Abstract
We subjected Staphylococcus aureus ATCC 29213 to serial passage in the presence of subinhibitory concentrations of magainin 2 and gramicidin D for several hundred generations. We obtained S. aureus strains with induced resistance to magainin 2 (strain 55MG) and gramicidin D (strain 55GR) that showed different phenotypic changes in membrane properties. Both exhibited a change in membrane phospholipid content and an increase in membrane rigidity, while an alteration in net charge compared to that of the control occurred only in the case of 55MG.
TEXT
The growing problem of resistance to conventional antibiotics in pathogens like Staphylococcus aureus and the need for new alternatives has stimulated interest in the development of antimicrobial peptides (AMPs) as human therapeutics (1). This has led to many AMPs, such as pexiganan (analogue of magainin), gramicidins, polymyxin, nisin, daptomycin, and defensin-mimetic molecule, being entered into clinical trials (2–6). However, it has been argued in several in vitro studies and recent reports that the use of AMPs in therapeutic amounts over an extended period of time might lead to reduced susceptibility due to adaptive changes in phenotypic and genotypic characteristics of the organism (7–13). Therefore, the aim of our study was to examine whether S. aureus bacteria, on continuous exposure to sublethal concentrations of certain well-studied AMPs (magainin 2 and gramicidin D), alter their susceptibility and whether resistant strains are selected. Since the putative mechanism of action of the AMPs studied involves targeting of the bacterial membrane, analyses of various cell membrane (CM) parameters were performed to understand their role in the in vitro-selected strains. (This study was presented in part at the 113th General Meeting, American Society for Microbiology, Denver, CO, 18 to 21 May 2013.)
To generate AMP-resistant strains, S. aureus ATCC 29213 was chosen and two cell lines were maintained for each peptide in Mueller-Hinton broth (MHB). Serial passage was done in two cell lines for each AMP, one in the presence of noninhibitory and increasing AMP concentrations (positive-selection line) and the other in the absence of peptide (control selection line), as described elsewhere (10). The experiment was conducted for 55 serial passages, constituting 600 to 700 bacterial generations. Each transfer was given a strain designation to indicate the serial passage number. After every 5 transfers, evolution of resistance in the positive-selection line against magainin 2 and gramicidin D was identified by determining the MIC in MHB, following NCCLS guidelines (14). Further confirmation of resistance was done by performing a bactericidal assay as described before (15, 16).
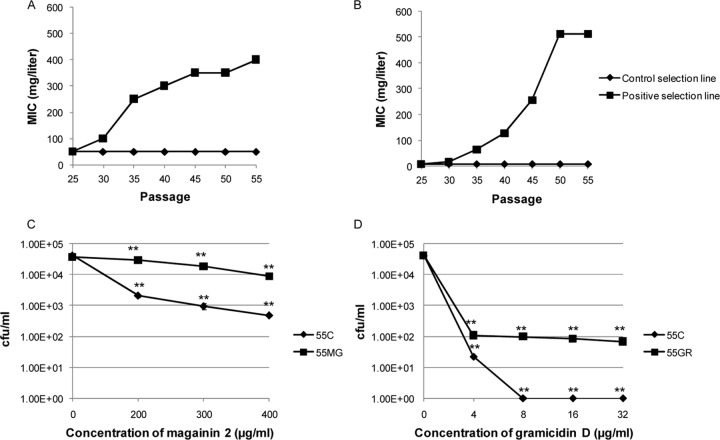
The development of resistance occurred for both of the AMPs studied, as indicated by a gradual rise in MIC values with increasing passage number in the positive-selection lines compared to the MICs in the respective control selection lines. At the 55th passage, the positive-selection strains showed an ≥8- and a 128-fold increase in MIC value for magainin 2 (strain 55MG) and gramicidin D (strain 55GR), respectively, compared to the MIC of the control (Fig. 1A and B). The MICs of the selected strains were stable after daily passaging on peptide-free medium for 10 consecutive transfers (data not shown). Furthermore, the in vitro bactericidal assay (Fig. 1C and D) showed a substantial increase in the number of CFU/ml in both selected strains (55MG and 55GR) compared to the growth of the control (55C) after treatment with different concentrations of the respective AMPs for 1 h.
Fig 1.
(A, B) MIC (mg/liter) values for S. aureus ATCC 29213 during serial passage in the absence of AMP (control selection line) and with increasing concentrations of AMPs (positive selection lines) magainin 2 (A) and gramicidin D (B). (C, D) CFU/ml in control and selected S. aureus strains exposed to different concentrations of magainin 2 (C) and gramicidin D (D). **, P ≤ 0.01 (one-way ANOVA; Minitab).
In order to understand the phenotypic modifications that the in vitro-selected strains had undergone, analyses of various membrane parameters were done. These included membrane order, total phospholipid content, flipping of the cationic phospholipid lysyl phosphatidylglycerol (LPG) to the outer membrane leaflet, and net cell charge. The membrane order of S. aureus cells was determined by measuring fluorescence polarization (Shimadzu RF-5301 PC spectrofluorimeter) using the fluorescent probe DPH (1,6-diphenyl-1,3,5-hexatriene) as described earlier (17, 18). The net charge values of the S. aureus strains were determined by measuring zeta potential on an electrophoresis instrument (ZC-2000; Microtec, Japan) as described elsewhere (19, 20). For membrane phospholipid (PL) compositional analysis, the major CM PLs of S. aureus, phosphatidylglycerol (PG), cardiolipin (CL), and LPG, were separated by 2-dimensional thin-layer chromatography (TLC) using silica 60 F254 HPTLC plates (Merck) and quantified as described before (21, 22). Outer-leaflet LPG was detected and measured using fluorescamine, a fluorescent probe, and quantified spectrophotometrically as detailed before (21, 22). All assays were done in triplicate and repeated in three independent experiments on different days, and the results were plotted as means ± standard deviations. Statistical analysis (multiple comparisons among data sets) was performed with one-way analysis of variance (ANOVA) using Minitab (15). A P value of ≤0.01 was considered significant.
Our study showed interesting but different phenotypic changes in the membrane parameters of magainin 2- and gramicidin D-resistant strains (55MG and 55GR, respectively). Although their membranes were found to be significantly more rigid than those of control bacteria (P ≤ 0.01), the two strains behaved differently. For example, 55MG showed a modest increase in rigidity (polarization value, 0.28 ± 0.02 [mean ± standard deviation]), whereas 55GR showed a substantial increase (polarization value, 0.32 ± 0.03) compared to that of the control, strain 55C (polarization value, 0.23 ± 0.02). This characteristic of altered membrane order was also observed in various S. aureus strains that are nonsusceptible to other cationic AMPs, such as daptomycin and tPMP-1 (23–25). It has been postulated that for each specific AMP and bacterial membrane interaction, there is an optimum relative membrane order at which AMPs exert maximum activity. Altered membrane order is an adaptation under the continuous selective pressure of the peptide, and this could be due to shifts in fatty acid unsaturation indices or branched-chain species (26).
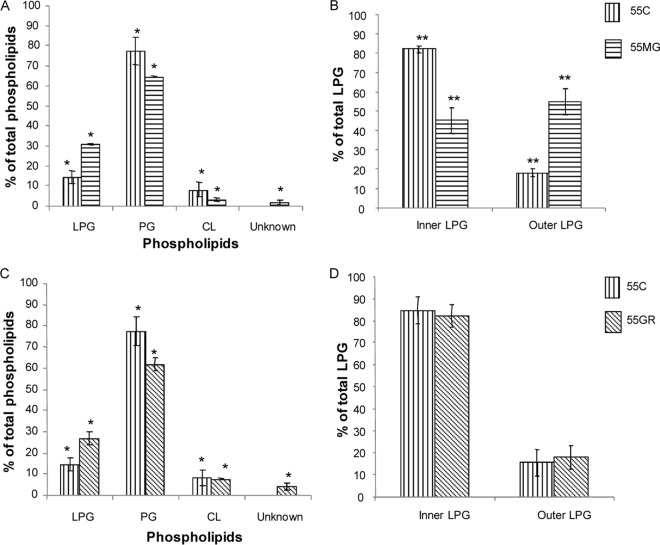
Another notable disparity observed was the relative net charge of the in vitro-selected strains as measured by zeta potential. As seen by the results shown in Fig. 2, strain 55MG exhibited a remarkable increase in its cationic charge (3 mV), while in strain 55GR, the zeta potential was almost the same (−14 mV) as that of the control (−18 mV). The difference in zeta potentials between the positive-selection and control S. aureus strains reached statistical significance (P ≤ 0.001). The increase in net charge in 55MG was manifested by a substantial increase in the synthesis of the cationic phospholipid LPG in its membrane compared to the synthesis of LPG in the membrane of the control strain 55C (Fig. 3A). In addition, ≥3-fold greater (P ≤ 0.01) translocation of LPG to the outer membrane leaflet was observed in 55MG than in the control (Fig. 3B). These findings are corroborated by previous reports that increased LPG content and its flipping to the outer membrane leaflet contribute to an increased positive charge in the staphylococcal cell surface (27). This underscored the potential for a charge-mediated repulsion of the peptide as one possibility for the magainin 2 resistance phenotype. On the contrary, translocation of the cationic phospholipid to the outer membrane leaflet in strain 55GR did not differ from that in the control (Fig. 3D), in spite of increased synthesis of total LPG (Fig. 3C). This could be one of the reasons why the net charge remained almost the same in 55GR as in the control. This phenomenon of increased LPG content without an effect on bacterial net charge was also reported by us and other scientists previously (17, 28). Liposome-based data suggested that, apart from surface charge regulation in AMP-CM interaction, LPG plays an additional role of stabilizing membrane integrity (29). It is also to be noted that, since gramicidin D is a neutral AMP (unlike magainin 2, having a net charge of +4), its mechanism of bactericidal action is not driven by electrostatic interaction. Rather, other factors, such as membrane rigidity, cell wall alteration, or hydrophobic interactions, might contribute to resistance to gramicidin D in S. aureus. It is postulated that the negatively charged phospholipids are required for the initial “docking” of AMPs within target CMs (30). Therefore, it was not surprising that the increase in LPG content in both 55MG and 55GR concomitantly reduced the proportion of negatively charged PG in our study.
Fig 2.
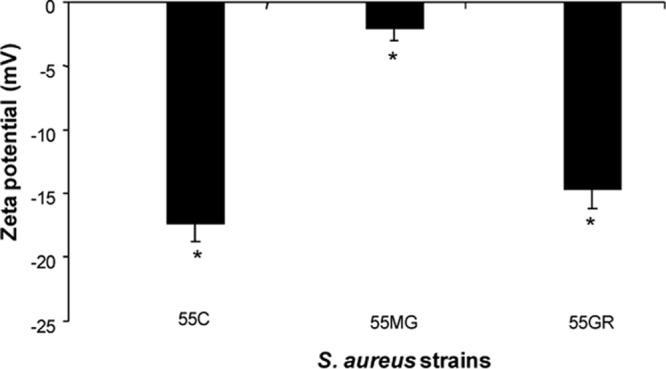
Zeta potentials of control and selected S. aureus strains. *, P ≤ 0.001 (one-way ANOVA; Minitab).
Fig 3.
(A, C) Phospholipid composition of 55MG (A) and 55GR (C) with respect to control (55C). (B, D) Asymmetry of LPG in 55MG (B) and 55GR (D) with respect to control (55C). *, P ≤ 0.001; **, ≤ P 0.01 (one-way ANOVA; Minitab).
In summary, the current study demonstrated the emergence of resistance in S. aureus under consistent in vitro exposure to magainin 2 and gramicidin D. S. aureus cells adapted differently to defend themselves from the lethal action of the two test AMPs. An increase in net charge along with a rigid membrane may account for resistance toward the cationic AMP magainin 2, while an increase in membrane rigidity combined with an alteration of membrane composition may contribute to the adaptive response to the neutral gramicidin D in S. aureus. Considering the urgent need for the introduction of AMPs as alternative therapies to combat bacterial infection, a study of this kind is of the utmost importance to enable minimization of the emergence of organisms resistant to AMPs and to develop AMPs as potentially useful antimicrobial agents.
ACKNOWLEDGMENTS
We are grateful to H. B. Bohidar of the School of Physical Sciences, JNU, and his team for helping out in measurements of zeta potential.
This research work was supported by grants to K.M. from the Indian Council of Medical Research and Department of Biotechnology, India. T.S. and M.S. acknowledge fellowships from the University Grants Commission, India.
Footnotes
Published ahead of print 15 July 2013
REFERENCES
- 1.Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 24:389–395 [DOI] [PubMed] [Google Scholar]
- 2.Hancock REW, Sahl H-G. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24:1551–1557 [DOI] [PubMed] [Google Scholar]
- 3.Jacob L, Zasloff M. 1994. Potential therapeutic applications of magainins and other antimicrobial agents of animal origin. Ciba Found. Symp. 186:197–223 [DOI] [PubMed] [Google Scholar]
- 4.Eckert R. 2011. Road to clinical efficacy: challenges and novel strategies for antimicrobial peptide development. Future Microbiol. 6:635–651 [DOI] [PubMed] [Google Scholar]
- 5.Giuliani A, Giovanna P, Nicoletto SF. 2007. Antimicrobial peptides: an overview of a promising class of therapeutics. Cent. Eur. J. Biol. 2:1–33 [Google Scholar]
- 6.Gruenheid S, Moual HL. 2012. Resistance to antimicrobial peptides in gram-negative bacteria. FEMS Microbiol. Lett. 330:81–89 [DOI] [PubMed] [Google Scholar]
- 7.Bell G, Gouyon P-H. 2003. Arming the enemy: the evolution of resistance to self-proteins. Microbiology 149:1367–1375 [DOI] [PubMed] [Google Scholar]
- 8.Walsh TR, Howe RA. 2002. The prevalence and mechanisms of vancomycin resistance in Staphylococcus aureus. Annu. Rev. Microbiol. 56:657–675 [DOI] [PubMed] [Google Scholar]
- 9.Kaatz GW, Lundstrom TS, Seo SM. 2006. Mechanisms of daptomycin resistance in Staphylococcus aureus. Int. J. Antimicrob. Agents 28:280–287 [DOI] [PubMed] [Google Scholar]
- 10.Perron GG, Zasloff M, Bell G. 2006. Experimental evolution of resistance to an antimicrobial peptide. Proc. Biol. Sci. 273:251–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollard JE, Snarr J, Chaudhary V, Jennings JD, Shaw H, Christiansen B, Wright J, Jia W, Bishop RE, Savage PB. 2012. In vitro evaluation of the potential for resistance development to ceragenin CSA-13. J. Antimicrob. Chemother. 67:2665–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuelsen Ø, Haukland HH, Jenssen H, Krämer M, Sandvik K, Ulvatne H, Vorland LH. 2005. Induced resistance to the antimicrobial peptide lactoferricin B in Staphylococcus aureus. FEBS Lett. 579:3421–3426 [DOI] [PubMed] [Google Scholar]
- 13.Tambe SM, Sampath L, Modak SM. 2001. In vitro evaluation of the risk of developing bacterial resistance to antiseptics and antibiotics used in medical devices. J. Antimicrob. Chemother. 47:589–598 [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th edition Approved standard M7-A6. NCCLS, Wayne, PA [Google Scholar]
- 15.Madhuri , Shireen T, Venugopal SK, Ghosh D, Gadepalli R, Dhawan B, Mukhopadhyay K. 2009. In vitro antimicrobial activity of alpha-melanocyte stimulating hormone against major human pathogen Staphylococcus aureus. Peptides 30:1627–1635 [DOI] [PubMed] [Google Scholar]
- 16.Singh M, Mukhopadhyay K. 2011. C-terminal amino acids of alpha-melanocyte stimulating hormone are requisite for its antibacterial activity against Staphylococcus aureus. Antimicrob. Agents Chemother. 55:1920–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shireen T, Singh M, Dhawan B, Mukhopadhyay K. 2012. Characterization of cell membrane parameters of clinical isolates of Staphylococcus aureus with varied susceptibility to alpha-melanocyte stimulating hormone. Peptides 37:334–339 [DOI] [PubMed] [Google Scholar]
- 18.Lakowicz JR. 2006. Principles of fluorescence spectroscopy, 3rd ed. Springer, New York, NY [Google Scholar]
- 19.Wilson WW, Wade MM, Holman SC, Champlin FR. 2001. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. Microbiol. Methods 43:153–164 [DOI] [PubMed] [Google Scholar]
- 20.Ouhara K, Komatsuzawa H, Yamada S, Shiba H, Fujiwara T, Ohara M, Sayama K, Hashimoto K, Kurihara H, Sugai M. 2005. Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, β-defensins and LL-37, produced by human epithelial cells. J. Antimicrob. Chemother. 55:888–896 [DOI] [PubMed] [Google Scholar]
- 21.Jones T, Yeamen MR, Sakoulas G, Yang S-J, Proctor RA, Sahl H-G, Schrenzel J, Xiong YQ, Bayer AS. 2008. Failures in clinical treatment of Staphylococcus aureus infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry and drug binding. Antimicrob. Agents Chemother. 52:269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukhopadhyay K, Whitmire W, Xiong YQ, Molden J, Jones T, Peschel A, Staubitz P, Adler-Moore J, McNamara PJ, Proctor RA, Yeamen MR, Bayer AS. 2007. In vitro susceptibility of Staphylococcus aureus to thrombin-induced platelet microbial protein-1(tPMP-1) is influenced by cell membrane phospholipid composition and asymmetry. Microbiology 153:1187–1197 [DOI] [PubMed] [Google Scholar]
- 23.Xiong YQ, Mukhopadhyay K, Yeamen MR, Adler-Moore J, Bayer AS. 2005. Functional interrelationships between cell membrane and cell wall in antimicrobial peptide-mediated killing of Staphylococcus aureus. Antimicrob. Agents Chemother. 49:3114–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayer AS, Prasad R, Chandra J, Koul A, Smriti M, Varma A, Skurray RA, Firth N, Brown MH, Koo S-P, Yeamen MR. 2000. In vitro resistance of Staphylococcus aureus to thrombin-induced microbicidal protein is associated with alterations in membrane fluidity. Infect. Immun. 68:3548–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra NN, Liu GY, Yeamen MR, Nast CC, Proctor RA, McKinnell J, Bayer AS. 2011. Carotenoid-related alteration of cell membrane fluidity impacts Staphylococcus aureus susceptibility to host defense peptides. Antimicrob. Agents Chemother. 55:526–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein W, Weber MHW, Marahiel MA. 1999. Cold shock response of Bacillus subtilis: isoleucine-dependent switch in the fatty acid branching pattern for membrane adaptation to low temperatures. J. Bacteriol. 181:5341–5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peschel A, Jack RW, Otto M, Collins LV, Staubitz P, Nicholson G, Kalbacher H, Nieuwenhuizen WF, Jung G, Tarkowski A, Kessel KPM, Strijp JAG. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor mprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 193:1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishra NN, McKinnell J, Yeaman MR, Rubio A, Nast CC, Chen L, Kreiswirth BN, Bayer AS. 2011. In vitro cross-resistance to daptomycin and host defense cationic antimicrobial peptides in clinical methicillin-resistant Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 55:4012–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilelee E, Pokorny A, Yeamen MR, Bayer AS. 2010. Lysyl-phosphatidylglycerol attenuates membrane perturbation rather than surface association of the cationic antimicrobial peptide 6W-RP-1 in a model membrane system: implications for daptomycin resistance. Antimicrob. Agents Chemother. 54:4476–4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishra NN, Bayer AS. 2013. Correlation of cell membrane lipid profiles with daptomycin resistance in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 57:1082–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]