Abstract
The present study aimed to investigate the presence, distribution, and persistence of Arcobacter spp. in an artisanal dairy plant and to test the isolates to determine their different genotypes in the processing plant and in foods. Samples were collected in an artisanal cheese factory on four occasions between October and December 2012. Food samples (raw milk, ricotta cheese, mozzarella cheese, and conditioning liquid), water samples, and environmental samples were analyzed by the culture method; isolates were identified by multiplex PCR and genotyped by pulsed-field gel electrophoresis (PFGE) analysis. Arcobacter butzleri was isolated from 29 out of 59 samples (46.6%), 22 of which were from environmental samples and 7 of which were from food samples. Cluster analysis divided the strains into 47 PFGE patterns: 14 PFGE clusters and 33 unique types. Our findings indicate that the plant harbored numerous A. butzleri pulsotypes and that the manual cleaning and sanitation in the studied dairy plant do not effectively remove Arcobacter. The recurrent isolation of A. butzleri suggests that the environmental conditions in the dairy plant constitute a good ecological niche for the colonization of this microorganism. In some cases, the presence of indistinguishable strains isolated from the same facilities on different sampling days showed that these strains were persistent in the processing environment.
INTRODUCTION
Interest in arcobacters in veterinary and human public health has increased from the first report of the isolation of arcobacters from food of animal origin; since then, studies worldwide have reported the occurrence of arcobacters on food and in food production animals and have highlighted possible routes of transmission, especially for Arcobacter butzleri, to the human population (1).
Arcobacter butzleri is the most important and prevalent species of the genus and has been classified as a serious hazard to human health by the International Commission on Microbiological Specifications for Foods (2) and as a significant zoonotic pathogen (3). A few surveys have investigated the presence of Arcobacter spp. in bulk tank raw cow's milk, reporting prevalence rates of 46% in Northern Ireland (4), 5.8% in Malaysia (5), and 48% in Italy (6). In Italy, A. butzleri was isolated in fecal samples and in-line milk filters on a water buffalo (WB) dairy farm (7, 8). Consumption of Arcobacter-contaminated food or water is regarded as the most probable route of transmission to humans and animals (9) following fecal contamination during the various stages of production processes (10). In the Mediterranean countries, there is a widespread tradition of raw milk cheese production, including, particularly in Italy, WB cheeses. A. butzleri demonstrated the ability to survive during the production process and shelf life of WB mozzarella cheese (11), and raw milk cheeses may be considered a potential source of human infection even though A. butzleri has not been isolated from dairy products. A. butzleri contamination of food processing plants may be an additional source of food contamination, as demonstrated in poultry slaughterhouses and spinach processing plants (12–17). To our knowledge, no study has hitherto investigated the presence of Arcobacter spp. in dairy plants. To trace the sources of contamination in a food processing plant, Arcobacter strains must be characterized by discriminative typing methods. Because of its high discriminatory power, pulsed-field gel electrophoresis (PFGE) is considered the gold standard and a valuable molecular tool for epidemiological analysis of Arcobacter strains (15). For these reasons, the aim of the present study was to investigate the presence, distribution, and persistence of A. butzleri in an artisanal dairy plant and to genotype by PFGE the isolates in order to assess the presence of different genotypes in the processing plant and in foods.
MATERIALS AND METHODS
Description of the cheese factory.
This survey investigated the presence of A. butzleri in one dairy plant processing, on alternate days, WB raw milk for WB mozzarella cheese production and bovine pasteurized (65°C for 30 min) milk for production of other cheeses; the whey from these cheeses is then steamed to make ricotta cheese. A maximum of 500 liters of milk is processed daily. Milk was purchased from one WB farm and one cow farm. The cheese factory was selected by taking into account the different aspects of organization and production so as to reflect artisanal cheese plants in Italy, specifically (i) the use of raw milk for cheese making; (ii) the limited amount of cheese production; (iii) the manual cleaning performed at the end of cheese production by rinsing with hot water, cleaning and sanitizing daily with GD90 (Golmar, Italy), rinsing with cold water, and, once a week, cleaning with Schiumaclor-12 (Golmar, Italy) and descaling with Scrost (Golmar, Italia); and (iv) the very low level of automation technology, with no automatic machines other than a mozzarella cheese molder and no pipes, except for a pump to unload the milk.
Sampling.
The cheese factory was sampled on four occasions between October and December 2012 exclusively on the WB milk processing days by collecting the following samples, which were placed into a Stomacher bag and delivered in a portable refrigerator (5 ± 3°C) to the laboratory.
Food samples were raw WB milk and raw cow's milk before the start of processing, WB mozzarella cheese, and the conditioning liquid of WB ricotta cheese.
Environmental samples were from different plant sites, including surfaces in contact with food before the start of processing (cleaned surfaces) and surfaces not in contact with food (e.g., the floor) before the start of processing. Samples were obtained by sponging an area of approximately 250 cm2, which represented the maximum area that could be sampled for all the sampling sites. Sponging within the area consisted of 10 passes vertically (up and down was considered 1 pass) and 10 passes horizontally (side to side was considered 1 pass) for each large side of the sponge.
Water samples, taken on each sampling day, were three samples of tap water (one from each of the three water sources available in the dairy plant).
Environmental and other samples were obtained at all sampling times, whereas food samples were obtained on three occasions. A total of 59 samples were collected: 32 environmental samples, 15 food samples, and 12 water samples (Table 1).
Table 1.
Isolation of A. butzleri from different sampling sites in one artisanal cheese factory
| Sample location or type | No. of samples analyzed | No. of samples positive for Arcobacter butzleri | % of samples that were positive |
|---|---|---|---|
| Surfaces in contact with product | |||
| Bulk tank valve | 4 | 4 | |
| Milk pump | 4 | 3 | |
| Cheese vat | 4 | 3 | |
| Drainage table | 4 | 2 | |
| Mozzarella cheese molding roller | 4 | 4 | |
| Curd cutter | 4 | ||
| Subtotal | 24 | 16 | 66.6 |
| Surfaces not in contact with product | |||
| Floor drains | 4 | 4 | |
| Cooler room floor | 4 | 2 | |
| Subtotal | 8 | 6 | 75 |
| Water | |||
| Tap water | 12 | 0 | |
| Subtotal | 12 | 0 | |
| Food samples | |||
| Raw cow milk | 3 | 3 | |
| Raw WB milk | 3 | 3 | |
| Ricotta cheese | 3 | 1 | |
| WB mozzarella cheese | 3 | 0 | |
| Mozzarella cheese conditioning liquid | 3 | 0 | |
| Subtotal | 15 | 7 | 46.6 |
| Total | 59 | 29 | 49.5 |
Detection and identification of Arcobacter butzleri.
Samples were transferred to the laboratory in refrigerated coolers at 5 ± 3°C and processed within 1 h of collection. Isolation was performed according to the method of Houf et al. (18): 25 ml of liquid samples or 25 g of solid samples or sponges was placed in a Stomacher bag and homogenized for 5 min in a Stomacher in 225 ml of Arcobacter broth (Oxoid, Basingstoke, United Kingdom) supplemented with 5% laked horse blood (Oxoid, Basingstoke, United Kingdom) and a mix of cefoperazone (16 mg/liter), amphotericin B (10 mg/liter), 5-fluorulacil (100 mg/liter), novobiocin (32 mg/liter), and trimethoprim (64 mg/liter) as a selective supplement. All antimicrobial substances were obtained as standard laboratory powders from Sigma (St. Louis, MO USA). After 48 h of incubation at 28 ± 1°C under microaerobic conditions, an aliquot of 10 μl of the enrichment broth was streaked onto selective agar plates prepared by suspending in water 24 g of Arcobacter broth (Oxoid, Basingstoke, United Kingdom) and 12 g of technical agar no. 3 (Oxoid, Basingstoke, United Kingdom) and supplemented with the selective antibiotic mixture described above. The plates were incubated at 28 ± 1°C microaerobically for 48 h. The microaerobic conditions were created by evacuating 80% of the normal atmosphere and introducing a gas mixture of 8% CO2, 8% H2, and 84% N2 into the jar. At least 10 colonies, if present, suspected of being Arcobacter spp. were picked from each plate, subcultured, and subjected to presumptive identification using tests that included growth under aerobic conditions and cellular morphology.
DNA extraction and Arcobacter multiplex PCR.
For the identification of the presumptive Arcobacter colonies, the isolates were subjected to DNA extraction using a REDExtract-N-Amp tissue PCR kit (Sigma, St. Louis, MO, USA) and identified by the multiplex PCR described by Douidah et al. (19). Two reference strains, A. butzleri DSM 8739T and Arcobacter cryaerophilus DSM 7289T (Leibniz Institute, DSMZ, Braunschweig, Germany), were used as controls; they were grown separately on nutrient agar supplemented with 5% laked horse blood (Oxoid, Basingstoke, United Kingdom) and incubated at 30°C for 48 h microaerobically. At least three isolates from each positive sample were confirmed by the multiplex PCR described by Houf et al. (20) and then genotyped by PFGE analysis.
PGFE.
Genomic DNA from at least three isolates from each positive sample, when available, was prepared for PFGE analysis. A PFGE protocol suitable for Arcobacter butzleri genotyping was set up based on previously reported protocols (16, 21). Agarose-embedded DNA was digested for 4 h at 37°C with 50 U of SacII (Fermentas, St. Leon-Rot, Germany). The restriction fragments were separated by PFGE (CHEF Mapper; Bio-Rad, Hercules, CA, USA) on 1.0% SeaKem gold agarose (Lonza, Rockland, ME, USA) in 0.5× Tris-borate-EDTA (TBE) buffer. Electrophoresis was performed with an initial switch time of 5 s, a final switch time of 40 s, a 120° angle, a gradient of 5.0 V/cm at 14°C, and a run time of 22.5 h. A lambda ladder PFG marker (New England BioLabs, MA, USA) was used as the size standard. One reference strain, A. butzleri strain DSM 8739T, was used as a positive-control strain in each PFGE gel. After electrophoresis, gels were stained in 3× GelRed (Biotium, Hayward, CA, USA) solution for 60 min. Gels were visualized with a UV gel documentation system (QuantityOne 4.5 software; Bio-Rad Laboratories, Hercules, CA, USA).
The PFGE patterns were analyzed by BioNumerics 6.6 software (Applied Maths, Keistraat, Belgium) using the Dice similarity index, and the dendrogram was constructed with the unweighted pair group method with arithmetic means (UPGMA). The optimization setting was 1.0%; the band position tolerance was 0.8%. Isolates showing a PFGE similarity level of 100% were grouped in the same PFGE cluster.
RESULTS
Arcobacter was isolated from 29 samples (49.5% of all samples), of which 22 were environmental and 7 were food samples; all 250 isolates were identified as A. butzleri. A higher percentage of samples from surfaces not in contact with food were contaminated with A. butzleri (75%) in comparison to samples from surfaces in contact with food (66.6%) and samples from food (46.6%) (all the raw milk samples and one ricotta cheese sample). All WB mozzarella cheese, conditioning liquid, and tap water samples were negative for Arcobacter. The numbers and sources of positive samples are summarized in Table 1.
A total of 124 isolates and two reference strains, A. butzleri DSM 8739T and Arcobacter cryaerophilus DSM 7289T, were characterized by PFGE, and all tested strains were typeable with SacII macrorestriction analysis.
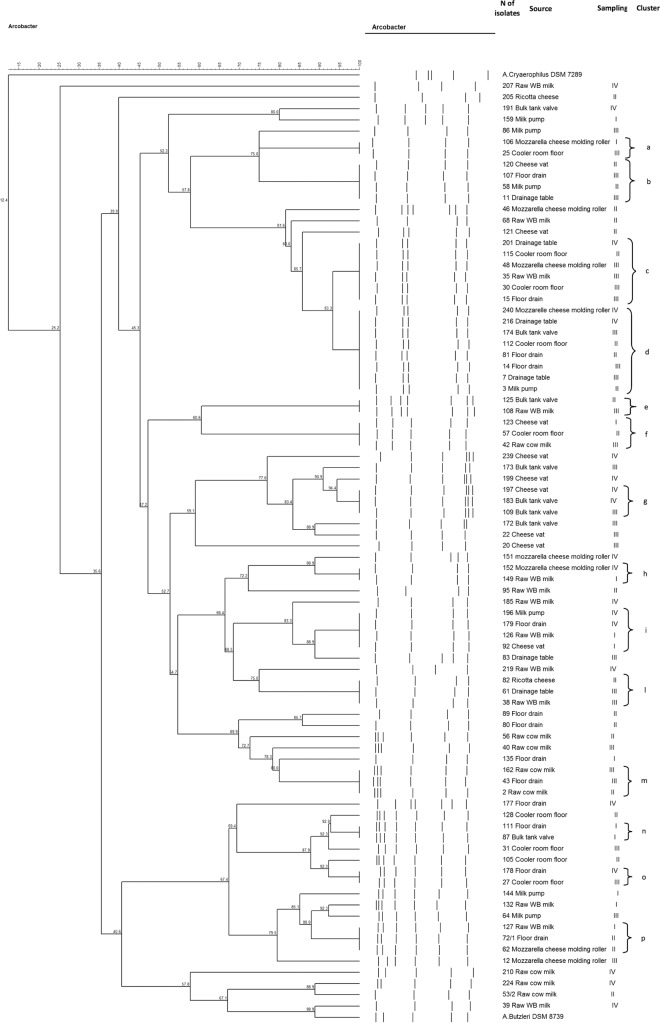
The cluster analysis of A. butzleri patterns revealed wide heterogeneity among these isolates. The SacII PFGE gel contained 4 to 7 bands, with strains clustering with similarities between 35.6% and 100%. Overall, the cluster analysis divided the strains into 47 PFGE patterns: 14 PFGE clusters (a to p) and 33 unique types (Fig. 1). Details on the source of isolation and sampling day of the isolates belonging to the clusters are reported in Fig. 1.
Fig 1.
Distribution of SacII digestion PFGE patterns of A. butzleri isolates. Information on the sources of isolates and sampling days (10 and 24 October 2012, 21 November 2012, and 5 December 2012, reported as I, II, III, and IV, respectively) of isolates belonging to the clusters is presented at the side of the dendrogram. The numbers on the horizontal axes indicate percentages of similarity, as determined by Dice correlation coefficient analysis and UPGMA clustering. Letters a to p represent the clusters. Isolates with the same PFGE pattern in the same sample are reported once.
DISCUSSION
We report the first isolation of A. butzleri from environmental samples collected in an artisanal cheese factory, in a ready-to-eat cheese produced for retail, and in raw WB milk. There was a high occurrence of A. butzleri in the artisanal cheese factory studied, with repeated detection of the microorganism and a frequently contaminated environment.
Raw milk can be considered a major source of A. butzleri contamination of the dairy plant but not the only source of A. butzleri contamination of cheese. As the present study isolated A. butzleri from all the raw milk samples tested, the microorganism is probably imported into the dairy plant with raw milk and spread to the environment during cheese processing. This suggestion is based on the detection of the same pulsotype in both the raw milk and the environmental samples (Fig. 1, clusters c, e, f, h, i, l, m, and p), even if the possibility of other sources of contamination cannot be excluded. Further evidence was found in the indistinguishable pulsotypes disclosed by PFGE analysis on different sampling days in samples from raw WB milk, the floor drain, the ricotta draining table, the mozzarella cheese molding roller, and the cooler room floor (Fig. 1, clusters c, f, h, i, and p). On the other hand, the high genetic heterogeneity among the A. butzleri raw milk isolates may be explained by multiple sources of milk contamination on the farm, multiple pulsotypes in a single dairy animal, and a high degree of genomic recombination, as suggested for poultry isolates (12).
The existence of indistinguishable pulsotypes in different surfaces areas, in contact or not in contact with food, on different sampling days about 1 month apart (Fig. 1, clusters a, b, d, g, n, and o) implies colonization of the plant by some A. butzleri strains and their persistence and distribution in different sites. As cross-contamination seems to be unavoidable due to the artisanal dairy plant logistics, several A. butzleri strains seem to have colonized the plant and were partially affected by repeated cleaning procedures, as previously demonstrated in a spinach processing plant (17). The persistence of an indigenous A. butzleri flora able to colonize a poultry abattoir was hypothesized (13, 14), and cross-contamination along the slaughter processing line, with the spread of isolates to different areas of the slaughterhouse, was demonstrated (16). A Belgian study (22) isolated arcobacters from boot samples, and boots and other tools (for example, trolleys) may be the cause of A. butzleri spreading from the processing area to other sites of the dairy plant in our study.
These considerations together with the ability of A. butzleri to adhere to different surfaces (23) and to form biofilm (16, 24) suggest two main sources of contamination in the investigated cheese factory: contaminated raw milk and resident strains able to survive in the wet dairy environment, characterized by large amounts of water and abundant organic material known to enhance the survival of A. butzleri (10).
Unlike other studies which identified processing water as a significant source of food and environmental contamination (13, 14), our study excluded the possibility of contamination by means of water, possibly due to the very simple water system in the investigated plant and to the exclusive use of chlorinated tap water for processing and cleaning.
The isolation of A. butzleri in the ricotta cheese confirms that dairy products are a potential source of human infection. Ricotta contamination is of particular interest for food safety, given ricotta's pH, which is close to neutrality (6.80 in the investigated cheese factory), and given the absence of any further antimicrobial treatment to control the replication of food-borne pathogens, including A. butzleri. We identified postprocessing as the most probable source of ricotta cheese contamination, as the investigated dairy plant produces the ricotta cheese by direct steaming of whey up to 90°C, and it is unlikely that A. butzleri can survive thermal treatment (25, 26). After the surfacing step, in which the whey proteins start to separate from the whey, surfacing as small flakes that gather on the surface (forming a layered white stratum), the ricotta is ladled into plastic molds on a steel draining table, and contact with equipment contaminated by A. butzleri is the most likely source of ricotta cheese contamination, as previously shown in poultry (13).
No contamination was observed in mozzarella cheese and in its conditioning liquid, despite the regular contamination of raw milk used for production and the contamination of food processing surfaces before and during operations. Due to the unfavorable pH (about 5.1 at the end of ripening in the investigated cheese factory [11]) and the stretching process during WB mozzarella cheese production, A. butzleri present in raw milk demonstrated the ability to survive only if present in high numbers (>7 log CFU/ml) (11). However, A. butzleri contamination of WB mozzarellas during postprocessing is unlikely, as the conditioning liquid in which the cheeses are submerged usually has a very low pH (2.80 in the studied dairy), well below the lower tolerated pH value (5.0) reported for A. butzleri (25).
The results of this study allow us to speculate that A. butzleri contamination may occur from a variety of sources in the observed dairy plant. The plant harbored numerous A. butzleri pulsotypes, and the manual cleaning and sanitation do not effectively remove Arcobacter from the plant. The recurrent A. butzleri isolation suggests that the environmental conditions in the dairy plant constitute a good ecological niche for the colonization of this microorganism. In some cases, the genetic identity of strains isolated from the same facilities on different sampling days showed that these strains were persistent in the processing environment. The presence of resident strains and their role in cheese safety should be investigated in industrial dairies.
Footnotes
Published ahead of print 23 August 2013
REFERENCES
- 1. Douidah L, de Zutter L, Baré J, De Vos P, Vandamme P, Vandenberg O, Van den Abeele, Houf K. 2012. Occurrence of putative virulence genes in Arcobacter species isolated from humans and animals. J. Clin. Microbiol. 50:735–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Commission on Microbiological Specifications for Foods (ICMSF) 2002. Microorganisms in foods, p 171 In Microbiological testing in food safety management Kluwer Academic/Plenum Publishers, New York, NY [Google Scholar]
- 3. Cardoen S, Van Huffel X, Berkvens D, Quoilin S, Ducoffre G, Saegerman C, Speybroeck N, Imberechts H, Herman L, Ducatelle R, Dierick K. 2009. Evidence-based semiquantitative methodology for prioritization of foodborne zoonoses. Foodborne Pathog. Dis. 6:1083–1096 [DOI] [PubMed] [Google Scholar]
- 4. Scullion R, Harrington CS, Madden RH. 2006. Prevalence of Arcobacter spp. in raw milk and retail raw meats in Northern Ireland. J. Food Prot. 69:1986–1990 [DOI] [PubMed] [Google Scholar]
- 5. Shah AH, Saleha AA, Murugaiyah M, Zunita Z, Memon AA. 2012. Prevalence and distribution of Arcobacter spp. in raw milk and retail raw beef. J. Food Prot. 75:1474–1478 [DOI] [PubMed] [Google Scholar]
- 6. Milesi S. 2010. Emerging pathogen Arcobacter spp. in food of animal origin. Ph.D. thesis University of Milan, Milan, Italy [Google Scholar]
- 7. Piva S, Serraino A, Florio D, Giacometti F, Pasquali F, Manfreda G, Zanoni RG. 2013. Isolation of Arcobacter species in water buffaloes (Bubalus bubalis). Foodborne Pathog. Dis. 10:475–477 [DOI] [PubMed] [Google Scholar]
- 8. Serraino A, Florio D, Giacometti F, Piva S, Mion D, Zanoni RG. 2013. Presence of Campylobacter and Arcobacter species in in-line milk filters of farms authorized to produce and sell raw milk and of a water buffalo dairy farm in Italy. J. Dairy Sci. 96:2801–2807 [DOI] [PubMed] [Google Scholar]
- 9. Collado L, Figueras MJ. 2011. Taxonomy, epidemiology, and clinical relevance of the genus Arcobacter. Clin. Microbiol. Rev. 24:174–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Driessche E, Houf K. 2008. Survival capacity in water of Arcobacter species under different temperature conditions. J. Appl. Microbiol. 105:443–451 [DOI] [PubMed] [Google Scholar]
- 11. Serraino A, Giacometti F, Daminelli P, Losio MN, Finazzi G, Marchetti G, Zambrini AV, Rosmini R. 2013. Survival of Arcobacter butzleri during production and storage of artisan water buffalo mozzarella cheese. Foodborne Pathog. Dis. 10.1089/fpd.2013.1485 [DOI] [PubMed] [Google Scholar]
- 12. Houf K, De Zutter L, Van Hoof J, Vandamme P. 2002. Occurrence and distribution of Arcobacter species in poultry processing. J. Food Prot. 65:1233–1239 [DOI] [PubMed] [Google Scholar]
- 13. Houf K, De Zutter L, Verbeke B, Van Hoof J, Vandamme P. 2003. Molecular characterization of Arcobacter isolates collected in a poultry slaughterhouse. J. Food Prot. 66:364–369 [DOI] [PubMed] [Google Scholar]
- 14. Gude A, Hillman TJ, Helps CR, Allen VM, Corry JE. 2005. Ecology of Arcobacter species in chicken rearing and processing. Lett. Appl. Microbiol. 41:82–87 [DOI] [PubMed] [Google Scholar]
- 15. Son I, Englen MD, Berrang ME, Fedorka-Cray PJ, Harrison MA. 2006. Genetic diversity of Arcobacter and Campylobacter on broiler carcasses during processing. J. Food Prot. 69:1028–1033 [DOI] [PubMed] [Google Scholar]
- 16. Ferreira S, Fraqueza MJ, Queiroz JA, Domingues FC, Oleastro M. 2013. Genetic diversity, antibiotic resistance and biofilm-forming ability of Arcobacter butzleri isolated from poultry and environment from a Portuguese slaughterhouse. Int. J. Food Microbiol. 162:82–88 [DOI] [PubMed] [Google Scholar]
- 17. Hausdorf L, Neumann M, Bergmann I, Sobiella K, Mundt K, Fröhling A, Schlüter O, Klocke M. 2013. Occurrence and genetic diversity of Arcobacter spp. in a spinach-processing plant and evaluation of two Arcobacter-specific quantitative PCR assays. Syst. Appl. Microbiol. 36:235–243 [DOI] [PubMed] [Google Scholar]
- 18. Houf K, Devriese LA, De Zutter L, Van Hoof J, Vandamme P. 2001. Development of a new protocol for the isolation and quantification of Arcobacter species from poultry products. Int. J. Food Microbiol. 71:189–196 [DOI] [PubMed] [Google Scholar]
- 19. Douidah L, De Zutter L, Vandamme P, Houf K. 2010. Identification of five human and mammal associated Arcobacter species by a novel multiplex-PCR assay. J. Microbiol. Methods 80:281–286 [DOI] [PubMed] [Google Scholar]
- 20. Houf K, Tutenel A, De Zutter L, Hoof JV, Vandamme P. 2000. Development of a multiplex PCR assay for the simultaneous detection and identification of Arcobacter butzleri, Arcobacter cryaerophilus and Arcobacter skirrowii. FEMS Microbiol. Lett. 193:89–94 [DOI] [PubMed] [Google Scholar]
- 21. Ribot EM, Fitzgerald C, Kubota K, Swaminathan B, Barrett TJ. 2001. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J. Clin. Microbiol. 39:1889–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Driessche E, Houf K, Vangroenweghe F, Nollet N, De Zutter L, Vandamme P, Van Hoof J. 2004. Occurrence and strain diversity of Arcobacter species isolated from healthy Belgian pigs. Res. Microbiol. 155:662–666 [DOI] [PubMed] [Google Scholar]
- 23. Assanta MA, Roy D, Lemay MJ, Montpetit D. 2002. Attachment of Arcobacter butzleri, a new waterborne pathogen, to water distribution pipe surfaces. J. Food Prot. 65:1240–1247 [DOI] [PubMed] [Google Scholar]
- 24. Kjeldgaard J, Jørgensen K, Ingmer H. 2009. Growth and survival at chiller temperatures of Arcobacter butzleri. Int. J. Food Microbiol. 131:256–259 [DOI] [PubMed] [Google Scholar]
- 25. D'Sa EM, Harrison MA. 2005. Effect of pH, NaCl content, and temperature on growth and survival of Arcobacter spp. J. Food Prot. 68:18–25 [DOI] [PubMed] [Google Scholar]
- 26. Hilton CL, Mackey BM, Hargreaves AJ, Forsythe SJ. 2001. The recovery of Arcobacter butzleri NCTC 12481 from various temperature treatments. J. Appl. Microbiol. 91:929–932 [DOI] [PubMed] [Google Scholar]