, Hongsiriwon S, Ngampiyaskul C, Limwongse C, Wittawatmongkol O, Aurpibul L, Kabat B, Toye M, Smith ME, Eksaengsri A, McIntosh K, Yogev R. 2011. Pharmacokinetics and safety of a new paediatric fixed-dose combination of zidovudine/lamivudine/nevirapine in HIV-infected children. Antivir. Ther. 16:1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasirye P, Kendall L, Adkison KK, Tumusiime C, Ssenyonga M, Bakeera-Kitaka S, Nahirya-Ntege P, Mhute T, Kekitiinwa A, Snowden W, Burger DM, Gibb DM, Walker AS. 2012. Pharmacokinetics of antiretroviral drug varies with formulation in the target population of children with HIV-1. Clin. Pharmacol. Ther. 91:272–280 [DOI] [PubMed] [Google Scholar]
- 12.Food and Drug Administration 2009. Office of Clinical Pharmacology Review for retrovir. Food and Drug Administration, Rockville, MD: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM253508.pdf [Google Scholar]
- 13.Capparelli EV, Englund JA, Connor JD, Spector SA, McKinney RE, Palumbo P, Baker CJ. 2003. Population pharmacokinetics and pharmacodynamics of zidovudine in HIV-infected infants and children. J. Clin. Pharmacol. 43:133–140 [DOI] [PubMed] [Google Scholar]
- 14.Hirt D, Warszawski J, Firtion G, Giraud C, Chappuy H, Lechenadec J, Benaboud S, Urien S, Blanche S, Tréluyer J-M. 11 March 2013. High exposure to zidovudine during the first 2 first weeks of life and concentration-toxicity relationships. J. Acquir. Immune Defic. Syndr. [Epub ahead of print.] 10.1097/QAI.0b013e3182908c00 [DOI] [PubMed] [Google Scholar]
- 15.McKinney RE, Jr, Maha MA, Connor EM, Feinberg J, Scott GB, Wulfsohn M, McIntosh K, Borkowsky W, Modlin JF, Weintrub P. 1991. A multicenter trial of oral zidovudine in children with advanced human immunodeficiency virus disease. The Protocol 043 Study Group. N. Engl. J. Med. 324:1018–1025 [DOI] [PubMed] [Google Scholar]
- 16.Volberding PA, Lagakos SW, Koch MA, Pettinelli C, Myers MW, Booth DK, Balfour HH, Jr, Reichman RC, Bartlett JA, Hirsch MS. 1990. Zidovudine in asymptomatic human immunodeficiency virus infection. A controlled trial in persons with fewer than 500 CD4-positive cells per cubic millimeter. The AIDS Clinical Trials Group of the National Institute of Allergy and Infectious Diseases. N. Engl. J. Med. 322:941–949 [DOI] [PubMed] [Google Scholar]
- 17.Moyle G. 2005. Mechanisms of HIV and nucleoside reverse transcriptase inhibitor injury to mitochondria. Antivir. Ther. 10(Suppl 2):M47–M52 [PubMed] [Google Scholar]
- 18.Moyle G. 2000. Toxicity of antiretroviral nucleoside and nucleotide analogues: is mitochondrial toxicity the only mechanism? Drug Saf. 23:467–481 [DOI] [PubMed] [Google Scholar]
- 19.Ross AC, Leong T, Avery A, Castillo-Duran M, Bonilla H, Lebrecht D, Walker UA, Storer N, Labbato D, Khaitan A, Tomanova-Soltys I, McComsey GA. 2012. Effects of in utero antiretroviral exposure on mitochondrial DNA levels, mitochondrial function and oxidative stress. HIV Med. 13:98–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pioger JC, Taburet AM, Colin JN, Colaneri S, Fillastre JP, Singlas E. 1989. Pharmacokinetics of zidovudine (AZT) and its metabolite (G-AZT) in healthy subjects and in patients with kidney failure. Therapie 44:401–404 (In French.) [PubMed] [Google Scholar]
- 21.Sim SM, Back DJ, Breckenridge AM. 1991. The effect of various drugs on the glucuronidation of zidovudine (azidothymidine; AZT) by human liver microsomes. Br. J. Clin. Pharmacol. 32:17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chappuy H, Tréluyer J-M, Jullien V, Dimet J, Rey E, Fouché M, Firtion G, Pons G, Mandelbrot L. 2004. Maternal-fetal transfer and amniotic fluid accumulation of nucleoside analogue reverse transcriptase inhibitors in human immunodeficiency virus-infected pregnant women. Antimicrob. Agents Chemother. 48:4332–4336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beal SL, Sheiner LB. 1998. NONMEM user guides parts I to VIII. Division of Clinical Pharmacology, University of California, San Francisco, CA [Google Scholar]
- 24.Ahn JE, Karlsson MO, Dunne A, Ludden TM. 2008. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J. Pharmacokinet. Pharmacodyn. 35:401–421 [DOI] [PubMed] [Google Scholar]
- 25.Foissac F, Urien S, Hirt D, Frange P, Chaix M-L, Treluyer J-M, Blanche S. 2011. Pharmacokinetics and virological efficacy after switch to once-daily lopinavir-ritonavir in treatment-experienced HIV-1-infected children. Antimicrob. Agents Chemother. 55:4320–4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. 2011. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 13:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beal SL. 2001. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 28:481–504 [DOI] [PubMed] [Google Scholar]
- 28.Panhard X, Legrand M, Taburet A-M, Diquet B, Goujard C, Mentré F. 2007. Population pharmacokinetic analysis of lamivudine, stavudine and zidovudine in controlled HIV-infected patients on HAART. Eur. J. Clin. Pharmacol. 63:1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fletcher CV, Balfour HH., Jr 1996. Variability in zidovudine serum concentrations. Pharmacotherapy 16:1154–1158 [PubMed] [Google Scholar]
- 30.Bazzoli C, Bénech H, Rey E, Retout S, Salmon D, Duval X, Tréluyer JM, Mentré F. 2011. Joint population pharmacokinetic analysis of zidovudine, lamivudine, and their active intracellular metabolites in HIV patients. Antimicrob. Agents Chemother. 55:3423–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gitterman SR, Drusano GL, Egorin MJ, Standiford HC. 1990. Population pharmacokinetics of zidovudine. The Veterans Administration Cooperative Studies Group. Clin. Pharmacol. Ther. 48:161–167 [DOI] [PubMed] [Google Scholar]
- 32.Zhou XJ, Sheiner LB, D'Aquila RT, Hughes MD, Hirsch MS, Fischl MA, Johnson VA, Myers M, Sommadossi JP. 1999. Population pharmacokinetics of nevirapine, zidovudine, and didanosine in human immunodeficiency virus-infected patients. The National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group Protocol 241 Investigators. Antimicrob. Agents Chemother. 43:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crémieux AC, Katlama C, Gillotin C, Demarles D, Yuen GJ, Raffi F. 2001. A comparison of the steady-state pharmacokinetics and safety of abacavir, lamivudine, and zidovudine taken as a triple combination tablet and as abacavir plus a lamivudine-zidovudine double combination tablet by HIV-1-infected adults. Pharmacotherapy 21:424–430 [DOI] [PubMed] [Google Scholar]
- 34.Vanhove GF, Kastrissios H, Gries JM, Verotta D, Park K, Collier AC, Squires K, Sheiner LB, Blaschke TF. 1997. Pharmacokinetics of saquinavir, zidovudine, and zalcitabine in combination therapy. Antimicrob. Agents Chemother. 41:2428–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mannu J, Jenardhanan P, Mathur PP. 2011. A computational study of CYP3A4 mediated drug interaction profiles for anti-HIV drugs. J. Mol. Model. 17:1847–1854 [DOI] [PubMed] [Google Scholar]