Abstract
The Gram-positive bacterium Clavibacter michiganensis subsp. michiganensis, causal agent of bacterial wilt and canker of tomato, is an economically devastating pathogen that inflicts considerable damage throughout all major tomato-producing regions. Annual outbreaks continue to occur in New York, where C. michiganensis subsp. michiganensis spreads via infected transplants, trellising stakes, tools, and/or soil. Globally, new outbreaks can be accompanied by the introduction of contaminated seed stock; however, the route of seed infection, especially the role of fruit lesions, remains undefined. In order to investigate the modes of seed infection, New York C. michiganensis subsp. michiganensis field strains were stably transformed with a gene encoding enhanced green fluorescent protein (eGFP). A constitutively eGFP-expressing virulent C. michiganensis subsp. michiganensis isolate, GCMM-22, was used to demonstrate that C. michiganensis subsp. michiganensis could not only access seeds systemically through the xylem but also externally through tomato fruit lesions, which harbored high intra- and intercellular populations. Active movement and expansion of bacteria into the fruit mesocarp and nearby xylem vessels followed, once the fruits began to ripen. These results highlight the ability of C. michiganensis subsp. michiganensis to invade tomato fruits and seeds through multiple entry routes.
INTRODUCTION
Seed-disseminated phytopathogens exemplify the adaptive nature of parasites by not only gaining access to seeds, surviving seed treatment processes, and colonizing emergent seedlings but also by attaining global distribution. Clavibacter michiganensis subsp. michiganensis, the causal agent of bacterial wilt and canker of tomato, continues to cause epidemics throughout all major tomato-producing regions, and thus ensuring healthy seed stock remains a top priority (1). C. michiganensis subsp. michiganensis was initially isolated from Michigan in 1909 and rapidly spread to nearby states, including New York (2). Unfortunately, annual outbreaks of C. michiganensis subsp. michiganensis continue to occur in New York, which has over 2,900 acres of fresh market tomato valued at 47.1 million dollars, representing the fourth most valuable vegetable in the state (3). Besides its impact on agriculture in the United States, C. michiganensis subsp. michiganensis is listed as a quarantine pest for Europe, Asia, the Caribbean, and Africa (1).
The worldwide dissemination of this Gram-positive bacterium is facilitated by contaminated seed stock, in which one infected seed in 10,000 is capable of initiating an epidemic (1, 2, 4). Currently, there are no resistant cultivars and few chemical controls are effective. Thus, sanitary practices are important for disease control (5, 6). C. michiganensis subsp. michiganensis survives in soil, on trellising stakes, greenhouse benches, and tools for months to years due to its ability to tolerate desiccation and cold temperatures (2, 4, 5). Small bacterial populations can rapidly increase during commercial transplant production by water splash and/or equipment (7). In addition, outbreaks frequently remain unnoticed for extended periods of time since latent infections and symptomless seedlings are relatively common (4, 5, 8).
The pathogenic nature of C. michiganensis subsp. michiganensis strain NCPPB382 is derived from its many putative serine proteases and cell-wall-degrading enzymes, which are encoded by two plasmids (pCM1 and pCM2) and a pathogenicity island located on the chromosome (9–11). C. michiganensis subsp. michiganensis enters the tomato epiphytically through natural openings and wounds or from infected seeds (1, 2, 7). Once inside a plant, the bacterium multiplies in the xylem vessels, forming extensive biofilm-like structures, which aid in pathogen colonization and movement (12). Systemic infection with high populations of >108 CFU/g, lead to the characteristic wilting, stem canker, and vascular discoloration (2, 13). In addition, the pathogen can ooze from cankers and hydathodes and, in combination with rain and wind, the pathogen spreads to distal leaves, fruits, and surrounding plants (2, 14). Bacteria present on the fruit surface can cause “bird's-eye” lesions that consist of small tan dots with white halos (2, 15).
To better understand the movement of C. michiganensis subsp. michiganensis in planta, strains have been previously transformed to express either the lux operon or the enhanced green fluorescent protein (eGFP) gene (12, 16). However, the initial eGFP transformation was transient and only remained stable for ∼1 month, whereas bioluminescent transformation inhibits the ability to track individual bacterial cells at low populations (12). In the present study, New York field strains were stably transformed with eGFP to better understand the natural routes of infection that could lead to contaminated tomato seeds.
Phytopathogens can access their host's seeds systemically through the vasculature or externally by penetrating the ovary wall or floral parts (17, 18). Since C. michiganensis subsp. michiganensis, is a systemic pathogen it is hypothesized that it accesses the developing seeds via the host vascular system (1). One of the earliest studies on bacterial canker looked at the systemic movement of the pathogen to the seeds; however, no detailed histopathological studies have clearly demonstrated this natural route of seed infection (2, 18), and the significance of bird's-eye lesions in fruit colonization remains unstudied. Our working hypothesis was that seed contamination by C. michiganensis subsp. michiganensis could occur through both systemic and external fruit inoculations. Our objectives here were to test the ability of C. michiganensis subsp. michiganensis to colonize developing tomato fruits and seeds either (i) systemically through the xylem and/or (ii) externally by entering the fruit through lesions on the exocarp.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The C. michiganensis subsp. michiganensis strains utilized in the present study were New York State field strains collected from multiple bacterial canker outbreaks (Table 1). The four individual field strains were chosen based on the highly aggressive nature observed on tomato. Depending on the assay, C. michiganensis subsp. michiganensis strains were incubated for 3 to 6 days at 27°C in Luria-Bertani (LB) (19), SB (20, 21), or D2ANX (22) media. When required, LB and SB media were supplemented with the antibiotic chloramphenicol (10 μg/liter; Fischer Scientific; Pittsburgh, PA).
Table 1.
C. michiganensis subsp. michiganensis strains used in this study
| Strain | County of origin | Yr collected | Voltage (kV/cm)a | Transformation efficiencyb | Source or reference |
|---|---|---|---|---|---|
| 04101-FS | Schoharie, NY | 2004 | 12.5 | 1 (2) | 25, 40 |
| 0690-FS | Rensselaer, NY | 2006 | 12.5 | 16 (18) | This study |
| 0767-FS | Oneida, NY | 2007 | 7.5 | 16 (27) | This study |
| 11015-FS | Albany, NY | 2011 | 12.5 | 3 (3) | This study |
Voltages shown are the electroporation settings used to transform field strains.
The optimized transformation efficiency is shown as transformants/μg of vector DNA. Numbers in parentheses indicate the total numbers of eGFP transformants collected.
Construction of the plasmid pKGT-GFP.
The entire coding region of egfp, with its upstream DNA fragment derived from the C. michiganensis subsp. michiganensis bacteriophage CMP1, was amplified with the primers GFP-SpeI-fow (TTGAACCACTAGTCAGTACTG) and GFP-SpeI-rev (ACGGGCACTAGTAGTGAG) using pK2-22 (the plasmid expressing eGFP) as a template (12). The generated 1,305-bp PCR product was cloned into pGEM-T Easy vector (Promega Corp., Madison, WI) to yield pGeStbGFP (see Fig. S1 in the supplemental material). The artificially created speI restriction sites included in the PCR primers were used to digest total plasmid DNA and generate a 1,294-bp speI DNA fragment. The latter was cloned into the unique speI restriction site of the Tn1409 transposon vector pKGT452Cβ (20) to yield pKGT-GFP (see Fig. S1 in the supplemental material). In this plasmid, speI is located downstream of the IRL (left inverted repeat) and 103 bp upstream of the chloramphenicol exporter gene, cmx. The pKGT-GFP was transferred into Escherichia coli JM109, and recombinant colonies with the green fluorescence phenotype were selected from LB agar plates containing chloramphenicol (10 μg/ml). Orientation of the eGFP insertion was verified with the PCR primers GFP-SpeI-fow and pKGT-Cmx-rev (AACACGAGAAGGCAAACT).
Transformation and isolation of eGFP-labeled C. michiganensis subsp. michiganensis.
DNA of pKGT-GFP was extracted from E. coli JM109 with an E.Z.N.A Fastfilter plasmid midi kit (Omega Bio-Tek, Norcross, GA) and subsequently electroporated into C. michiganensis subsp. michiganensis field strains. Competent cells were prepared as previously described by Stork et al. (21). Electroporation was performed with 50 μl of competent cells and 1 μg of the purified eGFP vector using the Bio-Rad Gene Pulser Xcell electroporation system (Hercules, CA) with the following settings: 0.2-cm electroporation cuvettes, 7.5 to 12.5 kV/cm (Table 1), 25 μF, and 600 Ω with a time constant between 12 and 16 ms. An increased transformation rate was observed when two pulses were applied with a 20-s interval between pulses (23). Cells were immediately mixed into SB medium and regenerated for 2 h at 27°C. Cells were spread onto SB agar plates containing chloramphenicol and incubated for 5 to 7 days (12).
Fifty eGFP-labeled mutants, derived from the four different field strains, were isolated and grown for 48 h in LB broth amended with chloramphenicol at 27°C. Triplicates of 100 μl of culture (optical density at 600 nm = 0.5) were screened for fluorescence emission using a 96-well black microtiter plate (Fisher Scientific, Pittsburgh, PA) and the Synergy 2 BioTek microplate reader (Winooski, VT) (12). Fluorescence units (FU) were normalized by subtracting the background florescence from control wells of wild-type C. michiganensis subsp. michiganensis grown in LB broth at the same concentration. eGFP-labeled mutants with >7,000 FU, at a sensitivity level set to 70, were further analyzed. The experiment was repeated three times for a total of nine fluorescence emission readings.
Pathogenicity assays.
Tomato seedlings (Lycopersicon esculentum), cultivar Mountain Fresh Plus, were grown in a Cornell potting mix (composed of peat, perlite, and vermiculite in a 4:1:1 ratio) with a 14-h light/8-h dark photoperiod in the greenhouse. The eGFP-C. michiganensis subsp. michiganensis isolates and parental field strains were grown for 48 to 72 h in LB medium (10 μg of chloramphenicol/liter was added to eGFP-labeled isolates) at 27°C, diluted to 108 CFU/ml, and inoculated onto ∼2-week-old tomato seedlings (n = 6/isolate) by the cotyledon clipping method (16). The wild-type field stains and sterile water were used as positive and negative controls, respectively. After the first week, tomato plants were screened daily for characteristic wilting and chlorosis. Ratings continued until all plants died or until 24 dpi. Disease incidence was used to compare the eGFP-labeled isolates to their respective field strains, in order to determine whether the isolates remained virulent following transformation. Five tomato plants comprised a replicate, and each treatment was replicated three times. The mean area under the disease progress curve (AUDPC) was calculated from disease incidence (24).
Approximately 10 days after inoculation, one of the six tomato seedlings from each treatment was harvested to quantify in planta bacterial populations. A 1-cm stem section above the inoculation site was aseptically removed from the harvested plants and homogenized in 400 μl of sterile 10 mM MgCl2 using a Retsch MM400 Tissuelyser (Newton, PA). After complete homogenization, 600 μl of sterile 10 mM MgCl2 was added for a final volume of 1 ml (25). The solution was spun down at 400 × g for 3 min (16). Serial dilutions were performed with the supernatant and plated onto either LB medium with chloramphenicol or D2ANX (for wild-type field strains and negative controls) and incubated for 4 to 6 days at 27°C. The C. michiganensis subsp. michiganensis populations (CFU/g of tissue) were calculated as follows: the weight of the sample × the volume plated × the number of colonies × the dilution factor. Significant differences among treatments for AUDPC and in planta populations were tested using analysis of variance (ANOVA; P < 0.05), followed by Dunnett and Tukey's studentized range post tests (P = 0.05) using SAS v4.3 (SAS Institute, Cary, NC). The experiment was repeated three times.
External inoculation of fruits.
Twenty Mountain Fresh Plus tomatoes were grown as described above and transplanted into one gallon pots at 3 weeks of age so the plants would mature and flower. The flowers were artificially pollinated with a hand-held vibrating tomato pollinator (model 5E846; Hydro-Gardens Worldwide, Inc., Colorado Springs, CO) multiple times per week. The eGFP-labeled isolate, GCMM-22, was prepared by growing the isolate at 27°C with shaking at 140 rpm for 48 to 72 h in liquid LB medium with chloramphenicol. The bacterial suspension was diluted to a density of 108 cells/ml and applied to the entire surface of immature green fruit 8 to 12 mm in diameter (n = 50) using a no. 2 horse-hair paintbrush as previously described (15). A similar number of tomato fruits (n = 41) were brushed with sterile-distilled water as a negative control. Twenty-one C. michiganensis subsp. michiganensis-inoculated fruits were harvested and aseptically dissected at the green, breaker, and turning stages of fruit development in agreement with the United States Standards for Fresh Tomatoes Color Classification (26). Within those 21 fruits, a total of 182 individual lesions were analyzed using confocal microscopy.
The fruits were collected and either dissected and analyzed immediately or stored at 4°C for no more than a week prior to dissection. The fruits were laterally sectioned first, and then transverse and lateral sections were made of the two halves. Individual lesions and pericarp tissues were targeted for analysis. All sections were hand sliced with sterile double-edged razors and visualized using an Olympus BX61 microscope connected to a confocal laser scanning microscope system (FluoView FV-300; Olympus, Melville, NY). An argon laser (488-nm excitation) and a green helium neon laser (543-nm excitation) was used to excite the eGFP-bacteria and induce plant autofluorescence, respectively (27). A total of 10 lesions from five fruits were excised and plated on antibiotic selective media to confirm that the C. michiganensis subsp. michiganensis cells observed during microscopy were viable (15). Negative control tomatoes were also harvested and analyzed.
Systemic inoculation of fruits.
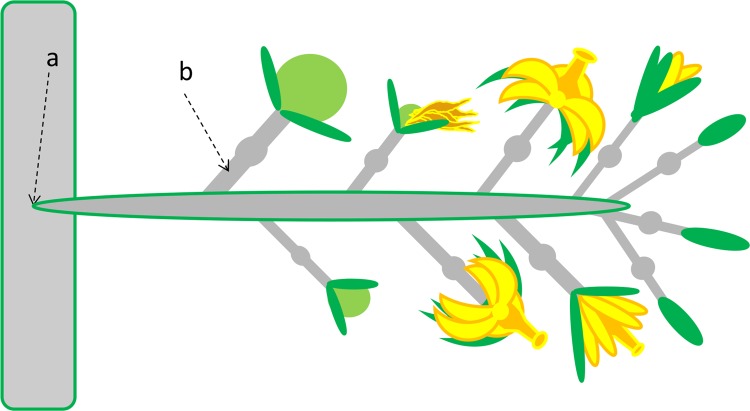
Flowering tomato plants and eGFP-labeled isolate GCMM-22 were both prepared as described above. For this experiment, 25 μl of bacterial suspension (108 cells/ml) was injected (using a syringe and a 26.5G needle) into the peduncle truss of 20 inflorescences (Fig. 1) (11). This was also performed with the negative control plants (n = 12), except sterile water was injected. In order to see the effects of inoculation at different stages of fruit development, inflorescences with floral units at various developmental stages (i.e., bud, flower, dead flower/emerging fruit, <1-cm fruit, or ≥1-cm fruit stage) were inoculated (Fig. 1). A total of 49 fruits, corresponding to 20 inoculated inflorescences, were harvested at various time points (days postinoculation [dpi]). Fruits were analyzed as described above, but instead of lesions, tissue slices at various distances below the calyx were screened for eGFP-C. michiganensis subsp. michiganensis. To confirm the C. michiganensis subsp. michiganensis that was observed during microscopy was viable, several 0.5-cm pedicel sections and colonized fruit vascular bundles were excised and plated onto antibiotic selective media (15). Negative control tomatoes were also harvested and analyzed.
Fig 1.
Schematic of systemic inoculation points. The inflorescence was injected with Clavibacter michiganensis subsp. michiganensis at either the stem/peduncle junction (peduncle truss) (a) or the pedicel (b). Several stages of fruit development were commonly present when inoculated: bud, flower, dead flower/emerging fruit, <1-cm fruit, or ≥1-cm fruit.
Individual pedicels on several inflorescences were also inoculated in a similar manner to the peduncle truss injections. Floral units (n = 7), at different stages of development, were selected to see the effects of infection in respect to flower/fruit stages (Fig. 1). Negative controls (n = 3) were also performed.
RESULTS
Transformation and characterization of eGFP-labeled C. michiganensis subsp. michiganensis isolates.
Transformation efficiency and electroporation voltages varied significantly among field strains (denoted by “-FS”); 11015-FS and 04101-FS were very difficult to transform, and only 3 and 2 eGFP-labeled isolates were obtained, respectively. However, a total of 27 and 18 eGFP-labeled isolates were obtained from the field strains 0767-FS and 0690-FS, respectively (Table 1). For the four wild-type field strains, a total of 50 eGFP-labeled isolates were collected and analyzed for fluorescence intensity. The average emitted fluorescence readings ranged from 58 to 17,323 FU, but only the most fluorescent eGFP-labeled isolates from each of the four field strains were further analyzed (Table 2). The two 04101-FS eGFP-labeled isolates both had fluorescence readings of ∼6,760 FU but were not included in the group selected for further analysis because of significantly reduced in vitro growth rates.
Table 2.
Virulence to tomato plants by eGFP isolates and parental C. michiganensis subsp. michiganensis strains
| Isolatea | FUb | Disease incidencec | AUDPCd | CFU/g in plantae |
|---|---|---|---|---|
| 11015-FS | 14/15 | 1,396.7A | 1.08 × 1010 | |
| GCMM-28 | 7,059 | 13/15 | 1,216.7ABC | 1.75 × 1010 |
| 0690-FS | 14/15 | 1,263.3AB | 1.16 × 1010 | |
| GCMM-22 | 8,252 | 15/15 | 1,340.0AB | 6.36 × 109 |
| GCMM-14 | 9,585 | 10/15 | 966.7BC | 1.02 × 1010 |
| GCMM-12 | 17,323 | 1/15 | 16.7D | 6.71 × 109 |
| GCMM-13 | 10,446 | 3/15 | 50.0D | 7.10 × 109 |
| GCMM-24 | 10,192 | 11/15 | 860.0C | 1.19 × 109 |
| 0767-FS | 14/15 | 1,223.3ABC | 1.38 × 1010 | |
| GCMM-26 | 14,535 | 15/15 | 1,543.3A | 2.31 × 1010 |
| GCMM-44 | 8,426 | 14/15 | 1,326.7AB | 2.33 × 1010 |
| GCMM-27 | 11,401 | 6/15 | 126.7D | 6.37 × 109 |
| GCMM-37 | 9,916 | 9/15 | 320.0D | 1.12 × 1010 |
| Water | 0/15 | 0.0D | 0 |
Wild-type field strains (-FS), along with their corresponding eGFP isolates (GCMM), are listed.
FU, fluorescence units. The mean normalized eGFP fluorescent reading is shown.
Expressed as the number of wilting plants/number of inoculated plants characterized at 17 to 24 dpi.
The mean area under the disease progress curve (AUDPC) for disease incidence is given. Significant differences among treatments were tested by ANOVA (P < 0.05), followed by Dunnett and Tukey's studentized range post tests (P = 0.05). AUDPC values followed by the same superscript letter are not significantly different.
Mean in planta population sizes recovered from 1 cm of stem tissue. No significant differences within field strains were observed when tested by ANOVA (P < 0.05).
The 10 selected eGFP-labeled isolates colonized the tomato stem at similar levels, 1 cm above the inoculation site, with CFU/g of tissue ranging from 109 to 1010. Within strains, the differences in the levels of colonization were not significantly different based on ANOVA (Table 2). In contrast to colonization, disease incidence varied among isolates. The mean AUDPC values for five of the ten eGFP-labeled isolates were significantly less than for the wild-type field strains used in the transformation, while the other five were similar to the wild-type field strains (Table 2). With four of the eGFP-labeled isolates, fewer than 10 of the 15 inoculated tomato plants wilted within 24 dpi (Table 2). Based upon these findings, eGFP-labeled isolate GCMM-22 was selected for fruit inoculation assays.
Fruit internalization of externally applied eGFP-labeled C. michiganensis subsp. michiganensis.
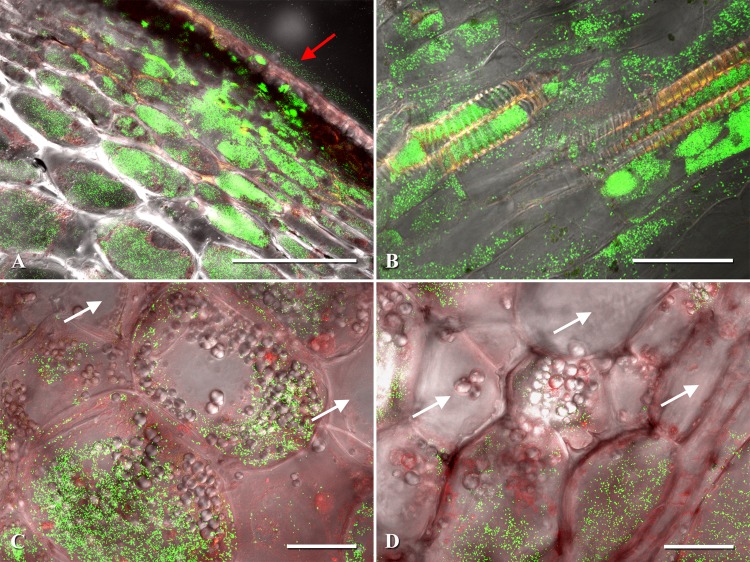
The tomato fruit pericarp is divided into three layers comprised of the exocarp, mesocarp (location of vascular bundles), and endocarp. After the external application of GCMM-22, lesions appeared over the entire fruit epidermis of all 50 inoculated fruits in as few as 3 days and did not increase appreciably in diameter after initial formation (Fig. 2A). Tomatoes at the green stage ranged in size from 2.7 to 5.8 cm in width, corresponding to 7 and 15 dpi, respectively (Table 3). All lesions viewed had heavy intra- and intercellular colonization of the exocarp and adjacent mesocarp cells (Fig. 3). Interestingly, C. michiganensis subsp. michiganensis appeared to remain constrained to these upper pericarp cell types throughout the green stage of fruit development (Table 3). However, as the fruits began to ripen, C. michiganensis subsp. michiganensis could be seen radiating outwards from the lesions in both lateral and basipetal directions at distances >18 to 25 parenchyma cells and even penetrating xylem vessels (Fig. 3B), thereby leading to the entry of C. michiganensis subsp. michiganensis into the vascular system.
Fig 2.
C. michiganensis subsp. michiganensis fruit colonization with different inoculation methods. (A to D) External inoculation. (A) Fruit lesions on the tomato exocarp; (B and C) heavy bacterial colonization of pericarp vascular bundles; (D) confocal image of aforementioned vascular bundle with eGFP-labeled C. michiganensis subsp. michiganensis colonization. (E to H) Systemic inoculation. (E) Fruit with discolored pedicel vasculature; (F and G) heavy bacterial colonization of pericarp vascular bundles; (H) vascular bundles colonized by eGFP-labeled C. michiganensis subsp. michiganensis. (I to L) Water inoculation. (I) Negative control fruit with no fruit lesions or pedicel vasculature discoloration; (J to L) negative control pericarp vascular bundles. All confocal microscopy images (D, H, and L) were generated by merging three channels (488 nm, 543 nm, and transmitted light). Red arrows indicate heavy vascular colonization by C. michiganensis subsp. michiganensis. White arrows indicate noncolonized vascular bundles. Scale bars: 1 mm in panels C, G, and K; 200 μm in panels D and H; 100 μm in panel L.
Table 3.
C. michiganensis subsp. michiganensis movement and colonization of tomato fruit tissue via external fruit inoculationa
| Tomato stage | Time (dpi) | No. of lesionsb | No. of lesions with C. michiganensis subsp. michiganensis colonizationc |
No. of fruit | No. of fruit with C. michiganensis subsp. michiganensis colonizationd |
|||
|---|---|---|---|---|---|---|---|---|
| Exocarp | Upper mesocarp | Endocarp | Xylem | Seede | ||||
| Green | 5–15 | 57 | 57 | 57 | 7 | 0 | 0 | 0 |
| Breaker | 31–34 | 56 | 56 | 56 | 7 | 3 | 2 | 1 |
| Turning | 32–41 | 69 | 69 | 69 | 7 | 3 | 3 | NA |
| Total | 182 | 182 | 182 | 21 | 6 | 5 | 1 | |
For the colonization analyses, each fruit was separated into five different tissue types: exocarp, mesocarp, xylem (located within mesocarp), endocarp, and seed.
Lesions were selected from seven fruit at each ripening stage and subsequently assessed for colonization with confocal microscopy.
Out of the total number of lesions assessed with confocal microscopy, the numbers noted represent how many lesions were colonized with C. michiganensis subsp. michiganensis in either the exocarp or upper mesocarp cells of the pericarp.
The number of fruit colonized by C. michiganensis subsp. michiganensis in the endocarp, xylem, or seed was assessed.
The number of fruit with visible seed colonization is given. NA, not applicable (no seeds were analyzed and viewed with the confocal at this late ripening stage due to the inability to effectively slice the seed in the gel matrix of the locular cavity).
Fig 3.
Examples of Clavibacter michiganensis subsp. michiganensis-infected pericarp cells of tomato. (A) Pericarp lesion expansion with eGFP-labeled C. michiganensis subsp. michiganensis colonizing the exocarp and mesocarp. (B) Fruit xylem and xylem parenchyma colonization by C. michiganensis subsp. michiganensis. (C) Intracellular colonization of mesocarp cells by C. michiganensis subsp. michiganensis. (D) Intracellular colonization of the endocarp and surrounding parenchyma cells. Confocal microscopy images were generated by merging three channels (488 nm, 543 nm, and transmitted light). The red arrow points to the lesion on the fruit surface. White arrows indicate noncolonized fruit cells. Scale bars: 200 μm in panel A; 50 μm in panels B, C, and D.
Entire locular cavities became colonized, and damaged vascular bundles could be visualized with the naked eye (Fig. 2A and B). It appeared that C. michiganensis subsp. michiganensis would unilaterally colonize fruits, as only individual locular cavities maintained heavily colonized xylem vessels. Fruits with heavy intravascular growth frequently supported C. michiganensis subsp. michiganensis populations in the pedicel. Further in planta spread was not explored. No pedicels were colonized from fruits lacking C. michiganensis subsp. michiganensis in fruit xylem vessels, regardless of ripening stage. In total, five fruits (two at the breaker and three at the turning stage) had heavily colonized xylem vessels and vascular bundles (Table 3, Fig. 2). All cultured lesions and vascular bundles had active bacterial growth. Negative control plants and fruits had no bacterial wilt symptoms, and no C. michiganensis subsp. michiganensis cells were observed during confocal microscopy.
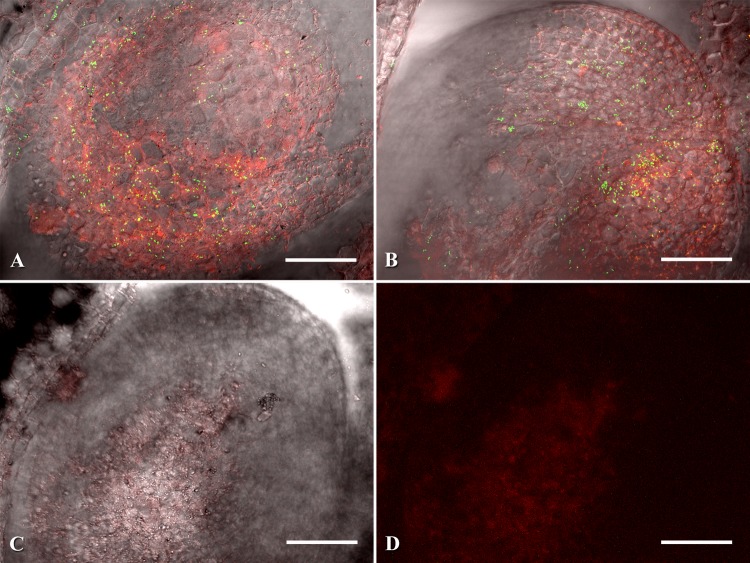
The entry of C. michiganensis subsp. michiganensis into the fruit xylem, after external inoculation, allowed for the eventual colonization of seeds (Table 3). Vascular discoloration leading to several developing seeds was observed in a fruit at the breaker stage and upon confocal analysis, C. michiganensis subsp. michiganensis was observed within three seeds of that fruit (Fig. 4). Small numbers of bacterial cells were observed colonizing the seeds near the xylem vessel attachment sites and endosperm (Fig. 4D).
Fig 4.
External inoculation of tomato fruits with subsequent seed colonization. (A and B) Vascular discoloration leading up to several discolored tomato seeds. (C) Thin section of the discolored seed shown in panel B with xylem leading into the seed from the funiculus. (D) Magnified view of seed in panel C with eGFP-labeled Clavibacter michiganensis subsp. michiganensis colonizing the cells surrounding the xylem vasculature. Section of seed corresponding to the same anatomical structure is labeled with a red “X”. White arrows are pointing to several C. michiganensis subsp. michiganensis. Red arrows point to discolored tomato seeds and vasculature. All confocal microscopy images (C and D) were generated by merging three channels (488 nm, 543 nm, and transmitted light). Scale bars: 1 mm in panel B; 200 μm in panel C; 50 μm in panel D.
Movement of eGFP-labeled C. michiganensis subsp. michiganensis inside fruit vasculature from a peduncle truss systemic inoculation.
Based on the sequential development of individual floral units on inflorescences, multiple stages of flower/fruit development could be observed on a single inflorescence (Fig. 1). Therefore, individual floral units were utilized to observe differences in fruit and/or seed infection rates based on the inoculation date. Development of extensive wilting and/or discoloration was not evident on any of the inoculated inflorescences; however, cankers could be seen developing at the site of inoculation, approximately 2 to 3 weeks postinoculation. No premature fruit abscission or external lesions occurred and infected fruit appeared similar (externally) to the negative controls with similar growth and ripening rates.
To assess the movement of C. michiganensis subsp. michiganensis within an inflorescence, fruits were collected at various time points (dpi) and processed at various distances below the calyx (Table 4, Fig. 2E to H). Not all fruits were infected after inoculation at the peduncle truss, because C. michiganensis subsp. michiganensis did not effectively colonize the peduncle of 6 of the 20 inoculated inflorescences. However, C. michiganensis subsp. michiganensis was consistently present ≤0.5 cm below the calyx, within the xylem and surrounding xylem parenchyma, in all 25 fruits examined from the 14 inflorescences that were colonized (Table 4). In one fruit, vascular bundles at the distal end near the columella, ∼5 cm below the calyx, became heavily concentrated with C. michiganensis subsp. michiganensis (Table 4, Fig. 2F to H). Negative control fruits had no vascular discolorations, abnormalities, or C. michiganensis subsp. michiganensis cells.
Table 4.
C. michiganensis subsp. michiganensis movement and colonization of tomato fruit tissue via systemic inoculation at the peduncle truss
| Time (dpi)a | No. of fruit | No. infected fruit | Infection rate (%) | No. of fruit with C. michiganensis subsp. michiganensis colonizationb |
|||
|---|---|---|---|---|---|---|---|
| ≤0.5 cm | 0.6–1.5 cm | Base of fruitc | Seedd | ||||
| 21–26 | 17 | 9 | 53 | 9 | 7 | 0 | 0 |
| 27–39 | 12 | 7 | 58 | 7 | 4 | 0 | 1 |
| 40–48 | 20 | 9 | 45 | 9 | 5 | 1 | NA |
| Total | 49 | 25 | 51 | 25 | 16 | 1 | 1 |
Days postinoculation (dpi) when harvested.
Of the infected fruit, the number in which C. michiganensis subsp. michiganensis was observed at multiple distances below the calyx or in the seed was assessed.
Vascular bundles colonized by C. michiganensis subsp. michiganensis at the base of the fruit represent the maximum distance bacteria travelled from the calyx.
This refers to C. michiganensis subsp. michiganensis seen surrounding, internalized, or colonizing the embryo vasculature. NA, not applicable (no seeds were analyzed and viewed with the confocal at this late fruit stage due to the inability to effectively slice the seed in the gel matrix of the locular cavity).
Colonization by the pathogen did cause extensive seed discoloration and darkening in some of the more mature fruits. A fruit that was harvested at 27 dpi had heavy xylem colonization, and C. michiganensis subsp. michiganensis could be seen colonizing the xylem vessels of the funiculus, directly leading to the developing seed (Fig. 5). Unfortunately, the late-ripening stages inhibited an extensive microscopic analysis of the collected seeds due to the inability to effectively dissect the seeds in the highly autofluorescent gel matrix of the locular cavities.
Fig 5.

Systemic inoculation of peduncle truss resulting in colonization of xylem vessels within the funiculus of a developing tomato seed. (A) Differential-interference-contrast image of xylem vessels leading into the developing seed. (B) Magnification of panel A showing xylem with eGFP-labeled Clavibacter michiganensis subsp. michiganensis colonization. The confocal image was acquired by merging three channels (488 nm, 543 nm, and transmitted light). Scale bars: 50 μm in panel A; 20 μm in panel B.
Pedicel systemic inoculation.
In addition to peduncle truss inoculations, the pedicels of individual floral units were also inoculated (Fig. 1) to more closely follow fruit infection by C. michiganensis subsp. michiganensis. Fruits harvested at 14 dpi appeared visually healthy, but extensive xylem colonization was observed (Table 5). Unlike peduncle inoculations, pedicel inoculations caused discoloration and premature abscission of the remaining vine-attached fruits within 4 to 5 weeks. Of the five green fruits that could be studied, one had been inoculated at the flower stage, and two each at the emerging fruit and ≥1-cm fruit stages. Large numbers of C. michiganensis subsp. michiganensis were seen throughout the pericarp xylem of all inoculated fruits, and at high numbers on multiple seeds of one fruit (Fig. 6). It appeared that C. michiganensis subsp. michiganensis colonized both the intra- and intercellular regions of the developing seeds. Negative control fruits had no discoloration or colonization by C. michiganensis subsp. michiganensis.
Table 5.
C. michiganensis subsp. michiganensis movement and colonization of tomato fruit tissue via systemic inoculation at the pedicel
| Inoculation stagea | No. of fruit | No. of infected fruit | Time (dpib) | No. of fruit with C. michiganensis subsp. michiganensis colonization |
||
|---|---|---|---|---|---|---|
| Xylem | Base of fruitc | Seedd | ||||
| Flower | 1 | 1 | 14 | 1 | 1 | 1 |
| Emerging fruit | 2 | 2 | 14 | 2 | 1 | 0 |
| ≥1-cm fruit | 2 | 2 | 14 | 2 | 0 | 0 |
| Total | 5 | 5 | 5 | 2 | 1 | |
Growth stage at which the pedicels were inoculated.
Days postinoculation (dpi) when harvested.
Vascular bundles colonized by C. michiganensis subsp. michiganensis at the base of the fruit represent the maximum distance bacteria traveled from the calyx.
The numbers of fruit with visible seed colonization are shown.
Fig 6.
Systemic inoculation of pedicel with subsequent tomato seed colonization. (A and B) eGFP-labeled Clavibacter michiganensis subsp. michiganensis colonization of developing seeds. (C) Negative control seed at same developmental stage. (D) Same image as panel C, but only two channels are shown (488 and 543 nm). Microscopy images (A to C) were generated by merging three channels (488 nm, 543 nm, and transmitted light). Scale bars: 50 μm.
DISCUSSION
Stable transformation of C. michiganensis subsp. michiganensis with constitutive eGFP expression permitted detailed in situ visualization of the entry routes used to colonize tomato fruits and seeds. Transformation efficiency was not equal among the four field strains, but differences in exopolysaccharide composition and electrocompetence may be partially responsible (28). Among the 10 eGFP-labeled isolates studied, differences in fluorescence and virulence were observed; however, all reached similar in planta populations at 1 cm above the inoculation point. In addition, two isolates were numerically (although not statistically) more aggressive than their wild-type field strain. The reason for these differences is unknown, but may due to the site of transposon integration.
Bird's-eye lesions were originally believed to be superficial blemishes, in a similar manner to bacterial speck or spot of tomato (2); however, eGFP-labeled C. michiganensis subsp. michiganensis actively grew within lesions on the exocarp of tomatoes and subsequently spread into the fruits when externally inoculated. Confinement of bacteria to the inter- and intracellular regions of the lesions during the earlier stages of fruit development was quickly lost once the fruit began to ripen (breaker stage), suggesting that the weakened cellular components facilitated bacterial spread into the thin-walled mesocarp cells and xylem of the pericarp. Once in the xylem, the pathogen spread through the fruit and entered the plant through the pedicel. C. michiganensis subsp. michiganensis is known to possess an arsenal of extracellular cell-wall-degrading enzymes, which could become more effective in combination with natural fruit softening processes (9, 10, 29). In one case, an externally inoculated fruit had mottling symptoms on an individual locular cavity, which appeared as a subepidermal “lesion” and was beginning to liquefy beneath the intact epidermis. This mottling symptom had been previously observed only in systematically infected greenhouse tomato fruits (30). These observations suggest that the external inoculation of a tomato fruit with C. michiganensis subsp. michiganensis can yield both (i) external bird's-eye lesions that ingress into the fruit pericarp and eventually to the seeds and (ii) systemically produced subepidermal “lesions” that egress from the inner fruit vasculature, in a similar manner to Xanthomonas campestris pv. pruni (30, 31).
The exact method of entry into the fruit from external sources is still unclear. Tomato fruits do not possess stomata, and wounds are not necessary for lesion development (15). Therefore, the pathogen is thought to access the fruit via glandular and nonglandular trichomes that cover developing green fruit. As the fruit begins to grow, it sheds trichomes, which exposes open trichome bases and provides points of entry, as has been shown for Pseudomonas syringae pv. tomato (2, 32). To our knowledge, the brush inoculation method does not produce wounds (15), but unidentified wounds could have been present on the fruit exocarp. However, the consistency of fruit xylem colonization, in addition to the movement of C. michiganensis subsp. michiganensis into the fruit mesocarp, suggests active pericarp infiltration. Lesion-to-vascular ingression most likely occurred near the distal end of the fruit, where vascular elements were noticeably closer to the epidermis. Fruit pericarp penetration has also been observed with Xanthomonas campestris pv. pruni (bacterial spot of Prunus) and Pseudomonas syringae pv. lachrymans (angular leaf spot of cucurbits), which actively penetrate several millimeters into fruit mesocarp (31, 33).
The systemic invasion of tomato fruits by C. michiganensis subsp. michiganensis has been previously observed (2, 30). However, no detailed histopathological study had been performed on the routes of seed infection (18). To see the impact of different stages of fruit development on fruit and seed infection by C. michiganensis subsp. michiganensis, floral units were systemically inoculated at various stages of growth. The highest fruit infection rate, via systemic inoculation, occurred during the dead flower/emerging fruit stage with a 78% infection rate; however, seed infections were more common when the floral units were between the bud and pre-anthesis flower stages. In peduncle truss inoculations, seed internalization was not directly visualized, but C. michiganensis subsp. michiganensis could be observed within the funiculus. Similarly, Xanthomonas campestris pv. campestris was rarely seen within the seeds of systemically inoculated cabbage, with most cases being confined to the funiculus, which dries and adheres to the seed coat (34). Conversely, pedicel inoculations led to intra- and intercellular colonization of the developing seeds. The ability of C. michiganensis subsp. michiganensis to access the xylem, funiculus, and seed, in combination with no external fruit or plant symptoms (as in the peduncle truss inoculations), highlights the difficulty in identifying diseased plants with potentially contaminated seeds.
The intracellular colonization of pericarp cells was consistently observed in fruits from both external and systemic inoculations. Intracellular colonization by phytobacteria is rarely observed, but it has been documented with Pseudomonas syringae pv. lachrymans and Xanthomonas campestris pv. cajani during seed infections (33, 35), as well as with Gram-positive phytobacteria such as Streptomyces ipomoes and S. turgidiscabies (36–38). In previous studies, C. michiganensis subsp. michiganensis had been primarily observed within the intercellular spaces of tomato leaves and stems, with intracellular populations occurring later in disease development (2, 7, 39), and yet intact fruit cells appeared to be colonized in the present study (Fig. 3).
Irrespective of the inoculation method (systemic or external), C. michiganensis subsp. michiganensis was observed within and around multiple developing tomato seeds. C. michiganensis subsp. michiganensis cells were located within the developing seed, endosperm, and funiculus, although at relatively low levels compared to the large numbers of cells observed in xylem and pericarp cells. These results highlight the difficulty in detecting and eradicating small initial pathogen populations within seed lots. However, as populations reach exponential growth either during transplant production or in the field, rapid spread will occur and visual symptoms will appear (5). The data presented here demonstrate that C. michiganensis subsp. michiganensis can actively infect tomato seeds both systemically through the xylem and externally via fruit lesions. However, complete comprehension of fruit pathogenesis by C. michiganensis subsp. michiganensis is still in its infancy since avirulent strains (negative in hypersensitive response assays) have been shown to produce external fruit lesions (15), and C. michiganensis subsp. michiganensis appears to be actively moving within the fruit pericarp, even though its purported to be nonmotile (2, 15). These and other questions will require further experimentation.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by grant IS-4403-11C from the United States-Israel Binational Agricultural Research and Development Fund. Support for M.A.T. was also provided by a fellowship from Cornell University College of Agriculture and Life Sciences and the National Science Foundation-Graduate Research Fellowships Program (grant DGE-1144153).
We thank Kent Loeffler for image organization and Lisa Jones, Holly Lange, and Amara Dunn for technical assistance.
Footnotes
Published ahead of print 6 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02495-13.
REFERENCES
- 1.De León L, Siverio F, López MM, Rodríguez A. 2011. Clavibacter michiganensis subsp. michiganensis, a seedborne tomato pathogen: healthy seeds are still the goal. Plant Dis. 95:1328–1338 [DOI] [PubMed] [Google Scholar]
- 2.Bryan MK. 1930. Studies on bacterial canker of tomato. J. Agric. Res. 41:825–851 [Google Scholar]
- 3.USDA 2013. Vegetables 2012: summary. US Department of Agriculture, Washington, DC [Google Scholar]
- 4.Chang R, Ries S, Pataky J. 1991. Dissemination of Clavibacter michiganensis subsp. michiganensis by practices used to produce tomato transplants. Phytopathology 81:1276–1281 [Google Scholar]
- 5.Werner N, Fulbright D, Podolsky R, Bell J, Hausbeck MK. 2002. Limiting populations and spread of Clavibacter michiganensis subsp. michiganensis on seedling tomatoes in the greenhouse. Plant Dis. 86:535–542 [DOI] [PubMed] [Google Scholar]
- 6.Sen Y, Feng Z, Vandenbroucke H, Van der Wolf J, Visser RGF, Van Heusden AW. 2013. Screening for new sources of resistance to Clavibacter michiganensis subsp. michiganensis (Cmm) in tomato. Euphytica 190:309–317 [Google Scholar]
- 7.Carlton WM, Braun EJ, Gleason ML. 1998. Ingress of Clavibacter michiganensis subsp. michiganensis into tomato leaves through hydathodes. Phytopathology 88:525–529 [DOI] [PubMed] [Google Scholar]
- 8.Gitaitis R, Beaver R, Voloudakis A. 1991. Detection of Clavibacter michiganensis subsp. michiganensis in symptomless tomato transplants. Plant Dis. 75:834–838 [Google Scholar]
- 9.Gartemann K-H, Abt B, Bekel T, Burger A, Engemann J, Flügel M, Gaigalat L, Goesmann A, Gräfen I, Kalinowski J, Kaup O, Kirchner O, Krause L, Linke B, McHardy A, Meyer F, Pohle S, Rückert C, Schneiker S, Zellermann E-M, Pühler A, Eichenlaub R, Kaiser O, Bartels D. 2008. The genome sequence of the tomato-pathogenic actinomycete Clavibacter michiganensis subsp. michiganensis NCPPB382 reveals a large island involved in pathogenicity. J. Bacteriol. 190:2138–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eichenlaub R, Gartemann K-H. 2011. The Clavibacter michiganensis subspecies: molecular investigation of gram-positive bacterial plant pathogens. Annu. Rev. Phytopathol. 49:445–464 [DOI] [PubMed] [Google Scholar]
- 11.Balaji V, Mayrose M, Sherf O, Jacob-Hirsch J, Eichenlaub R, Iraki N, Manulis-Sasson S, Rechavi G, Barash I, Sessa G. 2008. Tomato transcriptional changes in response to Clavibacter michiganensis subsp. michiganensis reveal a role for ethylene in disease development. Plant Physiol. 146:1797–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalupowicz L, Zellermann E-M, Fluegel M, Dror O, Eichenlaub R, Gartemann K-H, Savidor A, Sessa G, Iraki N, Barash I, Manulis-Sasson S. 2012. Colonization and movement of GFP-labeled Clavibacter michiganensis subsp. michiganensis during tomato infection. Phytopathology 102:23–31 [DOI] [PubMed] [Google Scholar]
- 13.Gartemann K-H, Kirchner O, Engemann J, Gräfen I, Eichenlaub R, Burger A. 2003. Clavibacter michiganensis subsp. michiganensis: first steps in the understanding of virulence of a Gram-positive phytopathogenic bacterium. J. Biotechnol. 106:179–191 [DOI] [PubMed] [Google Scholar]
- 14.Sharabani G, Manulis-Sasson S, Borenstein M, Shulhani R, Lofthouse M, Chalupowicz L, Shtienberg D. 2013. The significance of guttation in the secondary spread of Clavibacter michiganensis subsp. michiganensis in tomato greenhouses. Plant Pathol. 62:578–586 [Google Scholar]
- 15.Medina-Mora C, Hausbeck MK, Fulbright DW. 2001. Bird's eye lesions of tomato fruit produced by aerosol and direct application of Clavibacter michiganensis subsp. michiganensis. Plant Dis. 85:88–91 [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Miller SA, Baysal-Gurel F, Gartemann K-H, Eichenlaub R, Rajashekara G. 2010. Bioluminescence imaging of Clavibacter michiganensis subsp. michiganensis infection of tomato seeds and plants. Appl. Environ. Microbiol. 76:3978–3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal VK, Sinclair JB. 1997. Principles of seed pathology. CRC Press, Inc, New York, NY [Google Scholar]
- 18.Singh D, Mathur SB. 2004. Histopathology of seed-borne infections. CRC Press, Inc, New York, NY [Google Scholar]
- 19.Miller J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 20.Kirchner O, Gartemann KH, Zellermann EM, Eichenlaub R, Burger A. 2001. A highly efficient transposon mutagenesis system for the tomato pathogen Clavibacter michiganensis subsp. michiganensis. Mol. Plant-Microbe Interact. 14:1312–1318 [DOI] [PubMed] [Google Scholar]
- 21.Stork I, Gartemann K, Burger A, Eichenlaub R. 2008. A family of serine proteases of Clavibacter michiganensis subsp. michiganensis: chpC plays a role in colonization of the host plant tomato. Mol. Plant Pathol. 9:599–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadas R, Kritzman G, Klietman F, Gefen T, Manulis S. 2005. Comparison of extraction procedures and determination of the detection threshold for Clavibacter michiganensis ssp. michiganensis in tomato seeds. Plant Pathol. 54:643–649 [Google Scholar]
- 23.Laine MJ, Nakhei H, Dreier J, Lehtilä K, Meletzus D, Eichenlaub R, Metzler MC. 1996. Stable transformation of the gram-positive phytopathogenic bacterium Clavibacter michiganensis subsp. sepedonicus with several cloning vectors. Appl. Environ. Microbiol. 62:1500–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madden L, Hughes G, Van den Bosch F. 2007. The study of plant disease epidemics. American Phytopathological Society, St. Paul, MN [Google Scholar]
- 25.Balaji V, Smart CD. 2011. Overexpression of snakin-2 and extensin-like protein genes restricts pathogen invasiveness and enhances tolerance to Clavibacter michiganensis subsp. michiganensis in transgenic tomato (Solanum lycopersicum). Transgenic Res. 21:23–37 [DOI] [PubMed] [Google Scholar]
- 26.USDA 1991. United States standards for grades of fresh tomatoes. US Department of Agriculture, Washington, DC [Google Scholar]
- 27.Dunn AR, Fry BA, Lee TY, Conley KD, Balaji V, Fry WE, McLeod A, Smart CD. 2013. Transformation of Phytophthora capsici with genes for green and red fluorescent protein for use in visualizing plant-pathogen interactions. Australas. Plant Pathol. 42:583–593 [Google Scholar]
- 28.Yoshida T, Ayabe Y, Horinouchi M, Habe H, Nojiri H, Omori T. 2001. Improved conditions for the transformation by electroporation of the extracellular polysaccharide-producing methylotroph Methylobacillus sp. Biotechnol. Lett. 23:787–791 [Google Scholar]
- 29.Gross KC, Wallner SJ. 1979. Degradation of cell wall polysaccharides during tomato fruit ripening. Plant Physiol. 63:117–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Layne RE, Rainforth JR. 1966. A new symptom of bacterial canker resulting from systemic infection of tomato fruits and its implications in dissemination and seed transmission. Can. J. Plant Sci. 46:371–374 [Google Scholar]
- 31.Du Plessis HJ. 1990. Systemic invasion of plum seed and fruit by Xanthomonas campestris pv. pruni through stalks. J. Phytopathol. 130:37–45 [Google Scholar]
- 32.Getz S, Fulbright D, Stephens C. 1983. Scanning electron microscopy of infection sites and lesion development on tomato fruit infected with Pseudomonas syringae pv. tomato. Cytol. Histol. 73:39–43 [Google Scholar]
- 33.Wiles AB, Walker JC. 1951. The relation of Pseudomonas lachymans to cucumber fruits and seeds. Phytopathology 41:1059–1064 [Google Scholar]
- 34.Cook AA, Larson RH, Walker JC. 1952. Relation of the black rot pathogen to cabbage seed. Phytopathology 42:316–320 [Google Scholar]
- 35.Sharma M, Kumar D, Agarwal K, Singh T, Singh D. 2001. Colonization of pigeonpea seed by Xanthomonas campestris pv. cajani. J. Mycol. Plant Pathol. 31:216–219 [Google Scholar]
- 36.Joshi M, Rong X, Moll S, Kers J, Franco C, Loria R. 2007. Streptomyces turgidiscabies secretes a novel virulence protein, Nec1, which facilitates infection. Mol. Plant-Microbe Interact. 20:599–608 [DOI] [PubMed] [Google Scholar]
- 37.Clark C, Matthews S. 1987. Histopathology of sweet potato root infection by Streptomyces ipomoea. Phytopathology 77:1418–1423 [Google Scholar]
- 38.Hogenhout SA, Loria R. 2008. Virulence mechanisms of Gram-positive plant pathogenic bacteria. Curr. Opin. Plant Biol. 11:449–456 [DOI] [PubMed] [Google Scholar]
- 39.Wallis FM. 1977. Ultrastructural histopathology of tomato plants infected with Corynebacferium michiganense. Physiological Plant Pathol. 11:333–342 [Google Scholar]
- 40.Balaji V, Sessa G, Smart CD. 2011. Silencing of host basal defense response-related gene expression increases susceptibility of Nicotiana benthamiana to Clavibacter michiganensis subsp. michiganensis. Phytopathology 101:349–357 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.