Abstract
New approaches of empirical antifungal therapy (EAT) in selected hematological patients with persistent febrile neutropenia (PFN) have been proposed in recent years, but their cost-effectiveness has not been studied. The aim of this study was to compare the cost-effectiveness of two different approaches of EAT in hematological patients with PFN: the diagnosis-driven antifungal therapy (DDAT) approach versus the standard approach of EAT. A decision tree to assess the cost-effectiveness of both approaches was developed. Outcome probabilities and treatment pathways were extrapolated from two studies: a prospective cohort study following the DDAT approach and a randomized clinical trial following the standard approach. Uncertainty was undertaken through sensitivity analyses and Monte Carlo simulation. The average effectiveness and economic advantages in the DDAT approach compared to the standard approach were 2.6% and €5,879 (33%) per PFN episode, respectively. The DDAT was the dominant approach in the 99.5% of the simulations performed with average cost-effectiveness per PFN episode of €32,671 versus €52,479 in the EAT approach. The results were robust over a wide range of variables. The DDAT approach is more cost-effective than the EAT approach in the management of PFN in hematological patients.
INTRODUCTION
Invasive fungal infection (IFI) is a serious health problem in hematological patients and hematopoietic stem cell transplant (HSCT) recipients, resulting in a significant morbidity and mortality rate (1–3). Persistent febrile neutropenia (PFN) is a common clinical presentation of IFI in hematological patients. For this reason, during the last 3 decades, the standard approach recommended by the Infectious Diseases Society of America (IDSA) and other international societies for the management of persistent fever in neutropenic hematological patients has been indicating empirical antifungal therapy (EAT) in every patient, with the main objective of improving the outcome of IFI by prompt therapy (4, 5). However, the relatively low incidence of IFI in the whole hematological population, the risk of overtreatment with expensive drugs, and the potential appearance of adverse effects and antifungal resistance selection (6, 7) have made IFIs a safety and economic problem since the optimization of the health care budget is a major priority for all health care systems. Furthermore, the improvement of diagnostic procedures and the efficacy of new antifungal drugs have changed dramatically over the years (8, 9), allowing for new approaches for the management of this syndrome (8, 10–14), and even updated guidelines consider acceptable other more rational antifungal therapy approaches in a subset of high risk patients, although it is still considered as an experimental practice (4). The Andalusian Society of Infectious Diseases (SAEI) (12) proposed a diagnostic and therapeutic approach based on risk profile and driven by clinical criteria for selecting those patients with PFN who do not need antifungal therapy, avoiding overtreatment and toxic effects, with similar effectiveness to that reported in controlled trials. This approach has been validated prospectively even in a high-risk population (13, 14).
However, although pharmacoeconomic analyses comparing antifungal drugs used in treating PFN have been previously published (15, 16), the cost-effectiveness assessment of new approaches, such as the diagnosis-driven antifungal therapy (DDAT), has not been compared to the standard approach of EAT. Hence, the aim of our study was to evaluate the cost-effectiveness of the DDAT approach in hematological patients with PFN recommended by the SAEI (12) versus the EAT approach recommended by the IDSA guidelines (4).
MATERIALS AND METHODS
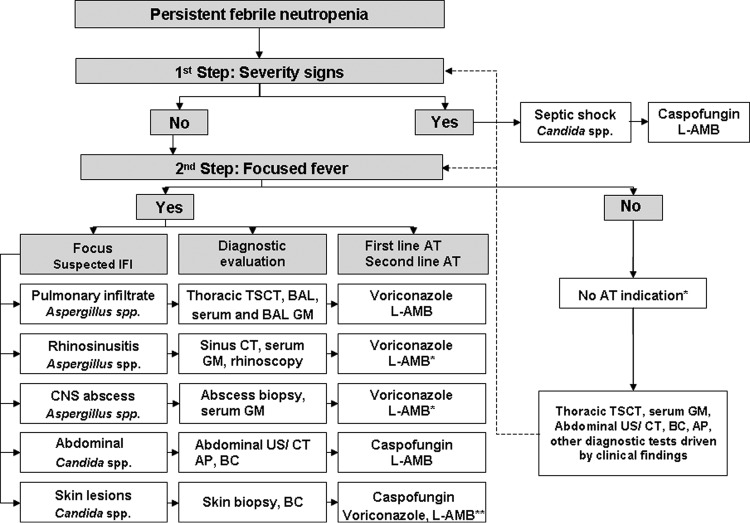
We used a pharmacoeconomic model (17) to assess the cost-effectiveness of two approaches for the management of PFN. The data were extrapolated from two earlier studies: a prospective cohort study (14) in which PFN episodes were managed following a DDAT approach (12). This DDAT approach included the indication of antifungal therapy following the results of a diagnostic work-up, including clinical signs and/or radiological imaging or microbiological results suggestive of IFI (Fig. 1), and a randomized clinical trial (7) in which patients with PFN received EAT according to the IDSA recommendations or standard approach (4).
Fig 1.
Algorithm of the DDAT approach for the management of PFN in patients with hematological malignancies and HSCT recipients proposed by the SAEI. TSCT, thin-section computed tomography; BAL, broncoalveolar lavage; GM, galactomannan antigen test; CT, computed tomography; CNS, central nervous system; US, ultrasound; AP, alkaline phosphatase; BC, blood cultures; AT, antifungal therapy. L-AMB, liposomal amphotericin B. “Voriconazole, L-AMB*”: voriconazole and L-AMB are used when Mucor spp. infection is suspected. Three boxes are marked with asterisks as follows. “Caspofungin, voriconazole, L-AMB**”: caspofungin, voriconazole, and/or liposomal amphotericin are the antifungal therapy of choice if Aspergillus, Scedosporium, or Fusarium sp. is suspected. “No AT indication*”: in patients who have neither severity signs nor clinical focus of fever, antifungal therapy is not initially indicated, and close clinical monitoring and further diagnostic tests are performed as clinically indicated until there is PFN resolution or a change in any of the above conditions.
The study was approved by the Ethics Committee and the Infections Committee (PI0068/2009) of the University Hospital Virgen del Rocío, Seville, Spain, and was performed in accordance with the Declaration of Helsinki. Written consent was not required.
Efficacy assessment.
The efficacy endpoint was an overall successful response to the first-line antifungal therapy (AT) determined by the five-component endpoint used in previous studies (7, 18–21). The overall response was considered successful if all five of the following criteria were met: the successful treatment of any baseline IFI, the absence of any breakthrough IFI during therapy or within 7 days after the completion of therapy, survival for 7 days after the completion of therapy, no premature discontinuation of AT because of drug related toxicity or lack of efficacy, and the resolution of fever (temperature below 38°C for at least 48 h) during neutropenia.
Perspective and time horizon.
Pharmacoeconomic analysis was performed from the perspective of the hospitals of the Andalusian Health System (AHS) from Spain, and the time horizon was 7 days after the end of the AT (the follow-up time to define an overall successful response or not).
Model structure.
A decision-making model was built to assess the results of the implementation of these two approaches for the management of PFN in two cohorts of hematological patients (Fig. 2).
Fig 2.
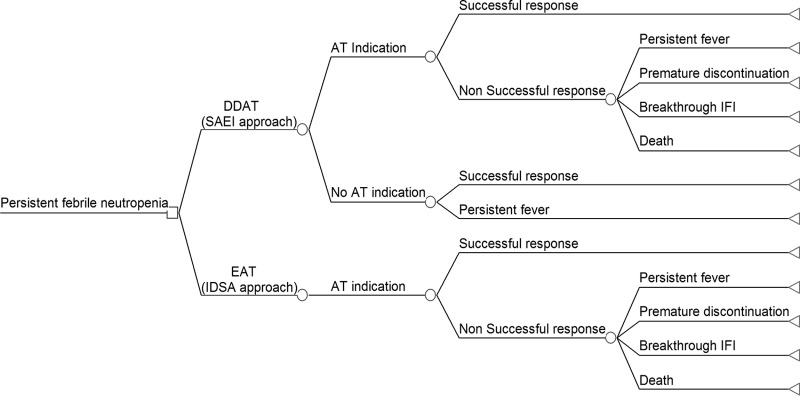
Structure of the decision tree to compare the cost-effectiveness of two approaches for the management of PFN in the hematological patient. SAEI approach, the SAEI approach or DDAT approach; AT, antifungal therapy; EAT, empirical antifungal therapy; IDSA approach, the IDSA approach or standard approach based on the indication of empirical antifungal therapy; IFI, invasive fungal infection.
The model included the possible pathways for each approach and whether the response was successful or not. The causes of failure included death, breakthrough IFI, premature discontinuation of AT (due to the lack of efficacy or toxicity), and persistent fever. Those patients in whom fever was not resolved within at least 48 h during neutropenia and who, following the EAT approach, did not receive AT were considered as a failure of response. In patients with a failure of response for reasons other than death, a second-line AT was administered. The second-line of AT in the DDAT approach arm was assumed according to the SAEI approach (Fig. 1). In the EAT arm, the second-line of AT assumed was caspofungin when liposomal amphotericin B (L-AMB) failed and vice versa. A duration of 12 days of the second-line AT was assumed in both approaches. In the case of switching to a second-line of AT after the failure of first-line AT, the second was considered successful.
Source data.
Data extrapolated from both studies included the baseline characteristics of patients, clinical outcomes, morbidity and mortality, the duration of first-line therapy, and the cause of failure and their probabilities (Table 1 and Table 2).
Table 1.
Baseline patients and persistent febrile neutropenia episodes characteristics
| Baseline characteristics | No. of subjects (%)b |
P | |
|---|---|---|---|
| DDAT approachc | Standard approachd | ||
| Patients | |||
| PFNa episodes/no. of patients | 85/72 | 1,095/1,095 | |
| Sex, male | 41 (56.9) | 610 (55.7) | NSe |
| Median age (range) | 47 (15–75) | 50 (16–83) | NS |
| Underlying disease | |||
| Acute myeloblastic leukemia | 25 (34.7) | 703 (64.2) | <0.001 |
| Acute lymphoblastic leukemia | 4 (4.7) | 107 (9.8) | NS |
| Lymphoma | 18 (25) | 120 (10.9) | <0.001 |
| Others | 25 (34.7) | 165 (15.1) | <0.001 |
| High-risk patients | 25 (34.7) | 268 (24.4) | NS |
| Allogeneic HSCT recipients | 15 (20.08) | 75 (6.8) | <0.001 |
| Relapse of acute leukemia | 10 (13.9) | 193 (17.6) | NS |
| Neutrophil count <100/mm3, no. (%) | 84 (98.8) | 806 (73.6) | <0.001 |
| First-line antifungal agents, no. (%) | 52 (61.2) | 1,095 (100) | <0.001 |
| Liposomal amphotericin | 4 (2.4) | 539 (49.2) | |
| Caspofungin | 24 (28.2) | 556 (50.8) | |
| Voriconazole | 23 (27.1) | ||
| Fluconazole | 2 (2.4) | ||
| No antifungal therapy | 33 (38.8) | ||
| Patients that developed IFI | 12* | 107† | NS |
PFN, persistent febrile neutropenia.
Values are presented as the “number of subjects (%)”except as noted otherwise in column 1. *, Eleven were baseline IFI and one was a breakthrough IFI; †, 54 were baseline IFI and the rest were breakthrough IFI.
For the DDAT approach, the results of EAT were extrapolated from the study cohort reported by Aguilar-Guisado M et al. (14) in which the hematological patients with PFN were managed according to SAEI guidelines (12).
For the standard approach, the results were extrapolated from the randomized trial reported by Walsh et al. (7). In this study, hematological patients with PFN were managed according to IDSA recommendations (4) indicating EAT.
NS, not significant.
Table 2.
Clinical outcomes and their probabilities
| Clinical outcome | No. of subjects (%) |
P | |||
|---|---|---|---|---|---|
| DDAT approacha |
Standard approachb (n = 1,095) | ||||
| –EAT (n = 33) | +EAT (n = 52) | Total (n = 85) | |||
| Overall therapeutic success | 16 (48.48) | 15 (28.85) | 31 (36.47) | 371 (33.88) | 0.62 |
| Therapeutic failure due toc: | 17 (51.51) | 37 (71.15) | 54 (63.53) | 724 (66.12) | 0.62 |
| Death | 11 (21.15) | 11 (12.94) | 99 (9.04) | 0.17 | |
| Breakthrough IFI | 1 (1.92) | 1 (1.18) | 53 (4.84) | 0.17 | |
| Premature discontinuation | 7 (13.46) | 7 (8.23) | 218 (19.91) | 0.007 | |
| Persistent fever | 17 (51.51) | 18 (34.61) | 35 (41.18) | 354 (32.33) | 0.02 |
The DDAT approach was the approach recommended by the SAEI (12). −EAT, Patients did not receive EAT; +EAT, patients received EAT.
The standard approach was the EAT approach recommended by the IDSA (4). All patients received EAT.
IFI, invasive fungal infection. Premature discontinuation involves the lack of efficacy and toxicity. Persistent fever was determined as the number of patients who failed therapy because of a reason other than persistent fever.
In both approaches, it was assumed that the patient monitoring and diagnostic tests included (i) a complete blood count and a liver and renal test every 48 h, (ii) a chest X-ray at the onset of the fever and thrice times a week, (iii) blood and nonblood cultures (i.e., stool, sputum and urine) twice a week, (iv) a serum galactomannan antigen test (GM) twice a week, and (v) a thoracic computed tomography (CT) exam between the fifth and seventh days of fever onset and every 2 weeks thereafter. Antibiotics and granulocyte colony-stimulating factor indications, other diagnostics tests, and intensive care unit management were considered not affected by the type of approach followed, and thus their frequency and nature were assumed to be the same in both approaches.
In the prospective cohort following the DDAT approach (14), the first-line AT was as follows (i) caspofungin at 70 mg on the first day, followed by a daily dose of 50 mg in 28.2% of episodes; (ii) L-AMB at 3 mg/kg/day in 4.7% of cases; (iii) voriconazole at 6 mg/kg/12 h on the first day, followed by 4 mg/kg/12 h and then 200 mg/12 h administered orally from the seventh day in 25.9% of cases; and (iv) fluconazole at 400 mg/12 h on the first day, followed by a daily dose of 400 mg/24 h for the remainder of days in 2.3% of cases. Antifungal therapy was not indicated in the rest of episodes (38.8%). The mean durations of the first-line AT were 11 days (range, 2 to 38 days) for caspofungin, 7.5 days (range, 1 to 17 days) for L-AMB, 19 days (range, 2 to 83 days) for voriconazole, and 8.5 days (range, 6 to 11 days) for fluconazole. In patients who did not receive AT, the average length of hospital stay was 14 days, similar to that described (14) for the mean duration of neutropenia.
In the randomized clinical trial following the EAT or standard approach (7), the first-line AT was caspofungin at 70 mg on the first day, followed by a daily dose of 50 mg in 50.8% of the patients, and then L-AMB at 3 mg/kg/day in the rest of the patients. The mean durations of AT in this study were 13 days (range, 1 to 90 days) and 12.5 days (range, 1 to 90 days), respectively. All of the antifungal drugs were intravenously (i.v.) administered except for voriconazole that switched to oral administration after the seventh day of therapy.
Costs.
The decision model was used to generate a weighted average cost per PFN episode managed. This was the sum product of the pathway treatment costs and their probabilities. The cost of the first-line AT was the cost of a complete course of the antifungal drug and the costs of the consumed resources (hospital stay and test costs). The cost of the second-line therapy was the cost of a complete course of the antifungal drug and the hospitalization costs. The cost of each failure pathway, except for death and persistent fever in patients who did not receive EAT, was the cost of the first- and second-line therapies added to the costs of the consumed resources (hospital stay and test costs). The cost of the failure pathway for persistent fever in patients who did not receive EAT was the cost of the consumed resources (hospital stay and test costs). The cost per successful response or deceased patient was calculated as a proportion of both: a complete course of first-line AT and the consumed resources (hospital stay and tests costs). Regarding antifungal drugs doses, patients were assumed to have an average weight of 70 kg (WHO Collaborating Centre for Drug Statistics Methodology [http://www.whocc.no/atc_ddd_publications/guidelines/]). Doses were rounded to the nearest vial size to reflect common hospital practice. The model made the assumption that patients switched to oral voriconazole after receiving voriconazole i.v. for 7 days.
Antifungal costs used were taken from the drug wholesale prices paid by Spanish public hospitals in 2011 (Ministerio de Sanidad, Servicios Sociales e Igualdad [http://www.msc.es/profesionales/farmacia/nomenclatorDI.htm]). Hospital stay and consumed resources costs were obtained from the Control and Management System of the Andalusian Health Service 2010-2011 (Servicio Andaluz de Salud–COAN [http://www.sas.junta-andalucia.es/principal/documentosAcc.asp?pagina=pr_coan&file=/contenidos/gestioncalidad/coan\manuales.htm]). Costs were adjusted in euros (€) for the year 2011 (Table 3). Patients did not incur any out-of-pocket costs and were covered by the AHS.
Table 3.
Resource costsa
| Inputb | U | U cost (€) |
|---|---|---|
| L-AMB | 50-mg i.v vial | 130.61 |
| Caspofungin | 70-mg i.v vial | 570.81 |
| 50-mg i.v. vial | 448.76 | |
| Voriconazole | 200-mg i.v. vial | 133.32 |
| 200-mg oral tablet | 35.67 | |
| Fluconazole | 400-mg i.v vial | 11.12 |
| Chest X-ray | One test | 9.19 |
| Thoracic CT scan | One test | 82.71 |
| Blood biochemistry test | One test | 2.28 |
| Complete blood count | One test | 1.4 |
| Blood culture | One test (two cultures) | 9.85 |
| Sputum culture | One test | 14.87 |
| Urine culture | One test | 5.44 |
| Stool culture | One test | 5.44 |
| Aspergillus GM serum antigen test | One test | 35.59 |
| Hospitalization | Inpatient per day | 401.30 |
Antifungal costs were extracted from the drug wholesale prices paid by Spanish public hospitals in 2011 (Ministerio de Sanidad, Servicios Sociales e Igualdad [http://www.msc.es/profesionales/farmacia/nomenclatorDI.htm]); hospital stay and consumed resource costs were obtained from the Control and Management System of the Andalusian Health Service, 2010-2011 (ServicioAndaluz de Salud-COAN [http://www.sas.junta-andalucia.es/principal/documentosAcc.asp?pagina=pr_coan&file=/contenidos/gestioncalidad/coan\manuales.htm]). All costs were adjusted in euros (€) for the year 2011.
L-AMB, liposomal amphotericin B; CT scan, computed tomography scan; GM, galactomannan.
Definitions.
PFN was defined as persistent fever (>96 h) refractory to empirical antibacterial therapy and without etiological diagnosis and neutropenia after chemotherapy or after HSCT (an absolute neutrophil count less than 0.5 × 109/liter) (19). Probable and proven IFIs were defined according to European Organization for Research and Treatment of Cancer/MSG criteria (22).
Statistical analysis.
Descriptive statistics are presented as percentages for discrete variables and means (range) for those continuous variables. The chi-square test or the Fisher exact test was used to compare the categorical data among the groups. Student t tests were performed for comparison of means, and the Mann-Whitney U-test was performed when appropriate. A two-sided P value of ≤0.05 was considered to be statistically significant. The statistical analysis was performed with software from the Statistical Package for the Social Sciences (SPSS, Inc., Chicago, IL) version 18.0.
A cost-effectiveness analysis to compare both approaches was performed through the TreeAge Pro Suite 2009 program (TreeAge Software, Inc., Williamstown, MA). The results of the analysis are presented as the average cost per PFN episode, cost difference, economic advantage, and average cost-effectiveness. If one approach was more efficacious and less expensive (cost-saving) than another, then the first one was termed dominant. In such a case, it was not necessary to calculate the incremental cost-effectiveness ratio, and this alternative would always lead to a better clinical outcome at a lesser cost and would therefore always be cost-saving (23). The costs considered acceptable for an effective treatment were €30,000 in all cases, (24), taking into account the complexity of the management of persistent fever in hematological patients and the high mortality related to IFI.
Sensitivity analysis was performed in order to evaluate the robustness of the study's conclusion. Deterministic and probabilistic sensitivity tests were produced by modifications of the baseline values of several key variables and assumptions, in relation to costs and probabilities (25). Baseline values were substituted by the highest and lowest values within a reasonable range of values (in the case of costs this was from 75 to 125% of the initial value, and in the case of duration of therapy a range of days reasonable in clinical practice and probabilities varied with a 95% confidence interval of their basal value). In cases in which substitution changed the study conclusion, values within the range replaced the baseline value. This was repeated until the exact variable value (or range of values) that changed the study outcome was identified. The effects of variations in the duration and costs of the first-line AT and in the costs of hospital stay in both approaches were analyzed. In the DDAT approach, the variation of the duration of voriconazole therapy, not switching to the oral route after the seventh day, and replacing the doses of L-AMB from 3 to 5 mg/kg/day were assessed. In both approaches, the impact of assumptions related to the duration of second-line AT (ranging between 10 to 14 days) and counting or not counting the diagnostic and monitoring tests were evaluated. Another two scenarios analyzed were: (i) replacing the probability of distribution of patients who received AT between the two arms (the DDAT approach and the EAT approach) of the decision-tree and (ii) replacing the probability of patient distribution in the EAT approach arm with that reported in a similar clinical trial where L-AMB and voriconazole were used (20).
The multivariate sensitivity analysis was carried out using a second-order probabilistic Monte Carlo simulation, which analyzed the degree of uncertainty of the parameters used in the model, especially their relationship to the effectiveness of the treatments and costs (see Table 4 for a description of the variation in the parameters and the distributions used in the univariate and multivariate analyses).
Table 4.
Summary of variables and distributions considered in sensitivity analyses and Monte Carlo simulations
| Model parametersa | Low | High | Distributionb |
|---|---|---|---|
| Treatment | |||
| L-AMB, 50 mg i.v., cost/vial (€) | 97.95 | 163.26 | Gamma (64.1, 0.12) |
| Caspofungin | |||
| 70 mg i.v., cost/vial (€) | 428.11 | 713.51 | Gamma (66.24, 0.11) |
| 50 mg i.v., cost/vial (€) | 336.57 | 560.95 | Gamma (64, 0.14) |
| Voriconazole | |||
| 200 mg i.v., cost/vial (€) | 99.99 | 166.65 | Gamma (64, 0.16) |
| Oral tablet, cost (€) | 26.75 | 44.59 | Gamma (64.05, 0.9) |
| Fluconazole, 200 mg i.v., cost/vial (€) | 8.34 | 13.9 | Gamma (64, 5.75) |
| Days of administration as FLT | |||
| L-AMB | 7 | 14 | Gamma (64, 8.53), gamma (64, 5.12) |
| Caspofungin | 7 | 14 | Gamma (64, 5.82), gamma (64, 4.92) |
| Voriconazole | 14 | 28 | Gamma (64, 5.33) |
| Fluconazole | 7 | 14 | Gamma (64, 7.53) |
| Cost of hospitalization per day (€) | 300.97 | 501.25 | Gamma (64, 0.16) |
| Probabilities | |||
| EAT indication* | 0.508 | 0.715 | Beta (24.83, 15.74) |
| Persistent fever* | 0.325 | 0.648 | Beta (32.896, 34.791) |
| Premature discontinuation* | 0.063 | 0.315 | Beta (51.904, 222.72) |
| Breakthrough IFI in patients* | 0 | 0.079 | Beta (62.27, 2,244.1) |
| Death* | 0.15 | 0.445 | Beta (44.93, 105.84) |
| Persistent fever† | 0.345 | 0.686 | Beta (31, 29.23) |
| Successful response‡ | 0.177 | 0.50 | Beta (43.06, 83.97) |
| Unsuccessful response* | 0.566 | 0.858 | Beta (18.48, 7.51) |
L-AMB, liposomal amphotericin B; FLT, first-linetherapy. *, That is, in selected patients who received AT following the DDAT approach; †, that is, in patients who not receive AT following the DDATapproach; ‡, that is, in patients managed using the EAT approach.
Where two values are given, the first value indicates the DDAT approach recommended by the SAEI, and the second value is the EAT approach recommended by the IDSA.
RESULTS
Clinical benefits.
The overall probability of a successful response associated with the DDAT approach was 36.5% compared to 33.9% of success probability for the EAT approach (see Table 2 for a description of the results for clinical outcome regarding the five composite endpoints in both study arms).
In the DDAT approach, AT was not indicated in 33 (38.8%) episodes of PFN, and 16 (48.5%) of these fulfilled the five composite endpoints considered for an overall successful response. Of the remaining episodes, 17 (51.5%) did not achieve a successful response because the fever was not resolved for at least 48 h during the neutropenia.
Costs.
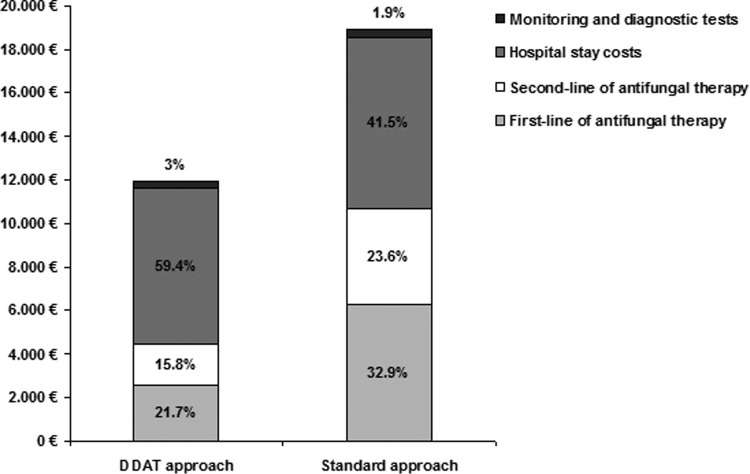
The average costs for the management of a PFN episode were €11,910 for the DDAT approach and €17,789 for the EAT approach. The economic advantage per PFN episode in the DDAT approach was €5,879 (33%) compared to the EAT approach. For both approaches, the main contributing costs to the overall therapy cost were the persistent fever (41.2% versus 32.3%) and the hospital stay (59% versus 41.5%) for the DDAT and EAT approaches, respectively (see Table 5 and Fig. 3 for the individual cost contributions to the overall cost for both approaches).
Table 5.
Proportional costs of both approaches to manage persistent febrile neutropenia in a hematological patient
| Therapy outcomea | DDAT approachb |
Standard approachc |
||||
|---|---|---|---|---|---|---|
| Proportion (%) | Cost (€)/patient | Weighted cost (€) | Proportion (%) | Cost (€)/patient | Weighted cost (€) | |
| Overall successful response | 36.5 | 8,309 | 3,033 | 33.9 | 11,692 | 3,964 |
| Successful response with EAT | 17. 2 | 10,845 | 1,911 | 33.9 | 11,692 | 3,964 |
| Successful response without EAT | 18.81 | 5,964 | 1,122 | |||
| Overall failure response | 63.5 | 13,976 | 8,875 | 66.1 | 20,915 | 13,825 |
| Failure response with EAT | 43.57 | 17,635 | 7,683 | 66.1 | 20,915 | 13,825 |
| Death | 12.9 | 10,288 | 1,327 | 9.04 | 11,726 | 1,060 |
| Breakthrough IFI | 1.17 | 17,575 | 207 | 4.84 | 22,390 | 1,084 |
| Premature discontinuation | 8.23 | 20,669 | 1,701 | 19.91 | 22,339 | 4,448 |
| Persistent fever | 21.18 | 20,963 | 4,440 | 32.33 | 22,394 | 7,240 |
| Failure response without AT | 19.98 | 5,964 | 1,191 | |||
| Total cost per patient | 11,910 | 17,789 | ||||
EAT, empirical antifungal therapy; IFI, invasive fungal infection. “Failure response without EAT” refers to all cases of failure response without receiving empirical antifungal therapy were due to the fever not being resolved for at least 48 h before the neutropenia resolution.
That is, the DDAT approach recommended by the SAEI (12).
That is, the standard EAT approach recommended by the IDSA (4).
Fig 3.
Contribution of different cost components to the overall therapy cost in two different approaches for the management of PFN in the hematological patient. The DDAT approach is the approach recommended by the SAEI (12). The standard approach is the EAT approach recommended by the IDSA (4).
Cost-effectiveness analysis.
The DDAT approach was the dominant approach, with an average cost-effectiveness per PFN episode of €32,671 versus €52,479 for the EAT approach. The cost advantage of the DDAT approach over the EAT approach was €19,808 (37.7%) per overall successful response of the PFN episode.
Sensitivity analysis.
The variations of the parameters used in the one-way sensitivity analysis indicated that the model was robust and not sensitive to changes. Thus, the DDAT approach was the dominant approach in all cases, and the incremental cost-effectiveness ratio was negative in all cases in favor of the DDAT approach.
We calculated the average cost difference between the two approaches when varying the duration of administration of L-AMB and caspofungin as the first-line AT to 14 days in both arms, obtaining economic advantages for the DDAT approach of €6,070 and €5,825 per PFN episode, respectively.
Not switching the i.v. voriconazole to oral administration after the seventh day of therapy and extending its administration to 60 days in the DDAT arm resulted in an economic advantage of €3,675 per overall successful response of the PFN episode for the DDAT approach. Also, when patients receiving voriconazole in the DDAT approach arm were removed and replaced by patients treated with caspofungin, an economic advantage of €7,656 per PFN episode managed was obtained in the DDAT arm. Similarly, when patients receiving a first-line treatment of caspofungin in the EAT arm were replaced by patients receiving voriconazole, an economic advantage for the DDAT arm of €6,833 per PFN episode managed was obtained. Replacing the 3-mg/kg/day doses of L-AMB, administered as first- and second-line AT treatments, with 5 mg/kg/day doses in the DDAT arm and varying the duration of administration of L-AMB and caspofungin as a second-line AT treatment to 10 to 14 days in both arms did not change the study conclusion. Also, an increase or decrease by 25% of the drug acquisition costs did not change the study's conclusion.
The main study conclusion also did not vary when the costs of monitoring and diagnostic tests were excluded in both approaches and also when the costs of serum GM tests were not considered in the EAT approach. An increase of 25% in the costs per day of hospitalization (from €401 to €501) offered an economic advantage of €6,068 per PFN episode to the DDAT approach.
When we varied the probability of AT indication from 0.612 to 0.715 in the arm of the DDAT approach and increased from 0.339 to 0.50 the probability of successful response in the arm of the EAT approach, the economic advantages per overall successful response of the PFN episode were €19,788 and €1,673, respectively, for the DDAT approach. Furthermore, when the probabilities for premature discontinuation, persistent fever, death, and breakthrough IFI in the DDAT arm were varied within their 95% confidence intervals, the study conclusions did not change.
We analyzed the cost-effectiveness results for both approaches in two different scenarios. First, the probability distribution of patients who received AT was replaced between the two arms of the study (from the DDAT arm to the EAT arm and vice versa) with an average cost-effectiveness per PFN episode of €31,197 in the DDAT approach versus €59,248 in the EAT approach. Second, the probability distribution of patients in the EAT arm was replaced by that previously described in the literature (20), with average cost-effectiveness values per PFN episode of €32,671 and €65,875 in the DDAT and EAT approaches, respectively.
Probabilistic sensitivity analysis resulting from Monte Carlo simulation demonstrated a 99.5% probability that the DDAT approach was cost-effective compared to the EAT approach, with a mean economic advantage of €5,896 per PFN episode. Based on the cost-saving probability distribution of both approaches and with a willingness to pay of €30,000, the DDAT approach represented a cost saving of 60% in the simulations, with a mean of €400 saved per PFN episode, and the EAT approach represented a cost saving in 2% of the simulations. The maximum expected cost saving for the DDAT approach was €5,070 and for the EAT approach was €500 per PFN episode (Fig. 4).
Fig 4.
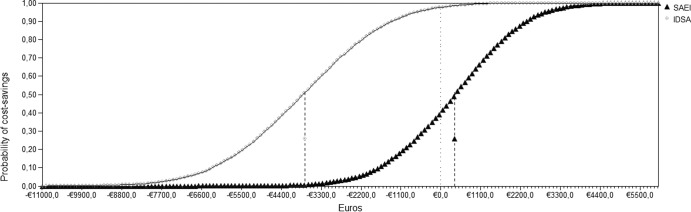
Cost-saving probability curves of two approaches for PFN—the DDAT approach proposed by the SAEI (12) and the EAT approach recommended by the IDSA (4)—for a willingness to pay of €30,000. Filled gray circles indicate the SAEI approach; filled black triangles indicate the IDSA approach.
DISCUSSION
This study demonstrates that the EAT indication in hematological patients with PFN driven by risk profile and clinical criteria (12) is more cost-effective than the standard approach of empirical AT (4, 5), with a cost advantage of 37.7% per unit of effectiveness. Furthermore, sensitivity analyses showed that the modification of key parameters and all of the established assumptions of the model had very little impact on the cost-effectiveness of the DDAT approach, demonstrating that it is the best approach and that the model is robust and stable. Our results emphasize the need to consider a tailored approach to achieve a more favorable balance between effectiveness and cost for the management of PFN away from the concept of the undifferentiated approach, as other authors have recently suggested (26).
This is the first study to investigate the pharmacoeconomics comparing two different approaches for the management of PFN. To date, several studies have been compared the cost-effectiveness of different antifungals used in the EAT approach in patients with PFN (15, 27–30). However, although new approaches have been proposed for the management of this syndrome (8, 11, 31), none of these studies compared the cost-effectiveness of these new approaches to the standard approach of EAT.
Although there are no significant differences in the effectiveness of both approaches, our results show that the DDAT is the dominant approach. In addition, it should be noted that the use of the composite endpoints as efficacy criteria places the DDAT approach in an unfavorable scenario, because this composite endpoint was applied to all patients, including patients who did not receive AT. Thus, episodes in which fever was not resolved for at least 48 h during the neutropenia were considered as a failure of response even when EAT was not indicated. In fact, whereas the proportion of failure response due to persistent fever was 48.9% in the EAT approach, it was 64.8% in the DDAT approach. However, the utility of the criteria of fever resolution as a marker of response (efficacy) of the EAT is controversial and has been previously debated in the literature (6). Nearly half of the cases of failure of response, in the DDAT approach, did not receive AT, and the PFN episode was resolved without IFI development or death, corroborating the nonspecificity of fever resolution for efficacy assessment.
The cost-saving of DDAT approach over the EAT approach could be due to several factors. First, there is a reduction of 38.8% of antifungal therapy, representing a direct cost saving. Second, costs derived from adverse effects and from the use of a second-line of AT, often resulting in a prolonged hospital stay (which was the main contributor to the overall cost for both approaches), are avoided in patients for whom EAT is not indicated. Another factor that may contribute to cost saving is the sequential use of oral voriconazole following i.v. voriconazole in the DDAT approach because oral voriconazole has an significantly lower acquisition cost (29.7%) than does caspofungin and L-AMB, from a Spanish perspective, and this allows an early patient discharge from hospital and the completion of therapy at home. Nevertheless, this fact could not have affected to our results because in our study the complete course of antifungal therapy was considered to be administered in-hospital. Although voriconazole failed a strict statistical assessment of noninferiority compared to L-AMB in EAT (20), many authors consider voriconazole to be a reliable alternative for PFN when invasive aspergillosis is suspected since voriconazole is the first-line therapy for this infection, and it may decrease the incidence of breakthrough IFI (14, 20, 32). Also, the updated guidelines of the IDSA recognize this antifungal between the therapeutic options for PFN (4).
The present study has several limitations. The fact that the decision-tree structure only allowed for a single switch to alternative AT is a limitation that may underestimate the cost in patients with multiple changes and discontinuations of antifungal therapy. Another limitation is that the use of the GM serum test was not included among the diagnostic tests of the clinical trial. However, it was an assumption of our model as part of current clinical practice without significant influence on costs by not determining the indication of EAT in the standard approach. A third limitation is that the costs caused by adverse effects of AT have not been considered, because most of the adverse effects are mild and involve a low cost, and also because severe adverse effects, such nephrotoxicity or hepatotoxicity, are difficult to assess in patients receiving multiple drug regimens. Moreover, a short time horizon was considered in the present study, because no long-term survival and quality-of-life data were available from the Walsh et al. (7) study. Therefore, these approaches cannot be evaluated over a long term, i.e., in terms of the life years gained as the primary outcome in pharmacoeconomic investigations (33). However, this was a minor limitation for the purpose of the present study, since long-term data have more relevance in chronic diseases involving a societal perspective, whereas the adoption of the hospital system perspective here is more appropriate for a critical condition such as IFI in neutropenic patients (34). Moreover, the present study was based on a comparison using a prospective cohort study with a small size (14) and a large randomized clinical trial (7). In spite of the small sample size, the prospective cohort study is the validation of a previous pilot study (13), in which similar results were reported. Also, it should be noted that although we assumed results of effectiveness in both approaches, data obtained from the randomized clinical trial are a measure of efficacy, placing in any case the results of the clinical trial in a better scenario than the results from the prospective cohort study that are derived from the clinical practice. Furthermore, both are prospective studies giving the best available clinical evidence to perform an economic evaluation, unlike other retrospective studies using data by chart review that may lead to a selection bias (30).
In addition, the assumptions in our model were validated by univariate sensitivity analysis and Monte Carlo simulation that revealed that the DDAT approach was the preferred approach in all cases. The limitations and assumptions of pharmacoeconomic models must be evaluated through sensitivity analysis that varies the potential values between a range of present variables that covers the most likely values and scenarios.
In conclusion, the DDAT approach is more cost-effective than the EAT approach for the management of PFN in hematological patients. These results may contribute to the reconsideration of the widespread use of empirical antifungal therapy in favor of a more rational prescription.
ACKNOWLEDGMENTS
We thank the Ministerio de Economía y Competitividad, Instituto de Salud Carlos III, cofinanced by the European Development Regional Fund “A Way to Achieve Europe” ERDF, as well as the Spanish Network for the Research in Infectious Diseases (REIPI RD12/0015) and the Consejería de Salud of the Junta de Andalucía (PI-0068/2009), for supporting this study.
I.E. has received speaking honoraria from Merck, Astellas Pharma, Gilead, and Pfizer, and J.M.C. has received speaking honoraria from Merck, Astellas Pharma, and Pfizer.
Footnotes
Published ahead of print 15 July 2013
REFERENCES
- 1.Pagano L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, Pastore D, Picardi M, Bonini A, Chierichini A, Fanci R, Caramatti C, Invernizzi R, Mattei D, Mitra ME, Melillo L, Aversa F, Van Lint MT, Falcucci P, Valentini CG, Girmenia C, Nosari A. 2006. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologicae 91:1068–1075 [PubMed] [Google Scholar]
- 2.Pizzo PA, Robichaud KJ, Gill FA, Witebsky FG. 1982. Empiric antibiotic and antifungal therapy for cancer patients with prolonged fever and granulocytopenia. Am. J. Med. 72:101–111 [DOI] [PubMed] [Google Scholar]
- 3.Vento S, Cainelli F. 2003. Infections in patients with cancer undergoing chemotherapy: etiology, prevention, and treatment. Lancet Oncol. 4:595–604 [DOI] [PubMed] [Google Scholar]
- 4.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young J-AH, Wingard JR, Infectious Diseases Society of America 2011. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 52:e56–e93 [DOI] [PubMed] [Google Scholar]
- 5.Link H, Böhme A, Cornely OA, Höffken K, Kellner O, Kern WV, Mahlberg R, Maschmeyer G, Nowrousian MR, Ostermann H, Ruhnke M, Sezer O, Schiel X, Wilhelm M, Auner HW. 2003. Antimicrobial therapy of unexplained fever in neutropenic patients—guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO), Study Group Interventional Therapy of Unexplained Fever, Arbeitsgemeinschaft Supportivmassnahmen in der Onkologie (ASO) of the Deutsche Krebsgesellschaft (DKG-German Cancer Society). Ann. Hematol. 82(Suppl 2):S105–S117 [DOI] [PubMed] [Google Scholar]
- 6.De Pauw BE, Sable CA, Walsh TJ, Lupinacci RJ, Bourque MR, Wise BA, Nguyen B-Y, DiNubile MJ, Teppler H. 2006. Impact of alternate definitions of fever resolution on the composite endpoint in clinical trials of empirical antifungal therapy for neutropenic patients with persistent fever: analysis of results from the Caspofungin Empirical Therapy Study. Transpl. Infect. Dis. 8:31–37 [DOI] [PubMed] [Google Scholar]
- 7.Walsh TJ, Teppler H, Donowitz GR, Maertens JA, Baden LR, Dmoszynska A, Cornely OA, Bourque MR, Lupinacci RJ, Sable CA, dePauw BE. 2004. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N. Engl. J. Med. 351:1391–1402 [DOI] [PubMed] [Google Scholar]
- 8.Maertens J, Theunissen K, Verhoef G, Verschakelen J, Lagrou K, Verbeken E, Wilmer A, Verhaegen J, Boogaerts M, Van Eldere J. 2005. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin. Infect. Dis. 41:1242–1250 [DOI] [PubMed] [Google Scholar]
- 9.Maertens J, Deeren D, Dierickx D, Theunissen K. 2006. Preemptive antifungal therapy: still a way to go. Curr. Opin. Infect. Dis. 19:551–556 [DOI] [PubMed] [Google Scholar]
- 10.Cherif H, Kalin M, Björkholm M. 2006. Antifungal therapy in patients with hematological malignancies: how to avoid overtreatment? Eur. J. Haematol. 77:288–292 [DOI] [PubMed] [Google Scholar]
- 11.Cordonnier C, Pautas C, Maury S, Vekhoff A, Farhat H, Suarez F, Dhédin N, Isnard F, Ades L, Kuhnowski F, Foulet F, Kuentz M, Maison P, Bretagne S, Schwarzinger M. 2009. Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: a randomized, controlled trial. Clin. Infect. Dis. 48:1042–1051 [DOI] [PubMed] [Google Scholar]
- 12.Cisneros JM, Espigado I, Rivero A, Lozano de León F, Parra J, Collado AR, Lomas JM, Pachón J. 2005. Empirical antifungal therapy in selected patients with persistent fever and neutropenia. Enferm. Infecc. Microbiol. Clin. 23:609–614 (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 13.Aguilar-Guisado M, Espigado I, Cordero E, Noguer M, Parody R, Pachón J, Cisneros JM. 2010. Empirical antifungal therapy in selected patients with persistent febrile neutropenia. Bone Marrow Transplant. 45:159–164 [DOI] [PubMed] [Google Scholar]
- 14.Aguilar-Guisado M, Martin-Pena A, Espigado I, Ruiz Perez de Pipaon M, Falantes J, De la Cruz F, Cisneros JM. 2012. Universal antifungal therapy is not needed in persistent febrile neutropenia: a tailored diagnostic and therapeutic approach. Haematologicae 97:464–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Badriyeh D, Liew D, Stewart K, Kong DCM. 2009. Cost-effectiveness evaluation of voriconazole versus liposomal amphotericin B as empirical therapy for febrile neutropenia in Australia. J. Antimicrob. Chemother. 63:197–208 [DOI] [PubMed] [Google Scholar]
- 16.O'Sullivan AK, Pandya A, Papadopoulos G, Thompson D, Langston A, Perfect J, Weinstein MC. 2009. Cost-effectiveness of posaconazole versus fluconazole or itraconazole in the prevention of invasive fungal infections among neutropenic patients in the United States. Value Health 12:666–673 [DOI] [PubMed] [Google Scholar]
- 17.Lilford RJ, Pauker SG, Braunholtz DA, Chard J. 1998. Decision analysis and the implementation of research findings. BMJ 317:405–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boogaerts M, Winston DJ, Bow EJ, Garber G, Reboli AC, Schwarer AP, Novitzky N, Boehme A, Chwetzoff E, De Beule K. 2001. Intravenous and oral itraconazole versus intravenous amphotericin B deoxycholate as empirical antifungal therapy for persistent fever in neutropenic patients with cancer who are receiving broad-spectrum antibacterial therapy: a randomized, controlled trial. Ann. Intern. Med. 135:412–422 [DOI] [PubMed] [Google Scholar]
- 19.Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, Pappas P, Seibel N, Greenberg RN, Dummer S, Schuster M, Holcenberg JS. 1999. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N. Engl. J. Med. 340:764–771 [DOI] [PubMed] [Google Scholar]
- 20.Walsh TJ, Pappas P, Winston DJ, Lazarus HM, Petersen F, Raffalli J, Yanovich S, Stiff P, Greenberg R, Donowitz G, Schuster M, Reboli A, Wingard J, Arndt C, Reinhardt J, Hadley S, Finberg R, Laverdière M, Perfect J, Garber G, Fioritoni G, Anaissie E, Lee J. 2002. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N. Engl. J. Med. 346:225–234 [DOI] [PubMed] [Google Scholar]
- 21.Winston DJ, Hathorn JW, Schuster MG, Schiller GJ, Territo MC. 2000. A multicenter, randomized trial of fluconazole versus amphotericin B for empiric antifungal therapy of febrile neutropenic patients with cancer. Am. J. Med. 108:282–289 [DOI] [PubMed] [Google Scholar]
- 22.Ascioglu S, Rex JH, De Pauw B, Bennett JE, Bille J, Crokaert F, Denning DW, Donnelly JP, Edwards JE, Erjavec Z, Fiere D, Lortholary O, Maertens J, Meis JF, Patterson TF, Ritter J, Selleslag D, Shah PM, Stevens DA, Walsh TJ. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7–14 [DOI] [PubMed] [Google Scholar]
- 23.Gold MR, Siegel JE, Russell LB, Weinstein MC. 1996. Cost-effectiveness in health and medicine. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 24.Sacristán JA, Oliva J, Del Llano J, Prieto L, Pinto JL. 2002. What is an efficient health technology in Spain? Gac Sanit. 16:334–343 (In Spanish). [DOI] [PubMed] [Google Scholar]
- 25.Briggs A. 1999. Economics notes: handling uncertainty in economic evaluation. BMJ 319:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maertens JA, Nucci M, Donnelly JP. 2012. The role of antifungal treatment in hematology. Haematologicae 97:325–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Badriyeh D, Liew D, Stewart K, Kong DCM. 2009. Economic impact of caspofungin as compared with liposomal amphotericin B for empirical therapy in febrile neutropenia in Australia. J. Antimicrob. Chemother. 63:1276–1285 [DOI] [PubMed] [Google Scholar]
- 28.Al-Badriyeh D, Liew D, Stewart K, Kong DCM. 2012. Pharmacoeconomic analysis of voriconazole versus caspofungin in the empirical antifungal therapy of febrile neutropenia in Australia. Mycoses 55:244–256 [DOI] [PubMed] [Google Scholar]
- 29.Stam WB, Aversa F, Kumar RN, Jansen JP. 2008. Economic evaluation of caspofungin versus liposomal amphotericin B for empiric antifungal treatment in patients with neutropenic fever in Italy. Value Health 11:830–841 [DOI] [PubMed] [Google Scholar]
- 30.Collins CD, Stuntebeck ER, DePestel DD, Stevenson JG. 2007. Pharmacoeconomic analysis of liposomal amphotericin B versus voriconazole for empirical treatment of febrile neutropenia. Clin. Drug Invest. 27:233–241 [DOI] [PubMed] [Google Scholar]
- 31.Tan BH, Low JGH, Chlebicka NL, Kurup A, Cheah FK, Lin RTP, Goh YT, Wong GC. 2011. Galactomannan-guided preemptive versus empirical antifungals in the persistently febrile neutropenic patient: a prospective randomized study. Int. J. Infect. Dis. 15:e350–356 [DOI] [PubMed] [Google Scholar]
- 32.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann J-W, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, De Pauw B. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408–415 [DOI] [PubMed] [Google Scholar]
- 33.Drummond MF, Sculpher MJ, Torrance GW. 2005. Methods for the economic evaluation of health care programmes. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 34.Sculpher M, Fenwick E, Claxton K. 2000. Assessing quality in decision analytic cost-effectiveness models: a suggested framework and example of application. Pharmacoeconomics 17:461–477 [DOI] [PubMed] [Google Scholar]