Abstract
Acyl coenzyme A (CoA) synthetase (ACS) enzymes catalyze the activation of free fatty acids (FAs) to CoA esters by a two-step thioesterification reaction. Activated FAs participate in a variety of anabolic and catabolic lipid metabolic pathways, including de novo complex lipid biosynthesis, FA β-oxidation, and lipid membrane remodeling. Analysis of the genome sequence of the filamentous fungus Neurospora crassa identified seven putative fatty ACSs (ACS-1 through ACS-7). ACS-3 was found to be the major activator for exogenous FAs for anabolic lipid metabolic pathways, and consistent with this finding, ACS-3 localized to the endoplasmic reticulum, plasma membrane, and septa. Double-mutant analyses confirmed partial functional redundancy of ACS-2 and ACS-3. ACS-5 was determined to function in siderophore biosynthesis, indicating alternative functions for ACS enzymes in addition to fatty acid metabolism. The N. crassa ACSs involved in activation of FAs for catabolism were not specifically defined, presumably due to functional redundancy of several of ACSs for catabolism of exogenous FAs.
INTRODUCTION
Lipids are essential components of all living cells. They are major components of biological membranes (e.g., phospholipids) and serve as energy reserves (e.g., triacylglycerols) as well as signaling molecules (e.g., sphingolipids and lysophosphatidic acid). In fungi, fatty acids (FAs) are synthesized de novo via the FA synthase complex (FAS type I) present in the cytosol (1) or in the mitochondria via a suite of enzymes, including an acyl carrier protein. These mitochondrial FAS type II enzymes are not present in a complex (2). Many fungi can also utilize exogenous FAs, both for incorporation into lipids and as a carbon source. In addition to incorporation into complex lipids in the endoplasmic reticulum (ER) (3, 4) and mitochondria (5), FAs can be remodeled by desaturation and elongation (6–8) and degraded for energy production in the peroxisome (glyoxysome) (9, 10) and in the mitochondria (9, 11).
One of the most important catalytic activities in lipid metabolism is activation of FAs by acyl coenzyme A (CoA) synthetase (ACS) (EC 6.2.1.3). Activated FAs participate in a number of cellular metabolic pathways, including synthesis of phospholipids, triacylglycerols, and cholesterol esters, FA elongation, FA desaturation, and β-oxidation. ACSs catalyze the two-step ATP-dependent reaction of FA activation, whereby first the FA substrate is adenylated to form an acyl-AMP intermediate and subsequently AMP is exchanged with CoA, forming a thioester bond to yield the activated acyl-CoA (12).
The number of carbons present in the FA substrate of ACSs ranges from 2 to more than 26. The characteristic length of the FA substrate defines subfamilies of ACSs as short-chain (SC; 2 to 4 carbons), medium-chain (MC; 4 to 12 carbons), long-chain (LC; 12 to 20 carbons), very-long-chain (VLC; 18 to 26 carbons), and “bubblegum” (14 to 24 carbons) FA activators (13). All ACSs contain a highly conserved ATP/AMP binding motif (14), which is conserved among all adenylate-forming enzymes. A second conserved motif (fatty acyl-CoA synthetase [FACS] signature motif) has been identified in ACSs activating long-chain FAs (15). Variations of the FACS motif have been described for ACSs activating other length classes of FA: medium chain (16), very long chain (17), and bubblegum (18). Structural determination of a long-chain ACS from the bacterium Thermus thermophilus provided insights into four conserved regions in fatty ACSs: adenine binding, linker, and gate motifs, as well as the phosphate binding site which corresponds to motif I (19). The linker motif, which links the N- and C-terminal domains of the protein, is believed to control the conformation of the substrate binding pocket and thus is likely important for substrate specificity. The linker motif, which is part of the FACS signature motif, has various consensus sequences for different-length class activators.
In the yeast Saccharomyces cerevisiae, six fatty ACSs have been identified (20). Four of these enzymes (Faa1p, Faa2p, Faa3p, and Faa4p) show activity toward medium-, long-, and very-long-chain FAs, and one (Fat1p) shows activity only toward very-long-chain FAs. An additional putative ACS (Fat2p) that is homologous to Fat1p has been shown to localize to the peroxisome; however, function and substrate specificity have not been defined. In addition to very-long-chain ACS activity, Fat1p is required for uptake of long-chain FAs. It is believed that exogenous FA utilization occurs via vectorial acylation, which requires the concerted activities of Fat1p for transport and either Faa1p or Faa4p for activation. While Faa1p has been shown to account for 90% of ACS activity in S. cerevisiae, Faa4p provides partially redundant activity. Faa2p has been shown to localize to the peroxisome and is believed to be involved in the β-oxidation of medium- and long-chain FAs. While the metabolic role of Faa3p has not been defined, this enzyme localizes to the cell surface and shows preference for unsaturated long-chain FAs and very-long-chain FAs.
The filamentous fungus Aspergillus nidulans is reported to contain six fatty ACSs; two (FaaA and FaaB) are homologs to the Faa proteins of S. cerevisiae, and four (FatA through FatD) are homologs to Fat proteins. Three additional predicted ACSs were identified computationally (21). The six fatty ACSs were interrogated for their involvement in FA activation for degradation by assessing induction of the associated genes during growth on a FA substrate as well as growth of individual deletion strains on FAs as a sole carbon source (21). Reiser and coworkers showed that FaaB functions as the major peroxisomal ACS responsible for activating a broad range of FAs for catabolism (21), while FaaA exhibited cytoplasmic localization. FatA, FatB, and FatD localized to the peroxisome. Strains containing a single deletion of a Fat gene, as well as the FatA, FatB, and FatC triple deletion strain, failed to show a difference in growth phenotype on FA substrates compared to the wild type (WT) (21). This result was attributed to either functional redundancy or misidentification by in silico analyses.
A distantly related filamentous ascomycete fungus, Neurospora crassa, has been used as a model system for studying the role of lipids, especially for understanding factors that control synthesis and composition of FAs and complex FAs containing lipids (7, 22–25). However, little is known with regard to activation of FAs to CoA esters. Only one N. crassa ACS has been reported to date, acetyl-CoA synthetase (encoded by acu-5 [NCU06836]), which has been implicated in the utilization of acetate (26). We hypothesized that N. crassa contains a number of additional ACS enzymes residing in various cellular compartments and exerting specific functions by generating FA-CoA esters that play a role in anabolic or catabolic reactions.
The possibility of perturbing FA metabolism to induce secretion of free FAs for the purpose of producing biodiesel precursors is an attractive alternative fuel strategy. In S. cerevisiae, strains containing a deletion of FAA1 and FAA4 exhibit a FA secretion phenotype, which was attributed to interrupted FA recycling (27, 28). We hypothesized that altering the intracellular pool of activated FAs by deleting fatty ACSs that function to activate FAs for specific pathways would disrupt FA equilibrium. In this work, we interrogated the N. crassa genome for fatty ACSs using sequence homology to known fungal ACSs. To explore the roles of these putative ACSs in FA activation, we characterized the respective gene deletion strains for the ability to activate FA for anabolic and catabolic lipid processes, as well as for variations in lipid composition. We constructed double mutants to assess functional redundancy among the ACSs. As a final line of evidence for metabolic function, cellular localization was determined.
MATERIALS AND METHODS
N. crassa strains and growth conditions.
The N. crassa wild-type (WT) strain FGSC 2489 and the gene deletion strains available from the N. crassa deletion collection (29) were obtained from the Fungal Genetics Stock Center (FGSC; University of Missouri, Kansas City, MO) (30). A his-3::H1-dsRed strain was used for fluorescence microscopy colocalization (31). A Δacs-5 homokaryotic strain was obtained through a cross of the heterokaryon available in the deletion collection (FGSC 13827) with FCSC 2489. A Δacs-5 homokaryotic strain was confirmed by PCR using a flank-specific primer and a primer to the hygromycin cassette used for deletion construction (29) (see Table S1 in the supplemental material). Double mutant strains were obtained from crosses between the single gene deletion strains of opposite mating types; double mutant genotypes were confirmed by PCR using a flank-specific primer and a primer to the hygromycin cassette used for deletion construction (29) (see Table S1 in the supplemental material). Crosses were performed on Westergaard's medium (32). Strains were precultured on Vogel's medium (VM) agar (33) supplemented with 2% sucrose for 8 days to obtain conidia for growth experiments.
For time course growth experiments, 1 × 106 conidia/ml were inoculated into 250-ml Erlenmeyer flasks containing 100 ml of VM supplemented with 2% (wt/vol) glucose or Tween 80 and cultured in constant light at 25°C and 200 rpm. Refreshed spent medium was prepared by using sterile filtered culture broth of a 2-day-old VM–2% glucose culture of the WT strain. Whole flasks were harvested and processed as follows. Duplicate 10-ml samples were filtered, washed, and dried to constant mass on preweighed filter paper for cell concentration measurements. Glucose remaining in the culture broth supernatant was determined via high-performance liquid chromatography (HPLC) using a Shimadzu HPLC equipped with an RFQ fast acid column (Phenomenex Inc.) run at 55°C with 0.01 N sulfuric acid pumped at 1 ml/min as the eluent. FA remaining in Tween 80 medium was determined by lipid analysis of culture broth supernatant as indicated below. Fifty milliliters of culture was washed with deionized water to remove the residual carbon source and medium salts and centrifuged at 4,500 rpm for 10 min at 4°C. The cell pellet was frozen in liquid nitrogen and lyophilized to complete dryness for lipid analysis (see below).
For the anabolic and catabolic pathway determination, 1 × 106 conidia/ml were inoculated into 24-well plates containing 3 ml/well of VM supplemented with 0.5% (wt/vol) lactose and/or even-chain saturated FAs ranging from 4 to 24 carbons at a final concentration of 25 mM carbon suspended in 1% Tergitol–NP-40 and cultured in constant light at 25°C and 200 rpm. The FA synthase inhibitor cerulenin was added to a final concentration of 10 μg/ml. Growth was determined by visual inspection.
Lipid analysis.
Total lipids were extracted and derivatized from lyophilized biomass via a direct transesterification process. Briefly, 2 ml of acidified methanol (MeOH; 5% [vol/vol] sulfuric acid) was added to 20 to 30 mg of lyophilized biomass, and the biomass was incubated at 95°C for 30 min. After cooling, FA methyl esters (FAMEs) were extracted in 2 ml of hexane via gentle agitation at 37°C for 45 min. Residual FAs in Tween 80 media were extracted and derivatized from liquid culture broth by saponification followed by methylation. Briefly, 1 ml of methanol saturated with KOH was added to 0.5 ml culture broth, and the mixture was incubated at 100°C for 2 h. Acid-catalyzed methylation was accomplished by adding 1.5 ml 1:1.2 6 N HCl-MeOH and incubating at 80°C for 6 h. FAMEs were extracted into 2 ml hexane via gentle agitation at 37°C for 45 min.
FAME extracts were analyzed directly by gas chromatography-flame ionization detection (GC-FID) using a Varian 3900 gas chromatograph. FAMEs were separated with an Omegawax 250 column (Supleco Inc.). Helium was used as the carrier gas at a flow rate of 1 ml/min. The column temperature program was as follows: hold at 120°C for 3 min, ramp from 120 to 250°C at 7°C/min, and hold at 250°C for 10 min. FAMEs were identified and quantified against a 37-component FAME mix external standard set (Supelco Inc.). Methyl tridecanote was spiked into samples prior to transesterification, and recovery of this internal standard was greater than 95%.
Molecular techniques and strain construction.
Standard methods for cloning and other molecular techniques were performed according to published methods (34). Genomic DNA from the N. crassa WT strain was extracted according to the method of Lee and Taylor (http://www.fgsc.net/fgn35/lee35.pdf). DNA amplification was performed using Phusion high-fidelity polymerase (Finnzymes) according to the manufacturer's instructions.
To facilitate creation of green fluorescent protein (GFP) gene fusion strains for colocalization and complementation studies, the pCSR1 vector (35), which allows positive selection of csr-1 targeted transformants, was modified to contain the Myceliophthora thermophila gpdA promoter, a multiple cloning site (MCS), the synthetic GFP (sGFP) gene, and the M. thermophila gpdA terminator (T. Starr and N. L. Glass, personal communication).
Restriction-free (RF) cloning (36) was used to insert acs into the pCSR1:gpd-gfp vector. The open reading frame of each of the ACS genes with the exception of the stop codon was amplified from genomic DNA of WT N. crassa (FGSC 2489) using the respective primer sets listed in Table S1 in the supplemental material. Primers were designed to contain a 24-base overlap with the vector at the desired insertion site, followed by 20 to 25 bases complementary to the gene of interest. PCR products were agarose gel purified and extracted using a gel purification kit (Zymo Research). Individual PCR products were used as a “megaprimer” to amplify the vector at the template/primer loading ratio of 40:400 nmol vector to megaprimer. Subsequently, DpnI was added to the reaction mixture to digest the methylated parental vector, and the reaction mixture was used directly to transform chemically competent Escherichia coli to repair the nicks and propagate the vector. All of the resulting plasmids were sequenced to verify correct construction.
Transformation of N. crassa by electroporation was performed as previously described (35). The sGFP gene fusions were transformed into the respective gene deletion strains. Positive transformants were verified by PCR genotyping using the primer set listed in Table S1 in the supplemental material.
Confocal microscopy.
Mycelia for microscopy were grown on VM agar supplemented with 2% sucrose at 25°C overnight. Mycelia were observed using a 100×, 1.4-numerical-aperture (NA) oil immersion objective on a Leica SD6000 microscope attached to a Yokogawa CSU-X1 spinning disc head with a 488-nm and/or 561- nm laser. Dual-color imaging was accomplished using automated acquisition of the two wavelengths in series with a time differential for emission filter changeover.
Bioinformatic analysis.
Candidate N. crassa ACS genes and proteins were identified using the Basic Local Alignment Search Tool (BLAST) algorithm (http://www.ncbi.nlm.nih.gov/BLAST) (37). The search strategy used queries of the S. cerevisiae ACS proteins (Faa1p to Faa4p, Fat1p, and Fat2p) to probe the N. crassa nonredundant protein database (BLASTp) and N. crassa nucleotide database (tBLASTn). N. crassa DNA and protein sequences were obtained from the assembled genome sequence at the Broad Institute (http://www.broadinstitute.org/annotation/genome/neurospora/MultiHome.html). The candidate ACS amino acid sequences were examined for the presence of the two highly conserved motifs aforementioned (i.e., motif I [AMP/ATP binding] and motif II [FACS signature]) using FIMO (http://meme.sdsc.edu/meme/cgi-bin/fimo.cgi). Approximately 1,000 bp of the predicted promoter regions of the candidate ACS genes was analyzed for the conserved binding sequence (CCTCGG) of the FarA and FarB transcription factors, which have been implicated in regulation of genes involved in fatty acid degradation in A. nidulans (38).
Putative ACS orthologs of select fungal species were identified using BLASTp against the respective protein database. Multiple protein alignment was performed using MUSCLE (http://www.ebi.ac.uk/Tools/msa/muscle/) (39); any sequences not including either motif 1 or 2 were discarded. Phylogenetic trees were generated using neighbor-joining analysis in MEGA5 (molecular evolutionary genetic analysis) program (40). Evolutionary distances were computed using the Poisson correction method. All positions containing gaps were eliminated from the data set. The robustness of the tree topology was evaluated by bootstrap analysis using a sampling size of 1,000.
RESULTS
Identification of genes encoding putative N. crassa acyl-CoA synthetases.
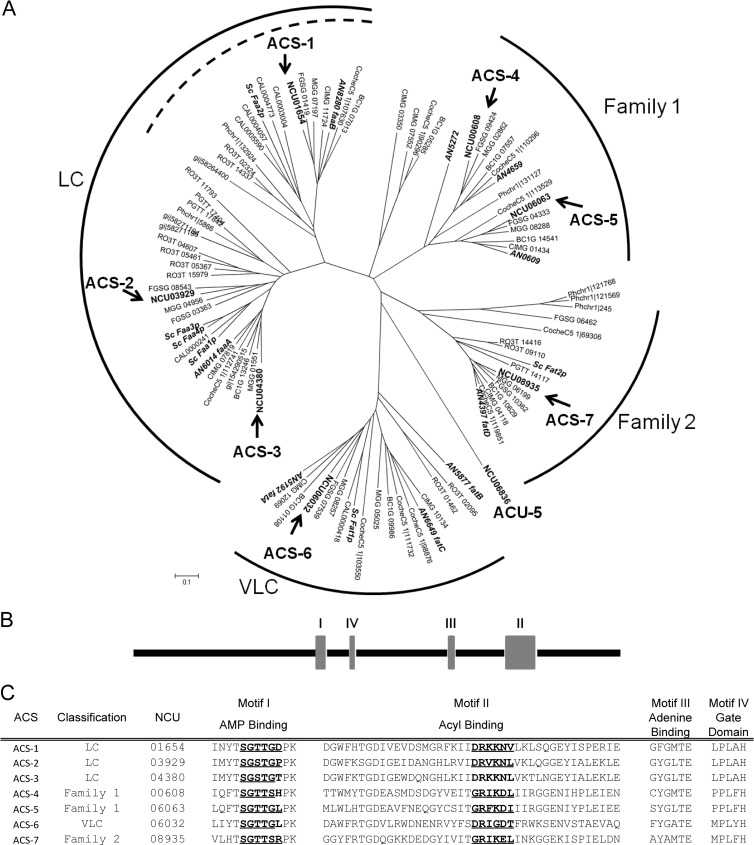
The amino acid sequences of six S. cerevisiae acyl-CoA synthetase proteins, Faa1p to Faa4p, Fat1p, and Fat2p, were used in BLASTp and tBLASTn searches of the N. crassa protein and nucleotide databases, respectively. Each identified ACS homolog was queried for the presence of the highly conserved AMP/ATP binding motif (14), as well as for the FACS signature motif (41); if either was absent, the identified homolog was eliminated. Seven N. crassa homologs of the S. cerevisiae ACS proteins were identified. The protein encoded by NCU01654 (ACS-1) showed significant identity (32.2%) to S. cerevisiae Faa2p, while two other N. crassa proteins, ACS-2 (encoded by NCU03929) and ACS-3 (encoded by NCU04380), showed high identity (40.6 to 47.8%) to S. cerevisiae proteins Faa1p, Faa3p, and Faa4p. Genes encoding four additional N. crassa proteins had high identity (26.8 to 52.2%) to genes encoding the FAT proteins: NCU06032 (encoding ACS-6) to the well-characterized Fat1p gene and NCU00608 (encoding ACS-4), NCU06063 (encoding ACS-5), and NCU08935 (encoding ACS-7) to the less-characterized Fat2p gene.
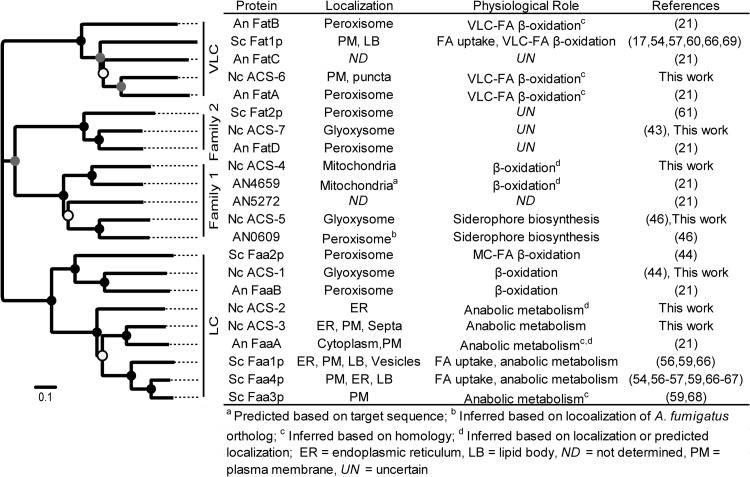
A previous phylogenetic analysis of human, mouse, zebrafish, fruit fly, nematode, and yeast ACSs revealed clades corresponding to FA substrate toward which the ACS was active (i.e., medium-, long-, or very-long-chain FAs) (42). To evaluate relationships among fungal ACSs, the genome sequences and protein databases of 11 additional fungi representing diversity across the fungal kingdom (Aspergillus nidulans, Botryotinia fuckeliana, Candida albicans, Cochliobolus heterostrophus, Coccidioides immitis, Cryptococcus neoformans, Fusarium graminearum, Magnaporthe grisea, Phanerochaete chrysosporium, Puccinia graminis, and Rhizopus oryzae) were interrogated for ACSs using the same search parameters as above. As many as 12 and as few as 3 putative ACS genes were identified in each fungal species. Protein sequences from the 94 identified fungal ACSs grouped into 4 clades (Fig. 1A; see Table S2 in the supplemental material). The clustering of the ACSs was consistent with the substrate specificity of characterized S. cerevisiae ACSs and predicted FA specificity for the long-chain (LC) and very-long-chain (VLC) clades. The FAA homologs, all predicted to have LC specificity, fell into two subclades. One subclade included N. crassa ACS-1 (Nc-ACS-1), S. cerevisiae Faa2p (Sc-Faa2p), and A. nidulans FaaB (An-FaaB), all of which localize to the peroxisome/glyoxysome (21, 43, 44). Within the second subclade, S. cerevisiae has two closely related paralogs (Sc-Faa3P and Sc-Faa4p), plus an additional ACS (Sc-Faa1p), while A. nidulans has only one (An-FaaA) in this entire subclade. In contrast, N. crassa has two predicted proteins, ACS-2 (encoded by NCU03929) and ACS-3 (encoded by NCU04380).
Fig 1.
Summary of acyl-CoA synthetases in fungi. (A) Phylogenetic tree of putative ACS proteins identified in 13 fungal species (Aspergillus nidulans, Botryotinia fuckeliana, Candida albicans, Cochliobolus heterostrophus, Coccidioides immitis, Cryptococcus neoformans, Fusarium graminearum, Magnaporthe grisea, Neurospora crassa, Phanerochaete chrysosporium, Puccinia graminis, Rhizopus oryzae, and Saccharomyces cerevisiae). N. crassa ACSs are in boldface, and gene names are indicated with arrows. A. nidulans and S. cerevisiae ACSs are in boldface and italicized. (B) Domain organization of the N. crassa ACSs. (C) Conserved amino acid sequences of putative N. crassa ACSs. Boldface and underlined amino acids within motifs I and II show the phosphate binding and linker domains, respectively. LC, long chain; VLC, very long chain.
The filamentous fungal homologs of Sc-Fat2p, for which substrate specificity is uncharacterized, formed two clades; we denote these as ACS families 1 and 2 (Fig. 1A). Family 2 comprised one S. cerevisiae protein, Fat2p, one A. nidulans protein, FatD, and N. crassa ACS-7 (encoded by NCU08935). An A. fumigatus homolog of one of the ACS proteins within family 1 in A. nidulans (AN0609), termed SidI, localized to the peroxisome and was recently characterized as a mevalonyl-CoA ligase involved in siderophore (triacetylfusarinine C [TAFC]) biosynthesis (45, 46). Two additional ACSs in family 1 (AN5272 and AN4659) were identified by in silico sequence analysis and are predicted to localize to the peroxisome (AN5272) or to mitochondria (AN4659); AN4659 was predicted to have short-chain FA specificity (21). In N. crassa, two predicted proteins, ACS-4 (encoded by NCU00608) and ACS-5 (encoded by NCU06063) cluster in family 1. In particular, ACS-5 is in the same clade as AN0609.
In addition to the AMP/ATP binding and the FACS signature motifs, four structurally significant domains have been defined for ACSs: phosphate binding, linker, adenine binding, and gate (19), where the phosphate binding domain is located within the AMP/ATP binding motif and the linker domain is located within the FACS signature motif. All seven of the predicted N. crassa ACS proteins contained sequences similar to those of all four domains; their alignment reveals a conserved domain structure (Fig. 1B and C). It has been proposed that the structural location of the linker motif determines the substrate specificity for ACS enzymes (19). Three similar consensus sequences have been suggested for short-chain and medium-chain (Gly-Arg-Xaa-Asp), long-chain (Asp-Arg-Xaa-Lys), and very-long-chain (Asp-Arg-Xaa-Gly) ACSs. As expected, the linker motif sequences for ACS-1, ACS-2, and ACS-3 match the consensus sequence for the long-chain ACS, while the linker motif sequence for ACS-6 matches the consensus sequence for the very-long-chain ACS. The linker motif for the Fat2p homologs (ACS-4, ACS-5, and ACS-7), however, is more similar to the short- and medium-chain ACS consensus motif. Our alignment showed that the S. cerevisiae and A. nidulans ACSs that fall within the same clades as the N. crassa ACSs share the respective short-/medium-, long-, and very-long-chain linker motifs.
In A. nidulans, two Zn2-Cys6 binuclear transcription factors, FarA and FarB, regulate genes involved in fatty acid catabolism; farAΔ mutants are unable to use fatty acids as a sole carbon source, while a farBΔ mutant is unable to utilize short-chain fatty acids but is unaffected in growth on longer-chain fatty acids (38). Homologs of farA and farB are present in the N. crassa genome (far-1, NCU08000, and far-2, NCU03643). The binding sites for A. nidulans FarA and FarB have been determined by in vitro analyses and are identical for both FarA and FarB: CCGAGG/CCTCGG (38). This binding site was used to search predicted promoter regions of acs-1 through acs-7. All but acs-4 contained one or more of the putative FAR-1/FAR-2 binding sites. Chromatin immunoprecipitation-DNA sequencing (ChIP-seq) analysis identified FAR-1 binding within promoter regions of acs-1, acs-5, and acs-6 and FAR-2 binding sites within predicted promoters of acs-5 and acs-7 (E. L. Bredeweg and M. Freitag, personal communication).
Phenotypic analysis of Δacs strains grown on glucose.
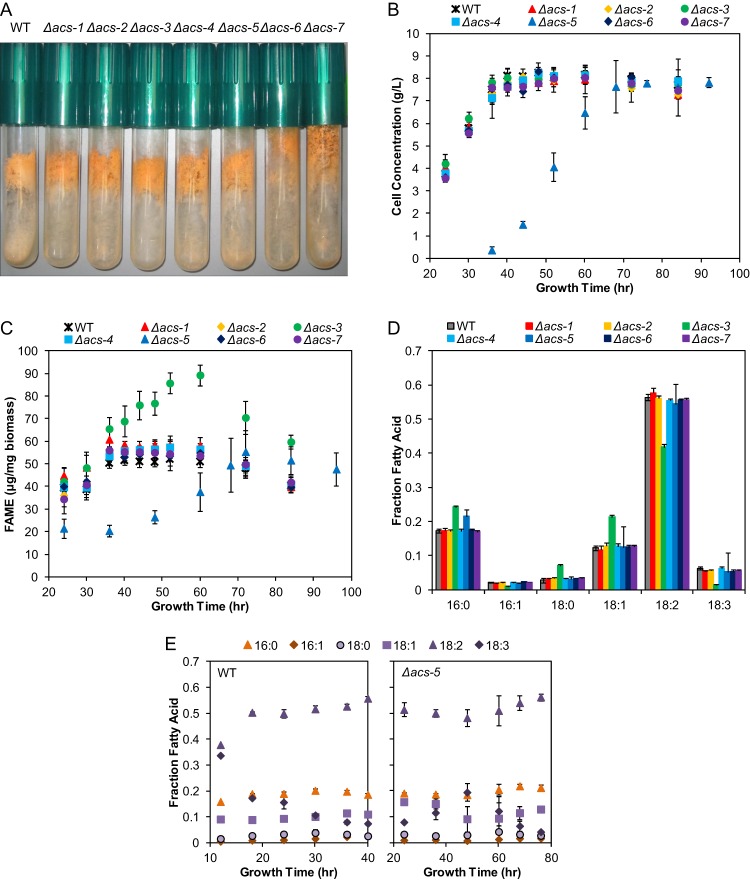
The macroscopic phenotype and growth of individual strains containing deletions in each of the ACS genes were evaluated on agar medium containing glucose. The Δacs-1, Δacs-2, Δacs-3, Δacs-4, and Δacs-5 deletion strains were morphologically indistinguishable from their wild-type isogenic parental strain. However, the Δacs-6 and Δacs-7 strains displayed slightly longer aerial hyphae than the wild-type strain (Fig. 2A). The maximal linear growth rates of all of the mutant strains were equivalent to that of the wild-type strain on agar with Vogel's medium (VM)–2% glucose (data not shown). However, the ability to grow in submerged liquid VM–2% glucose was not consistent among all strains. For example, the Δacs-5 mutant exhibited a significant growth defect (Fig. 2B) and displayed a 20-h lag in biomass accumulation during submerged growth compared to the wild type. The maximal growth rate (0.31 g/liter/h between 36 to 60 h) and accumulated cell mass (8 g/liter), however, were unaffected.
Fig 2.
Phenotypic analysis of N. crassa Δacs strains on glucose. (A) Macroscopic morphology of the WT (FGSC 2489) and Δacs strains. (B) Biomass accumulation in submerged liquid culture in VM–2% glucose. (C) Gas chromatography-flame ionization detection (GC-FID) quantification of fatty acid methyl esters (FAMEs) derived from total lipids during time course in VM–2% glucose. Lipid was quantified per mg of lyophilized biomass. (D) Fatty acid (FA) composition of total cellular lipid of N. crassa Δacs strains. Individual FAMEs derived from total lipids were quantified using GC-FID. Fatty acids are given as a mass fraction of total derivatized FA at the 60-h growth point for all strains, except Δacs-5, which was analyzed at 72 h. Ninety-seven percent of total cellular FAs comprise C16:0, C16:1, C18:0, C18:1, C18:2, and C18:3. (E) FA profile for WT and Δacs-5 strains during early time points. All values are representative of quadruplicate biological samples. Error bars indicate standard deviations.
To further investigate the physiological role of ACS-5, we assessed whether the growth defect in the Δacs-5 mutant was a result of a germination defect in liquid media by evaluating the frequency of conidial germination in liquid VM–2% glucose for the wild type. We also included the Δacs-3 and Δacs-4 mutants, as they displayed an altered phenotype compared to the wild-type strain. The frequency of germination for the wild-type strain at 4 h postinoculation was 79%. The Δacs-4 and Δacs-5 mutants germinated with the same frequency as the wild type (78% and 76%, respectively), while the Δacs-3 mutant exhibited slightly increased germination frequency (88%) at 4 h.
To determine if an arrest in growth occurred in the Δacs-5 mutant after germination, the number of colonies that developed from individual conidia was determined and compared to that for the wild type, as well as to those for the Δacs-3 and the Δacs-4 mutants. Approximately 200 conidia per strain were plated onto Vogel's medium containing sorbose and incubated at 30°C for 2 days. For the wild-type strain, 100% of plated conidia formed viable, macroscopic colonies. The Δacs-3, Δacs-4, and Δacs-5 mutants produced 65%, 96%, and 0.5% viable colonies, respectively. Colony formation was reassessed after 7 days, and no additional colonies of the Δacs-3 and Δacs-4 strains were observed. However, 70% of Δacs-5 conidia formed viable, macroscopic colonies. This result suggests that the Δacs-5 strain has a defect postgermination that resulted in an extreme lag phase. These data for the Δacs-5 strain are consistent with previously reported growth phenotypes for conidia that have lost the siderophore ferricrocin (47), including an inoculum-dependent population effect (48). In A. fumigatus, a homolog of acs-5, termed sidI, was shown to be essential for formation of the TAFC siderophore (45), and recently it was shown that introduction of Nc-acs-5 into an A. nidulans mutant lacking PTS1-dependent peroxisomal import (ΔpexE) increased TAFC production (46).
N. crassa secretes the hydroxamate cyclic peptide siderophore coprogen under iron-limited conditions, but also under normal laboratory culture conditions (47). To evaluate whether ACS-5 is involved in siderophore biosynthesis in N. crassa, wild-type and Δacs-5 strains were grown in iron-replete medium (VM–2% glucose supplemented with1.5 mM FeSO4). Similar conditions allowed recovery of growth phenotypes associated with disruption of extracellular siderophore biosynthesis in A. fumigatus (49). The lag phase in the Δacs-5 mutant was completely abolished under these conditions (see Fig. S1 in the supplemental material). Previously, it has been shown that the addition of spent medium from a wild-type culture reverted the growth phenotype of N. crassa siderophore mutants, consistent with the presence of an extracellular siderophore (47, 50). As predicted, the addition of spent medium from a wild-type culture to fresh medium inoculated with the Δacs-5 mutant also abolished the lag phase (see Fig. S1 in the supplemental material). These data indicate that, as for SidI in A. fumigatus, ACS-5 is involved in siderophore biosynthesis in N. crassa.
Lipid phenotype of ACS mutants.
Since ACSs play a pivotal role in the majority of the lipid metabolic pathways, we determined whether any of the N. crassa ACS deletion mutants had lipid perturbations. As a proxy for lipid perturbation, we evaluated lipid-derived FA methyl esters (FAMEs) using gas chromatography-flame ionization detection (GC-FID). Lipids were extracted, derivatized, and quantified from lyophilized biomass of wild-type and Δacs cultures grown under submerged conditions on VM–2% glucose over a period of 5 days (Fig. 2C). In the wild-type strain, the lipid content increased until carbon source depletion (36 h). Intracellular lipid content remained constant for an additional period of 25 h, after which lipid stores began to be utilized, as indicated by the decrease in lipid content after 70 h. The lipid profiles by FAME analyses of the Δacs-1, Δacs-2, Δacs-4, Δacs-6, and Δacs-7 mutants were indistinguishable from that of the wild-type strain. Consistent with the growth phenotype in liquid culture, lipid accumulation in the Δacs-5 mutant showed a prolonged lag phase and reached wild-type levels only after 70 h of growth. Most notably, the Δacs-3 strain accumulated 75% more lipid per cell mass than the wild-type strain, with the highest lipid accumulation at 60 h (Fig. 2C). However, no evidence of FA secretion was detected via FAME analysis of culture broth supernatant for any strain.
FAME analysis was used to determine the profile of FAs in wild-type and the Δacs strains at the 60-h time point, with the exception of the Δacs-5 mutant, which was analyzed at the 72-h time point (Fig. 2D). Like most other fungi, N. crassa produces predominantly 16- and 18-carbon saturated and unsaturated FAs, with 18:2 accounting for 50% (wt/wt) of the total FA. A small amount of 24-carbon FAs was detected as a part of the sterol ester class of lipid (data not shown). Analysis of lipid distribution by FAME analysis in the Δacs-3 mutant (60 h) revealed a decrease in unsaturated FAs (16:1, 18:2, and 18:3), with a corresponding increase in saturated 16-carbon (16:0) and saturated and monounsaturated 18-carbon FAs (18:0 and 18:1) (Fig. 2D). This result suggests that ACS-3 is involved in activating FAs for desaturation.
The Δacs-1, Δacs-2, Δacs-4, Δacs-6, and Δacs-7 mutants showed a lipid profile identical to that of the wild-type strain (Fig. 2D). At the 72-h time point, the Δacs-5 mutant has more 16:0 but was otherwise more similar to the wild type for the other lipid classes. However, at earlier time points the lipid accumulation in the Δacs-5 mutant was significantly different (Fig. 2C). We therefore compared FA composition in the Δacs-5 mutant to that in the wild type over a time course of early growth (12 to 24 h for WT and 24 to 60 h for the Δacs-5 mutant) (Fig. 2E). During the lag phase of growth in the wild-type strain (up to 12 h), 70% of the total FA comprised 18:2 and 18:3 lipids in equivalent amounts. As the culture aged to stationary phase, the fraction of 18:3 decreased to 5% and 18:2 increased to 60% of the total lipids. For the Δacs-5 strain, 18:2 constituted 50% or more of the total FAs at all time points in the culture, although at ∼50 h of growth, the Δacs-5 mutant showed a rise in 18:3 FAs accompanied by a dip 18:1 FAs.
Characterization of the role of FA activation for anabolic and catabolic pathways.
The activated FAs synthesized by the ACSs are used in both anabolic pathways (i.e., complex lipid assembly, FA elongation, FA desaturation) and catabolic pathways (i.e., β-oxidation). ACS activity is not required for activation of de novo FAs since fatty acyl-CoA esters are the end product of FA biosynthesis; however, ACSs will be active on endogenous FAs obtained from complex lipid remodeling or exogenous FAs obtained from the culture medium. In this study, we probed ACS activity against exogenous FA.
To assay for FA activation for catabolism, even-number 6- to 24-carbon saturated FAs were provided as a sole carbon source to the wild type and the Δacs deletion mutants. Strains that exhibited a growth defect under these conditions are deficient in activating a FA for catabolism. As shown in Table 1 and in Fig. S2 in the supplemental material, no strain, including the wild type, was able to grow on 6- to 10-carbon FAs as the sole carbon source, indicating that 6- to 10-carbon FAs are not activated by any of the ACSs. Similarly, slower growth was observed for all strains on very-long-chain FAs (20:0, 22:0, and 24:0). Growth on the FAs 12:0, 14:0, 16:0, 18:0 of the Δacs-2, Δacs-4, Δacs-5, Δacs-6, and Δacs-7 mutants was identical to that of the wild type. However, the Δacs-1 and Δacs-3 mutants did not grow on 12-carbon FAs. These results indicate that the medium-chain FA 12:0 is preferentially activated for catabolism by ACS-1 and ACS-3. The fact that no mutant was significantly different from the wild type in utilization of long- and very-long-chain FAs suggests that there is functional redundancy among the ACSs of N. crassa for activation of these FAs for catabolism.
Table 1.
Growth phenotype of N. crassa Δacs mutants in the presence of fatty acids
| Medium and strain | Phenotypea for: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No FA | 4:0 | 6:0 | 8:0 | 10:0 | 12:0 | 14:0 | 16:0 | 18:0 | 20:0 | 22:0 | 24:0 | |
| VM–25 mM carbon (fatty acid) | ||||||||||||
| WT | NG | LG | NG | NG | NG | G | G | G | G | LG | VLG | VLG |
| Δacs-1 | NG | LG | NG | NG | NG | NG | G | G | G | LG | VLG | VLG |
| Δacs-2 | NG | LG | NG | NG | NG | G | G | G | G | LG | VLG | VLG |
| Δacs-3 | NG | LG | NG | NG | NG | NG | G | G | G | LG | VLG | VLG |
| Δacs-4 | NG | LG | NG | NG | NG | G | G | G | G | LG | VLG | VLG |
| Δacs-5 | NG | LG | NG | NG | NG | G | G | G | G | LG | VLG | VLG |
| Δacs-6 | NG | LG | NG | NG | NG | G | G | G | G | LG | VLG | VLG |
| Δacs-7 | NG | LG | NG | NG | NG | G | G | G | G | LG | VLG | VLG |
| VM–0.5% lactose, 25 mM carbon (fatty acid) | ||||||||||||
| WT | G | VLG | NG | NG | NG | VLG | G | G | G | LG | LG | LG |
| Δacs-1 | G | VLG | NG | NG | NG | NG | G | G | G | LG | LG | LG |
| Δacs-2 | G | VLG | NG | NG | NG | VLG | G | G | G | LG | LG | LG |
| Δacs-3 | G | VLG | NG | NG | NG | VLG | G | G | G | LG | LG | LG |
| Δacs-4 | G | VLG | NG | NG | NG | VLG | G | G | G | LG | LG | LG |
| Δacs-5 | G | VLG | NG | NG | NG | VLG | G | G | G | LG | LG | LG |
| Δacs-6 | G | VLG | NG | NG | NG | VLG | G | G | G | LG | LG | LG |
| Δacs-7 | G | VLG | NG | NG | NG | VLG | G | G | G | LG | LG | LG |
| VM–0.5% lactose, 25 mM carbon (fatty acid), 10 μg/ml cerulenin | ||||||||||||
| WT | VLG | VLG | NG | NG | NG | VLG | G | G | G | LG | LG | LG |
| Δacs-1 | VLG | VLG | NG | NG | NG | NG | G | G | G | LG | LG | LG |
| Δacs-2 | VLG | VLG | NG | NG | NG | NG | G | G | G | LG | LG | LG |
| Δacs-3 | VLG | NG | NG | NG | NG | NG | VLG | LG | LG | NG | NG | NG |
| Δacs-4 | VLG | VLG | NG | NG | NG | NG | G | G | G | LG | LG | LG |
| Δacs-5 | VLG | VLG | NG | NG | NG | NG | G | G | G | LG | LG | LG |
| Δacs-6 | VLG | VLG | NG | NG | NG | NG | G | G | G | LG | LG | LG |
| Δacs-7 | VLG | VLG | NG | NG | NG | NG | G | G | G | LG | LG | LG |
NG, no growth; VLG, very low growth; LG, low growth; G, growth. Boldface indicates differences from the respective control.
To assay for activation for anabolic pathways, the growth phenotype between two feeding regimens was evaluated for each strain: FA and lactose-supplemented Vogel's medium in the presence or absence of cerulenin, an inhibitor of FA biosynthesis (Table 1; see Fig. S3 and S4 in the supplemental material). In the absence of cerulenin, all strains should grow on all FAs unless the FA is inhibitory; this condition serves as the growth control. For FA media plus lactose and cerulenin, a growth defect in the Δacs strains indicates a deficiency in activating the FA for anabolic pathways. If growth is observed under these conditions, the ACS is not needed for anabolic pathways or the activity is compensated for by another ACS. As shown in Table 1 and in Fig. S3 in the supplemental material, wild-type N. crassa is unable to grow in the presence of 6:0 to 10:0 FAs, even in the presence of lactose. Because N. crassa was also unable to activate these FAs for catabolism (Table 1; see Fig. S2 in the supplemental material), these results indicate that these FAs inhibit de novo biosynthesis of FAs of appropriate chain length for membrane formation. This result is consistent with findings where medium-length FAs inhibited FA biosynthesis and prevented synthesis of full-length membrane lipids in A. nidulans and insect eggs (11, 51). In addition, the Δacs-1 mutant was unable to grow in the presence of 12:0 and lactose (Table 1; see Fig. S3 in the supplemental material). Among the ACS mutants, only the Δacs-3 mutant exhibited differential growth in the presence of cerulenin, lactose, and 14:0 to 24:0 FAs (Table 1; see Fig. S4 in the supplemental material). These data indicate that ACS-3 is the main activating ACS of exogenous FAs. Other ACSs, however, provide some functional redundancy toward long-chain FAs because the Δacs-3 strain exhibited some growth on 14:0 to 18:0 FAs.
Phenotypic analysis of the Δacs-3 strain grown in the presence of FAs.
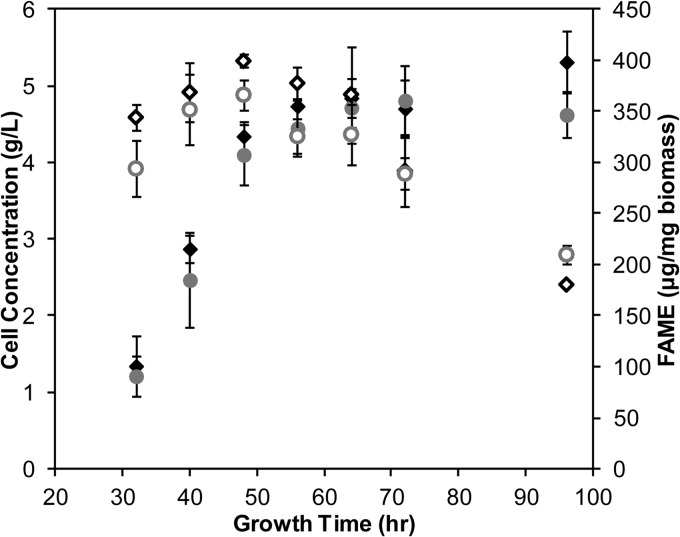
To further probe the underlying physiological role of ACS-3, we assessed the growth and lipid phenotypes of the wild type and the Δacs-3 mutant when grown on Tween 80. This nonionic surfactant composed of PEGylated sorbitan esterified with oleic acid (18:1) (25% by weight of Tween 80) is an ideal source for FA supplementation for filamentous fungi (24). N. crassa secretes lipases that cleave the FA from the polysorbitan backbone. Both the FA and polysorbitan component can be used as a carbon source by N. crassa, as is indicated by the growth yield (Fig. 3). Because Tween 80 is soluble in water and can be fully used as a carbon source by N. crassa, we can assess the influence on the lipid profile of FA-supplemented media in the wild type versus the mutant.
Fig 3.
Phenotypic analysis of N. crassa Δacs-3 mutant strain compared to the wild type with FA-supplemented medium. Open and solid diamonds represent data for the wild type, while open and solid circles represent data for the Δacs-3 mutant. Solid diamonds and circles represent biomass accumulation in submerged liquid culture growth in VM–2% Tween 80. Open diamonds and circles represent GC-FID quantification of FAMEs derived from total lipids over the growth time course. Lipid was quantified per mg of lyophilized biomass. All values are representative of triplicate biological samples. Error bars indicate standard deviations.
Both the wild-type and Δacs-3 strains exhibited a 40% reduction in linear growth rate (0.19 g/liter/h) on VM–2% Tween 80 compared to growth on glucose media (Fig. 3). The lipid content of the wild-type versus that of the Δacs-3 culture grown on VM supplemented with Tween 80 was assessed via FAME analysis (Fig. 3). For the wild-type strain, the overall lipid content at its maximum was 6-fold higher than that for growth on glucose as the sole carbon source (compare Fig. 2C with 3). This result indicates that, under an excess of FA, N. crassa is either storing FA for later use or internally compartmentalizing the FAs to mediate the toxicity of the hydrophobic environment. As the strains enter stationary phase, lipid content decreases, indicating that the amount of FA present in the media is no longer in excess and can be efficiently utilized. Interestingly, although the Δacs-3 mutant accumulated significantly more lipid under glucose conditions (Fig. 2C), it showed a lipid profile similar to that of the wild type when grown in media with exogenously supplied FAs (Fig. 3). These data indicate that exogenously supplied FA is sufficient to repress FA biosynthesis and overaccumulation of lipid in this strain.
Subcellular localization of ACSs.
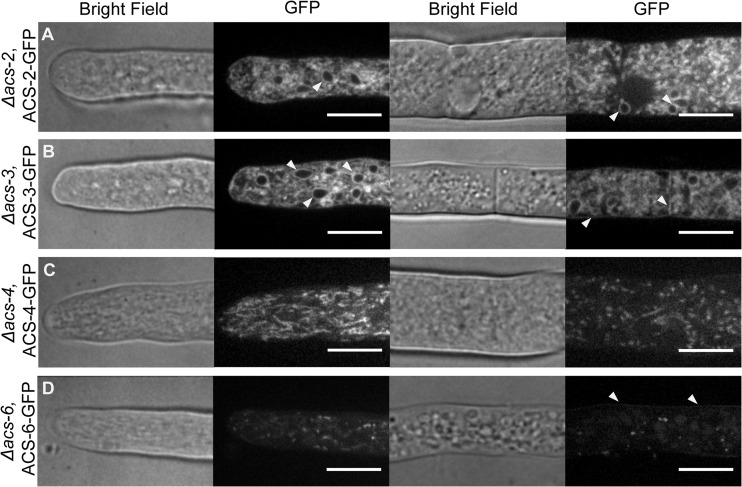
To investigate the subcellular localization of the N. crassa ACSs, C-terminal sGFP fusion constructs, driven by the constitutive Myceliophthora thermophila gpdA promoter, were prepared and transformed into the csr-1 locus (35) of Δacs-2, Δacs-3, Δacs-4, and Δacs-6 mutants (see Materials and Methods). Three independent clones were evaluated for each construct by spinning disc confocal microscopy. Trunk and branch hyphae, as well as apical and distal hyphal regions, were analyzed for marker fluorescence. ACS-1 and ACS-7 were not tagged, as they were previously localized to the proteome of the peroxisome/glyoxysome (43). Similarly, a GFP-tagged ACS-5 was shown to localize to peroxisomes when introduced into A. nidulans (46).
Confocal fluorescence microscopy revealed that ACS-2 and ACS-3 have similar localization patterns (Fig. 4A and B) and localized to membrane-bound structures that resemble the endoplasmic reticulum (ER). Colocalization of H1::dsRed (i.e., dsRed-labeled histone 1, nuclear marker) with either ACS-2–GFP or ACS-3–GFP verified ER localization of ACS-2 and ACS-3 (Fig. 5A and B). Additionally, ACS-3 localized to the plasma membrane (PM) and septa, consistent with our finding that ACS-3 is the major activator of exogenous FAs. The ACS-4–GFP fusion localized to mitochondria (Fig. 4C), which in the apical tip region appear as long tubular structures, while in subapical regions, the structures were shorter and more diffuse; ACS-4–GFP colocalized with the MitoTracker Red FM stain (Fig. 5C). Finally, the Δacs-6 strain expressing ACS-6–GFP showed localization to the plasma membrane in distal hyphal regions, as well as to puncta throughout the hypha, but did not colocalize with the glyoxysome (data not shown). While localization to the plasma membrane was not observed in all hyphae (Fig. 4D), this localization pattern is expected for ACS-6, which is the homolog to the S. cerevisiae long-chain FA transporter, Fat1p.
Fig 4.
Localization of ACS-GFP fusion proteins. ACS-GFP fusions were expressed in the background strain of the respective Δacs strain. Bright-field and GPF fluorescence images were obtaining by spinning disc confocal microscopy (see Materials and Methods). The two columns of panels on the left show localization in the tip region of a hypha, while the two right columns show localization in subapical regions. Arrows indicate putative endoplasmic reticulum (ER) localization for ACS-2–GFP and ER, plasma membrane, and septal localization for ACS-3–GFP. For ACS-6–GFP, arrows show plasma membrane localization. Bars = 10 μm.
Fig 5.
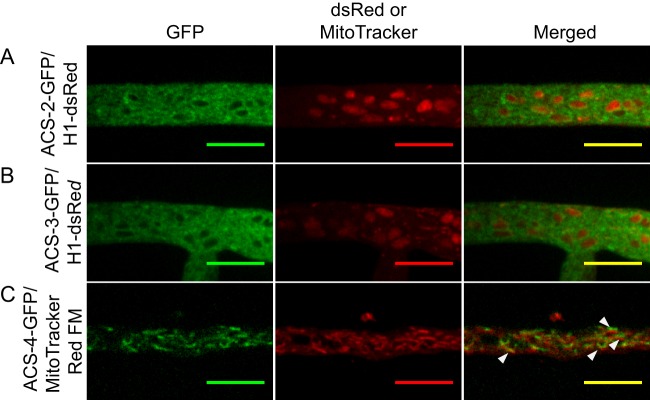
Colocalization of ACS proteins with fluorescent markers for different cellular structures. (A) Forced heterokaryon of Δacs-2 ACS-2–sGFP plus Δrid H1-dsRed strains. (B) Forced heterokaryon of Δacs-3 ACS-3–sGFP plus Δrid H1-dsRed strains. ACS-2–GFP and ACS-3–GFP localizes to the membrane surrounding the nuclei, as indicated by the H1-dsRed fluorescence, indicating endoplasmic reticulum localization. (C) Δacs-4 strain ACS-4–GFP colocalizes with the mitochondria, as indicated by MitoTracker Red FM dye. Fluorescence images were obtaining by spinning disc confocal microscopy (see Materials and Methods). Bars = 10 μm.
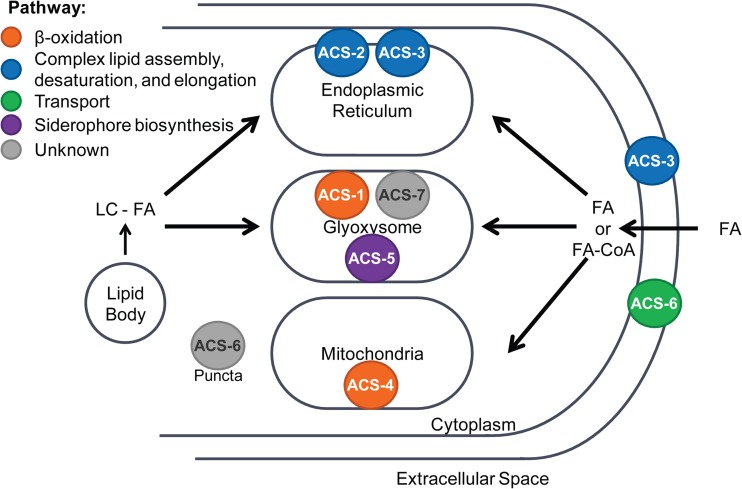
Subcellular localization of each ACS gives an indication of the pathways for which they provide activated FAs. For example, the glyoxysomal localization of ACS-1 and ACS-7 and the mitochondrial localization of ACS-4 support their role in providing activated FA for the glyoxysomal and mitochondrial β-oxidation pathways, respectively. ACS-2 and ACS-3, which exhibited ER localization, are likely involved in activating FAs for anabolic pathways such as FA elongation, desaturation, and complex lipid assembly, as the ER houses other enzymes involved in these pathways. Since most of the Δacs mutant strains did not exhibit phenotypes different from that of the wild-type strain, we were unable to determine functional complementation. However, the wild-type FA phenotype was restored in the Δacs-3 mutant by the introduction of the ACS-3–GFP fusion construct (see Fig. S5 in the supplemental material).
Double mutant analysis.
To gain further insight into the physiological role of specific ACSs and to elucidate possible functional redundancy, double deletion mutants of the long-chain clade acs genes were constructed and evaluated for FA composition: Δacs-1; Δacs-2, Δacs-1; Δacs-3, and Δacs-3; Δacs-2 mutants. We were unable to produce the triple deletion strain (Δacs-1; Δacs-3; Δacs-2), suggesting that a strain bearing all three of these lesions is not viable. The Δacs-1; Δacs-2 and Δacs-1; Δacs-3 mutants exhibited a wild-type growth phenotype on solids and in liquid medium, while the Δacs-3; Δacs-2 mutant strain lacked conidia and displayed a 58% reduction in linear growth rate compared to the wild type. The total FA contents and compositional profiles are given in Table 2.
Table 2.
Fatty acid composition at 60 h growth on VM–2% glucose
| Strain | FAME amt (μg/mg biomass) | % (of total FAs) of FA: |
|||||
|---|---|---|---|---|---|---|---|
| 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | 18:3 | ||
| WT | 51.04 ± 2.7 | 17.26 ± 0.57 | 2.27 ± 0.09 | 2.79 ± 0.72 | 12.3 ± 0.63 | 56.41 ± 0.82 | 6.33 ± 0.49 |
| Δacs-1 | 58.22 ± 3.39 | 17.34 ± 0.78 | 1.91 ± 0.14 | 3.12 ± 0.30 | 11.76 ± 1.15 | 57.67 ± 1.41 | 5.38 ± 0.30 |
| Δacs-2 | 55.58 ± 1.54 | 17.14 ± 0.42 | 2.16 ± 0.05 | 3.44 ± 0.34 | 12.83 ± 0.94 | 56.11 ± 0.83 | 5.66 ± 0.25 |
| Δacs-3 | 86.81 ± 4.42 | 24.28 ± 0.38 | 1.06 ± 0.08 | 7.12 ± 0.36 | 21.26 ± 0.78 | 41.88 ± 0.89 | 1.52 ± 0.19 |
| Δacs-1 Δacs-2 | 57.55 ± 1.71 | 16.67 ± 0.57 | 2.01 ± 0.04 | 2.91 ± 0.19 | 13.57 ± 0.53 | 57.74 ± 0.69 | 5.68 ± 0.28 |
| Δacs-1 Δacs-3 | 68.13 ± 1.56 | 19.63 ± 0.49 | 1.02 ± 0.03 | 4.01 ± 0.17 | 17.18 ± 1.07 | 54.66 ± 0.84 | 2.35 ± 0.3 |
| Δacs-3 Δacs-2 | 84.83 ± 21.05 | 33.52 ± 0.31 | 1.59 ± 0.03 | 5.61 ± 0.36 | 18.05 ± 0.20 | 38.53 ± 0.30 | 0.5 ± 0.04 |
Combinatorial deletions of the acs genes led to a spectrum of fatty acid phenotypes. The Δacs-1; Δacs-2 mutant strain exhibited nearly wild-type levels and composition of fatty acids, while the Δacs-3; Δacs-2 mutant accumulated Δacs-3 levels of fatty acids, with an even greater increase in 16:0 and concomitant decrease in polyunsaturated 18:2 and 18:3 FAs (Table 2). These data support the hypothesis of functional redundancy of ACS-2 and ACS-3 in activating FAs for desaturation and elongation. Surprisingly, the Δacs-1; Δacs-3 mutant strain gave an intermediate fatty acid phenotype compared to the Δacs-3 single deletion strain (compare Fig. 2C and D with Table 2). Thus, contrary to expectations, the deletion of the major peroxisomal ACS, presumably activating FA for β-oxidation, in the Δacs-3 background resulted in a decrease in FA accumulation. No evidence of FA secretion was detected in the extracellular medium for any of the double deletion strains.
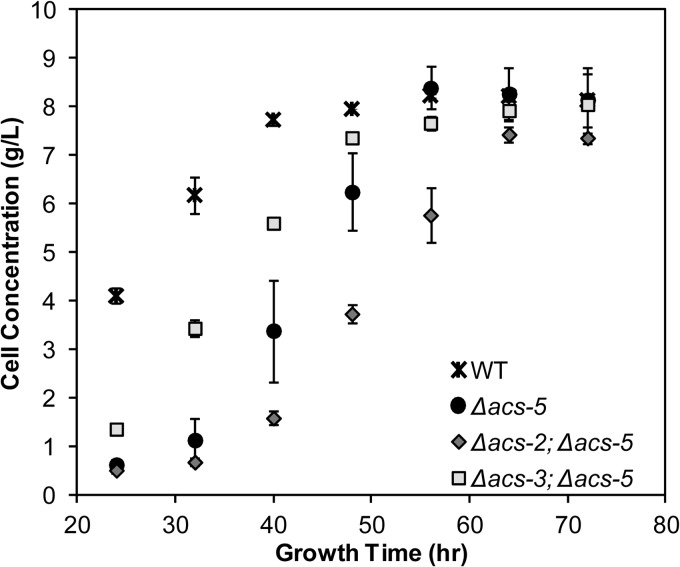
As a means to determine how further perturbations in ACS activity affected the Δacs-5 strain, double deletion strains with Δacs-2 and Δacs-3 were constructed and evaluated for growth lag in liquid culture growth. As shown in Fig. 6, the Δacs-2; Δacs-5 mutant exhibited a prolonged lag phase similar to the single Δacs-5 deletion strain. However, the Δacs-3; Δacs-5 mutant showed partial recovery of the lag in growth phenotype (Fig. 6) and exhibited nearly the same FA levels as the Δacs-3 mutant (data not shown). These data are consistent with the reduction in lag phase when the Δacs-5 mutant was grown in fatty acid-supplemented media (see Fig. S6 in the supplemental material).
Fig 6.
Biomass accumulation of Δacs-5 single- and multiple-gene deletion strains in VM–2% glucose. All values are representative of triplicate biological samples. Error bars indicate standard deviations.
DISCUSSION
Fatty ACSs play an important role in lipid metabolism by catalyzing the formation of acyl-CoA esters. In addition to activating FAs for degradation via β-oxidation or for biosynthesis of glycerolipids, acyl-CoA esters have been reported to act as regulators of various intracellular functions and in cell signaling (52). In this work, we identified seven putative fatty ACSs encoded in the genome of N. crassa; these enzymes are predicted to activate specific FA pools for various intracellular processes. We took a global perspective on the role of ACS in lipid metabolism by characterizing the FA content and composition in acs deletion strains in N. crassa and assessed cellular localization, thus providing further insights for FA elongation and desaturation pathways (Table 3). Studies in other fungi have taken a more focused approach to analyze ACS involvement in N-myristoylation (44, 53), FA import (54), and β-oxidation (21).
Table 3.
Overview of putative acyl-CoA synthetases
| Protein | Gene | Localization | Anabolic/catabolic pathway | Growth phenotype of Δacs strain on: |
Lipid phenotype of Δacs strain on: |
||
|---|---|---|---|---|---|---|---|
| Glucose | FA | Glucose | FA | ||||
| ACS-1 | NCU01654 | Glyoxysomea | Catabolicc | WT | No growth on 12:0 | WT | NDg |
| ACS-2 | NCU03929 | ERe | Anabolicd | WT | WT | WT | ND |
| ACS-3 | NCU04380 | ER, PM,f septa | Anabolicc | WT | No growth on 12:0 | ↑75%; ↓polyunsat FAh | WT |
| ACS-4 | NCU00608 | Mitochondria | Catabolicd | WT | WT | WT | ND |
| ACS-5 | NCU06063 | Peroxisomeb | Anabolic (siderophore) | Prolonged lag | WT | Different early time 18:3 | ND |
| ACS-6 | NCU06032 | PM, puncta | Uncertain | WT | WT | WT | WT |
| ACS-7 | NCU08935 | Glyoxysomea | Catabolicd | WT | WT | WT | ND |
Subcellular localization provides an excellent starting point for deciphering the physiological role of ACSs, as it reflects the location of the pathway for which it is providing activated FAs. To further support our finding for the physiological role of the N. crassa ACSs and broadly define the role of ACSs in fungal metabolism, we compared our results with S. cerevisiae and A. nidulans ACS homologs (Fig. 7). ACS homologs were identified from these three species in all clades, with the exception of the absence of a representative member from S. cerevisiae in family 1 (Fig. 1A and 7). While originally these clades were named based on known or predicted FA specificity of the ACS, we find a high conservation of subcellular localization (Fig. 7).
Fig 7.
Comparison of localization patterns and physiological roles of N. crassa, A. nidulans, and S. cerevisiae ACSs that clustered by phylogeny. See Materials and Methods for tree construction. Black circles, ≥95% bootstrap support; gray circles, 80 to 95%; white circles, 50 to 80%.
Three of the four clades contain members that localize to the peroxisome or glyoxysome, where the glyoxysome contains only a subset of the peroxisomal proteins. However, both of these organelles house the machinery required for FA β-oxidation. N. crassa, A. nidulans, and S. cerevisiae each have one ACS within the LC clade that localizes to the peroxisome/glyoxysome (Fig. 7 and 8). Sc-Faa2p activates MC FAs for degradation within the peroxisome (55), while an A. nidulans ΔfaaB strain exhibits a growth defect on short-, medium-, and long-chain FAs (21). The N. crassa Δacs-1 strain exhibited a growth defect on lauric acid (12:0) as the sole carbon source, even when supplemented with lactose, both with and without FA biosynthesis inhibition. These results indicate that ACS-1 mediates toxicity resistance to lauric acid by activating it for degradation, similar to FaaB (21).
Fig 8.
Cellular localization and schematic representation of fatty acid activation by ACSs in N. crassa for anabolic and catabolic lipid metabolic pathways. ACS-5 likely activates mevalonate for siderophore biosynthesis.
S. cerevisiae and N. crassa contain several additional ACSs within the LC clade (Sc-Faa1p, Sc-Faa3p, Sc-Faa4p, Nc-ACS-2, and Nc-ACS-3), while A. nidulans only has one (An-FaaA). ACSs in this subclade exhibit broad cellular localization (Fig. 7). S. cerevisiae faa1Δ and faa4Δ strains exhibit reduced β-oxidation of oleic acid (18:1) compared to the wild-type strain, indicating a role for Faa1p and Faa4p in FA catabolism (54). Although Sc-Faa1p and -Faa4p provide FA-CoA for β-oxidation, the more central physiological roles of these proteins is in FA uptake via vectorial acylation and activation of exogenous FA for anabolic lipid metabolic processes such as N-myristoylation (53, 54, 56, 57). Here, we showed that ACS-2 and ACS-3 were broadly distributed throughout the fungal cell in the cytoplasm and membrane-bound compartments, particularly the ER, as well as the plasma membrane and septa. The ER localization pattern indicates that ACS-2 and ACS-3 are FA activators for anabolic lipid metabolic pathways, since the ER is the site of complex lipid assembly, FA desaturation, and FA elongation (Fig. 8). The N. crassa Δacs-2 strain did not exhibit any growth or lipid phenotype compared with the wild-type strain, while the N. crassa Δacs-3 strain displayed the most pronounced lipid phenotype of all of the N. crassa Δacs mutants. Like Sc-Faa1p, Nc-ACS-3 provides the prominent ACS activity for exogenous FA fed into anabolic pathways, which is also consistent with its plasma membrane localization. The A. nidulans member of this clade, encoded by faaA, was not evaluated for a role in anabolic activation; we predict that the A. nidulans ΔfaaA strain would exhibit a phenotype similar to that of the N. crassa Δacs-3 mutant.
One of the most prominent phenotypes observed in the N. crassa Δacs-3 strain was the overaccumulation of FAs. Because a catabolism phenotype was not observed for the Δacs-3 mutant on exogenous FA, the cause for increased lipid accumulation in the Δacs-3 strain must be increased FA synthesis. The increased content of saturated and shorter FAs of the Δacs-3 mutant and even more so of the Δacs-3; Δacs-2 double mutant suggests the roles of ACS-2 and ACS-3 in FA activation for desaturation and elongation, consistent with a findings on a homologous protein in the oleaginous yeast Yarrowia lipolytica (58). Increased FA biosynthesis may result from relief of feedback inhibition by acyl-CoA esters or possibly an attempt by the cell to fill an unmet need for unsaturated FAs. However, when the Δacs-3 strain was grown in media supplemented with FA, the mutant no longer accumulated lipid above wild-type levels, supporting the hypothesis of feedback inhibition relief. Our results differ from the FA secretion phenotype observed in the S. cerevisiae Δfaa1 and Δfaa1 Δfaa4 strains (27, 28). This indicates a different capacity of N. crassa to store excess lipid, which is more consistent with oleaginous microorganisms (58).
The partial compensation of activation of VLC FAs for anabolic pathways is observed in the N. crassa Δacs-3 strain, and we predict that ACS-6 provides this activity. The most well-characterized member of the VLC clade of ACS is S. cerevisiae Fat1p. In S. cerevisiae, Fat1p was shown both to activate VLC FAs for β-oxidation and to be required for uptake of LC FAs from the environment; both functions are supported by subcellular localization of Fat1p to the plasma membrane and lipid bodies (59, 60). However, N. crassa ACS-6 was not required for growth on exogenously supplied FA, similar to A. nidulans ΔfatA, ΔfatB, and ΔfatC mutants (21). ACS-6–GFP localized to the plasma membrane in some hyphae but was more frequently observed in puncta. While the plasma membrane localization is consistent with that of Fat1p, the puncta did not colocalize with the glyoxysome. These results indicate either that ACS-6 is nonfunctional for FA transport or that another FA transport protein(s) exists.
The family 2 clade, which contains S. cerevisiae Fat2p, A. nidulans FatD, and N. crassa ACS-7, is poorly characterized. Sc-Fat2p, An-FatD and Nc-ACS-7 all localize to the peroxisome/glyoxysome. However, the natural substrate of Sc-Fat2p has yet to be identified (61). The A. nidulans fatDΔ (21) and N. crassa Δacs-7 mutants also do not exhibit a growth phenotype in any of the conditions evaluated, nor did we observe a lipid phenotype of the Δacs-7 mutant when grown on either glucose or FA. Further work is needed to define the physiological role and biochemical specificity of these ACSs.
In many eukaryotic cells, FA β-oxidation occurs in both the peroxisome (or glyoxysome) and the mitochondria. S. cerevisiae lacks mitochondrial β-oxidation, and until recently this was assumed to be true across the entire fungal kingdom. The characterization of both peroxisomal and mitochondrial β-oxidation pathways in A. nidulans (11) led to a comparative genomic study of β-oxidation pathways across the fungal kingdom, which revealed that both pathways exist in the majority of fungi (9, 62). The mitochondrial β-oxidation pathway is specific for short-chain FAs and has also been implicated in breakdown of the amino acids isoleucine and valine (63, 64). Although enzyme activities for β-oxidation were not identified in the mitochondrial fraction of N. crassa cell lysates (10), our data support the mitochondrial β-oxidation hypothesis. Subcellular localization of ACS-4 to mitochondria indicates an involvement of this protein in mitochondrial β-oxidation and is consistent with previous data of oleate-induced mitochondrial ACS activity in N. crassa (65). In A. nidulans, FA degradation in the mitochondria is limited to short-, medium-, and long-chain FAs and is essential for growth on short-chain FAs. The A. nidulans homolog of ACS-4 (AN4659) was suggested by in silico analyses to be a putative short-chain ACS that localizes to mitochondria (21). N. crassa was unable to grow on most short- and medium-chain FAs, and for those that Neurospora is able to utilize, the Δacs-4 strain did not exhibit a growth defect. One possible explanation for these observations is ACS-4 is active against endogenous pools of short- and/or medium-chain FAs resulting from partial β-oxidation of long-chain FAs in the glyoxysome.
Within the family 1 ACS subclade, ACS-5 clusters with A. nidulans AN0609, whose gene is an ortholog of A. fumigatus sidl, which is required for the TAFC siderophore biosynthesis (45). Growth phenotypes observed for the Δacs-5 mutant strain are consistent with ACS-5 involvement in siderophore biosynthesis in N. crassa. Supplementation experiments further supported this hypothesis and specifically the role in biosynthesis of the N. crassa extracellular iron scavenger coprogen (47). Thus, members of ACS family 1 exhibit divergent functions, and some do not function primarily to activate FAs.
Cellular localization through proteomics (43) and microscopy reveal that, in N. crassa, ACSs are found in glyoxysomes, microsomes, PM, ER, and mitochondria, which reflects the pathway for which the ACSs are providing activated substrate: complex lipid biosynthesis and FA modification in the ER and FA β-oxidation in the glyoxysome and mitochondria (Fig. 8). It is clear that there is functional redundancy among the seven ACSs in N. crassa for activation of exogenous FA for degradation; however, one, ACS-3, is the predominant FA activator for anabolic pathways. In addition to elucidating lipid metabolic pathways specific for ACS function, we identified candidate Δacs strains that exhibited a disruption in FA equilibrium, altered FA profile, and overaccumulation of FAs. These results can inform future work toward tailoring FA hyperaccumulation for applications such as biofuel and biochemical production.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the Energy Biosciences to D.S.C., H.W.B., and N.L.G. C.M.R. is supported in part by the Department of Energy Office of Science Graduate Fellowship Program, made possible in part by the American Recovery and Reinvestment Act of 2009, administered by ORISE-ORAU under contract no. DE-AC05-06OR23100. We acknowledge use of materials generated by P01 GM068087 (Functional Analysis of a Model Filamentous Fungus).
Footnotes
Published ahead of print 19 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00079-13.
REFERENCES
- 1.Chopra A, Khuller GK. 1984. Lipid metabolism in fungi. Crit. Rev. Microbiol. 11:209–271 [DOI] [PubMed] [Google Scholar]
- 2.Hiltunen JK, Schonauer MS, Autio KJ, Mittelmeier TM, Kastaniotis AJ, Dieckmann CL. 2009. Mitochondrial fatty acid synthesis type II: more than just fatty acids. J. Biol. Chem. 284:9011–9015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czabany T, Athenstaedt K, Daum G. 2007. Synthesis, storage and degradation of neutral lipids in yeast. BBA. Molecular and cell biology of lipids 1771:299–309 [DOI] [PubMed] [Google Scholar]
- 4.Coleman RA, Lee DP. 2004. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 43:134–176 [DOI] [PubMed] [Google Scholar]
- 5.Zinser E, Sperka-Gottlieb CD, Fasch EV, Kohlwein SD, Paltauf F, Daum G. 1991. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J. Bacteriol. 173:2026–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tehlivets O, Scheuringer K, Kohlwein SD. 2007. Fatty acid synthesis and elongation in yeast. Biochim. Biophys. Acta 1771:255–270 [DOI] [PubMed] [Google Scholar]
- 7.McKeon T, Goodrich-Tanrikulu M, Lin J-T, Stafford A. 1997. Pathways for fatty acid elongation and desaturation in Neurospora crassa. Lipids 32:1–5 [DOI] [PubMed] [Google Scholar]
- 8.Zensen R, Husmann H, Schneider R, Peine T, Weiss H. 1992. De novo synthesis and desaturation of fatty acids at the mitochondrial acyl-carrier protein, a subunit of NADH:ubiquinone oxidoreductase in Neurospora crassa. FEBS Lett. 310:179–181 [DOI] [PubMed] [Google Scholar]
- 9.Shen Y-Q, Burger G. 2009. Plasticity of a key metabolic pathway in fungi. Funct. Integr. Genomics 9:145–151 [DOI] [PubMed] [Google Scholar]
- 10.Kionka C, Kunau WH. 1985. Inducible beta-oxidation pathway in Neurospora crassa. J. Bacteriol. 161:153–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maggio-Hall LA, Keller NP. 2004. Mitochondrial β-oxidation in Aspergillus nidulans. Mol. Microbiol. 54:1173–1185 [DOI] [PubMed] [Google Scholar]
- 12.Watkins PA. 1997. Fatty acid activation. Prog. Lipid Res. 36:55–83 [DOI] [PubMed] [Google Scholar]
- 13.Soupene E, Kuypers FA. 2008. Mammalian long-chain acyl-CoA synthetases. Exp. Mol. Med. 233:507–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babbitt PC, Kenyon GL, Martin BM, Charest H, Slyvestre M, Scholten JD, Chang KH, Liang PH, Dunaway-Mariano D. 1992. Ancestry of the 4-chlorobenzoate dehalogenase: analysis of amino acid sequence identities among families of acyl:adenyl ligases, enoyl-CoA hydratases/isomerases, and acyl-CoA thioesterases. Biochemistry 31:5594–5604 [DOI] [PubMed] [Google Scholar]
- 15.Black PN, DiRusso CC, Metzger AK, Heimert TL. 1992. Cloning, sequencing, and expression of the fadD gene of Escherichia coli encoding acyl coenzyme A synthetase. J. Biol. Chem. 267:25513–25520 [PubMed] [Google Scholar]
- 16.Mikolajczyk S, Brody S. 1990. De novo fatty acid synthesis mediated by acyl-carrier protein in Neurospora crassa mitochondria. Eur. J. Biochem. 187:431–437 [DOI] [PubMed] [Google Scholar]
- 17.Watkins PA, Lu J-F, Steinberg SJ, Gould SJ, Smith KD, Braiterman LT. 1998. Disruption of the Saccharomyces cerevisiae FAT1 gene decreases very long-chain fatty acyl-CoA synthetase activity and elevates intracellular very long-chain fatty acid concentrations. J. Biol. Chem. 273:18210–18219 [DOI] [PubMed] [Google Scholar]
- 18.Steinberg SJ, Morgenthaler J, Heinzer AK, Smith KD, Watkins PA. 2000. Very long-chain acyl-CoA synthetases. J. Biol. Chem. 275:35162–35169 [DOI] [PubMed] [Google Scholar]
- 19.Hisanaga Y, Ago H, Nakagawa N, Hamada K, Ida K, Yamamoto M, Hori T, Arii Y, Sugahara M, Kuramitsu S, Yokoyama S, Miyano M. 2004. Structural basis of the substrate-specific two-step catalysis of long chain fatty acyl-CoA synthetase dimer. J. Biol. Chem. 279:31717–31726 [DOI] [PubMed] [Google Scholar]
- 20.Black PN, DiRusso CC. 2007. Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation. Biochim. Biophys. Acta 1771:286–298 [DOI] [PubMed] [Google Scholar]
- 21.Reiser K, Davis M, Hynes M. 2010. Aspergillus nidulans contains six possible fatty acyl-CoA synthetases with FaaB being the major synthetase for fatty acid degradation. Arch. Microbiol. 192:373–382 [DOI] [PubMed] [Google Scholar]
- 22.Elovson J. 1975. Purification and properties of the fatty acids synthetase complex from Neurospora crassa, and the nature of the fas-mutation. J. Bacteriol. 124:524–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stafford AE, McKeon TA, Goodrich-Tanrikulu M. 1998. Conversion of palmitate to unsaturated fatty acids differs in a Neurospora crassa mutant with impaired fatty acid synthase activity. Lipids 33:303–306 [DOI] [PubMed] [Google Scholar]
- 24.Goodrich-Tanrikulu M, Jacobson DJ, Stafford AE, Lin JT, McKeon TA. 1999. Characterization of Neurospora crassa mutants isolated following repeat-induced point mutation of the beta subunit of fatty acid synthase. Curr. Genet. 36:147–152 [DOI] [PubMed] [Google Scholar]
- 25.Goodrich-Tanrikulu M, Stafford AE, Lin JT, Makapugay MI, Fuller G, McKeon TA. 1994. Fatty acid biosynthesis in novel ufa mutants of Neurospora crassa. Microbiology 140(Pt 10):2683–2690 [DOI] [PubMed] [Google Scholar]
- 26.Connerton IF, Fincham JRS, Sandeman RA, Hynes MJ. 1990. Comparison and cross-species expression of the acetyl-CoA synthetase genes of the ascomycete fungi, Aspergillus nidulans and Neurospora crassa. Mol. Microbiol. 4:451–460 [DOI] [PubMed] [Google Scholar]
- 27.Michinaka Y, Shimauchi T, Aki T, Nakajima T, Kawamoto S, Shigeta S, Suzuki O, Ono K. 2003. Extracellular secretion of free fatty acids by disruption of a fatty acyl-CoA synthetase gene in Saccharomyces cerevisiae. J. Biosci. Bioeng. 95:435–440 [DOI] [PubMed] [Google Scholar]
- 28.Scharnewski M, Pongdontri P, Mora G, Hoppert M, Fulda M. 2008. Mutants of Saccharomyces cerevisiae deficient in acyl-CoA synthetases secrete fatty acids due to interrupted fatty acid recycling. FEBS J. 275:2765–2778 [DOI] [PubMed] [Google Scholar]
- 29.Colot HV, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, Weiss RL, Borkovich KA, Dunlap JC. 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. U. S. A. 103:10352–10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCluskey K. 2003. The Fungal Genetics Stock Center: from molds to molecules. Adv. Appl. Microbiol. 52:245–262 [DOI] [PubMed] [Google Scholar]
- 31.Simonin A, Palma-Guerrero J, Fricker M, Glass NL. 2012. Physiological significance of network organization in fungi. Eukaryot. Cell 11:1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westergaard M, Mitchell HK. 1947. Neurospora V. A synthetic medium favoring sexual reproduction. Am. J. Bot. 34:573–577 [Google Scholar]
- 33.Vogel HJ. 1956. A convenient growth medium for Neurospora (medium N). Microb. Genet Bull. 13:42–43 [Google Scholar]
- 34.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 35.Bardiya N, Shiu PKT. 2007. Cyclosporin A-resistance based gene placement system for Neurospora crassa. Fungal Genet. Biol. 44:307–314 [DOI] [PubMed] [Google Scholar]
- 36.van den Ent F, Lowe J. 2006. RF cloning: a restriction-free method for inserting target genes into plasmids. J. Biochem. Biophys. Methods 67:67–74 [DOI] [PubMed] [Google Scholar]
- 37.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 38.Hynes MJ, Murray SL, Duncan A, Khew GS, Davis MA. 2006. Regulatory genes controlling fatty acid catabolism and peroxisomal functions in the filamentous fungus Aspergillus nidulans. Eukaryot. Cell 5:794–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Black PN, Zhang Q, Weimar JD, DiRusso CC. 1997. Mutational analysis of a fatty acyl-coenzyme A synthetase signature motif identifies seven amino acid residues that modulate fatty acid substrate specificity. J. Biol. Chem. 272:4896–4903 [DOI] [PubMed] [Google Scholar]
- 42.Watkins PA, Maiguel D, Jia Z, Pevsner J. 2007. Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. J. Lipid Res. 48:2736–2750 [DOI] [PubMed] [Google Scholar]
- 43.Managadze D, Würtz C, Wiese S, Meyer HE, Niehaus G, Erdmann R, Warscheid B, Rottensteiner H. 2010. A proteomic approach towards the identification of the matrix protein content of the two types of microbodies in Neurospora crassa. Proteomics 10:3222–3234 [DOI] [PubMed] [Google Scholar]
- 44.Knoll LJ, Johnson DR, Gordon JI. 1994. Biochemical studies of three Saccharomyces cerevisiae acyl-CoA synthetases, Faa1p, Faa2p, and Faa3p. J. Biol. Chem. 269:16348–16356 [PubMed] [Google Scholar]
- 45.Yasmin S, Alcazar-Fuoli L, Gründlinger M, Puempel T, Cairns T, Blatzer M, Lopez JF, Grimalt JO, Bignell E, Haas H. 2012. Mevalonate governs interdependency of ergosterol and siderophore biosyntheses in the fungal pathogen Aspergillus fumigatus. Proc. Natl. Acad. Sci. U. S. A. 109:497–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gründlinger M, Yasmin S, Lechner BE, Geley S, Schrettl M, Hynes M, Haas H. 2013. Fungal siderophore biosynthesis is partially localized in peroxisomes. Mol. Microbiol. 88:862–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horowitz NH, Charlang G, Horn G, Williams NP. 1976. Isolation and identification of the conidial germination factor of Neurospora crassa. J. Bacteriol. 127:135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Charlang G, Williams NP. 1977. Germination-defective mutant of Neurospora crassa that responds to siderophores. J. Bacteriol. 132:1042–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schrettl M, Bignell E, Kragl C, Sabiha Y, Loss O, Eisendle M, Wallner A, Arst HN, Jr, Haynes K, Haas H. 2007. Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog. 3:1195–1207. 10.1371/journal.ppat.0030128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Charlang GW, Horowitz NH. 1971. Germination and growth of Neurospora at low water activities. Proc. Natl. Acad. Sci. U. S. A. 68:260–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Juarez MP, Napolitano R. 2000. Effects of organic acids on lipid synthesis and ecdysis in Triatoma infestans eggs. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 125:503–510 [DOI] [PubMed] [Google Scholar]
- 52.Faergeman NJ, Knudsen J. 1997. Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem. J. 323:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson DR, Knoll LJ, Levin DE, Gordon JI. 1994. Saccharomyces cerevisiae contains four fatty acid activation (FAA) genes: an assessment of their role in regulating protein N-myristoylation and cellular lipid metabolism. J. Cell Biol. 127:751–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Færgeman NJ, Black PN, Zhao XD, Knudsen J, DiRusso CC. 2001. The acyl-CoA synthetases encoded within FAA1 andFAA4 in Saccharomyces cerevisiae function as components of the fatty acid transport system linking import, activation, and intracellular utilization. J. Biol. Chem. 276:37051–37059 [DOI] [PubMed] [Google Scholar]
- 55.Hettema EH, van Roermund CW, Distel B, van den Berg M, Vilela C, Rodrigues-Pousada C, Wanders RJ, Tabak HF. 1996. The ABC transporter proteins Pat1 and Pat2 are required for import of long-chain fatty acids into peroxisomes of Saccharomyces cerevisiae. EMBO J. 15:3813–3822 [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson DR, Knoll LJ, Rowley N, Gordon JI. 1994. Genetic analysis of the role of Saccharomyces cerevisiae acyl-CoA synthetase genes in regulating protein N-myristoylation. J. Biol. Chem. 269:18037–18046 [PubMed] [Google Scholar]
- 57.Zou Z, Tong F, Færgeman NJ, Børsting C, Black PN, DiRusso CC. 2003. Vectorial acylation in Saccharomyces cerevisiae: Fat1p and fatty acyl-CoA synthetase are interacting components of a fatty acid import complex. J. Biol. Chem. 278:16414–16422 [DOI] [PubMed] [Google Scholar]
- 58.Wang J, Zhang B, Chen S. 2011. Oleaginous yeast Yarrowia lipolytica mutants with a disrupted fatty acyl-CoA synthetase gene accumulate saturated fatty acid. Process Biochem. 46:1436–1441 [Google Scholar]
- 59.Natter K, Leitner P, Faschinger A, Wolinski H, McCraith S, Fields S, Kohlwein SD. 2005. The spatial organization of lipid synthesis in the yeast Saccharomyces cerevisiae derived from large scale green fluorescent protein tagging and high resolution microscopy. Mol. Cell. Proteomics 4:662–672 [DOI] [PubMed] [Google Scholar]
- 60.Obermeyer T, Fraisl P, DiRusso CC, Black PN. 2007. Topology of the yeast fatty acid transport protein Fat1p: mechanistic implications for functional domains on the cytosolic surface of the plasma membrane. J. Lipid Res. 48:2354–2364 [DOI] [PubMed] [Google Scholar]
- 61.Blobel F, Erdmann R. 1996. Identification of a yeast peroxisomal member of the family of AMP-binding proteins. Eur. J. Biochem. 240:468–476 [DOI] [PubMed] [Google Scholar]
- 62.Cornell MJ, Alam I, Soanes DM, Wong HM, Hedeler C, Paton NW, Rattray M, Hubbard SJ, Talbot NJ, Oliver SG. 2007. Comparative genome analysis across a kingdom of eukaryotic organisms: specialization and diversification in the fungi. Genome Res. 17:1809–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maggio-Hall LA, Lyne P, Wolff JA, Keller NP. 2008. A single acyl-CoA dehydrogenase is required for catabolism of isoleucine, valine and short-chain fatty acids in Aspergillus nidulans. Fungal Genet. Biol. 45:180–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hynes MJ, Murray SL, Khew GS, Davis MA. 2008. Genetic analysis of the role of peroxisomes in the utilization of acetate and fatty acids in Aspergillus nidulans. Genetics 178:1355–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hii V, Courtright JB. 1982. Induction of acyl coenzyme A synthetase and hydroxyacyl coenzyme A dehydrogenase during fatty acid degradation in Neurospora crassa. J. Bacteriol. 150:981–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Athenstaedt K, Zweytick D, Jandrositz A, Kohlwein SD, Daum G. 1999. Identification and characterization of major lipid particle proteins of the yeast Saccharomyces cerevisiae. J. Bacteriol. 181:6441–6448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ashrafi K, Farazi TA, Gordon JI. 1998. A role for Saccharomyces cerevisiae fatty acid activation protein 4 in regulating protein N-myristoylation during entry into stationary phase. J. Biol. Chem. 273:25864–25874 [DOI] [PubMed] [Google Scholar]
- 68.Huh W-K, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O'Shea EK. 2003. Global analysis of protein localization in budding yeast. Nature 425:686–691 [DOI] [PubMed] [Google Scholar]
- 69.Choi J-Y, Martin CE. 1999. The Saccharomyces cerevisiae FAT1 gene encodes an acyl-CoA synthetase that is required for maintenance of very long shain fatty acid levels. J. Biol. Chem. 274:4671–4683 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.