Abstract
Leguminous plants establish symbiosis with nitrogen-fixing alpha- and betaproteobacteria, collectively called rhizobia, which provide combined nitrogen to support plant growth. Members of the inverted repeat-lacking clade of legumes impose terminal differentiation on their endosymbiotic bacterium partners with the help of the nodule-specific cysteine-rich (NCR) peptide family composed of close to 600 members. Among the few tested NCR peptides, cationic ones had antirhizobial activity measured by reduction or elimination of the CFU and uptake of the membrane-impermeable dye propidium iodide. Here, the antimicrobial spectrum of two of these peptides, NCR247 and NCR335, was investigated, and their effect on the transcriptome of the natural target Sinorhizobium meliloti was characterized. Both peptides were able to kill quickly a wide range of Gram-negative and Gram-positive bacteria; however, their spectra were only partially overlapping, and differences were found also in their efficacy on given strains, indicating that the actions of NCR247 and NCR335 might be similar though not identical. Treatment of S. meliloti cultures with either peptide resulted in a quick downregulation of genes involved in basic cellular functions, such as transcription-translation and energy production, as well as upregulation of genes involved in stress and oxidative stress responses and membrane transport. Similar changes provoked mainly in Gram-positive bacteria by antimicrobial agents were coupled with the destruction of membrane potential, indicating that it might also be a common step in the bactericidal actions of NCR247 and NCR335.
INTRODUCTION
The introduction of antibiotics into medical practices during the first half of the last century revolutionized the treatment of infectious diseases caused by bacteria. However, the intensive use of these drugs led to the widespread emergence of antibiotic-resistant bacteria, and nowadays the general presence of these multiresistant microbes has become a major problem in public health therapy (1). This necessitates urgent development of new generations of antibacterial agents with novel modes of action that can be effective also in the case of multiresistant microbes (2).
Nearly all organisms, including bacteria, fungi, plants, and animals, produce antimicrobial peptides (AMPs) that are ribosomally synthesized natural antibiotics. AMPs are the effector molecules of the innate immunity in plants and animals and are able to kill microbes; however, they can fulfill signaling functions as well. The most general mode of their antimicrobial action is the disruption of the microbial membranes or the formation of pores that ultimately will also lead to cell lysis. However, a growing body of evidence suggests that AMPs, by entering the cell, can have intracellular targets (3). A recent development in genomics (high-throughput sequencing of genomes and transcriptomes) revealed large numbers of genes (up to several hundred) coding for AMP-like proteins/peptides in different eukaryotic genomes, especially in plants.
The genome of legumes like alfalfa, pea, or lentil belonging to the inverted repeat-lacking clade (IRLC) in the Fabaceae/Leguminosae family harbors a gene family that codes for secreted nodule-specific cysteine-rich (NCR) peptides that are reminiscent of as well as distinct from defensins, the largest group of plant AMPs (4). The common features of the two families are the small size of peptides and the disulfide bridges that stabilize their structure, while the differences include the number of cysteines and the charge of the peptides. NCRs can be anionic, neutral, and cationic and contain 4 or 6 cysteines, compared to 8 cysteines in plant and 6 cysteines in vertebrate defensins, which are all cationic molecules (5, 6). In the model legume Medicago truncatula (a diploid relative of the cultivated alfalfa, Medicago sativa), there are more than 500 NCR genes (7). These small genes usually contain two exons: the first one codes for a relatively conserved signal peptide, while the second one codes for the mature active peptide. The peptides are highly divergent in amino acid composition; only 4 or 6 cysteines at given positions are conserved. These AMP-like peptides are produced solely in a symbiotic organ, the root nodule, which is formed in symbiosis by legume plants to host Rhizobium soil bacteria. In the nodules, the plant cells are invaded with rhizobia, which by maturation of the symbiotic cell differentiate to nitrogen-fixing bacteroids, reducing atmospheric nitrogen to ammonia and providing nitrogen nutrient for the plant (8). Expression of NCR genes requiring the presence of the endosymbiont Sinorhizobium meliloti is activated only in the symbiotic cells. In M. truncatula, NCR transcripts represent about 5% of the nodule transcriptome (9). Earlier, it was shown that NCR peptides direct an irreversible, terminal differentiation process of bacteria resulting in enlargement and branching of nitrogen-fixing bacteroids coupled to their genome amplification, membrane permeabilization, and irreversible loss of their cell division ability (10). The ex planta bactericidal effects of certain cationic NCRs have also been demonstrated on the free-living S. meliloti cultures.
The aims of this study were to determine whether cationic NCRs have antibacterial activities on bacteria other than rhizobia, including Gram-negative and Gram-positive human/animal and plant pathogens, and to get an insight into the NCR-provoked global gene expression changes in S. meliloti.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains were grown on LB plates or in liquid medium: S. meliloti strain 1021, Listeria monocytogenes, Xanthomonas campestris, Clavibacter michiganensis, Agrobacterium tumefaciens, Pseudomonas aeruginosa, and Pseudomonas syringae at 30°C and Enterococcus faecalis, Salmonella enterica serovar Typhimurium, Staphylococcus aureus, Escherichia coli, Bacillus megaterium, and Bacillus cereus at 37°C. For the transcriptome analysis, S. meliloti was grown and treated in LSM (11) liquid medium from which methionine and arginine were omitted but was supplemented with 0.01% (wt/vol) yeast extract.
Measurement of in vitro NCR peptide activities.
Bacterial cultures in the exponential growth phase were rediluted and grown to early logarithmic phase (optical density at 600 nm [OD600] = 0.1) and then collected, washed, and resuspended in 10 mM potassium-phosphate buffer (pH = 7.0) to the same optical density.
To investigate the bactericidal effect of the chemically synthetized mature NCR247 (NH2-RNGCIVDPRCPYQQCRRPLYCRRR, pI = 10.15) and NCR335 (NH2-RLNTTFRPLNFKMLRFWGQNRNIMKHRGQKVHFSLILSDCKTNKDCPKLRRANVRCRKSYCVPI, pI = 11.22) peptides, serial dilutions of the bacterial suspensions treated at different concentrations for various time periods were plated to determine the number of surviving cells.
To check whether the peptides affect membrane integrity, we followed the uptake of the membrane-impermeable DNA-binding dye propidium iodide (PI). Bacteria were treated in Fluotrac 200 (Greiner Bio-One) microtiter plates in the presence of 5 μg/ml PI. PI uptake was detected by the fluorescence (excitation of 530 nm, emission of 600 nm) of its DNA-bound form measured in a fluorescence plate reader (FLUOstar Optima from BMG Labtech).
Preparation of RNA samples.
Bacterial cultures in the exponential growth phase were rediluted and grown to early logarithmic phase (OD600 = 0.1) in the modified LSM medium in a 40-ml volume, and then sterile water (untreated control), NCR247, or NCR335 was added to a final concentration of 10 μg/ml. Three biological replicates from each treatment were incubated with vigorous shaking for 10 and 30 min. Total RNA was purified from samples using the RiboPure-Bacteria kit (Ambion). Residual DNA was removed by using RQ1 RNase-free DNase (Promega). Each RNA sample was divided: one half was kept for the validation experiments, while the other part was processed for the transcriptome analysis.
Transcriptome analysis by RNA-Seq.
For sequencing, the RNAs from the three biological replicates were pooled. Before library preparation, ribosomal RNAs were removed using the Ribo-Zero rRNA removal kit for Gram-negative bacteria (Epicentre). Library preparation and RNA sequencing (RNA-Seq) were performed by using the dedicated kits and the SOLiD4 sequencer (Life Technologies), respectively. We generated 20 to 25 million 50-nucleotide-long reads per sample, from which approximately 45% proved to be quality data and thus could be mapped onto the S. meliloti genome (12).
Bioinformatic analysis.
Basic bioinformatic analyses (mapping of reads to the reference genome, normalization, calculation of expression values) were performed with the help of the CLC Genomic Workbench software. Reads mapping to tRNA and rRNA were removed from further analysis. We omitted the genes from further analysis if their expression was lower than 10 reads per 1 million mRNA reads. To identify up- and downregulated genes, RPKM values (reads per kilobase of gene model per million mapped reads) were compared. For pathway analysis, we used the KEGG database (http://www.genome.jp/kegg/).
Validation of the sequencing data.
To validate the sequencing results, quantitative reverse transcription-PCR (qRT-PCR) was performed on selected genes by using the primers shown in Table S1 in the supplemental material and rRNA as a reference, and then the results were compared to the sequencing data. RNA was reverse transcribed by the high-capacity cDNA reverse transcription kit (Life Technologies). PCR amplification was performed using the Power SYBR green kit (Life Technologies) and detected by the incorporation of the SYBR green dye in a StepOne real-time PCR system using StepOne software version 2.1 (Life Technologies). Two technical replicates were performed on all biological replicates.
RNA-Seq data accession number.
The RNA-Seq data have been deposited into the Gene Expression Omnibus database under accession number GSE47447 (www.ncbi.nlm.nih.gov/geo).
RESULTS
Antimicrobial spectrum of the NCR peptides.
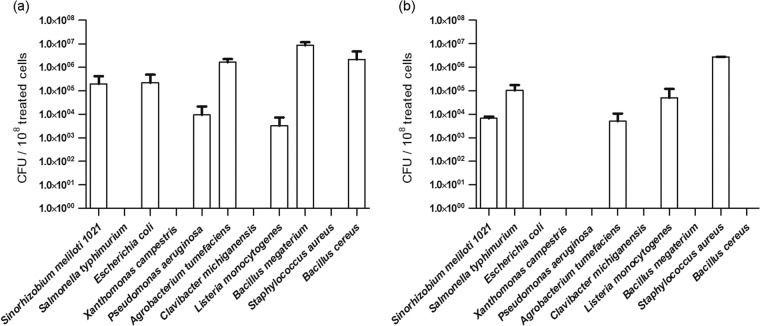
Medicago NCR247 and NCR335 peptides decreased the living cell number of the symbiotic S. meliloti bacteria by three and four orders of magnitude, respectively, when ∼107 bacteria were exposed in vitro to the peptides at the concentration of 50 μg/ml for 3 h (Fig. 1). To investigate whether these compounds have an antibacterial effect on different Gram-negative (E. coli, S. Typhimurium, A. tumefaciens, P. aeruginosa, X. campestris) and Gram-positive (B. megaterium, B. cereus, C. michiganensis, S. aureus, L. monocytogenes) bacteria, including human/animal and plant pathogens, these microorganisms were treated similarly with 50 μg/ml of the NCR247 and NCR335 peptides, and the number of surviving cells was determined by counting the CFU. Figure 1 shows that both peptides, although having different spectrums, decreased the living cell number of all tested bacteria from at least one order of magnitude to their complete elimination. Two plant-pathogenic microorganisms, X. campestris and C. michiganensis, were particularly sensitive toward both peptides, while A. tumefaciens and L. monocytogenes expressed moderate resistance against them, as 0.01 to 5% of the cells survived the exposure to the peptides. S. Typhimurium and S. aureus were effectively killed by NCR247, while B. megaterium, E. coli, B. cereus, and P. aeruginosa were sensitive to NCR335.
Fig 1.
Antibacterial activity of NCR247 (a) and NCR335 (b) peptides. Cell survival is shown as the number of colonies formed (CFU) from 108 cells treated for 3 h with peptides at a concentration of 50 μg/ml.
Transcriptome analysis of NCR-treated cells.
To investigate the early global gene expression changes in S. meliloti, the natural target of the NCR peptides, in response to NCR247 or NCR335 in vitro, genome-wide transcriptome profiling was performed by the RNA-Seq method (13). Exponentially growing cells were subjected to the peptides at a concentration of 10 μg/ml for 10 and 30 min. At these periods of treatments, NCR247 exposure did not affect colony-forming (surviving or cell division) ability, while NCR335 treatments reduced the CFU value to 66 and 57% of that of the untreated cultures, indicating that the antirhizobial potential of NCR335 is higher than that of NCR247. A total of 120 min of exposure, however, severely affected the colony-forming rate (NCR247, 8.1%; NCR335, 9.5%). At 30 min, the uptake of the membrane-impermeable dye propidium iodide (PI) was not detected in the case of NCR247 (see Fig. S1 in the supplemental material), indicating that drastic alteration or disintegration of the bacterial membranes had not started yet.
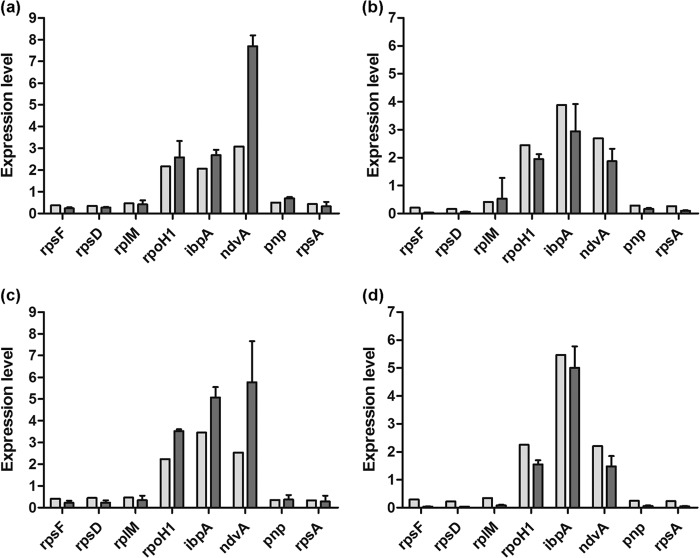
RNA samples isolated from the bacteria after the 10 and 30 min of treatments were used for RNA-Seq. In total, differential expression of 879 genes (representing 14% of the predicted protein-coding sequences in the S. meliloti 1021 genome) could be observed at a 2-fold cutoff: 366 genes were downregulated, while 543 genes were upregulated (see Table S2 in the supplemental material). NCR335 affected a higher number of genes than NCR247, causing down- and upregulation of 319 and 418 genes, respectively. In the case of NCR247 treatment, 153 genes were downregulated and 242 genes were upregulated. The numbers of genes that differentially expressed in single or multiple samples are shown in the Venn diagrams of Fig. 2. To validate the RNA-Seq data, real-time qRT-PCR experiments were performed using the RNA samples that had been used for sequencing. The results of the qRT-PCR experiments confirmed the up- or downregulation of the selected genes (Fig. 3).
Fig 2.
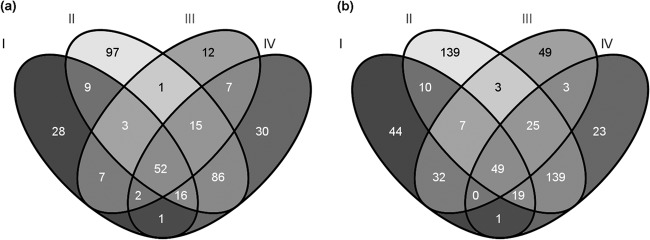
The number of the at-least-two-times downregulated (a) and upregulated (b) genes after 10 (I, II) and 30 (III, IV) min of treatment with the NCR247 (I, III) and NCR335 (II, IV) peptides.
Fig 3.
Validation of the RNA-Seq results. Expression of selected genes relative to the rRNA level in RNA samples from bacteria treated with NCR247 (a, c) and NCR335 (b, d) for 10 (a, b) and 30 (c, d) min was determined by qRT-PCR (dark-gray columns) and compared to values obtained by RNA-Seq (light-gray columns).
No overlap between the up- and downregulated genes was noticed, i.e., none of the genes upregulated by one peptide was downregulated by the other one at any time point. First, we looked for genes that were specifically regulated by one of the peptides. For this purpose, genes were investigated individually by comparing their expression in both peptide treatments at both time points. In the case of most responding genes, the treatments resulted in similar expression changes, though the threshold was not crossed in all four samples. Such genes, i.e., those that showed an above-threshold increase or decrease in certain samples and between 1.3- and 2-fold up- or downregulation in the others, were discussed among the generally down- and upregulated functions (Table 1).
Table 1.
Selected S. meliloti genes regulated differentially by the NCR peptides
| Category and gene | Putative function | Fold change |
|||
|---|---|---|---|---|---|
| 10 min |
30 min |
||||
| NCR247 | NCR335 | NCR247 | NCR335 | ||
| Genes encoding proteins involved in transcriptional regulation | |||||
| SMa1207 | Crp family transcriptional regulator | 0.45 | 0.17 | 0.50 | 0.44 |
| SMc01468 | Chemotaxis protein | 0.73 | 0.39 | 0.62 | 0.47 |
| SMc02521 | Glycerol-3-phosphate regulon repressor transcription regulator protein | 1.91 | 2.48 | 1.72 | 2.15 |
| SMc03806 | Nitrogen regulatory protein PII 2 | 1.54 | 4.66 | 2.01 | 3.56 |
| SMa0160 | GntR family transcriptional regulator | 3.21 | 2.87 | 2.39 | 1.92 |
| SM_b20215 | Transcriptional regulator protein | 1.65 | 3.20 | 1.91 | 2.38 |
| SMa0961 | Response regulator | 0.70 | 1.69 | 2.03 | 2.99 |
| SMa1056 | Transcriptional regulator | 0.86 | 1.98 | 2.13 | 2.67 |
| SMa5007 | Transcriptional regulator | 1.41 | 3.25 | 1.87 | 3.47 |
| SMc00129 | Sensor histidine kinase | 1.98 | 1.99 | 2.12 | 2.13 |
| SMc00329 | Iron response regulator protein | 1.25 | 2.10 | 1.44 | 2.22 |
| SMc00458 | Transcription regulator protein | 2.45 | 2.86 | 2.31 | 2.29 |
| SMc02584 | Transcription regulator protein | 1.17 | 2.11 | 1.38 | 2.43 |
| SMc02876 | Transcription regulator protein | 1.59 | 2.23 | 2.13 | 2.30 |
| SMc02888 | Transcription regulator protein | 2.85 | 2.08 | 3.05 | 2.00 |
| SMc04203 | RNA polymerase sigma factor FECI protein | 1.49 | 1.91 | 2.55 | 2.68 |
| SMc04242 | Zinc uptake regulation protein | 6.04 | 5.23 | 2.44 | 2.98 |
| SM_b20344 | Transcriptional regulator protein | 1.06 | 3.41 | 0.73 | 2.08 |
| SM_b21115 | Response regulator protein | 0.90 | 2.25 | 1.06 | 2.03 |
| SM_b21706 | LacI family transcriptional regulator | 0.75 | 2.32 | 1.04 | 2.03 |
| SMa0402 | GntR family transcriptional regulator | 1.15 | 2.51 | 1.04 | 2.06 |
| SMc00562 | Transcription regulator protein | 1.95 | 10.61 | 1.08 | 6.65 |
| SMc00653 | Two-component receiver domain protein | 1.01 | 2.19 | 0.81 | 2.22 |
| SMc03169 | Transcription regulator protein | 1.74 | 4.39 | 0.79 | 2.48 |
| SMc03824 | Transcription regulator protein | 1.29 | 3.16 | 0.73 | 2.28 |
| SMc03949 | Nitrogen regulatory protein | 1.19 | 2.13 | 0.98 | 2.76 |
| SMc00646 | RNA polymerase factor sigma-32 | 2.16 | 2.44 | 2.23 | 2.25 |
| Genes encoding proteins involved in transcription | |||||
| SMc01285 | DNA-directed RNA polymerase subunit alpha | 0.48 | 0.19 | 0.42 | 0.22 |
| SMc01316 | DNA-directed RNA polymerase subunit beta′ | 0.54 | 0.41 | 0.46 | 0.38 |
| SMc01317 | DNA-directed RNA polymerase subunit beta | 0.47 | 0.29 | 0.44 | 0.36 |
| SMc02408 | DNA-directed RNA polymerase subunit omega | 0.81 | 0.39 | 0.62 | 0.32 |
| SMc01322 | Transcription antitermination protein NusG | 0.60 | 0.32 | 0.88 | 0.42 |
| SMc02796 | Transcription termination factor Rho | 0.76 | 0.35 | 0.61 | 0.35 |
| Genes encoding proteins involved in conformational modification, metabolism, or maturation of RNA | |||||
| SMa0126 | Cold shock family protein | 0.92 | 0.32 | 1.04 | 0.31 |
| SMc01428 | Cold shock transcription regulator protein | 1.06 | 0.37 | 1.26 | 0.47 |
| SMc04234 | Cold shock-like transcription regulator protein | 0.96 | 0.42 | 1.02 | 0.43 |
| SMc04318 | Cold shock transcription regulator protein | 0.85 | 0.46 | 0.77 | 0.37 |
| SMc00324 | Polynucleotide phosphorylase/polyadenylase | 0.51 | 0.28 | 0.35 | 0.24 |
| SM_b20880 | ATP-dependent RNA helicase | 0.71 | 0.21 | 0.57 | 0.21 |
| SMc00522 | ATP-dependent RNA helicase | 0.54 | 0.25 | 0.57 | 0.34 |
| SMc01720 | RNase P | 0.57 | 0.24 | 0.41 | 0.34 |
| SMc01336 | RNase E protein | 1.00 | 0.46 | 0.81 | 0.39 |
| SMc01327 | tRNA/rRNA methyltransferase | 0.73 | 0.41 | 0.68 | 0.47 |
| Genes encoding subunits of and proteins involved in biogenesis of ribosomes | |||||
| SMc01049 | GTP-binding protein | 0.72 | 0.42 | 0.74 | 0.42 |
| SMc02695 | GTP-dependent nucleic acid-binding protein EngD | 0.64 | 0.41 | 0.59 | 0.50 |
| SM_b20995 | GTP-binding protein EngA | 0.48 | 0.39 | 0.61 | 0.45 |
| SMc00323 | 30S ribosomal protein S15 | 0.48 | 0.28 | 0.48 | 0.32 |
| SMc00335 | 30S ribosomal protein S1 | 0.43 | 0.26 | 0.33 | 0.23 |
| SMc00363 | 50S ribosomal protein L35 | 0.49 | 0.25 | 0.83 | 0.36 |
| SMc00364 | 50S ribosomal protein L20 | 0.51 | 0.19 | 0.40 | 0.24 |
| SMc00485 | 30S ribosomal protein S4 | 0.35 | 0.16 | 0.45 | 0.22 |
| SMc00565 | 50S ribosomal protein L9 | 0.31 | 0.19 | 0.23 | 0.21 |
| SMc00567 | 30S ribosomal protein S18 | 0.35 | 0.16 | 0.36 | 0.23 |
| SMc00568 | 30S ribosomal protein S6 | 0.37 | 0.21 | 0.41 | 0.29 |
| SMc00704 | 50S ribosomal protein L28 | 0.51 | 0.29 | 0.57 | 0.37 |
| SMc01152 | 30S ribosomal protein S20 | 0.45 | 0.24 | 0.58 | 0.25 |
| SMc01283 | 50S ribosomal protein L17 | 0.57 | 0.24 | 0.43 | 0.22 |
| SMc01286 | 30S ribosomal protein S11 | 0.50 | 0.18 | 0.41 | 0.19 |
| SMc01287 | 30S ribosomal protein S13 | 0.53 | 0.26 | 0.61 | 0.32 |
| SMc01290 | 50S ribosomal protein L15 | 0.37 | 0.23 | 0.30 | 0.22 |
| SMc01291 | 50S ribosomal protein L30 | 0.36 | 0.16 | 0.22 | 0.16 |
| SMc01292 | 30S ribosomal protein S5 | 0.36 | 0.17 | 0.23 | 0.19 |
| SMc01293 | 50S ribosomal protein L18 | 0.34 | 0.25 | 0.27 | 0.22 |
| SMc01294 | 50S ribosomal protein L6 | 0.34 | 0.23 | 0.29 | 0.21 |
| SMc01295 | 30S ribosomal protein S8 | 0.29 | 0.16 | 0.25 | 0.17 |
| SMc01296 | 30S ribosomal protein S14 | 0.32 | 0.19 | 0.26 | 0.17 |
| SMc01297 | 50S ribosomal protein L5 | 0.33 | 0.16 | 0.30 | 0.22 |
| SMc01298 | 50S ribosomal protein L24 | 0.30 | 0.15 | 0.31 | 0.24 |
| SMc01299 | 50S ribosomal protein L14 | 0.40 | 0.20 | 0.32 | 0.23 |
| SMc01300 | 30S ribosomal protein S17 | 0.31 | 0.30 | 0.27 | 0.23 |
| SMc01301 | 50S ribosomal protein L29 | 0.38 | 0.26 | 0.28 | 0.21 |
| SMc01302 | 50S ribosomal protein L16 | 0.34 | 0.24 | 0.28 | 0.20 |
| SMc01303 | 30S ribosomal protein S3 | 0.29 | 0.27 | 0.25 | 0.25 |
| SMc01304 | 50S ribosomal protein L22 | 0.30 | 0.26 | 0.20 | 0.23 |
| SMc01305 | 30S ribosomal protein S19 | 0.25 | 0.21 | 0.22 | 0.19 |
| SMc01306 | 50S ribosomal protein L2 | 0.28 | 0.19 | 0.21 | 0.18 |
| SMc01307 | 50S ribosomal protein L23 | 0.28 | 0.23 | 0.29 | 0.22 |
| SMc01308 | 50S ribosomal protein L4 | 0.27 | 0.23 | 0.20 | 0.23 |
| SMc01309 | 50S ribosomal protein L3 | 0.27 | 0.16 | 0.21 | 0.16 |
| SMc01310 | 30S ribosomal protein S10 | 0.29 | 0.16 | 0.21 | 0.19 |
| SMc01313 | 30S ribosomal protein S7 | 0.37 | 0.17 | 0.32 | 0.22 |
| SMc01314 | 30S ribosomal protein S12 | 0.41 | 0.17 | 0.41 | 0.21 |
| SMc01318 | 50S ribosomal protein L7/L12 | 0.36 | 0.23 | 0.35 | 0.23 |
| SMc01319 | 50S ribosomal protein L10 | 0.35 | 0.20 | 0.36 | 0.19 |
| SMc01320 | 50S ribosomal protein L1 | 0.41 | 0.21 | 0.42 | 0.23 |
| SMc01321 | 50S ribosomal protein L11 | 0.43 | 0.16 | 0.41 | 0.22 |
| SMc01369 | 50S ribosomal protein L33 | 0.67 | 0.27 | 0.59 | 0.31 |
| SMc01803 | 30S ribosomal protein S9 | 0.43 | 0.44 | 0.45 | 0.57 |
| SMc01804 | 50S ribosomal protein L13 | 0.46 | 0.41 | 0.46 | 0.34 |
| SMc02101 | 30S ribosomal protein S2 | 0.46 | 0.31 | 0.44 | 0.36 |
| SMc02692 | 50S ribosomal protein L25 | 0.34 | 0.20 | 0.42 | 0.24 |
| SMc03770 | 50S ribosomal protein L21 | 0.56 | 0.27 | 0.73 | 0.36 |
| SMc03772 | 50S ribosomal protein L27 | 0.45 | 0.23 | 0.63 | 0.39 |
| SMc03859 | 30S ribosomal protein S16 | 0.56 | 0.33 | 0.54 | 0.36 |
| SMc03863 | 50S ribosomal protein L19 | 0.55 | 0.28 | 0.40 | 0.34 |
| SMc03881 | 50S ribosomal protein L32 | 0.54 | 0.24 | 0.63 | 0.36 |
| SMc03990 | 50S ribosomal protein L31 | 0.59 | 0.16 | 0.62 | 0.25 |
| SMc04003 | 50S ribosomal protein L36 | 0.55 | 0.21 | 0.72 | 0.33 |
| SMc04003 | 50S ribosomal protein L36 | 0.55 | 0.21 | 0.72 | 0.33 |
| SMc04320 | 30S ribosomal protein S21 | 1.07 | 0.37 | 1.01 | 0.36 |
| SMc04434 | 50S ribosomal protein L34 | 0.54 | 0.20 | 0.86 | 0.25 |
| Genes encoding proteins involved in translation | |||||
| SMc00357 | Elongation factor P | 0.51 | 0.22 | 0.51 | 0.24 |
| SMc01311 | Elongation factor Tu | 0.35 | 0.23 | 0.27 | 0.20 |
| SMc01312 | Elongation factor G | 0.31 | 0.19 | 0.26 | 0.22 |
| SMc01326 | Elongation factor Tu | 0.36 | 0.22 | 0.28 | 0.21 |
| SMc02100 | Elongation factor Ts | 0.50 | 0.35 | 0.33 | 0.30 |
| SMc02310 | Translation initiation factor IF-1 | 0.65 | 0.38 | 0.73 | 0.44 |
| SMc02914 | Translation initiation factor IF-2 | 0.78 | 0.50 | 0.61 | 0.38 |
| SMc00362 | Translation initiation factor IF-3 | 0.80 | 0.39 | 0.87 | 0.42 |
| SMc02050 | Trigger factor | 0.52 | 0.22 | 0.46 | 0.26 |
| SMc01700 | Peptidyl-prolyl cis-trans isomerase A signal peptide protein | 0.83 | 0.42 | 0.78 | 0.45 |
| Genes encoding proteins involved in purine/pyrimidine metabolism | |||||
| SM_b21284 | Uricase | 0.66 | 2.95 | 1.48 | 2.48 |
| SM_b21286 | Xanthine dehydrogenase | 0.72 | 2.12 | 0.92 | 2.03 |
| SMc02386 | AMP nucleosidase | 1.33 | 2.27 | 1.11 | 2.16 |
| SMc01215 | Carbamoyl phosphate synthase large subunit | 0.50 | 0.39 | 0.61 | 0.51 |
| SMc01569 | Carbamoyl phosphate synthase small subunit | 0.55 | 0.36 | 0.57 | 0.49 |
| SMc00488 | Phosphoribosylformylglycinamidine synthase II | 0.62 | 0.49 | 0.63 | 0.46 |
| SMc00495 | Phosphoribosylaminoimidazole-succinocarboxamide synthase | 0.59 | 0.27 | 0.56 | 0.30 |
| SMc00508 | Adenylosuccinate lyase | 0.56 | 0.46 | 0.61 | 0.47 |
| SMc00615 | Phosphoribosylaminoimidazole synthetase | 0.51 | 0.46 | 0.59 | 0.44 |
| SMc00494 | Phosphoribosylformylglycinamidine synthase subunit PurS | 0.56 | 0.23 | 0.48 | 0.30 |
| SMc01288 | Adenylate kinase | 0.56 | 0.40 | 0.72 | 0.37 |
| SMc00595 | Nucleoside diphosphate kinase | 0.43 | 0.22 | 0.47 | 0.21 |
| SMc02099 | Uridylate kinase | 0.49 | 0.49 | 0.78 | 0.59 |
| SMc01815 | Dihydropyrimidine dehydrogenase | 4.07 | 7.42 | 2.96 | 4.05 |
| Genes encoding proteins involved in oxidative phosphorylation and electron transport | |||||
| SMc00187 | Ubiquinol-cytochrome c reductase iron-sulfur subunit protein | 0.58 | 0.28 | 0.57 | 0.46 |
| SMc00188 | Cytochrome b transmembrane protein | 0.55 | 0.36 | 0.56 | 0.41 |
| SMc00189 | Cytochrome c1 protein | 0.54 | 0.47 | 0.45 | 0.35 |
| SMc02897 | Cytochrome c transmembrane protein | 0.68 | 0.45 | 0.66 | 0.41 |
| SMc00868 | FoF1 ATP synthase subunit B | 0.51 | 0.31 | 0.52 | 0.34 |
| SMc00869 | FoF1 ATP synthase subunit B′ | 0.49 | 0.34 | 0.54 | 0.39 |
| SMc00870 | FoF1 ATP synthase subunit C | 0.36 | 0.31 | 0.49 | 0.32 |
| SMc00871 | FoF1 ATP synthase subunit A | 0.36 | 0.27 | 0.57 | 0.33 |
| SMc02498 | FoF1 ATP synthase subunit delta | 0.67 | 0.30 | 0.79 | 0.46 |
| SMc02499 | FoF1 ATP synthase subunit alpha | 0.50 | 0.38 | 0.40 | 0.38 |
| SMc02500 | FoF1 ATP synthase subunit gamma | 0.44 | 0.44 | 0.37 | 0.36 |
| SMc02501 | FoF1 ATP synthase subunit beta | 0.49 | 0.57 | 0.43 | 0.41 |
| SMc02502 | FoF1 ATP synthase subunit epsilon | 0.53 | 0.52 | 0.46 | 0.47 |
| SMc03239 | Inorganic pyrophosphatase | 0.85 | 0.44 | 0.74 | 0.43 |
| Genes encoding proteins involved in fatty acid synthesis and metabolism and membrane modification | |||||
| SMa0335 | 3-Ketoacyl-ACP reductase | 1.27 | 5.13 | 2.99 | 5.69 |
| SMc00262 | Acetyl-coenzyme A (CoA) acetyltransferase | 2.20 | 2.38 | 1.87 | 2.22 |
| SMc00976 | Enoyl-CoA hydratase | 2.33 | 2.04 | 1.51 | 1.22 |
| SMc02162 | Long-chain fatty-acid–CoA ligase | 2.47 | 2.42 | 1.95 | 1.64 |
| SMc02227 | Enoyl-COA hydratase | 3.45 | 5.33 | 1.56 | 2.21 |
| SMc02228 | Acetyl-CoA acetyltransferase | 3.17 | 4.00 | 1.53 | 1.67 |
| SMc02229 | Acyl-CoA dehydrogenase | 3.21 | 3.48 | 1.36 | 1.36 |
| SMc03836 | Acyl-CoA thioesterase i protein | 1.42 | 4.58 | 1.77 | 4.67 |
| SMc04041 | Lysophospholipase L2 protein | 1.71 | 3.07 | 1.87 | 2.97 |
| SMc01784 | Glycerol-3-phosphate acyltransferase PlsX | 0.76 | 0.42 | 0.69 | 0.47 |
| SMc01270 | Glutathione-dependent formaldehyde dehydrogenase | 0.59 | 0.44 | 0.74 | 0.42 |
| SMc00005 | Enoyl-ACP reductase | 0.52 | 0.28 | 0.62 | 0.32 |
| SMc00573 | Acyl carrier protein | 0.59 | 0.49 | 0.61 | 0.47 |
| SMc00574 | 3-Oxoacyl-ACP synthase | 0.65 | 0.46 | 0.71 | 0.49 |
| SMc01344 | Acetyl-CoA carboxylase biotin carboxyl carrier protein subunit | 0.90 | 0.39 | 0.74 | 0.45 |
| SMc04278 | Acyl carrier protein | 0.59 | 0.40 | 0.60 | 0.45 |
| SMc02520 | Glycerol-3-phosphate dehydrogenase | 2.84 | 2.53 | 2.03 | 2.07 |
| SMc02645 | Cyclopropane-fatty-acyl-phospholipid synthase | 1.03 | 3.50 | 1.23 | 2.53 |
| Genes encoding proteins involved in membrane transport | |||||
| SM_b20989 | Stomatin-like protein | 1.26 | 2.42 | 1.21 | 8.27 |
| SM_b20345 | Efflux protein | 0.99 | 3.98 | 0.65 | 2.38 |
| SM_b20346 | Efflux protein | 1.31 | 4.32 | 0.85 | 2.42 |
| SM_b20506 | l-Arabinose transporter permease | 0.94 | 2.29 | 1.20 | 2.04 |
| SMc00563 | Transporter | 1.00 | 6.50 | 1.13 | 4.08 |
| SMc00564 | Transporter | 0.80 | 4.59 | 1.22 | 3.21 |
| SMc03167 | Multidrug efflux system protein | 1.40 | 5.57 | 0.97 | 3.10 |
| SMc03168 | Multidrug efflux system protein | 2.21 | 5.77 | 0.87 | 2.95 |
| SMc03825 | Transporter | 0.95 | 3.17 | 1.12 | 2.53 |
| SM_b21432 | Iron uptake ABC transporter substrate-binding protein precursor | 2.67 | 0.98 | 2.62 | 1.21 |
| SMc03845 | Conserved extracellular hypothetical protein | 2.44 | 0.89 | 2.20 | 0.80 |
| SMc02085 | Biopolymer transport transmembrane protein | 3.02 | 1.47 | 3.34 | 1.54 |
| SMc02084 | Biopolymer transport transmembrane protein | 2.81 | 1.57 | 2.57 | 1.47 |
| SMc04128 | Heavy metal-transporting ATPase (Cd export) | 0.11 | 0.27 | 0.23 | 0.32 |
| SMc04167 | Histidine-rich transporter transmembrane protein (Cd export) | 0.26 | 0.46 | 0.45 | 0.67 |
| SMc04175 | Transmembrane protein (cation transport) | 0.48 | 0.43 | 0.63 | 0.46 |
| SM_b20903 | Sugar uptake ABC transporter permease | 0.59 | 0.48 | 0.51 | 0.41 |
| SMc04317 | Iron-binding periplasmic protein | 0.58 | 0.37 | 0.51 | 0.37 |
| SMc04454 | ABC transporter ATP-binding protein | 0.59 | 0.47 | 0.56 | 0.49 |
| SMc01179 | Spermidine export protein MdtJ | 0.49 | 0.38 | 0.82 | 0.41 |
| SMc01652 | Spermidine/putrescine transport system substrate-binding protein | 0.59 | 0.80 | 0.42 | 0.35 |
| SMc01510 | Hemin importer ATP-binding subunit | 2.46 | 1.96 | 2.74 | 2.15 |
| SMc01511 | Hemin transport system permease transmembrane protein | 3.41 | 2.09 | 3.54 | 2.13 |
| SMc01512 | Hemin-binding periplasmic transmembrane protein | 5.59 | 2.46 | 4.54 | 2.06 |
| SMc01513 | Hemin transport protein | 5.04 | 3.05 | 4.74 | 2.80 |
| SMc01657 | Ferrioxamine B receptor precursor protein | 3.36 | 2.11 | 3.27 | 2.06 |
| SMc01659 | Ferrichrome/ferrioxamine B periplasmic transporter | 2.40 | 2.09 | 2.99 | 1.87 |
| SMc03807 | Ammonium transporter | 1.44 | 4.21 | 1.79 | 3.21 |
| SMc03900 | Cyclic beta-1,2-glucan ABC transporter | 3.08 | 2.69 | 2.54 | 2.20 |
| SMc04350 | Multidrug efflux system transmembrane protein | 2.35 | 2.74 | 2.08 | 2.69 |
| SMc04351 | Transmembrane ATP-binding ABC transporter protein | 2.62 | 2.89 | 2.70 | 2.87 |
| SMc02514 | Glycolipoprotein (GLP) periplasmic binding ABC transporter protein | 10.82 | 11.19 | 5.89 | 6.96 |
| SMc02515 | GLP hypothetical protein | 4.37 | 5.51 | 2.15 | 2.78 |
| SMc02516 | GLP transport system permease ABC transporter protein | 5.88 | 7.31 | 2.47 | 3.90 |
| SMc02517 | GLP transport system permease ABC transporter protein | 5.46 | 5.85 | 2.37 | 2.93 |
| SMc02518 | GLP ABC transporter ATP-binding protein | 4.54 | 4.27 | 2.56 | 2.73 |
| SMc02519 | GLP ABC transporter ATP-binding protein | 3.87 | 3.29 | 2.54 | 2.24 |
| SMc00265 | Periplasmic binding protein | 4.06 | 2.53 | 2.03 | 1.61 |
| SMc02171 | Periplasmic binding ABC transporter protein | 2.00 | 2.54 | 1.58 | 1.87 |
| SMc02219 | Amino acid-binding periplasmic protein | 1.00 | 2.49 | 2.10 | 3.82 |
| SMc02616 | Permease transmembrane protein | 0.85 | 1.62 | 2.40 | 2.83 |
| SMc02873 | Periplasmic binding (signal peptide) ABC transporter protein | 3.31 | 2.17 | 2.04 | 1.32 |
| SMc03121 | Periplasmic binding ABC transporter protein | 2.50 | 2.20 | 1.60 | 1.38 |
| SMc03167 | Multidrug efflux system protein | 1.40 | 5.57 | 0.97 | 3.10 |
| SMc04202 | Transmembrane protein | 1.41 | 2.76 | 1.63 | 2.42 |
| SM_b20117 | Sugar transferase | 1.10 | 3.16 | 1.41 | 4.33 |
| SM_b20611 | C4-dicarboxylate transporter DctA | 3.88 | 2.38 | 2.25 | 1.68 |
| SM_b20633 | Sugar uptake ABC transporter permease | 0.99 | 2.30 | 1.66 | 3.39 |
| SM_b21604 | Sugar uptake ABC transporter substrate-binding protein precursor | 8.07 | 16.69 | 8.27 | 4.83 |
| SMa0155 | Tripartite ATP-independent periplasmic transporter (TRAP-T) family | 3.10 | 2.03 | 2.18 | 2.42 |
| SMa0157 | ABC transporter substrate-binding protein | 6.15 | 4.53 | 3.27 | 2.18 |
| SMc04243 | High-affinity zinc uptake system membrane ABC transporter protein | 4.48 | 3.72 | 2.66 | 2.69 |
| SMc04244 | High-affinity zinc uptake system ATP-binding ABC transporter protein | 4.92 | 3.85 | 2.39 | 2.73 |
| SMc04245 | High-affinity zinc uptake system ABC transporter protein | 18.43 | 14.64 | 4.35 | 5.63 |
| SMc04246 | Transmembrane signal peptide protein | 3.98 | 3.21 | 2.77 | 2.07 |
| Genes encoding stress-related proteins | |||||
| SM_b21294 | Hsp20 family heat shock protein | 0.87 | 4.43 | 1.43 | 6.37 |
| SM_b21295 | Hsp20 family heat shock protein | 0.88 | 4.21 | 1.30 | 5.27 |
| SM_b22023 | Molecular chaperone GroES | 1.25 | 1.92 | 2.25 | 2.69 |
| SMa1894 | Methionine sulfoxide reductase B | 1.34 | 2.39 | 1.37 | 2.56 |
| SMa1896 | Methionine sulfoxide reductase A | 1.41 | 2.46 | 1.41 | 2.29 |
| SMc00646 | RNA polymerase factor sigma-32 | 2.16 | 2.44 | 2.23 | 2.25 |
| SMc01106 | Small heat shock protein | 1.92 | 3.71 | 2.30 | 4.04 |
| SMc01280 | Protease | 1.19 | 4.40 | 1.73 | 2.96 |
| SMc01758 | Chaperonin GroEL | 1.10 | 2.36 | 1.54 | 2.72 |
| SMc02433 | ATP-dependent protease | 1.94 | 4.85 | 2.92 | 5.10 |
| SMc02575 | ATP-dependent protease peptidase subunit | 1.67 | 3.63 | 2.65 | 3.94 |
| SMc02576 | Acetyltransferase | 1.59 | 2.41 | 2.12 | 3.26 |
| SMc02858 | Chaperone protein DnaJ | 1.30 | 2.04 | 1.44 | 2.42 |
| SMc02885 | Methionine sulfoxide reductase A | 2.18 | 3.20 | 2.23 | 3.27 |
| SMc04040 | Heat shock protein | 2.06 | 3.88 | 3.45 | 5.46 |
| Genes encoding proteins involved in modification of SH groups | |||||
| SMc01238 | Glutathione S-transferase | 0.89 | 1.86 | 2.04 | 6.91 |
| SMc01443 | Glutathione S-transferase | 1.08 | 2.72 | 2.07 | 2.04 |
| SMc02443 | Glutaredoxin | 1.50 | 2.37 | 2.03 | 2.79 |
| SMc02761 | Thioredoxin | 2.06 | 4.60 | 2.12 | 3.82 |
| Genes encoding proteins involved in glycine cleavage | |||||
| SMc02047 | Glycine cleavage system amino methyl transferase T | 1.07 | 3.51 | 1.10 | 2.89 |
| SMc02048 | Glycine cleavage system protein H | 1.18 | 3.06 | 1.21 | 2.76 |
| SMc02049 | Glycine dehydrogenase | 1.17 | 2.31 | 1.12 | 2.11 |
Peptide-specific up- and downregulated genes.
NCR247 specifically downregulated the expression of the SMc04329 gene coding for a conserved ferredoxin-like hypothetical protein and upregulated 11 genes coding, for example, for sulfite oxidase subunits YedYZ, the substrate-binding protein precursor of an iron uptake ABC transporter, as well as the ExbBD proteins that, in complex with TonB, transduce energy to TonB-dependent transporters, facilitating mainly the uptake of iron complexes (14). NCR335 specifically downregulated the expression of seven open reading frames (ORFs) coding for hypothetical proteins, one gene coding for a fatty acid desaturase, the mucR gene coding for a transcription factor, and five ORFs coding for proteins involved in RNA metabolism, such as the RNA chaperons Csp1, -4, -A2, -A8, and RNaseP. The 59 genes that were specifically upregulated by NCR335 have the coding capacity for 32 hypothetical proteins, one stomatin-like membrane protein, one oxidoreductase domain-containing protein, one plasmid stability protein, and one cyclopropane-fatty-acyl-phospholipid synthase involved in membrane modifications. Of the remaining encoded proteins, three enzymes are involved in the degradation of AMP to urate, and three proteins compose the glycine cleavage system that contributes to the pool of compounds containing only one carbon. The largest groups of the NCR335-induced genes encoding proteins with known/predicted roles belong to functional categories of transcriptional regulation (9 genes) and membrane transport (8 genes). Interestingly, four genes coding for transcriptional regulators (TRs) were linked to operons/genes involved in the production of seven (ABC-type) membrane transporters (MTs) that were also induced by the peptide, raising the possibility that these transcription factors directly regulate the expression of the neighboring genes.
Generally downregulated functions.
We looked for enriched functional categories and pathways among the products of those genes that showed differential expression. It is worth mentioning that NCR335, which is more potent in its antirhizobial activity than NCR247, often caused more pronounced expression changes than the other peptide. A good portion of the downregulated functions affected genetic information processing (Table 1). The expression of genes coding for proteins involved in transcription, such as the RNA-polymerase subunits (RpoABCZ) and the transcription terminator (Rho) and antiterminator (NusG) proteins, was reduced. Factors involved in translation, like translation initiation factors (IF-1, -2, -3), elongation factors (G, P, Tu1, Tu2, Ts), the ribosome-associated chaperone trigger factor (Tig), and genes coding for all (Rps, Rpm, and Rpl) ribosomal subunits and proteins predicted to function in ribosome biogenesis (HflX, EngD), also showed decreased transcript levels. In addition, proteins participating in conformational modification, metabolism, or maturation of RNA molecules (ATP-dependent RNA helicases RhlE1 and RhlE2, RNase E, polynucleotide phosphorylase/polyadenylase Pnp) also had decreased expression. The expression of genes coding for enzymes participating in the early, common steps of purine and thiamine biosynthesis was also reduced, as well as the transcription of the ndk gene encoding the nucleoside-diphosphate kinase catalyzing the exchange of phosphate groups between different nucleoside diphosphates. Further major biochemical functions that are downregulated by the peptides are oxidative phosphorylation and fatty acid biosynthesis: all genes coding for the elements of the FoF1 ATP synthase and cytochrome bc1 complex, as well as six genes coding for fatty acid biosynthetic enzymes, showed decreased expression. Certain ABC transporter genes, including the ones implicated in heavy metal (cadmium) and spermidine/putrescine export, were also inhibited (Table 1).
Generally upregulated functions.
Among the upregulated genes, 15 sequences coding for stress-related functions were identified: the highest induction was observed in the case of the ibpA, rpoH1, and msrA1 genes coding for a heat shock protein, an RNA polymerase sigma factor, and methionine sulfoxide reductase A (Table 1). The msrA and the clpB and hslV genes encoding ATP-dependent proteases were also induced. The upregulation of other heat shock proteins (Hsp20s, GroES, GroEL, DnaJ, SMc01106) and protease (DegP3)-coding genes were less pronounced; though NCR247 increased their transcription, this increase crossed the threshold only in the case of NCR335 treatment. Similarly to the NCR335-specific changes, a high number of upregulated genes encoded MTs (35 genes) as well as TRs (15 genes), and four TR-coding genes were linked to peptide-induced MT operons/genes. We do not know the substrate for most of the upregulated membrane transporters, except for ZnuABC mediating high-affinity zinc uptake (regulated by the induced Zur transcription factor) and FoxA-FhuFP-HmuSTUV proteins involved in ferrioxamine transport. The genes coding for the redox proteins thioredoxin and glutaredoxin as well as glutathione S-transferases, carbon monoxide dehydrogenase subunits, and enzymes involved in fatty acid oxidation were also induced by the peptides.
DISCUSSION
Antimicrobial peptides are considered to be potential candidates for a novel generation of antibiotics. In this study, potent antimicrobial properties of two nodule-specific cysteine-rich symbiotic peptides were demonstrated, indicating the potential for their use in the human/animal health care, plant protection, and food industry. Both cationic peptides NCR247 and NCR335 were able to quickly kill a wide range of Gram-negative and Gram-positive human/animal- and plant-pathogenic bacteria, though with a different spectrum and from moderate to very high efficiency. This ability further distinguishes NCRs and plant defensins, because the latter ones are active against fungi (5, 15). At present, the mode(s) of their action is not known, although the uptake of PI (data not shown) indicated that the peptides changed the permeability of the bacterial membranes. In addition, the reason for the observed differences in the sensitivity of the strains is not known. One possibility is that the two peptides do not recognize the same bacterial target(s) or have different affinity toward a common target which might be present in different amounts or might be differentially accessible in the various species. Another possible explanation, which does not exclude the previous ones, is the different sensitivity of the peptides toward the degrading activity of the various proteases produced by the bacteria.
Since the Medicago genome codes for almost 600 NCR peptides and roughly half of them are cationic, the nodules on the roots of this species represent a treasure of potential antibiotics. One step of this exploration is to test the target spectrum of the individual peptides. Another important question is their mode of action. How do these peptides kill bacteria? Do they simply destroy the bacterial membranes or have specific targets that inhibit bacterial functions? As a consequence, can the bacteria develop resistance against these NCRs? This probability is less plausible if multiple bacterial targets and pathways are affected.
Here, the potential targets and possible bacterial mechanisms affected upon in vitro exposure to the cationic NCR peptides were studied by transcriptome analysis of S. meliloti bacteria. Very few peptide-specific gene expression changes could be observed, indicating similar modes of action of the two peptides in this species. The more cationic NCR335 peptide often induced more pronounced up- or downregulation of the affected genes than NCR247, mirroring its more potent antirhizobial activity. Treatment of bacteria with the peptides resulted, within 10 min, in downregulation of genes involved in basic cellular functions, such as transcription-translation and energy production. We compared these results with the limited data available on the effects of other antimicrobial peptides and agents on other bacteria. A similar downregulation of the transcription-translation machinery has been observed in the Gram-positive S. aureus cells exposed to daptomycin, an acidic cyclic lipopeptide antibiotic (16) known to act via the obstruction of peptidoglycan biosynthesis and cell membrane depolarization (17). The spermidine/putrescine transporter-encoding genes were also inhibited; however, the expression of genes coding for the ATP synthetase subunits was not affected by daptomycin treatment. Interestingly, the effects of the membrane-depolarizing agent carbonyl cyanide m-chlorophenylhydrazone (CCCP) on S. aureus were more similar to the NCR-induced changes in Sinorhizobium, because CCCP caused the downregulation of transcription-translation-related and ATP synthetase genes. Though the Lactococcus bacteriocin nisin (18) attacks also the peptidoglycan biosynthesis (19) and forms pores in bacterial membranes (20), most of the gene expression changes characteristic for daptomycin or CCCP treatments in S. aureus were not observed or were less pronounced after challenging the bacteria with this agent (17). In Streptococcus pneumoniae, nisin induced moderate transcriptome variations. Similarly to NCR peptides in S. meliloti, the human AMP, the cathelicidin-derived linear LL-37 peptide (21), downregulated the S. pneumoniae genes coding for RNA polymerases and helicases, ribosomal subunits, and translation initiation and elongation factors (22). The effects of AMPs and AMP-like peptides on gene expression are similar to those caused by exposure to hydrogen peroxide, as was demonstrated in Bradyrhizobium japonicum by both fulminant shock and prolonged exposure to this chemical (23). The upregulated genes also show some similarities. In all cases, one can observe the induction of genes encoding stress-related chaperonins, proteases, methionine sulfoxide reductases, and sigma factors, as well as proteins involved in oxidative stress response, mainly thioredoxins but also glutaredoxins and glutathione S-transferases. To further facilitate their adaptation to the stress conditions, the cells also upregulated the expression of a large number of transcriptional regulators and membrane transporters. In the case of the NCR peptides, quite a few upregulated genes coding for transcription factors were linked to upregulated genes/operons that code for membrane transporter proteins, indicating possible concerted actions.
Based on these observations, it seems that the defensin-like NCR peptides, that in planta govern the differentiation of bacteria, exert their antimicrobial activity in vitro by affecting bacterial cell membranes, probably via forming pores and destroying the membrane potential. The impairment of membrane integrity was confirmed by detecting the uptake of the membrane-impermeable dye PI after treatment of the cells with the peptides. The loss of membrane integrity and membrane potential then might lead to stress responses which include the slowdown of the cell metabolism and the arresting of cell division. Higher concentrations of and/or longer exposure to the stressor might then lead to the death of the cells. In the plant cells, where different cocktails of cationic as well as anionic and neutral NCRs are produced in the different developmental stages, though most likely in much lower concentrations than in our experiments, the effects of peptides are less dramatic. Rhizobia isolated from the nodules slowly take up propidium iodide, indicating membrane permeabilization; however, in planta, the activity of peptides results only in the loss of cell division capacity but not in the death of wild-type bacteria. Interestingly, bacA mutant S. meliloti cells, which are more sensitive toward the peptides, are quickly eliminated from the NCR-producing plant cells as a result of the peptides' antibacterial action (24). This indicates that the plant cells produce peptides in an amount that is close to the lethal concentrations. The bacterial cells in symbiosis still had active metabolism, but interestingly, the expression of genes coding for proteins involved in transcription, translation, and ATP synthesis was downregulated (25). In addition, the expression of a high number of membrane transporter-coding genes changed in symbiosis; however, those involved in iron acquisition were downregulated in contrast to their upregulation we observed in vitro. The observed differences between the in vitro and in planta effects might be the consequences of different concentrations of NCRs, the presence of various sets of peptides in the plant cells, and/or the different environmental/physiological conditions. The main mode of action of the peptides can be the disruption of membrane potential or the opening of pores for the transport of other peptides and molecules, but they, especially the acidic and neutral ones, might affect intracellular targets, as well, such as the inhibition of the synthesis of storage compounds like polyhydroxybutyrate (PHB) or the uncoupling of DNA synthesis and cell division observed in symbiosis and in vitro by using a low concentration of peptides (26). Getting a deeper insight into the roles of the NCR molecules during the development of bacteria in planta necessitates the (transcriptome) analysis of rhizobia that can establish effective symbiosis both with NCR-producing plants and with legumes that have no coding capacity for these peptides.
In conclusion, it was demonstrated that plant-derived developmental regulators of bacteria can serve as antimicrobial agents against a wide range of Gram-negative and Gram-positive bacteria. Transcriptome analysis of cells treated with the peptides revealed characteristic gene expression changes that are accompanied with the stress caused by the disruption of bacterial membrane potential, which can cause the death of bacterial cells.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the “SYM-BIOTICS” Advanced Grant of the European Research Council (grant number 269067) and by the Hungarian National Office for Research and Technology and Agence Nationale de la Recherche (NSPEPBAC: TÉT_09-1-2010-0009/ANR-09-BLAN-0396-01).
Footnotes
Published ahead of print 30 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01791-13.
REFERENCES
- 1. Yoshikawa TT. 2002. Antimicrobial resistance and aging: beginning of the end of the antibiotic era? J. Am. Geriatr. Soc. 50:S226–S229 [DOI] [PubMed] [Google Scholar]
- 2. Chopra I. 2013. The 2012 Garrod lecture: discovery of antibacterial drugs in the 21st century. J. Antimicrob. Chemother. 68:496–505 [DOI] [PubMed] [Google Scholar]
- 3. Maroti G, Kereszt A, Kondorosi E, Mergaert P. 2011. Natural roles of antimicrobial peptides in microbes, plants and animals. Res. Microbiol. 162:363–374 [DOI] [PubMed] [Google Scholar]
- 4. Mergaert P, Nikovics K, Kelemen Z, Maunoury N, Vaubert D, Kondorosi A, Kondorosi E. 2003. A novel family in Medicago truncatula consisting of more than 300 nodule-specific genes coding for small, secreted polypeptides with conserved cysteine motifs. Plant Physiol. 132:161–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carvalho AD, Gomes VM. 2009. Plant defensins—prospects for the biological functions and biotechnological properties. Peptides 30:1007–1020 [DOI] [PubMed] [Google Scholar]
- 6. Cederlund A, Gudmundsson GH, Agerberth B. 2011. Antimicrobial peptides important in innate immunity. FEBS J. 278:3942–3951 [DOI] [PubMed] [Google Scholar]
- 7. Young ND, Debelle F, Oldroyd GED, Geurts R, Cannon SB, Udvardi MK, Benedito VA, Mayer KFX, Gouzy J, Schoof H, Van de Peer Y, Proost S, Cook DR, Meyers BC, Spannagl M, Cheung F, De Mita S, Krishnakumar V, Gundlach H, Zhou SG, Mudge J, Bharti AK, Murray JD, Naoumkina MA, Rosen B, Silverstein KAT, Tang HB, Rombauts S, Zhao PX, Zhou P, Barbe V, Bardou P, Bechner M, Bellec A, Berger A, Berges H, Bidwell S, Bisseling T, Choisne N, Couloux A, Denny R, Deshpande S, Dai XB, Doyle JJ, Dudez AM, Farmer AD, Fouteau S, Franken C, Gibelin C, Gish J, Goldstein S, Gonzalez AJ, Green PJ, Hallab A, et al. 2011. The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature 480:520–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kereszt A, Mergaert P, Maroti G, Kondorosi E. 2011. Innate immunity effectors and virulence factors in symbiosis. Curr. Opin. Microbiol. 14:76–81 [DOI] [PubMed] [Google Scholar]
- 9. Maunoury N, Redondo-Nieto M, Bourcy M, Van de Velde W, Alunni B, Laporte P, Durand P, Agier N, Marisa L, Vaubert D, Delacroix H, Duc G, Ratet P, Aggerbeck L, Kondorosi E, Mergaert P. 2010. Differentiation of symbiotic cells and endosymbionts in Medicago truncatula nodulation are coupled to two transcriptome-switches. PLoS One 5:e9519. 10.1371/journal.pone.0009519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mergaert P, Uchiumi T, Alunni B, Evanno G, Cheron A, Catrice O, Mausset AE, Barloy-Hubler F, Galibert F, Kondorosi A, Kondorosi E. 2006. Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium-legume symbiosis. Proc. Natl. Acad. Sci. U. S. A. 103:5230–5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liang J, Shah DM, Wu YS, Rosenberger CA, Hakimi S. November 2001. Antifungal polypeptide from alfalfa and methods for controlling plant-pathogenic fungi. US patent 6316407 B1
- 12. Galibert F, Finan TM, Long SR, Puhler A, Abola P, Ampe F, Barloy-Hubler F, Barnett MJ, Becker A, Boistard P, Bothe G, Boutry M, Bowser L, Buhrmester J, Cadieu E, Capela D, Chain P, Cowie A, Davis RW, Dreano S, Federspiel NA, Fisher RF, Gloux S, Godrie T, Goffeau A, Golding B, Gouzy J, Gurjal M, Hernandez-Lucas I, Hong A, Huizar L, Hyman RW, Jones T, Kahn D, Kahn ML, Kalman S, Keating DH, Kiss E, Komp C, Lelaure V, Masuy D, Palm C, Peck MC, Pohl TM, Portetelle D, Purnelle B, Ramsperger U, Surzycki R, Thebault P, Vandenbol M, Vorholter FJ, Weidner S, Wells DH, Wong K, Yeh KC, Batut J. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668–672 [DOI] [PubMed] [Google Scholar]
- 13. Pinto AC, Melo-Barbosa HP, Miyoshi A, Silva A, Azevedo V. 2011. Application of RNA-seq to reveal the transcript profile in bacteria. Genet. Mol. Res. 10:1707–1718 [DOI] [PubMed] [Google Scholar]
- 14. Noinaj N, Guillier M, Barnard TJ, Buchanan SK. 2010. TonB-dependent transporters: regulation, structure, and function. Annu. Rev. Microbiol. 64:43–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Osborn RW, Desamblanx GW, Thevissen K, Goderis I, Torrekens S, Vanleuven F, Attenborough S, Rees SB, Broekaert WF. 1995. Isolation and characterization of plant defensins from seeds of Asteraceae, Fabaceae, Hippocastanaceae and Saxifragaceae. FEBS Lett. 368:257–262 [DOI] [PubMed] [Google Scholar]
- 16. Tally FP, Zeckel M, Wasilewski MM, Carini C, Berman CL, Drusano GL, Oleson FB., Jr 1999. Daptomycin: a novel agent for Gram-positive infections. Expert Opin. Invest. Drugs 8:1223–1238 [DOI] [PubMed] [Google Scholar]
- 17. Muthaiyan A, Silverman JA, Jayaswal RK, Wilkinson BJ. 2008. Transcriptional profiling reveals that daptomycin induces the Staphylococcus aureus cell wall stress stimulon and genes responsive to membrane depolarization. Antimicrob. Agents Chemother. 52:980–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Breukink E, de Kruijff B. 1999. The lantibiotic nisin, a special case or not? Biochim. Biophys. Acta 1462:223–234 [DOI] [PubMed] [Google Scholar]
- 19. Breukink E, Wiedemann I, van Kraaij C, Kuipers OP, Sahl H, de Kruijff B. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361–2364 [DOI] [PubMed] [Google Scholar]
- 20. Hasper HE, Kramer NE, Smith JL, Hillman JD, Zachariah C, Kuipers OP, de Kruijff B, Breukink E. 2006. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 313:1636–1637 [DOI] [PubMed] [Google Scholar]
- 21. Sorensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS, Borregaard N. 2001. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97:3951–3959 [DOI] [PubMed] [Google Scholar]
- 22. Majchrzykiewicz JA, Kuipers OP, Bijlsma JJ. 2010. Generic and specific adaptive responses of Streptococcus pneumoniae to challenge with three distinct antimicrobial peptides, bacitracin, LL-37, and nisin. Antimicrob. Agents Chemother. 54:440–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jeon JM, Lee HI, Donati AJ, So JS, Emerich DW, Chang WS. 2011. Whole-genome expression profiling of Bradyrhizobium japonicum in response to hydrogen peroxide. Mol. Plant Microbe Interact. 24:1472–1481 [DOI] [PubMed] [Google Scholar]
- 24. Haag AF, Baloban M, Sani M, Kerscher B, Pierre O, Farkas A, Longhi R, Boncompagni E, Herouart D, Dall'Angelo S, Kondorosi E, Zanda M, Mergaert P, Ferguson GP. 2011. Protection of Sinorhizobium against host cysteine-rich antimicrobial peptides is critical for symbiosis. PLoS Biol. 9:e1001169. 10.1371/journal.pbio.1001169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Becker A, Berges H, Krol E, Bruand C, Ruberg S, Capela D, Lauber E, Meilhoc E, Ampe F, de Bruijn FJ, Fourment J, Francez-Charlot A, Kahn D, Kuster H, Liebe C, Puhler A, Weidner S, Batut J. 2004. Global changes in gene expression in Sinorhizobium meliloti 1021 under microoxic and symbiotic conditions. Mol. Plant Microbe Interact. 17:292–303 [DOI] [PubMed] [Google Scholar]
- 26. Van de Velde W, Zehirov G, Szatmari A, Debreczeny M, Ishihara H, Kevei Z, Farkas A, Mikulass K, Nagy A, Tiricz H, Satiat-Jeunemaitre B, Alunni B, Bourge M, Kucho K, Abe M, Kereszt A, Maroti G, Uchiumi T, Kondorosi E, Mergaert P. 2010. Plant peptides govern terminal differentiation of bacteria in symbiosis. Science 327:1122–1126 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.