Abstract
Human norovirus (huNoV) and hepatitis A virus (HAV) have been involved in several produce-associated outbreaks and identified as major food-borne viral etiologies. In this study, the survival of huNoV surrogates (murine norovirus [MNV] and Tulane virus [TV]) and HAV was investigated on alfalfa seeds during storage and postgermination. Alfalfa seeds were inoculated with MNV, TV, or HAV with titers of 6.46 ± 0.06 log PFU/g, 3.87 ± 0.38 log PFU/g, or 7.01 ± 0.07 log 50% tissue culture infectious doses (TCID50)/g, respectively. Inoculated seeds were stored for up to 50 days at 22°C and sampled during that storage period on days 0, 2, 5, 10, and 15. Following storage, virus presence was monitored over a 1-week germination period. Viruses remained infectious after 50 days, with titers of 1.61 ± 0.19 log PFU/g, 0.85 ± 0.21 log PFU/g, and 3.43 ± 0.21 log TCID50/g for MNV, TV, and HAV, respectively. HAV demonstrated greater persistence than MNV and TV, without a statistically significant reduction over 20 days (<1 log TCID50/g); however, relatively high levels of genomic copies of all viruses persisted over the testing time period. Low titers of viruses were found on sprouts and were located in all tissues as well as in sprout-spent water sampled on days 1, 3, and 6 following seed planting. Results revealed the persistence of viruses in seeds for a prolonged period of time, and perhaps of greater importance these data suggest the ease of which virus may transfer from seeds to sprouts and spent water during germination. These findings highlight the importance of sanitation and prevention procedures before and during germination.
INTRODUCTION
With the increasing consumption of sprouted seeds due to health benefits (1), sprouts have been found associated with at least 55 food-borne outbreaks occurring worldwide, resulting in a total of 15,233 illnesses (2). In 2011, the large outbreak in Europe associated with fenugreek seeds contaminated by Escherichia coli O104:H4 (3) renewed awareness for sprout and seed safety. Alfalfa sprouts historically have been a major player in food-borne outbreaks. According to the U.S. Food and Drug Administration (FDA), since 1990 there have been more than 30 reported outbreaks linked to the consumption of raw or lightly cooked alfalfa sprouts in North America, where E. coli O157:H7 and various serotypes of Salmonella were identified as the major bacterial etiologies (4). It is known that sprouts have the potential for bacterial pathogen growth during germination, which provides a warm, humid, and nutrient-abundant environment for sprouting. Recently, the FDA Food Safety Modernization Act (FSMA) Proposed Produce Safety Rule addressed the importance of sprout safety by requiring treatment immediately before sprouting to reduce microorganisms and specific bacterial monitoring, including testing of sprouts and spent irrigation waters.
Many research studies have been conducted in attempts to better understand the interaction of bacterial pathogens with seeds and sprouts (5–12). If the seeds were contaminated prior to germination, bacterial pathogens such as E. coli O157:H7, Vibrio cholerae O1, and Salmonella enterica serovar Typhi may grow and are more likely to be transferred to outer surfaces and inner tissues (5, 6). Many factors that affect bacterial attachment were identified, such as characteristics of surfaces, types of bacterial pathogens, and methods of disinfection. It was found that wrinkled/rough or damaged alfalfa seeds were likely to harbor more bacteria, and this bacterial contamination was also more resistant to sanitizers compared to that of smooth and healthy seeds (8, 13). Barak et al. (7, 10) found that different serovars of S. enterica and plant-associated bacteria attached to alfalfa sprouts significantly better than E. coli O157:H7 during rinsing steps, probably due to the presence of curli. Other factors affecting bacterial growth and survival on seeds were also identified, such as homogenization methods, rinsing methods, soaking times, temperature, use of surfactants, irrigation systems, and sprouting devices (11, 12). However, little knowledge is known about the risk and survival associated with the viruses on seeds and sprouts. It is likely that viruses may be present in these moist environments that have previously been found to harbor contamination with pathogenic bacteria; however, the lack of epidemiological evidence is likely due to the lack of testing of foods and fecal samples for norovirus or other food-borne viruses.
Viruses are a great concern for produce safety, as viruses may be introduced from the preharvest environment at the farm, at the sprouting facility, and during preparation via infected food handlers or cross-contamination in restaurant/food establishments (14–17). It was estimated that viruses cause over 5 million food-borne illnesses each year in the United States, and human norovirus (huNoV) and hepatitis A virus (HAV) are identified as the most common viral etiologies of food-borne illnesses (18, 19). The low infectious dose of both huNoV and HAV, with estimated averages of 10 to 100 virus particles, means that even a small amount of contamination has the potential to cause illness (20–22).
Currently, there is no cell culture available for huNoVs in the laboratory; therefore, surrogates like murine norovirus (MNV) are used to predict norovirus behavior in environmental persistence studies (23). MNV was the first norovirus to be propagated in cell culture and shares similar genetic and structural features with human norovirus (23). Tulane virus (TV), a newly discovered calicivirus, belongs to the genus Recovirus and is another potential surrogate (24, 25). TV has significant genetic diversity compared with MNV but is capable of binding histo-blood group antigens (HBGA), which indicates that it shares structure similarity with huNoVs (26). Therefore, it is interesting to compare the survival of these two huNoV surrogates in environmental settings.
In this study, the behaviors of MNV, TV, and HAV were investigated on intentionally contaminated alfalfa seeds during storage and on sprouts after a 7-day germination period. The degree of virus transfer to spent irrigation water was also investigated. Lastly, the distribution of viruses on contaminated sprouts was investigated. This study is important for determining the persistence of viruses on the seed surface and for evaluating the potential risk associated with sprouting and irrigation water after seed contamination.
MATERIALS AND METHODS
Virus cultivation and infectivity.
Murine norovirus (MNV-1) (a gift from Herbert Virgin, Washington University School of Medicine, St. Louis, MO) was cultured in RAW 264.7 cells (ATCC TIB-71) in Dulbecco's modified Eagles medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Mediatech, Manassas, VA), 100 U/ml penicillin-streptomycin-0.25 μg/ml amphotericin B (HyClone, Logan, UT), 2 mM l-alanine–l-glutamine (Gibco, Carlsbad, CA), and 1 mM sodium bicarbonate (Cellgro, Manassas, VA). Tulane virus (a gift from Xi Jiang, University of Cincinnati College of Medicine, Cincinnati, OH) was propagated in LLC-MK2 cells (ATCC CCL-7) in medium 199 (HyClone, Logan, UT) supplemented with 10% FBS and 100 U/ml penicillin G-streptomycin-0.25 μg/ml amphotericin B. After typically 48 h of infection of 80 to 90% confluent monolayers for both MNV and TV, complete cytopathic effect (CPE) was observed. Hepatitis A virus (HAV) strain HM175 (ATCC VR-1402) was propagated in fetal rhesus monkey kidney cells (FRhK-4) (ATCC CRL-1688) in DMEM supplemented with 10% FBS, 100 U/ml penicillin G-streptomycin-0.25 μg/ml amphotericin B, and 1 mM sodium bicarbonate. HAV was then infected in an 80 to 90% confluent monolayer of FRhK-4 cells for typically 7 days to observe CPE. Viruses were obtained following three cycles of freeze-thawing infected cells and centrifugation at 2,000 × g for 15 min. The supernatant was filtered through by a 0.2-μm-pore-size membrane filter (Thermo, Rochester, NY) before storing viruses at −80°C until use.
MNV and TV plaque assays were performed similarly to previous studies with slight modifications (23, 24). In brief, RAW 264.7 and LLC-MK2 cells were grown to 80 to 90% confluence in 6-well plates (Costar; Corning, NY), and 100 μl of 10-fold serial dilutions of each virus sample prepared in Hanks' balanced salt solution (HBSS) (Cellgro, Manassas, VA) was dispensed over monolayers in duplicate. The plates were incubated at 37°C with 5% CO2 for 1 h with gentle agitation every 15 min followed by the addition of 2-ml overlays. MNV-1 overlays consisted of 1.5% agarose (Lonza SeaPlaque; Rockland, ME) with complete Eagles medium (MEM) (HyClone, Logan, UT) supplemented with 2% FBS, 100 U/ml penicillin G-streptomycin-0.25 μg/ml amphotericin B, 2 mM l-alanine–l-glutamine, and 1 mM sodium pyruvate. TV overlays consisted of 1.5% agarose with complete medium 199 supplemented with 2% FBS and 100 U/ml penicillin G-streptomycin-0.25 μg/ml amphotericin B. After the incubation period (typically 48 h for MNV and TV), 1 ml of 0.2 g/liter neutral red (Fisher, Fair Lawn, NJ) was added into each well, followed by 2 to 5 h of incubation. Titers of virus were determined and expressed by PFU.
The titer of HAV was determined by using the 50% tissue culture infectious dose (TCID50) in fetal rhesus monkey kidney cells (FRhK-4) (27). Cell monolayers were allowed to grow in 96-well plates containing complete DMEM supplemented with 2% FBS, 100 U/ml penicillin G-streptomycin-0.25 μg/ml amphotericin B, and 1 mM sodium bicarbonate. Virus samples (100 μl) in 10-fold serial dilutions (eight replicates for each dilution) were inoculated onto confluent cells at 37°C with 5% CO2 for typically 15 days, and CPE was observed microscopically. Virus titers were determined and expressed by TCID50 using the Reed-Muench method (27).
Virus genome quantification by real-time RT-PCR.
The presence of MNV, TV, and HAV genomic copies was detected on seeds, sprouts, and water samples. To generate a standard curve for each virus type, 1 ml of virus stock with known genomic copies (107 genomic copies/ml for both HAV and MNV and 106 genomic copies/ml for TV) was 10-fold serially diluted with HBSS. RNA was extracted and reverse transcribed into cDNA by using the QIAamp viral RNA minikit (Qiagen, Valencia, CA) and Omniscript reverse transcription (RT) kit (Qiagen) as reference protocols, respectively. Three sets of primers were used for each type of virus: forward primer (5′-CAGCACATCAGAAAGGTGAG-3′) and reverse primer (5′-CTCCAGAATCATCTCCAAC-3′) for HAV (28), forward primer (5′-CCAGCTTGATGTAGGCGATT-3′) and reverse primer (5′-CTCAGCCATTGCACTCAAAG-3′) for TV (26), forward primer (5′-TCTTCGCAAGACACGCCAATTTCAG-3′) and reverse primer (5′-GCATCACAATGTCAGGGTCAACTC-3′) for MNV (29). Real-time PCRs were performed in a total reaction volume of 20 μl containing 10 μl SYBR green PCR master mix (Qiagen), 2 μl cDNA, and the same set of primers with the protocol from the QuantiTect SYBR green PCR kit (Qiagen) on 384-well plates. Reactions were run on the Applied Biosystems 7900 HT sequence detection system (Applied Biosystems, Foster City, CA) with the following thermal conditions: 95°C for 10 min followed by 40 cycles of 94°C for 15 s, annealing temperature of each virus for 30 s (59°C for MNV, 56°C for HAV, 59°C for TV), followed by a dissociation step at 60°C for 15 s and 90°C for 15 s. SYBR green signals were read in every cycle, and the logarithm of the increment in fluorescence was plotted versus the cycle number with a fixed threshold level for all runs. Virus quantity was then determined by comparison to a standard curve and expressed as genomic copies. The standard curve was generated in duplicate for each quantitative PCR (qPCR) run. The detection limit for all virus types was determined to be ∼100 genomic copies/ml. MNV, TV, and HAV in HBSS served as positive controls, and negative controls consisted of the environmental sample (seed, sprout, or water) without virus.
Alfalfa seed preparation and storage.
Alfalfa seeds (Johnny's Selected Seeds, Winslow, ME) were sterilized by submerging seeds in 70% ethanol for 5 min, followed by soaking them in a 10% bleach solution for 20 min. Seeds were rinsed with deionized water and then dried under the laminar flow hood at room temperature overnight before being divided into 1-g samples in 1.5-ml microcentrifuge tubes prior to inoculation. After treatment, little effect was observed visually on sprouting percentage compared with untreated sprout seeds. Every 1 g of seed sample was individually inoculated with 200 μl of MNV, TV, or HAV and was stored for up to 50 days at 22°C in individual closed tubes. Seed samples were collected on sampling days (0, 2, 5, 10, 15, 20, 30, and 50), and every 1 g of seeds was carefully placed into 1 ml of HBSS and vortexed for 1 min, and the solution was retained for infectivity assays or/and real-time RT-PCR.
Alfalfa sprout germination and irrigation water collection.
On sampling days (0, 2, 5, 10, and 15), another set of inoculated seed samples were germinated in the sprout growth chambers (Victorio, Orem, UT). The growth chamber had three trays: the top tray was empty and used for watering, the middle tray had rings to distribute seeds evenly and was used for germination, and the bottom tray was a holding container to collect spent irrigation water. During the 7-day germination period, 500 ml municipal tap water was added daily on the top tray. Water was then siphoned over seeds/sprout and finally drained and collected in the bottom tray. The humidity and temperature inside growth chambers containing uninoculated seeds/sprouts were measured and recorded daily by a Traceable Therm./Clock/Humidity monitor (Fisher, Pittsburgh, PA). Spent irrigation water samples (1 ml, duplicates) were collected on days 1, 3, and 6 following initial seed sprouting for each sample and were processed for quantification of virus collected in the spent irrigation water. Sprouts (approximately 12 g sprouts from 1 g seeds after 7-day germination) were collected in 50-ml centrifuge tubes containing 10 ml HBSS and vortexed for 1 min to elute the virus from the sprout for virus detection. In addition, 10 alfalfa sprouts germinated from inoculation day 0 seeds were randomly collected. The portions of sprouts, including primary root, hypocotyl, true leaves, and seed coat, were cut separately by using scissors and collected with forceps. The scissors and forceps were soaked in 10% bleach (Clorox, Oakland, CA) and neutralized in 5% sodium thiosulfate (Fisher, Fair Lawn, NJ) every time after being used to prevent cross-contamination. The presence of virus genomic copies from each portion of sprouts was determined.
Statistical analysis.
Experiments were conducted in triplicate. Results are reported as means and standard deviations. Data were analyzed by analysis of variance (ANOVA) on JMP software (version 9.0; SAS Institute Inc., Cary, NC), and significance was indicated if P values were <0.05.
RESULTS
Virus recovery on the surface of alfalfa seeds after inoculation.
Initial titers of viruses inoculated on seeds were determined to be 6.46 ± 0.06 log PFU/g (7.15 ± 0.50 log genomic copies/g) for MNV, 3.87 ± 0.38 log PFU/g (5.92 ± 0.45 log genomic copies/g) for TV, and 7.01 ± 0.07 log TCID50/g (7.90 ± 0.37 log genomic copies/g) for HAV. After seeds were visibly dried after inoculation (approximately an hour) on day 0, MNV, TV, and HAV were recovered from seeds with titers of 6.55 ± 0.24 log PFU/g (7.44 ± 0.06 log genomic copies/g), 3.43 ± 0.07 log PFU/g (5.73 ± 1.19 log genomic copies/g), and 5.60 ± 0.19 log TCID50/g (6.55 ± 0.15 log genomic copies/g), respectively. Log reductions were listed on day 0 (Table 1). The results showed significant reductions of HAV and TV on the surface of seeds after drying, with values of 1.41 ± 0.19 TCID50/g and 0.44 ± 0.07 log PFU/g (P < 0.05), respectively. MNV was an exception, where little reduction was observed.
Table 1.
Infectivity reduction of HAV, MNV, and TV on alfalfa seeds and in HBSS stored at 22°C for up to 50 days
| Virus | Matrix | Infectivity reduction of virus (log PFU/g or log TCID50/g)a on day: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 5 | 10 | 15 | 20 | 30 | 50 | ||
| HAV | Seeds | a 1.41 ± 0.19 A | a 1.44 ± 0.32 A | a 2.02 ± 0.93 A | a 1.61 ± 0.09 A | a 1.78 ± 0.14 A | a 2.15 ± 0.39 A | a 3.68 ± 0.00 B | a 3.58 ± 0.21 B |
| HBSS | b 0.00 ± 0.00 A | b 0.05 ± 0.00 A | a 0.80 ± 0.35 B | a 1.80 ± 0.35 C | a 1.80 ± 0.35 C | a 2.38 ± 0.00 CD | a 2.80 ± 0.35 D | a 4.22 ± 0.24 E | |
| MNV | Seeds | a −0.09 ± 0.24 A | a 0.76 ± 0.63 B | a 2.18 ± 0.03 C | a 2.46 ± 0.04 CD | a 2.86 ± 0.14 DE | a 3.14 ± 0.21 EF | a 3.90 ± 0.22 F | a 4.85 ± 0.19 G |
| HBSS | a 0.00 ± 0.00 A | a −0.32 ± 0.43 A | a 2.24 ± 0.11 B | b 2.97 ± 0.03 C | a 3.19 ± 0.10 C | a 3.82 ± 0.14 CD | a 4.22 ± 0.13 D | a 4.94 ± 0.31 E | |
| TV | Seeds | a 0.44 ± 0.07 A | a 0.57 ± 0.12 B | a 0.81 ± 0.04 B | a 1.53 ± 0.08 C | a 2.01 ± 0.17 D | a 2.15 ± 0.16 D | a 2.19 ± 0.09 D | a 2.58 ± 0.21 E |
| HBSS | b 0.00 ± 0.00 A | a 0.03 ± 0.34 A | a 0.77 ± 0.28 B | b 0.91 ± 0.03 BC | b 1.00 ± 0.06 BC | b 1.24 ± 0.03 C | b 1.69 ± 0.01 D | b 1.84 ± 0.08 D | |
Values are means ± standard deviations (SD) from three replicates; values in rows with the same preceding letter indicate no significant difference (P > 0.05) between seeds and HBSS for each virus; values in rows with the same following letter indicate no significant difference (P > 0.05) in virus survival between different sampling days.
Survival of viruses on alfalfa seeds and in HBSS during a 50-day storage at 22°C.
The survival rates of infectious virus particles for MNV, TV, and HAV on seeds after inoculation as well as in HBSS were determined at 21°C (ranging from 17.9 to 23.4°C) for up to 50 days. The infectivity reductions for each virus with log PFU or log TCID50 were determined (Table 1). All viruses remained infectious on seeds for up to 50 days, with various trends in reduction. Generally, the reductions observed in infectivity increased with extended storage time both on alfalfa seeds and in HBSS.
After an initial decrease of 1.5 log TCID50/g after drying, HAV persisted with no significant reduction on the surface of alfalfa seeds (<1 log TCID50/g) over 20 days (P < 0.05) and decreased approximately 2 log TCID50/g within 50 days; however, both MNV and TV were reduced significantly within the first 2 days (P < 0.05) on seeds. A greater reduction in MNV (almost 5 log PFU/g) was observed on the seed surface than in TV (approximately 2 log PFU/g) after 50 days.
There was no significant reduction in HBSS within the first 2 days; however, a significant decrease was observed after 5 days (P < 0.05), regardless of virus type. TV was relatively stable in HBSS with less than a 2 log PFU/g reduction after 50 days, whereas a ∼4 to 5 log PFU/g or TCID50/g reduction was found in both MNV and HAV.
Differences in virus survival were observed based on matrices (either seeds or HBSS). The reductions in virus infectivity from seeds and in HBSS were similar over this storage period for MNV, and no significant difference (P > 0.05) was observed between seeds and HBSS over the storage period on day 10. TV decreased more quickly starting on day 0 in seeds, and significantly greater reductions (P < 0.05) were found in seeds beginning at day 10. In addition, after a reduction of approximately 1.5 log TCID50/g on day 0, HAV persisted on seeds and in HBSS.
The genomic copies of all the viruses were also determined over the time period, and the data were displayed in Fig. 1 and 2. The numbers of genomic copies for HAV and MNV were relatively constant in both matrices, resulting in an ∼2-log reduction over 50 days. No significant differences in genomic copies of HAV and MNV were detected on seeds within the first 30 and 15 days, respectively. However, this trend was not observed for TV. The genomic copies of TV had trends similar to those of the plaque assay results and significantly decreased after 10 days in both matrices. The reduction of TV genomic copies in HBSS was lower than that on seeds, which matched the plaque assay data as well.
Fig 1.
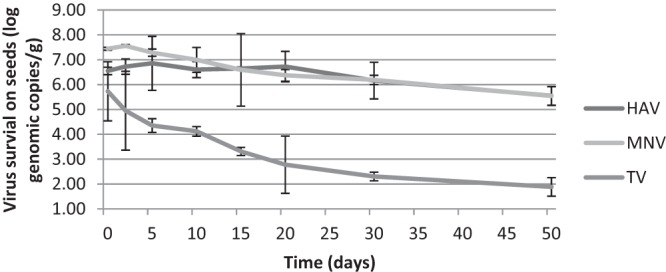
Genomic copies of HAV, MNV, and TV present on alfalfa seeds stored at 22°C for up to 50 days.
Fig 2.
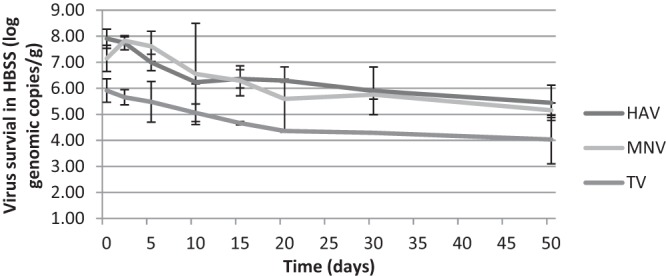
Genomic copies of HAV, MNV, and TV present in HBSS stored at 22°C for up to 50 days.
Survival of viruses on alfalfa sprout after 7 days of germination.
The seeds were allowed to germinate for 0, 2, 5, 10, and 15 days postinoculation with daily watering. After a 7-day germination period, the approximate weight of sprouts germinated from 1 g of seeds was ∼12 g, and viruses were detected on sprouts from seeds that were artificially contaminated. The humidity of sprouts was measured mainly above 55% in the growth chamber, ranging from 36% to >90%. The levels of viruses detected on sprouts depended largely on the amounts of viruses that survived on seeds initially (Table 2). As there was no significant reduction of HAV on seeds within the first 15 days (P > 0.05), similar levels of HAV ranging from 2.43 to 3.46 log TCID50 were detected on the sprouts after germination for all the samples selected within this period, approximately 2.5 log TCID50 lower than the initial titers on the inoculated seeds. In addition, the levels of MNV and TV found on sprouts decreased corresponding to the decreasing titers over time. Interestingly the titers of TV associated with sprouts were <1 log PFU lower than that on seeds before germination, whereas more reductions were observed with HAV and MNV. Again, the numbers of genomic copies were similar with small fluctuations, which were ∼1 to 2 logs higher than that determined by infectivity assays for all the sprout samples. As observed previously, the genomic copies of HAV and MNV declined but were persistent on sprouts. However, the TV genomic copies were found to be relatively stable over the course of the experiment.
Table 2.
Infectivity and genomic copies of HAV, MNV, and TV on alfalfa sprouts germinated (1 g seeds) on days 0, 2, 5, 10, and 15 after inoculation
| Day | Virus survival on sprouts from inoculated seeds after storage timea |
|||||
|---|---|---|---|---|---|---|
| HAV |
MNV |
TV |
||||
| Infectivity (log TCID50) | No. of genomic copies | Infectivity (log PFU) | No. of genomic copies | Infectivity (log PFU) | No. of genomic copies | |
| 0 | 3.46 ± 0.71 A | 4.25 ± 0.47 AB | 3.10 ± 0.08 A | 4.73 ± 0.51 A | 2.19 ± 0.06 A | 3.36 ± 0.22 A |
| 2 | 3.04 ± 0.59 A | 4.57 ± 0.07 A | 2.73 ± 0.36 AB | 3.64 ± 0.69 AB | 2.13 ± 0.09 A | 3.58 ± 0.65 A |
| 5 | 2.71 ± 0.35 A | 4.19 ± 0.20 AB | 2.31 ± 0.36 B | 3.43 ± 0.50 B | 2.08 ± 0.03 A | 3.94 ± 0.36 A |
| 10 | 2.54 ± 0.12 A | 4.34 ± 0.65 AB | 2.16 ± 0.05 B | 3.29 ± 0.19 B | 1.26 ± 0.12 B | 3.49 ± 0.05 A |
| 15 | 2.43 ± 0.04 A | 3.58 ± 0.64 B | 1.14 ± 0.05 C | 2.78 ± 0.91 B | 1.09 ± 0.12 B | 3.47 ± 0.15 A |
Values are means ± SD from three replicates; values in columns with the same letter indicate no significant difference (P > 0.05) comparing virus survival by infectivity assay or number of genomic copies on sprouts from inoculated seeds with storage periods of 0, 2, 5, 10, and 15 days.
Virus was distributed within the alfalfa sprout. The anatomy of a sprout (Fig. 3), including four parts, primary root, hypocotyl, true leaves, and seed coat, shows all portions of sprouts which were identified at least twice to be contaminated by each virus RNA genome (Table 3).
Fig 3.
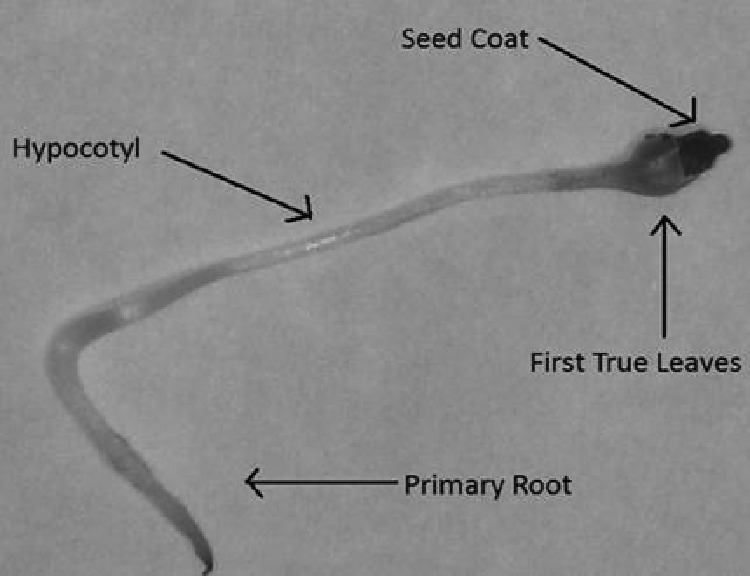
Anatomy of an alfalfa sprout. The presence of viruses on each portion of the sprout was determined after a 7-day period of germination.
Table 3.
Localization of HAV, MNV, and TV in the alfalfa sproutsa
| Virus | No. of positive samples/total no. |
|||
|---|---|---|---|---|
| Primary root | Hypocotyl | First true leaves | Seed coat | |
| HAV | 3/3 | 3/3 | 3/3 | 3/3 |
| MNV | 2/3 | 3/3 | 3/3 | 3/3 |
| TV | 2/3 | 3/3 | 3/3 | 3/3 |
Each sample represents a pool of 10 sprouts. Sprouts were germinated from seeds at inoculation day 0.
Presence of viruses in irrigation water during sprout germination.
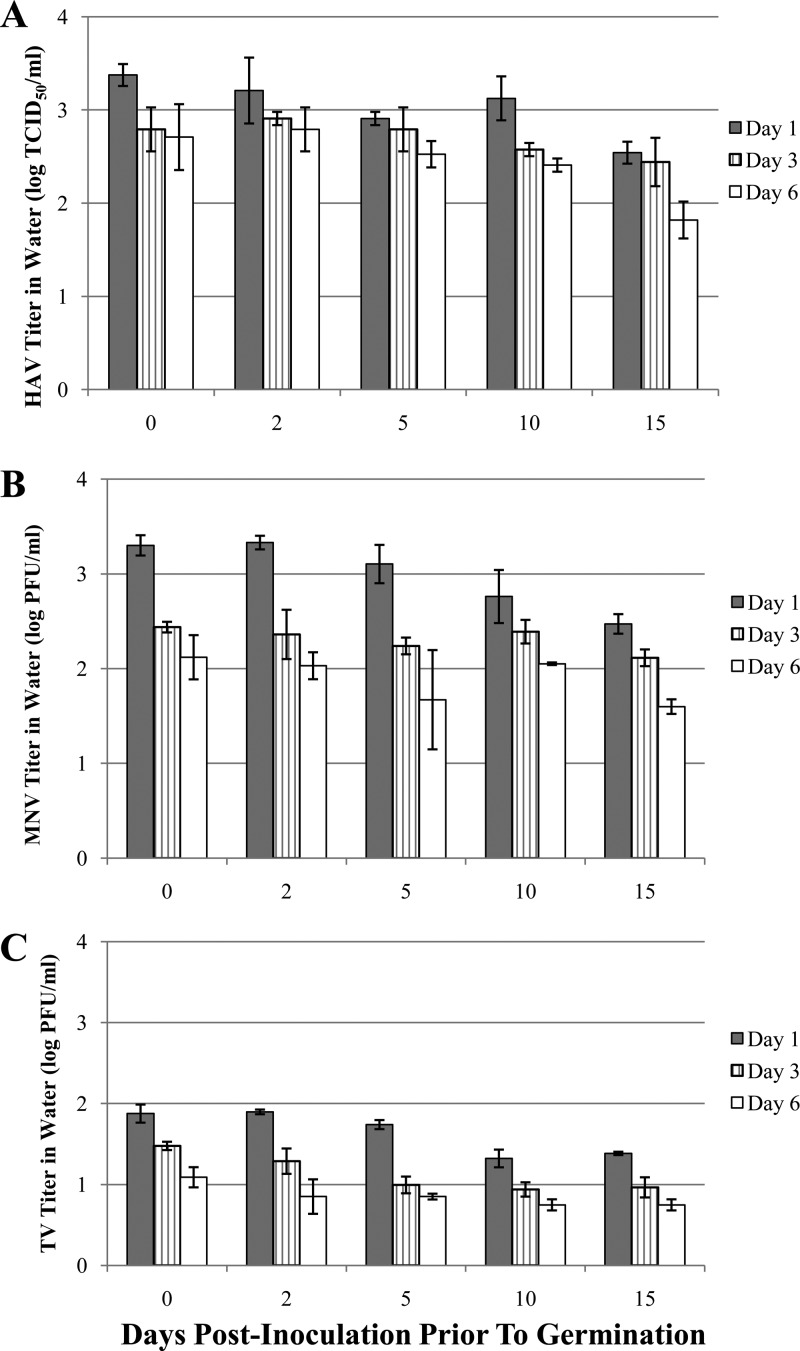
The alfalfa seeds were watered daily, and irrigation water was collected on days 1, 3, and 6 during a 7-day germination period to determine the presence of each virus. The levels of HAV, MNV, and TV transferred from seeds/sprouts to irrigation water are shown in Fig. 4A, B, and C. Viruses were detected in all the irrigation water samples over the germination period. Due to the initial inoculum levels, the levels of MNV and HAV were higher than that of TV, with approximately 2 log PFU and 2 log TCID50, respectively, in irrigation water on germination day 1 within this 15-day storage period, whereas more than 1 log PFU of TV was found on germination day 1 for all samples. A general trend of decreasing number of viruses in irrigation water from day 1 to day 6 was observed during sprouting, and in most cases significantly larger amounts of viruses were detected on day 1 than on days 3 and 6 (P < 0.05). As well, the titers of each virus in water on the same germination day decreased with extending time. Little reduction of HAV and MNV was observed in water on each germination day, and the levels of TV were likely similar on day 6. The numbers of genomic copies of each virus were found relatively persistent, at ∼1 to 2 logs higher than that determined by infectivity assays.
Fig 4.
Presence of HAV (A), MNV (B), and TV (C) in spent irrigation water collected on days 1, 3, and 6 during alfalfa seed germination from seeds inoculated on day 0 and stored for up to 15 days.
DISCUSSION
Alfalfa sprouts may become contaminated from a number of sources, including contaminated seeds, water, or mishandling/cross-contamination during food preparation (30–32). Contaminated seeds were previously identified as the major cause for sprout-associated outbreaks (17). In this study, we demonstrated that MNV, TV, and HAV can persist on the surface of alfalfa seeds for a prolonged period, and these viruses could contaminate sprouts after germination and be transferred to spent irrigation water. This result is not surprising and is supported by previous viral infectivity studies at room temperature in tap water/seawater/groundwater, which demonstrated long-term infectivity of MNV (>30 days), TV (>30 days), huNoV (>61 days), and HAV (>60 days) (25, 33, 34).
Virus survival varied depending on virus types and matrices. Different survival patterns were observed on seeds and in HBSS for all the viruses. HAV was relatively persistent over the first 20 days, followed by small reductions within 50 days on seeds, which confirmed the conclusion that HAV persisted better under dry conditions, as stated in other studies (35). On day 0, viruses were recovered from seeds after drying, and for both HAV and TV, recoveries from seeds after inoculation decreased significantly. Previous studies showed that HAV did not lose infectivity after drying in plasma or culture medium (36). Observed reductions may be explained by differences in recovery, which reinforces the strong attachment between alfalfa seeds and viruses. The influential factors, including electrostatic and hydrophobic forces, as well as isoelectric point (pI) of the capsid proteins, environmental conditions (e.g., pH, ionic strength, humidity, darkness, and temperature), were identified to be involved in virus binding to similar matrices (34, 37–41). Little additional reduction was observed after MNV was recovered from seeds on day 0, which revealed relatively weak attachment.
It appears that alfalfa seeds can provide niches for virus survival and protect viruses from harsh conditions. With their oval shape, the surfaces of alfalfa seeds are relatively uniform. The seed surface contains small hills and narrow valleys which are not likely to allow entrapment of bacteria (8); however, these valleys might offer spots for virus attachment, as viral particles are much smaller than bacterial pathogens. It is possible that viruses harbored within seed coat crevices may escape the environmental effects of light, temperature, and pH fluctuations. Additionally, surface crevices could also prevent removal or inactivation of food-borne pathogens by washing and reduce contact with disinfectants, resulting in ineffective virus removal and inactivation.
The survival rates of MNV and TV were slightly different on seeds as well as in HBSS. However, recent studies showed more similar patterns of both MNV and TV survival in tap water over 25 days at 20°C (25). In most cases, viruses tend to survive better at lower temperatures (25, 35, 42, 43), and the temperature fluctuations could result in virus inactivation. In addition, alfalfa sprouts provided a neutral pH (44), and pH ranging from 6 to 8 has been shown to be preferable for virus survival, with decreased rates of inactivation (25, 42, 43, 45). HBSS contains salts and provides a stable pH at ∼7.25, which is within that range. The difference observed here may be in part explained by the levels of initial inoculums. The initial titer of TV was much lower than that of MNV, and it is possible that the virus could persist for a long period of time at low levels. Moreover, it was shown that HAV survival was inversely proportional to the level of relative humidity (35), whereas MNV acted in the opposite manner (42).
Attempts to correlate virus infectivity with the number of genomic copies as determined by real-time RT-PCR provided useful information on the relative stability of the virus itself. The genomic copies of HAV and MNV on seeds were relatively persistent regardless of their infectivity, whereas for TV, the genomic copies decreased in a manner similar to the number of infective virus as determined by plaque assay. This may indicate loss of HAV and MNV infectivity as a result of capsid changes rather than from denaturation that could impact genome integrity. In addition, the inactivation of TV may lead to degradation of RNA more easily. However, the genomic materials detected in this study were small segments for each virus, and it should be noted that these do not represent the whole genome. On the contrary, the levels of TV genomic copies detected on sprouts and spent water were stable without significant difference (P < 0.05), probably due to the high level of humidity.
As huNoV surrogates, TV may be more environmentally robust than MNV, with less reduction in infectivity observed both on seeds and in HBSS, and the genomic copies were capable of persisting in HBSS regardless of infectivity. This indicates that TV could be another possible surrogate for huNoV in environmental studies, especially under the conditions of high humidity. Sinclair et al. provided the criteria for surrogate selection to conduct risk assessment in the environment, emphasizing both surrogate attributes (e.g., practical, biological, and environmental attributes) and experimental context (46). In order to determine if TV is the ideal surrogate, it is necessary that the characteristics of TV are similar or very close to that of huNoV in natural or engineered systems. TV is cultivable in cell culture and still genetically related to huNoVs, although not as similar as MNV is. The most interesting property of TV is its functional morphology to bind HBGA (26). In this study, TV displayed environmental attributes similar to those of MNV, which was relatively tolerant at room temperature and had a neutral pH regardless of humidity, and TV generally exhibited greater resistances than MNV in infectivity. Considering both attributes, TV can be selected as a tentative surrogate of huNoVs in environmental survival studies. However, one surrogate might not be able to present the full properties of huNoVs under different environmental conditions or treatments, and the number of genomic copies of TV in drying conditions was very persistent and decreased in a pattern similar to that of its infectivity. Therefore, it remains necessary to employ several surrogates for study to better understand the potential behavior of huNoVs, as surrogates exhibit slight differences in each attribute.
Virus transmission in water is an important concern for the sprout industry, based on this study. The seeds were watered daily for germination, and the rates of virus survival on seeds were significantly reduced after the first watering, but the viruses spread through water to contaminate the entire batch of sprouts, including all the portions of sprouts. Three types of viruses all survived and were still infectious in the irrigation water during the process of germination, and the virus titers depended on the initial levels on the seeds. TV survival in the germination water was found to be less than that of HAV and MNV due to the original lower inoculum. A similar conclusion was drawn from previous reports (47–49). It was previously observed that viruses in contaminated water could be easily absorbed by vegetables after being immersed in water, and viruses persisted during storage (50). Washing without any application of disinfectant or sanitizers can result in reduction but does not guarantee a complete decontamination (50). In view of the fact that alfalfa sprouts are most likely consumed raw or may be just slightly cooked as an ingredient for different recipes, adequate hygienic measures both in production and during preparation are necessary to reduce food-borne illness.
Studies showed the presence of viruses in used irrigation water at room temperature stored for a short period and that viruses could be transmitted to produce by being washed with contaminated water or could be internalized via roots (50–52). Wastewater or irrigation water can be another source of contamination if reused (53). The risks can be increased by virus persistence as well as by the heterogeneous distribution of viruses (54). It is advisable to test the irrigation water to obtain an indication of the amount of contamination of sprouts grown from seeds and avoid cross-contamination (55). Other techniques to reduce contamination on seeds can be utilized, such as high-pressure processing (56), irradiation (57), heat, and calcium hypochlorite (58).
In this study, alfalfa seeds were selected as a model to understand the behavior of food-borne viruses during a prolonged storage, as well as the interaction of viruses with sprouted seeds and their transfer to irrigation water during germination. These findings suggest that viruses may survive for a relatively long period of time on seeds and reveal the ease with which viruses may transfer and spread during the germination process. Thus, it is imperative to apply appropriate disinfectants to remove pathogens from seeds and to implement good agricultural and manufacturing practices, including worker hygiene and sanitation, during sprouting to limit contamination and cross-contamination. Attention should be paid to the reuse of irrigation water, which could be a potential source of pathogens.
ACKNOWLEDGMENT
This project was funded in part by USDA NoroCORE grant number 2011-68003-30395.
Footnotes
Published ahead of print 6 September 2013
REFERENCES
- 1.Schrader WL. 2002. Sprout production in California, publication 8060. University of California Department of Agriculture and Natural Resources, Davis, CA [Google Scholar]
- 2.Erdozain G, Erdozain MS, Powell D. 2011. Sprouts associated outbreaks. Bites, Manhattan, KS [Google Scholar]
- 3.Buchholz U, Bernard H, Werber D, Böhmer MM, Remschmidt C, Wilking H, Deleré Y, an der Heiden M, Adlhoch C, Dreesman J, Ehlers J, Ethelberg S, Faber M, Frank C, Fricke G, Greiner M, Höhle M, Ivarsson S, Jark U, Kirchner M, Koch J, Krause G, Luber P, Rosner B, Stark K, Kühne M. 2011. German outbreak of Escherichia coli O104:H4 associated with sprouts. N. Engl. J. Med. 365:1763–1770 [DOI] [PubMed] [Google Scholar]
- 4.International Food Safety Network 25 April 2009, posting date Sprout associated outbreaks in North America, 1990–2009. IFSN, Manhattan, KS: http://foodsafety.ksu.edu/en/article-details.php?a=3&c=10&sc=74&id=865 [Google Scholar]
- 5.Itoh Y, Sugita-Konishi Y, Kasuga F, Iwaki M, Hara-Kudo Y, Saito N, Noguchi Y, Konuma H, Kumagai S. 1998. Enterohemorrhagic Escherichia coli O157:H7 present in radish sprouts. Appl. Environ. Microbiol. 64:1532–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro-Rosas J, Escartín EF. 2000. Survival and growth of Vibrio cholerae O1, Salmonella typhi, and Escherichia coli O157:H7 in alfalfa sprouts. J. Food Sci. 65:162–165 [Google Scholar]
- 7.Barak JD, Whitehand LC, Charkowski AO. 2002. Differences in attachment of Salmonella enterica serovars and Escherichia coli O157: H7 to alfalfa sprouts. Appl. Environ. Microbiol. 68:4758–4763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fransisca L, Feng H. 2012. Effect of surface roughness on inactivation of Escherichia coli O157:H7 87-23 by new organic-acid surfactant combinations on alfalfa, broccoli, and radish seeds. J. Food Prot. 75:261–269 [DOI] [PubMed] [Google Scholar]
- 9.Gorski L, Palumbo JD, Nguyen KD. 2004. Strain-specific differences in the attachment of Listeria monocytogenes to alfalfa sprouts. J. Food Prot. 67:2488–2495 [DOI] [PubMed] [Google Scholar]
- 10.Barak JD, Gorski L, Naraghi-Arani P, Charkowski AO. 2005. Salmonella enterica virulence genes are required for bacterial attachment to plant tissue. Appl. Environ. Microbiol. 71:5685–5691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu FM, Beuchat LR, Wells JG, Slutsker L, Doyle MP, Swaminathan B. 2001. Factors influencing the detection and enumeration of Escherichia coli O157: H7 on alfalfa seeds. Int. J. Food Microbiol. 71:93–99 [DOI] [PubMed] [Google Scholar]
- 12.Fu TJ, Reineke KF, Chirtel S, Vanpelt OM. 2008. Factors influencing the growth of Salmonella during sprouting of naturally contaminated alfalfa seeds. J. Food Prot. 71:888–896 [DOI] [PubMed] [Google Scholar]
- 13.Charkowski AO, Sarreal CZ, Mandrell RE. 2001. Wrinkled alfalfa seeds harbor more aerobic bacteria and are more difficult to sanitize than smooth seeds. J. Food Prot. 64:1292–1298 [DOI] [PubMed] [Google Scholar]
- 14.Hall AJ, Eisenbart VG, Etingüe AL, Gould LH, Lopman BA, Parashar UD. 2012. Epidemiology of foodborne norovirus outbreaks, United States, 2001–2008. Emerg. Infect. Dis. 18:1566–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeWaal CS, Bhuiya F. 2007. Outbreaks by the numbers: fruits and vegetables 1990–2005. Poster presentation P3-03. 94th Annual Meeting of the International Association for Food Protection, Lake Buena Vista, FL, 8 to 11 July 2007 [Google Scholar]
- 16.Wei J, Kniel KE. 2010. Pre-harvest viral contamination of crops originating from fecal matter. Food Environ. Virol. 2:195–206 [Google Scholar]
- 17.National Advisory Committee on Microbiological Criteria for Foods 1999. Microbiological safety evaluations and recommendations on sprouted seeds. Int. J. Food Microbiol. 52:123–153 [DOI] [PubMed] [Google Scholar]
- 18.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M-A, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koopmans M, Duizer E. 2004. Foodborne viruses: an emerging problem. Int. J. Food Microbiol. 90:23–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel MM, Hall AJ, Vinjé J, Parashar UD. 2009. Noroviruses: a comprehensive review. J. Clin. Virol. 44:1–8 [DOI] [PubMed] [Google Scholar]
- 21.Teunis PFM, Moe CL, Liu P, Miller ES, Lindesmith L, Baric RS, Le Pendu J, Calderon RL. 2008. Norwalk virus: how infectious is it? J. Med. Virol. 80:1468–1476 [DOI] [PubMed] [Google Scholar]
- 22.Sair AI, D'Souza DH, Jaykus LA. 2002. Human enteric viruses as causes of foodborne disease. Compr. Rev. Food Sci. Food Saf. 1:73–89 [DOI] [PubMed] [Google Scholar]
- 23.Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW. 2004. Replication of norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2:2076–2084. 10.1371/journal.pbio.0020432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farkas T, Sestak K, Wei C, Jiang X. 2008. Characterization of a rhesus monkey calicivirus representing a new genus of Caliciviridae. J. Virol. 82:5408–5416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirneisen KA, Kniel KE. 2013. Comparing human norovirus surrogates: murine norovirus and tulane virus. J. Food Prot. 76:139–143 [DOI] [PubMed] [Google Scholar]
- 26.Farkas T, Cross RW, Hargitt E, Lerche NW, Morrow AL, Sestak K. 2010. Genetic diversity and histo-bood group antigen interactions of rhesus enteric caliciviruses. J. Virol. 84:8617–8625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. (Lond.) 27:493–497 [Google Scholar]
- 28.Schwab KJ, De Leon R, Baric RS, Sobsey MD. 1991. Detection of rotaviruses, enteroviruses, and hepatitis A virus by reverse transcriptase-polymerase chain reaction, p 475–491 In Advances in water analysis and treatment. Proceedings of the AWWA Water Quality Technology Conference, Orlando, FL, 10 to 14 November 1991 American Water Works Association, Denver, CO [Google Scholar]
- 29.Hsu CC, Wobus CE, Steffen EK, Riley LK, Livingston RS. 2005. Development of a microsphere-based serologic multiplexed fluorescent immunoassay and a reverse transcriptase PCR assay to detect murine norovirus 1 infection in mice. Clin. Diagn. Lab. Immun. 12:1145–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erdozain MS, Allen KJ, Morley KA, Powell DA. 2013. Failures in sprouts-related risk communication. Food Control 30:649–656 [Google Scholar]
- 31.Howard MB, Hutcheson SW. 2003. Growth dynamics of Salmonella enterica strains on alfalfa sprouts and in waste seed irrigation water. Appl. Environ. Microbiol. 69:548–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greig JD, Todd ECD, Bartleson CA, Michaels BS. 2007. Outbreaks where food workers have been implicated in the spread of foodborne disease. Part 1. Description of the problem, methods, and agents involved. J. Food Prot. 70:1752–1761 [DOI] [PubMed] [Google Scholar]
- 33.Chung H, Sobsey MD. 1993. Comparative survival of indicator viruses and enteric viruses in seawater and sediment. Water Sci. Technol. 27:425–428 [Google Scholar]
- 34.Seitz SR, Leon JS, Schwab KJ, Lyon GM, Dowd M, McDaniels M, Abdulhafid G, Fernandez ML, Lindesmith LC, Baric RS, Moe CL. 2011. Norovirus infectivity in humans and persistence in water. Appl. Environ. Microbiol. 77:6884–6888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mbithi JN, Springthorpe VS, Sattar SA. 1991. Effect of relative humidity and air temperature on survival of hepatitis A virus on environmental surfaces. Appl. Environ. Microbiol. 57:1394–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terpstra FG, van den Blink AE, Bos LM, Boots AGC, Brinkhuis FHM, Gijsen E, van Remmerden Y, Schuitemaker H, van't Wout AB. 2007. Resistance of surface-dried virus to common disinfection procedures. J. Hosp. Infect. 66:332–338 [DOI] [PubMed] [Google Scholar]
- 37.Vega E, Smith J, Garland J, Matos A, Pillai SD. 2005. Variability of virus attachment patterns to butterhead lettuce. J. Food Prot. 68:2112–2117 [DOI] [PubMed] [Google Scholar]
- 38.Vega E, Garland J, Pillai SD. 2008. Electrostatic forces control nonspecific virus attachment to lettuce. J. Food Prot. 71:522–529 [DOI] [PubMed] [Google Scholar]
- 39.Kukavica-Ibrulj I, Darveau A, Jean J, Fliss I. 2004. Hepatitis A virus attachment to agri-food surfaces using immunological, virological and thermodynamic assays. J. Appl. Microbiol. 97:923–934 [DOI] [PubMed] [Google Scholar]
- 40.Langlet J, Gaboriaud F, Gantzer C, Duval JFL. 2008. Impact of chemical and structural anisotropy on the electrophoretic mobility of spherical soft multilayer particles: the case of bacteriophage MS2. Biophys. J. 94:3293–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Guyader FS, Atmar RL. 2008. Binding and inactivation of viruses on and in food, with a focus on the role of the matrix, p 189–208 In Doyle MP, Koopmans MPG, Cliver DO, Bosch AB. (ed), Foodborne viruses: progress and challenges. ASM Press, Washington, DC [Google Scholar]
- 42.Cannon JL, Papafragkou E, Park GW, Osborne J, Jaykus LA, Vinje J. 2006. Surrogates for the study of norovirus stability and inactivation in the environment: a comparison of murine norovirus and feline calicivirus. J. Food Prot. 69:2761–2765 [DOI] [PubMed] [Google Scholar]
- 43.Davidson I, Nagar S, Haddas R, Ben-Shabat M, Golender N, Lapin E, Altory A, Simanov L, Ribshtein I, Panshin A, Perk S. 2010. Avian influenza virus H9N2 survival at different temperatures and pHs. Avian Dis. 54:725–728 [DOI] [PubMed] [Google Scholar]
- 44.Weagant SD, Bound AJ. 2001. Evaluation of techniques for enrichment and isolation of Escherichia coli O157:H7 from artificially contaminated sprouts. Int. J. Food Microbiol. 71:87–92 [DOI] [PubMed] [Google Scholar]
- 45.Feng YY, Ong SL, Hu JY, Tan XL, Ng WJ. 2003. Effects of pH and temperature on the survival of coliphages MS2 and Q beta. J. Ind. Microbiol. Biotechnol. 30:549–552 [DOI] [PubMed] [Google Scholar]
- 46.Sinclair RG, Rose JB, Hashsham SA, Gerba CP, Haas CN. 2012. Criteria for selection of surrogates used to study the fate and control of pathogens in the environment. Appl. Environ. Microbiol. 78:1969–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahon BE, Ponka A, Hall WN, Komatsu K, Dietrich SE, Siitonen A, Cage G, Hayes PS, Lambert-Fair MA, Bean NH, Griffin PM, Slutsker L. 1997. An international outbreak of Salmonella infections caused by alfalfa sprouts grown from contaminated seeds. J. Infect. Dis. 175:876–882 [DOI] [PubMed] [Google Scholar]
- 48.Breuer T, Benkel DH, Shapiro RL, Hall WN, Winnett MM, Linn MJ, Neimann J, Barrett TJ, Dietrich S, Downes FP, Toney DM, Pearson JL, Rolka H, Slutsker L, Griffin PM, Investigation Team 2001. A multistate outbreak of Escherichia coli O157:H7 infections linked to alfalfa sprouts grown from contaminated seeds. Emerg. Infect. Dis. 7:977–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohle-Boetani JC, Farrar JA, Werner SB, Minassian D, Bryant R, Abbott S, Slutsker L, Vugia DJ, Investigation Team 2001. Escherichia coli O157 and Salmonella infections associated with sprouts in California, 1996–1998. Ann. Intern. Med. 135:239–247 [DOI] [PubMed] [Google Scholar]
- 50.Croci L, De Medici D, Scalfaro C, Fiore A, Toti L. 2002. The survival of hepatitis A virus in fresh produce. Int. J. Food Microbiol. 73:29–34 [DOI] [PubMed] [Google Scholar]
- 51.Baert L, Uyttendaele M, Vermeersch M, Van Coillie E, Debevere J. 2008. Survival and transfer of murine norovirus 1, a surrogate for human noroviruses, during the production process of deep-frozen onions and spinach. J. Food Prot. 71:1590–1597 [DOI] [PubMed] [Google Scholar]
- 52.DiCaprio E, Ma YM, Purgianto A, Hughes J, Li JR. 2012. Internalization and dissemination of human norovirus and animal caliciviruses in hydroponically grown romaine lettuce. Appl. Environ. Microbiol. 78:6143–6152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamilton AJ, Stagnitti F, Xiong XZ, Kreidl SL, Benke KK, Maher P. 2007. Wastewater irrigation: the state of play. Vadose Zone J. 6:823–840 [Google Scholar]
- 54.Petterson SR, Ashbolt NJ. 2001. Viral risks associated with wastewater reuse: modeling virus persistence on wastewater irrigated salad crops. Water Sci. Technol. 43:23–26 [PubMed] [Google Scholar]
- 55.Fu T, Stewart D, Reineke K, Ulaszek J, Schlesser J, Tortorello M. 2001. Use of spent irrigation water for microbiological analysis of alfalfa sprouts. J. Food Prot. 64:802–806 [DOI] [PubMed] [Google Scholar]
- 56.Neetoo H, Ye M, Chen HQ. 2008. Potential application of high hydrostatic pressure to eliminate Escherichia coli O157:H7 on alfalfa sprouted seeds. Int. J. Food Microbiol. 128:348–353 [DOI] [PubMed] [Google Scholar]
- 57.Waje C, Kwon JH. 2007. Improving the food safety of seed sprouts through irradiation treatment. Food Sci. Biotechnol. 16:171–176 [Google Scholar]
- 58.Suslow TV, Wu JC, Fett WF, Harris LJ. 2002. Detection and elimination of Salmonella Mbandaka from naturally contaminated alfalfa seed by treatment with heat or calcium hypochlorite. J. Food Prot. 65:452–458 [DOI] [PubMed] [Google Scholar]