Abstract
The genome of the ascomycete Neurospora crassa encodes CAO-1 and CAO-2, two members of the carotenoid cleavage oxygenase family that target double bonds in different substrates. Previous studies demonstrated the role of CAO-2 in cleaving the C40 carotene torulene, a key step in the synthesis of the C35 apocarotenoid pigment neurosporaxanthin. In this work, we investigated the activity of CAO-1, assuming that it may provide retinal, the chromophore of the NOP-1 rhodopsin, by cleaving β-carotene. For this purpose, we tested CAO-1 activity with carotenoid substrates that were, however, not converted. In contrast and consistent with its sequence similarity to family members that act on stilbenes, CAO-1 cleaved the interphenyl Cα-Cβ double bond of resveratrol and its derivative piceatannol. CAO-1 did not convert five other similar stilbenes, indicating a requirement for a minimal number of unmodified hydroxyl groups in the stilbene background. Confirming its biological function in converting stilbenes, adding resveratrol led to a pronounced increase in cao-1 mRNA levels, while light, a key regulator of carotenoid metabolism, did not alter them. Targeted Δcao-1 mutants were not impaired by the presence of resveratrol, a phytoalexin active against different fungi, which did not significantly affect the growth and development of wild-type Neurospora. However, under partial sorbose toxicity, the Δcao-1 colonies exhibited faster radial growth than control strains in the presence of resveratrol, suggesting a moderate toxic effect of resveratrol cleavage products.
INTRODUCTION
Carotenoid cleavage oxygenases (CCOs) are a family of nonheme iron enzymes found in all taxa, usually involved in the oxidative cleavage of double bonds in the conjugated carbon chain of carotenoids (1–4). Carotenoids are a large group of terpenoid pigments produced by all photosynthetic species and also by many fungi and nonphotosynthetic bacteria (5, 6). Investigation of CCO sequences in noncarotenogenic microorganisms led to the finding of a subgroup of CCOs that are inactive on carotenoids but are able to cleave similar double bonds in biphenolic compounds from the stilbene family that may derive from lignin degradation (7). Indeed, lignostilbene-degrading enzymes are the longest known members of this enzyme family (8). The stilbene resveratrol is a phytoalexin found in the fruits of different plants, such as grapes, peanuts, or cotton (9). Resveratrol is accumulated in considerable amounts in grape skin, and it is well known because of its presence in red wine. Increasing data are revealing numerous beneficial effects of resveratrol on human health, including anticarcinogenic properties (reviewed in reference 10).
The cleavage of carotenoids by CCOs leads to apocarotenoids, a group of compounds that fulfill important functions in many organisms. Among them, the precursors of the plant hormones abscisic acid (11) and strigolactones (12) and the rhodopsin-chromophore retinal (13) stand out. Rhodopsins are a widespread group of photoreactive proteins with diverse light-absorbing functions (reviewed in reference 14, 15). Rhodopsins are classified in two groups. Type I rhodopsins include the archaeal bacteriorhodopsin and halorhodopsin ion pumps that use light as the sole energy source (16) and similar proteins with diverse light-dependent functions in other microbial groups (14). Type II rhodopsins are found in animals, where they play a central role in vision (17). Rhodopsins exhibit a conserved tertiary organization consisting of seven transmembrane domains and an internal pocket, which contains a highly conserved lysine residue to which retinal is covalently bound (15). Retinal is produced in animals by a CCO enzyme that catalyzes the symmetrical cleavage of dietary β-carotene (13), while cyanobacteria utilize longer apocarotenoids, already cleaved carotenoids, to produce retinal and retinal-like compounds (18, 19).
CCOs are also present in filamentous fungi. For instance, the zygomycete Phycomyces blakesleeanus employs two CCOs, CarS and AcaA, to sequentially convert β-carotene into the C18 ketone β-apo-13-carotenone via the C25 intermediate β-apo-12′-carotenal (20, 21) in the pathway leading to the trisporic acid pheromones. The ascomycetes Neurospora crassa (referred to hereinafter as Neurospora) and Fusarium fujikuroi produce neurosporaxanthin, a C35 apocarotenoic acid. The genes needed for the synthesis of this xanthophyll have been identified in both fungi (reviewed in reference 22, 23), including those encoding the CCO enzyme CAO-2 in Neurospora (24) and its ortholog CarT in F. fujikuroi (25), which catalyze the formation of a C35 apocarotenal that is further oxidized to neurosporaxanthin by the aldehyde dehydrogenase YLO-1 (26) or CarD (27). cao-2 and other genes involved in the neurosporaxanthin biosynthesis are regulated by light, as can be expected from the assumed photoprotective function of this pigment.
Neurospora is a leading model organism for investigating many biological processes, particularly those related to the molecular mechanisms of light responses (28). Neurospora has a gene for a putative photoactive rhodopsin, nop-1 (29), and it is therefore expected to contain a retinal-forming enzyme. This is the case for F. fujikuroi, where the gene carX (30) encodes a CCO that cleaves β-carotene symmetrically to produce retinal (31), the presumed chromophore of the nop-1 ortholog OpsA (32) and of CarO, a further rhodopsin encoded in the genome of this fungus (33). Underscoring the functional interrelation of the encoded enzymes, carO is linked and transcriptionally coregulated with carX and with the genes carRA and carB, needed for the synthesis of the CarX substrate β-carotene as a side product of the neurosporaxanthin biosynthetic pathway. Therefore, the gene cluster formed by carX, carRA, carB, and carO is self-sufficient to produce functional CarO rhodopsin. Targeted mutations of either nop-1 in Neurospora or carO or opsA in F. fujikuroi did not cause detectable phenotypic alterations under laboratory conditions, hindering the understanding of their biological functions.
Putative rhodopsin-encoding genes are also found in other fungal genomes (34), including Ustilago maydis. Formerly, we described the CCO enzymes Cco1 and Rco1 of this fungus. Cco1 is a CarX ortholog that cleaves β-carotene to produce retinal (35). Accordingly, the overexpression or deletion of cco1 resulted in decreased or enhanced accumulation of β-carotene, respectively. Retinal formation may be particularly relevant in U. maydis, which contains three presumptive rhodopsin-encoding genes. Unexpectedly, the second CCO enzyme, Rco1, showed no activity on carotenoid substrates but cleaved resveratrol (36). Similar resveratrol-cleaving enzymes were identified in the fungi Aspergillus fumigatus, Chaetomium globosum, and Botryotinia fuckeliana (36). The biological relevance for resveratrol-cleaving enzymes in these species remains to be elucidated.
The NOP-1 rhodopsin of Neurospora binds retinal in vitro (37) and also shows a photochemical reaction cycle (38). Therefore, it is expected that Neurospora, as shown for F. fujikuroi and U. maydis, contains a retinal-forming enzyme. Besides the torulene-cleaving enzyme CAO-2, the genome of Neurospora encodes a further CCO, called CAO-1 (NCU07008), for which a retinal biosynthetic function was formerly postulated (24). Here, we show that CAO-1 is not a carotenoid or apocarotenoid cleavage enzyme. We heterologously expressed CAO-1 in Escherichia coli and assayed its enzymatic activity in vivo and in vitro. CAO-1 did not convert β-carotene or any of the apocarotenoids tested; however, it efficiently cleaved resveratrol and its derivative piceatannol. Correspondingly, cao-1 mRNA levels were not affected by light but increased dramatically in the presence of resveratrol.
MATERIALS AND METHODS
Strains and growth conditions.
Neurospora crassa wild-type strain 74-OR23-1 (mating type A) (FGSC 2489), the Δmus52-bar (mating type a) mutant (FGSC 9719), and the Δnop-1 (mating type A) mutant (FGSC 15897) were obtained from the Fungal Genetics Stock Center (39). Genomic DNA and total RNA were extracted from mycelial samples grown for 3 days in petri dishes at 30°C in 25 ml Vogel's broth (40) in the dark or after illumination at 5 W m−2 white light. To obtain retarded colonial growth, the stringency of the sorbose agar (40) was reduced using 5 g liter−1 sorbose, 2 g liter−1 glucose, and 2 g liter−1 fructose.
At the times indicated in the figures, resveratrol (Sigma, St. Louis, MO) was added to the medium from a 100 mg ml−1 ethanol solution. As a control for the different experiments, the same volume of ethanol was added to the medium without resveratrol. To check the effect of resveratrol on gene expression, the compound was added to reach final concentrations of either 50 or 200 mg liter−1 (0.22 or 0.88 mM, respectively). For resveratrol toxicity assays, the compound was added to reach a final concentration of 1 g liter−1 (4.38 mM). The toxicity of resveratrol was checked by growing the strains in Vogel's agar with sorbose (as indicated above). Three replicates per plate of every strain were incubated at 30°C, and the diameters of the colonies were measured after 24 h.
To test whether resveratrol can be used as a carbon source, 60-mm-diameter petri dishes with 7 ml of Vogel's medium containing 1 g liter−1 sucrose, 1 g liter−1 resveratrol, or no carbon source were used. The plates were incubated at 30°C for 48 h.
To test the effect of resveratrol on sexual capability, crosses between the wild-type strain and the Δcao-1 mutant or between the wild-type strain and the mus-52 mutant were achieved on synthetic crossing medium (40) supplemented with 1 g liter−1 resveratrol. Parallel crosses were carried out in control plates without resveratrol. Strains of the opposite mating type were inoculated in the diametrical edges of the plates and incubated at 25°C for 3 weeks.
To test the ability of Δcao-1 to grow on wood, 1-cm-thick slices from a ca. 4-cm-diameter dry branch of Platanus hispanica were submerged in sterile distilled water for 3 min and placed in an empty petri dish. A drop with 107 conidia either from the wild type or one of the two Δcao-1 mutants was added to the center of each premoistened slice, and the plates were incubated at 30°C for 1 week on water-embedded filter paper in a closed container to avoid desiccation. Growth tests without ensuring high-humidity conditions resulted in no apparent mycelial growth.
For testing the ability of Δcao-1 to grow on grapes, 1 μl with 105 conidia was inoculated directly on the grapes (commercial Red globe variety) or after pricking their skin. The grapes were incubated under illumination at 30°C for 7 days.
cao-1 targeted deletion.
The cao-1 knockout strain was created using the yeast recombinational cloning protocol (41). The 5′ cao-1 flanking segment was amplified with primers 5′-GTAACGCCAGGGTTTTCCCAGTCACGACGGTACACCTCTAGAGCCATCG-3′ (cao1-5F) and 5′-ATCCACTTAACGTTACTGAAATCTCCAACAGTCGACTACGACGTTATCC-3′ (cao1-6R), and the 3′ cao-1 flanking segment was amplified with primers 5′-CTCCTTCAATATCATCTTCTGTCTCCGACACCACCTCTACGCTTATACC-3′ (cao1-4F) and 5′-GCGGATAACAATTTCACACAGGAAACAGCGTAGAGGGAGAATTGGTACG-3′ (cao1-3R). The hygromycin resistance cassette, consisting of the hygromycin B phosphotransferase gene hph (42) driven by the trpC promoter, was amplified with the primers 5′-GTCGGAGACAGAAGATGATATTGAAGGAGC-3′ and 5′-GTTGGAGATTTCAGTAACGTTAAGTGGAT-3′ from plasmid pCSN44 (43). The PCR products were cloned into the Saccharomyces cerevisiae strain FY834 (44) together with EcoRI/XhoI-digested plasmid pRS426 (45). This yeast vector contains the URA3 gene, and selection was done on medium lacking uracil. Plasmid DNA isolated from pooled yeast transformants served as the template for specific amplification of the deletion cassette, using the primers cao1-5F and cao1-3R.
Molecular techniques.
Genomic DNA was isolated from frozen mycelium samples with the GeneElute plant genomic miniprep kit (Sigma). Total RNA was extracted from mycelia lysed with a FastPrep homogenizer prior to using the RNeasy plant minikit (Qiagen, Chatsworth, CA). For Southern blot analysis, genomic DNA was isolated from mycelia as previously described (46). PCR analyses were performed with ca. 50 ng genomic DNA, 0.2 mM deoxynucleoside triphosphates (dNTPs), 1 μM forward and reverse primers, and 0.2 U μl−1 Expand high-fidelity polymerase (Roche, Mannheim, Germany). The reaction mixtures were heated at 94°C for 2 min, followed by 35 cycles of denaturation (94°C for 20 s), annealing (50 to 65°C for 20 s, according to the melting temperature of the primers), and polymerization (72°C for 1 to 4 min, depending on the length of the expected product) and by a final polymerization step at 72°C for 5 min. Southern blot hybridization was performed using standard protocols (47). The 5′ flanking cao-1 segment obtained with primers cao1-5F and cao1-5R was radioactively labeled and used as a probe.
Neurospora transformation.
Amounts of 10 μg DNA of the deletion cassette and 5 × 105 conidia were mixed into 50 μl of 1 M sorbitol and incubated on ice for 5 min. Conidia were electroporated in an ECM 630 Electro Cell Manipulator precision pulse system (BTX Harvard Apparatus) using a charging voltage of 1.5 kV and a resistance of 600 Ω. After the electroshock, 1 ml of Vogel's minimal medium supplemented with 2% yeast extract was added, and the conidia were incubated at 30°C for 3 h. Two hundred microliters of this suspension was spread on solid medium containing 300 μg ml−1 hygromycin. Hygromycin-resistant colonies were isolated and crossed with the wild-type strain on synthetic cross medium following standard Neurospora protocols (40). Ascospores were activated at 60°C for 45 min and grown in hygromycin-supplemented Vogel's medium. The resulting colonies were isolated and tested for cao-1 integrity by PCR and Southern blotting. For PCR analyses, internal fragments of cao-1 and al-1, respectively, were amplified with the primer sets 5′-ATGGCTGAATACGTCTTCTCCG-3′ and 5′-ACACGTCACCTCATCCCGTC-3′, resulting in a 1,706-bp product, and 5′-ACTTACAGACAAAATGGCTG-3′ and 5′-AACCCTACCTCACAAATAGC-3′, resulting in a 1,990-bp product. The occurrence of the targeted cao-1 replacement was checked with the primer set cao1-5F/cao1-3R, mentioned above. This primer set gives rise to a 4,835-bp product for the wild-type cao-1 gene and a 3,726-bp product for the hygR Δcao-1 allele.
Expression analyses.
Real-time reverse transcription RT-PCR (RT-PCR) expression analyses were performed using DNase-pretreated total RNA samples as the template. The reactions were carried out in 25-μl volumes in an ABI 7500 (Applied Biosystems, Branchburg, NJ) and started with a 30-min reverse transcription step at 48°C, followed by 10 min at 95°C, 40 cycles of 95°C denaturation for 15 s and 60°C polymerization for 1 min. Dissociation steps were achieved afterwards. The primers used for the reactions were 5′-TTCGTCCGTGAGGCAGAAG-3′ and 5′-GACTAGGTCGGTGTATCTG-3′ for cao-1, 5′-TCAAGGGACTGAGAGAGCCG-3′ and 5′-CGTTGACGTTGTTGTGCCAC-3′ for cao-2, and 5′-CGCTATCGCCTACCCCATT-3′ and 5′-CGACGAGGAAGCCTGTTTG-3′ for al-2. The primers were designed with Primer Express version 2.0.0 (Applied Biosystems) from intronless sequences from each gene and synthesized (high-performance liquid chromatography [HPLC] grade) by StabVida (Oeiras, Portugal). Optimization of MgCl2 and primer concentrations and annealing temperatures for RT-PCR experiments was done as recommended by the manufacturer, using 50 ng of RNA and 5 mM each primer. The β-tubulin gene of Neurospora crassa (NCU04054, primers 5′-CGTCCATCAGCTCGTTGAGA-3′ and 5′-CGCCTCGTTGTCAATGCA-3′) was used as a control for constitutive expression. Relative gene expression was calculated with the cycle threshold (2−ΔΔCT) method with Sequence Detection Software version 1.2.2 (Applied Biosystems). Each RT-PCR analysis was performed four times (duplicate samples from two independent experiments), and standard deviations calculated to ensure statistical accuracy.
Protein expression and in vitro assays.
For in vitro analysis, the cao-1 coding sequence was amplified from cDNA obtained from Neurospora total RNA using the SuperScript III first-strand synthesis system for RT-PCR (Invitrogen, Paisley, United Kingdom) and primers 5′-ATGGCTGAATACGTCTTCTCCG-3′ (cao1-1F) and 5′-ACACGTCACCTCATCCCGTC-3′ (cao1-1R). The amplified product was cloned into pSC (StrataClone PCR cloning kit; Stratagene), leading to plasmid pSC-cao1, and the cDNA was sequenced to confirm its integrity. The cDNA was then excised using EcoRI and introduced into accordingly digested and alkaline phosphatase-pretreated pGEX-5X-1 (Amersham Biosciences, NJ) to yield plasmid pGEX-cao1. E. coli BL21 was transformed with pGEX-cao1, grown, and induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) to purify CAO-1 protein as previously described (18, 48). For enzyme assays, β-apo-8′-carotenal, 3-hydroxy-β-apo-8′-carotenal, and apo-8′-lycopenal were kindly provided by BASF (Ludwigshafen, Germany). These substrates were purified on thin-layer chromatography (TLC) silica gel plates (Merck, Darmstadt, Germany) run in petroleum benzene-diethyl ether-acetone (40:10:7, vol/vol/vol). The substrates were then scraped off under dim light and eluted with acetone. Highly pure stilbene, resveratrol, piceatannol, rhapontin, 3,4-dihydroxybenzaldehyde, and 4-hydroxybenzaldehyde were purchased from Sigma. 3-Hydroxystilbene and pinosylvin were obtained from Sequoia Research Products Ltd. (Pangbourne, United Kingdom), and trismethoxy-resveratrol from BIOZOL Diagnostica Vertrieb GmbH (Eching, Germany). For enzyme assays, substrates were mixed with an ethyl alcohol solution of octyl-β-glucoside (1%, wt/vol), dried in a vacuum centrifuge, and resuspended in water. The final concentration of the substrates was 100 μM for stilbenoids and 40 μM for apocarotenoids. The incubation buffer contained 1 mM TCEP [Tris(2-carboxyethyl)phosphine hydrochloride], 0.3 mM FeSO4, and 1 mg ml−1 catalase (Sigma) in 200 μl of 100 mM HEPES-NaOH, pH 8.0. After a 30-min incubation at 27°C, assays were stopped by adding 1 volume of acetone, extracted with petroleum benzene-diethyl ether (1:4, vol/vol), and subjected to HPLC analysis. Assay mixtures with apocarotenoids were incubated for at least 2 h.
In vivo test.
For in vivo assays, the cao-1 coding sequence was cloned into pBAD/TOPO using the ThioFusion expression kit (Invitrogen) according to the manufacturer's recommendations. For this purpose, the cDNA was amplified from pSC-cao1 using the proofreading PfuUltra II fusion HS DNA polymerase (Stratagene) in the buffer provided and the primers cao1-1F and cao1-1R. The PCR product was purified using the GFX PCR DNA and gel band purification kit (Amersham Biosciences) and ligated into the expression plasmid, yielding pThio-cao1. Lycopene-, β-carotene-, and zeaxanthin-accumulating E. coli XL1-Blue cells, harboring the required biosynthetic genes from Erwinia herbicola, were transformed with pThio-cao1 and the empty plasmid pBAD-Thio. Cultures were grown at 37°C to reach an optical density at 600 nm (OD600) of 0.5 and induced overnight at 28°C with 0.2% (wt/vol) arabinose. The bacteria were harvested and extracted with acetone, and the extracts were subjected to HPLC analysis.
Analytical methods.
Substrates were quantified spectrophotometrically at their individual λmax (wavelength of maximum absorption) using extinction coefficients calculated from E1% [extinction coefficient calculated for a 1% (wt/vol) solution] as described previously (49) or as determined with the synthetic compounds (BASF). The protein concentration was determined with the Bio-Rad protein assay kit (Bio-Rad, CA). For HPLC, a Waters system (Eschborn, Germany) equipped with a photodiode array detector (model 996) was used. A C30 (YMC Europe, Schermbeck, Germany) phase column was used for carotenoid separation as described previously (19). For analyses of in vitro assays with piceatannol, the column was developed using a solvent system as follows. Solvent B was methanol–water–tert-butylmethyl ether (50:50:10, vol/vol/vol), with the water containing 2.5 g liter−1 ammonium acetate, and solvent A was methanol–tert-butylmethyl ether–water (50:50:10, vol/vol/vol); solvents were used at a flow-rate of 1 ml min−1 with a linear gradient from 100% B to 40% B within 15 min and then to 0% B within 10 min. The flow rate was then increased within 1 min to 2 ml min−1 100% A, maintaining these final conditions for 12 min. The analyses of in vitro assays with other stilbenes and the detection of resveratrol were performed with the following solvent system. Solvent B was MeOH-water (35:65, vol/vol), and solvent A was MeOH–tert-butylmethyl ether–water (500:500:100, vol/vol/vol). The column was developed with B from a flow rate of 1 to 1.4 ml min−1 within 10 min, followed by a linear gradient from 100% B to 50% B within 10 min at a flow rate of 1.4 ml min−1, and then to 100% A at a flow rate of 2 ml min−1 within 4 min, maintaining these final conditions for another 4 to 24 min at a flow rate of 2 ml min−1.
Germination assay.
Eppendorf tubes containing 1 ml Vogel's broth supplemented with 0.2 g liter−1 (0.88 mM) resveratrol or only with the same volume of ethanol were inoculated with 107 wild-type or Δcao-1 mutant conidia. The tubes were incubated at 30°C and 40 rpm for 8 h. After this time, the germinated spores were observed and photographed under a microscope (model DM 1000; Leica Microsystems, Germany).
Sequence analyses.
Sequence alignments were achieved with the Clustal X 1.83 program (50). Corrections for multiple substitutions were applied for phylogenetic analysis. P. blakesleeanus CarS and AcaA sequences were obtained from its genome database (http://genome.jgi-psf.org/cgi-bin/searchGM?db=Phybl2).
RESULTS
CAO-1 is not associated with carotenoid metabolism.
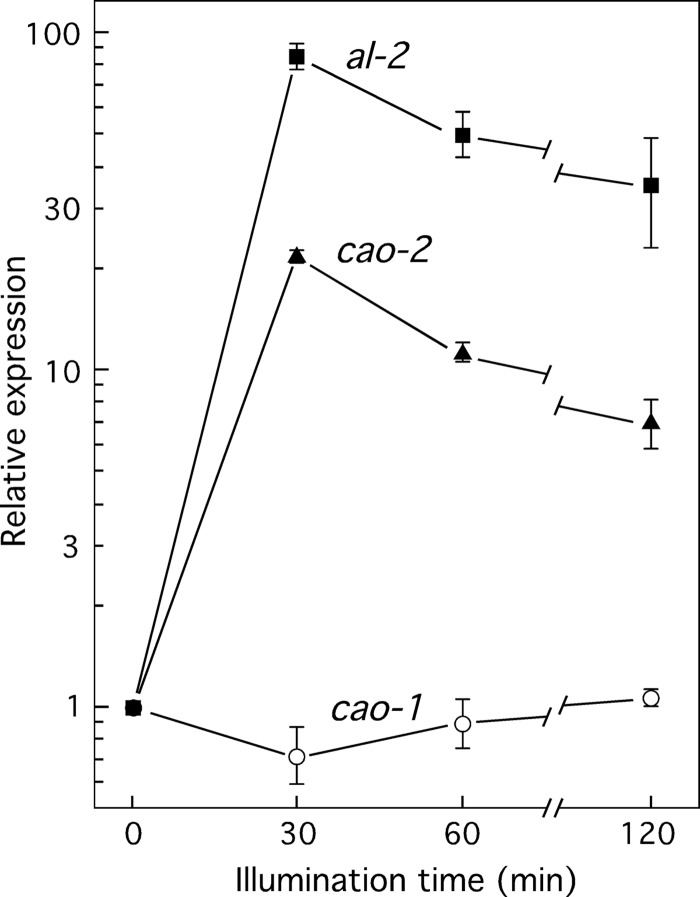
Besides CAO-2, CAO-1 is the only clear member of the CCO-1 family encoded in the Neurospora genome, and therefore, it is the likely candidate for retinal biosynthesis. Comparison with CarX of F. fujikuroi shows that CAO-1 is smaller in size (526 residues versus 696), and a CLUSTAL alignment revealed 130 coincident positions between both proteins. Hence, we have investigated a possible relation of CAO-1 with carotenoid metabolism. The gene carX is strongly induced by light in F. fujikuroi (30). Since carotenoid biosynthesis in Neurospora mycelia is induced by light, we first investigated the effect of light on cao-1 regulation. Mycelia grown in the dark were exposed to white light for different times, and their mRNA contents were determined by RT-PCR. The results showed no significant variation of cao-1 mRNA levels under illumination (Fig. 1). In contrast, rapid induction was found for the genes cao-2 and al-2, coding for enzymes of the carotenoid pathway, formerly reported to be stimulated by light (24).
Fig 1.
Effect of light on cao-1, cao-2, and al-2 transcript levels. Real-time RT-PCR analyses of cao-1, cao-2, and al-2 mRNA in total-RNA samples of the wild type grown in the dark or exposed to light for 15 min, 30 min, 1 h, or 2 h. Levels are relative to the value for each gene in the dark. All data show averages and standard deviations for four measurements from two independent experiments.
To check a possible activity of CAO-1 as a retinal-forming enzyme, its cDNA was cloned and expressed as a thio-fusion protein in E. coli strains engineered to produce different carotenoids. This in vivo assay proved to be effective for the β-carotene-cleaving activity of CarX from F. fujikuroi (31). The expression of cao-1 in a β-carotene-producing E. coli strain produced no bleaching of the cultures, indicating a lack of β-carotene degradation. Moreover, no change was observed in β-carotene levels in HPLC analyses (see Fig. S1 in the supplemental material) and no retinal was detected under appropriate detection wavelengths. Similar results were obtained upon the expression of cao-1 in E. coli cells producing lycopene and zeaxanthin as representative examples of linear or hydroxylated carotenoids, providing further support for the lack of activity of CAO-1 on carotenoid substrates.
As a further approach to test a possible activity of CAO-1 on carotenoids, crude lysates of thioredoxin-CAO-1-overexpressing cells (described below) were incubated in vitro with different carotenoid substrates that are cleaved by other CCO enzymes (20, 31). No traces of enzymatic activity could be detected after HPLC separation of the carotenoids extracted from the reaction mixtures (see Fig. S1 and S2 in the supplemental material), reinforcing the lack of activity of CAO-1 on carotenoid substrates.
CAO-1 belongs to the Rco1 CCO subfamily.
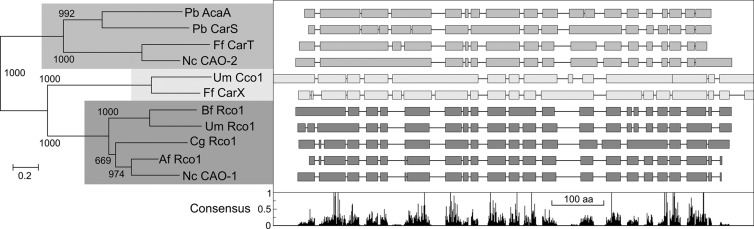
The lack of activity of CAO-1 on carotenoids and its low similarity to CarX prompted us to carry out a deeper analysis of its sequence features. Sequence alignments between functionally characterized fungal CCOs revealed three different groups (Fig. 2). The enzymes CAO-2 of Neurospora and CarT of F. fujikuroi are clustered in the phylogenetic tree with CarS and AcaA, two CCOs recently discovered in the zygomycete P. blakesleeanus (20, 21). CAO-2 and CarT cleave the monocyclic carotene torulene, and CarS cleaves β-carotene to produce the substrate for AcaA, β-apo-12′carotenal. The activities of the four enzymes have in common the asymmetrical cleavage of different carotenoid substrates. Despite the taxonomic distance between the ascomycete F. fujikuroi and the basidiomycete U. maydis and the apparent differences in gene organization between CarX and Cco1, these β-carotene-cleaving enzymes form a separate group in the phylogenetic tree. CarX and Cco1 recognize the same substrate as CarS, namely, β-carotene, but they cleave it symmetrically. Finally, the four resveratrol-cleaving CCO enzymes recently described in U. maydis, A. fumigatus, C. globosum, and B. fuckeliana form a consistent separate group. CAO-1 is unambiguously located in this group, suggesting a putative resveratrol-cleaving activity. The gene organizations revealed by the protein alignments are consistent with the independent evolution of these enzymatic CCO subgroups (Fig. 2).
Fig 2.
Sequence analysis of CAO-1 (NCU07008) and other already characterized fungal CCOs. (Left) Neighbor-joining phylogram of 11 proteins, as follows: AcaA and CarS from Phycomyces blakesleeanus (genes 77754 and 79747, respectively, in its genome database), CarT (accession number CAL90971) and CarX (CAH70723) from Fusarium fujikuroi, CAO-2 from Neurospora (NCU11424), Cco1 (UM00965) and Rco1 (UM05084) from Ustilago maydis, and Rco1 from Botryotinia fuckeliana (XP_001548426), Chaetomium globosum (XP_001219451), and Aspergillus fumigatus (XP_746307). The shaded areas distinguish three major groups according to the enzymatic reaction. Top, four enzymes that cleave asymmetrically diverse carotenoid substrates; middle, two enzymes that cleave β-carotene symmetrically; bottom, resveratrol-cleaving enzymes with no known activity on carotenoid substrates. (Right) Simplified representation of the Clustal comparison between the proteins indicated on the left. Interruptions indicate gaps introduced by the Clustal program to facilitate alignment. The diagram below plots the proportion for the presence of the consensus amino acid (aa) at each position.
The CAO-1 enzyme cleaves resveratrol.
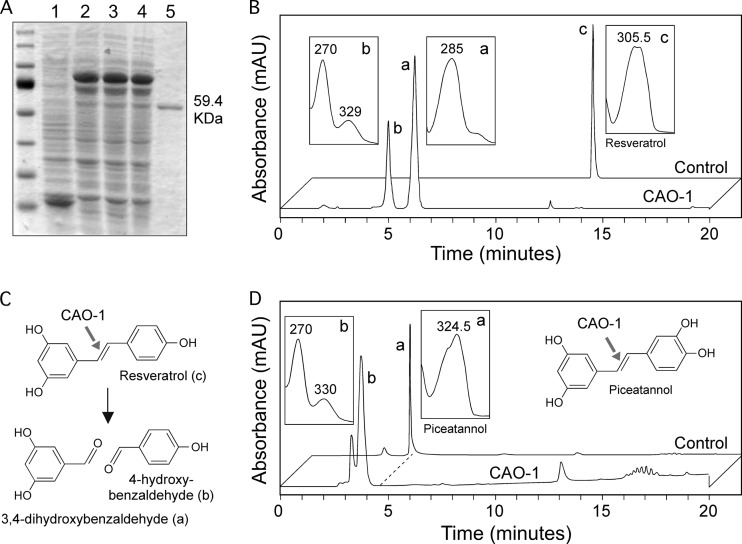
Because of the sequence similarity to fungal CCOs cleaving biphenolic substrates, we tested whether CAO-1 cleaves resveratrol. For this purpose, glutathione S-transferase (GST)-CAO-1 fusion protein was expressed in E. coli cells, purified with glutathione-Sepharose, and released by the protease factor Xa, resulting in a protein of the expected size (Fig. 3A). Incubation of the recombinant CAO-1 protein in vitro with resveratrol resulted in the formation of two products (Fig. 3B, insets a and b) with elution times and UV spectrum maximal absorptions identical to those of 3,4-dihydroxybenzaldehyde and 4-hydroxybenzaldehyde, the compounds resulting from the resveratrol cleavage activity by U. maydis Rco1 (36). Liquid chromatography-mass spectrometry (LC-MS) analyses (data not shown) confirmed the identity of both products and proved the oxidative cleavage at the interphenyl double bond of resveratrol (Fig. 3C).
Fig 3.
Purification and in vitro activity of CAO-1 on resveratrol and piceatannol. (A) Coomassie-stained SDS acrylamide gel showing CAO-1 purification. Lanes: 1, total lysate of control cells expressing GST (control); 2, total lysate of cells expressing GST-CAO-1; 3, sample 2 after removal of inclusion bodies; 4, nonbinding supernatant of sample 3; 5, eluted CAO-1 protein. The size of the only visible protein in lane 5 is indicated. (B) HPLC analyses of the in vitro assay products obtained after incubation of CAO-1 with resveratrol; UV spectra of the indicated peaks are shown in insets. (C) Resveratrol cleavage reaction and the products identified. (D) HPLC analyses of the in vitro assay products obtained after incubation of CAO-1 with piceatannol; UV spectra of the indicated peaks are shown in insets. mAU, milli-absorbance unit.
To further elucidate the substrate specificity of CAO-1, purified enzyme was incubated with other stilbenes. Cleaving activity was detected with piceatannol (Fig. 3D), a compound that differs from resveratrol only in the occurrence of an additional hydroxyl group, while the other substrates tested, i.e., trans-stilbene, 4-monohydroxy-trans-stilbene, 3,5-dihydroxy-trans-stilbene (pinosylvin), trismethoxy-resveratrol, and 3,3′,5-trihydroxy-4′-methoxystilbene-3-O-β-d-glucoside, were not converted (see Fig. S2 in the supplemental material). This result indicates that stilbenes converted by CAO-1 substrates must have at least three unmodified hydroxyl groups (see Fig. S2).
The cao-1 gene is regulated by resveratrol.
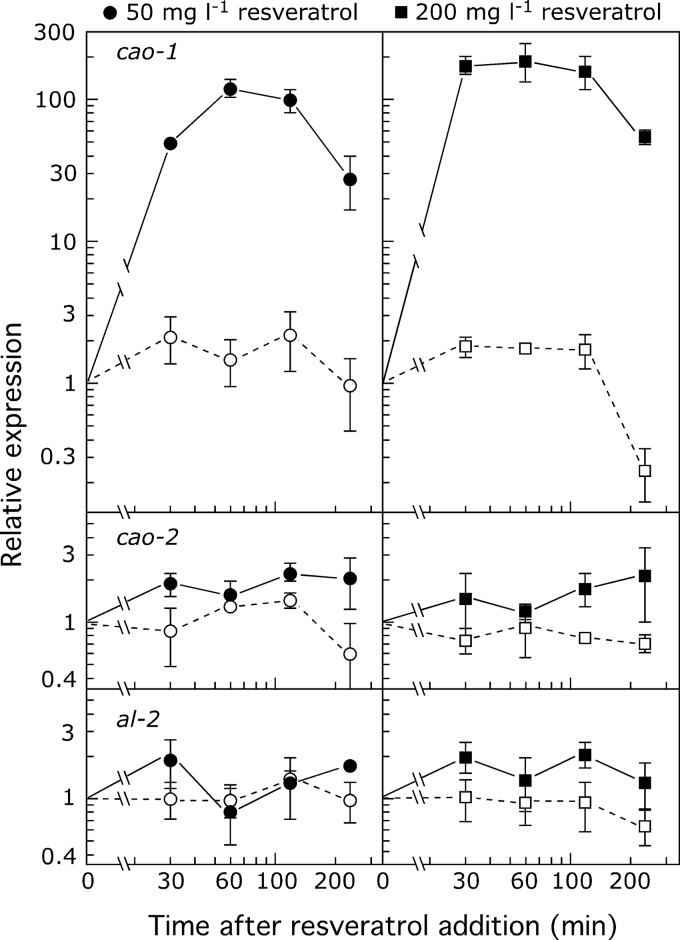
Given that resveratrol is the natural substrate of CAO-1, it might be expected that the presence of this compound does impact the transcript levels of the cao-1 gene. Indeed, adding resveratrol at two different concentrations resulted in a rapid increase of cao-1 mRNA, reaching up to 200-fold after 1 h of incubation with 200 mg liter−1 resveratrol (Fig. 4). After reaching its maximum under the two concentrations tested, the mRNA level decreased in the following hours. Because of its low solubility in water, resveratrol was added dissolved in ethanol. The addition of the same amount of ethanol to control cultures did not cause any significant change of cao-1 mRNA content, excluding the induction being caused by the organic solvent. In contrast to cao-1, adding resveratrol did not alter the mRNA levels of the carotenogenic genes cao-2 and al-2, indicating their unrelated function.
Fig 4.
Effect of resveratrol on cao-1, cao-2, and al-2 expression. Real-time RT-PCR analyses of cao-1, cao-2, and al-2 mRNA in the wild type grown in the absence of resveratrol or exposed to the indicated concentrations of resveratrol for 15 min, 30 min, 1 h, 2 h, and 4 h. The effect of parallel addition of the equivalent amount of ethanol is shown as a control (open symbols). Levels are relative to the value of each gene before the addition of resveratrol or ethanol. All data show averages and standard deviations of four measurements from two independent experiments.
The cao-1 deletion does not cause apparent phenotypic alteration.
To investigate the biological role of cao-1 in Neurospora, targeted deletion of this gene was performed with the standard protocols available for this fungus. Knockout strains were obtained in a mus-52 genetic background (51), because of its efficient homologous recombination. Five hygromycin-resistant colonies obtained upon transformation with the cao-1 disruption cassette were crossed with the wild type, and one colony of the progeny from each cross was chosen for further analyses. Two of the five descendants contained a wild-type mus-52 gene, and three contained the bar marker of the mus-52 deletion cassette, as deduced from the blasticidin resistance phenotype conferred by the bar marker (51). Southern blot analysis showed the replacement of the wild-type cao-1 gene by the deletion cassette in the five strains (see Fig. S3A in the supplemental material). The lack of the cao-1 sequence was confirmed by PCR assays (see Fig. S3B), and the five transformant strains are hereinafter referred to as Δcao-1 mutants.
The Δcao-1 mutants did not differ from the wild type under the usual growth conditions in the laboratory, either in slant or in Erlenmeyer cultures (slant cultures of representative strains are shown in Fig. S4A in the supplemental material). The morphology and pigmentation of the mutant strains were the same as those of the wild type, their mycelia grew at similar rates in race tubes (data not shown), and they formed mature perithecia when crossed with a wild-type strain of opposite sex, irrespective of the presence of resveratrol in the medium (see Fig. S5). Since Neurospora is able to grow on autoclaved wood (52), we tested whether mutants differ in this feature. Both wild-type and mutants were equally capable of growth on a wood substrate with no supplemental nutrients (see Fig. S4B). Because of the known presence of resveratrol in grapes, the ability of Neurospora to grow on this fruit was also assayed. However, no growth was observed after inoculation of wild-type or Δcao-1 conidia either on intact or punctured grape skin.
Resveratrol exerts low toxicity against Neurospora.
The addition of resveratrol to the culture medium produced only minor alterations in the aspect of the wild type or the mus-52 mutant in slant cultures. As the only visible difference, a larger mass of aerial mycelia could be observed in the tubes, but the Δcao-1 mutants and the control strains exhibited the same phenotypic pattern (see Fig. S4A in the supplemental material). Resveratrol was reported to induce severe morphological alterations in actively growing cells in U. maydis, including swelling and vacuolization (36). Such alterations were hardly apparent in germinating Neurospora conidia at the same resveratrol concentration, 200 μM, especially in those of the Δcao-1 mutants (see Fig. S6).
The transcriptional activation of the cao-1 gene could be explained by a possible use of resveratrol as a carbon source. Culturing of wild-type Neurospora in Vogel's medium without a carbon source results in very sparse hyphal development (see Fig. S7A in the supplemental material), explained by conidial reserves, the minor amount of biotin in the Vogel's formulation, or trace contaminants in the medium components. A similar hyphal density was observed upon the addition of 1 g liter−1 resveratrol irrespective of the Δcao-1 mutation. However, the addition of 1 g liter−1 sucrose (a concentration 15 times lower than that of the standard Vogel's medium) led to dense mycelial development. This result indicates that resveratrol is not a carbon source for Neurospora.
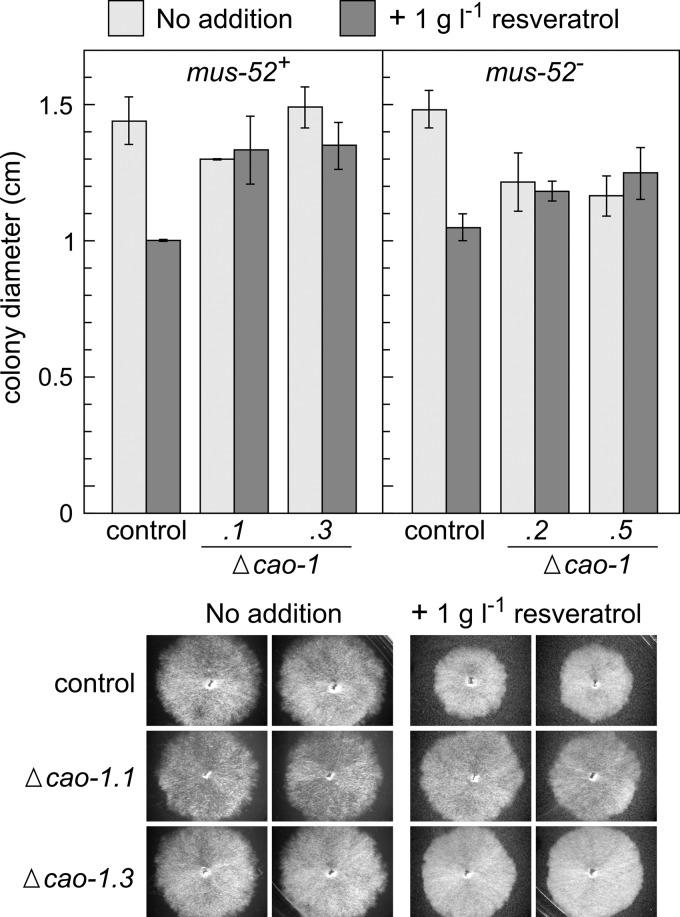
The rapid expansion of the Neurospora colonies on standard agar medium did not allow the detection of growth differences between the Δcao-1 mutants and their control strains, with or without the addition of resveratrol. Neither could differences be observed in sorbose medium, under which conditions sorbose toxicity results in the formation of very small colonies. However, a relaxation of the stringency conditions of the sorbose medium allowed the observation of a resveratrol-dependent effect of the Δcao-1 mutation on the Neurospora growth rate. After 24 h of incubation under these partially restrictive culture conditions, the size of the Δcao-1 colonies was significantly larger than the size of colonies of the control strains and similar to the size observed in the absence of resveratrol (Fig. 5). The Δnop-1 mutant, unable to produce retinal-binding Nop-1 rhodopsin (29, 37), exhibited the same sensitivity to resveratrol as the wild type (see Fig. S8 in the supplemental material). These results confirm the low toxicity of resveratrol on Neurospora but indicate a certain toxicity of the resveratrol degradation products, 3,4-dihydroxybenzaldehyde and 4-hydroxy-benzaldehyde. Because of the low solubility of resveratrol, a large proportion, up to 30 mg liter−1 (Sigma product sheet), of the added resveratrol precipitates, an effect observed even at low magnification with a stereoscopic microscope (see Fig. S7A and S8). No degradation halo was observed around the wild-type colonies (see Fig. S7B), suggesting that the CAO-1 enzyme is not secreted into the medium, as found for a resveratrol-degrading laccase produced by Botrytis cinerea (53).
Fig 5.
Effect of resveratrol on growth of Δcao-1 mutants and their respective wild type and mus-52− controls. The strains were incubated on low-stringency sorbose-containing agar for 24 h. Data show averages and standard deviations for at least three independent colonies. (Bottom) Representative examples of mus-52+ colonies.
DISCUSSION
A previous analysis of the Neurospora genome revealed the occurrence of two genes encoding presumptive CCO proteins (24). Anticipating a hypothetical role in carotenoid metabolism of this fungus, they were called CAO-1 and CAO-2. The prediction was confirmed for CAO-2, it being responsible for the cleavage step in neurosporaxanthin biosynthesis and coregulated with other genes of the carotenoid pathway (24), but not for CAO-1, for which no relation with carotenoid metabolism could be found. In contrast to the carX gene, mediating retinal formation in F. fujikuroi, or to the structural genes of the F. fujikuroi and Neurospora carotenoid pathways, cao-1 mRNA levels were not affected by light. Moreover, heterologously expressed CAO-1 showed no activity with the different carotenoids or apocarotenoids tested, including the usual retinal precursor, β-carotene, or other substrates cleaved by the CarX enzyme, such as β-apo-8′-carotenal (31). The ability of the NOP-1 rhodopsin to bind retinal in vitro (37) suggests the occurrence of a retinal-forming enzyme in Neurospora. However, our results demonstrate that this enzyme is not CAO-1.
Thus far, retinal biosynthetic activity has only been found in certain members of the CCO family, either in fungi or in higher organisms. The lack of clear orthologs for CCO enzymes in the Neurospora genome, apart from CAO-1 and CAO-2, led us to consider two alternative hypotheses for retinal biosynthesis in this fungus, as follows.
(i) Retinal may be produced by a non-CCO biosynthetic activity. At least, there is a second enzyme group able to cleave β-carotene, although retinal formation has not been demonstrated: the fungus Lepista irina excretes a peroxidase enzyme (Q8J1S4) that degrades β-carotene to generate apocarotenoid-related compounds. One of them was identified as β-apo-10′-carotenal (54). We could not find a clear ortholog for Q8J1S4 in the Neurospora genome. The best match was the cytochrome c peroxidase NCU03297, with a size similar to that of Q8J1S4 (368 and 358 aa, respectively) but only 19% coincident residues. Similar β-carotene-degrading activities were found in other fungi (55) but have not been described in Neurospora. A different possibility is the occurrence of a nonorthodox CCO protein. Neurospora genes encoding proteins with alternative CCO-related sequences are under investigation.
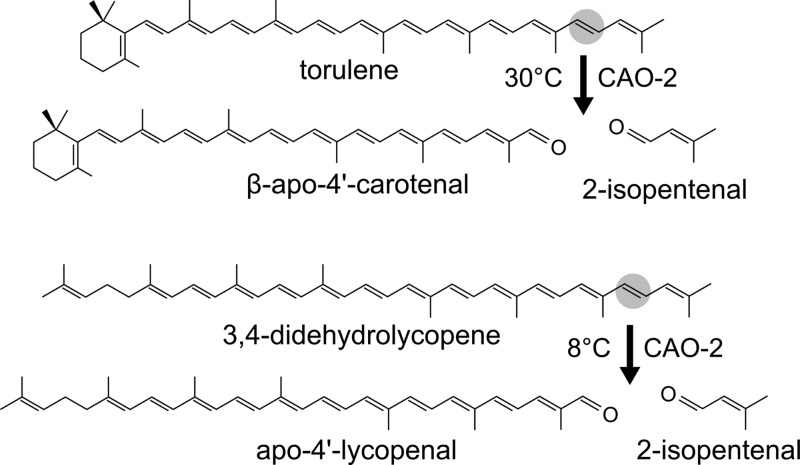
(ii) Despite its ability to bind retinal (20 carbon atoms, abbreviated C20), the physiological NOP-1 chromophore may be a nonretinal molecule. Albeit they are larger in size, we cannot discard for this role β-apo-4′-carotenal (C35) or apo-4′-lycopenal (C35), i.e., the aldehyde products resulting from the oxidative cleavage of either torulene or 3,4-didehydrolycopene by CAO-2 (Fig. 6). Their availability depends on the growth conditions: β-apo-4′-carotenal (C35) is preferentially used by Neurospora as an intermediate of the pathway under illumination at 30°C, and apo-4′-lycopenal is preferentially used under illumination at cold temperature (56). Because of its similarity to retinal and its formation at a physiological temperature, β-apo-4′-carotenal is a more-plausible candidate. In support of this hypothesis, retinoid analogues may functionally replace retinal in visual opsins (57). Moreover, β-apo-12′-carotenal (C25) and n-hexenal (C6) can replace retinal as the chromophore in the rhodopsin responsible for phototaxis in the chytridiomycete Allomyces reticulatus (58). n-Hexenal is particularly efficient, although it produces a shift in the response toward the near-UV region of the spectrum. Interestingly, n-hexenal is similar in size to the small side product of CAO-2 activity, 3-methyl-2-butenal (Fig. 6, 2-isopentenal). The function of NOP-1 in Neurospora has not yet been established, and therefore, no information is available on the wavelength for its putative response. An investigation of the effect of near-UV exposure on the wild type and nop-1 mutants of Neurospora could provide unexpected clues to the function of this protein.
Fig 6.
Oxidative cleavage reactions catalyzed by CAO-2 in Neurospora. Each reaction is predominant at the indicated temperature (56).
The sequence similarities of CAO-1 with fungal resveratrol-cleaving enzymes led us to suspect the same enzymatic function for this protein. The resveratrol-cleaving activity of CAO-1 was fully confirmed in vitro and correlated with a marked induction of cao-1 expression in vivo by the addition of resveratrol to the medium. Plants produce phytoalexins as a defense mechanism. Resveratrol displays antibiotic activity against numerous bacteria and fungi (reviewed in reference 59), including potential human pathogens, such as Candida albicans (60). In this fungus, resveratrol blocks the yeast-mycelial transition induced by serum, required for its pathogenic activity, and produces an arrest of cell cycle progression at the S phase. Resveratrol is also active against S. cerevisiae and against some filamentous fungi, such as Aspergillus flavus, Aspergillus oryzae, Penicillium expansum, and B. cinerea (61, 62). The sensitivity to resveratrol has received special attention in B. cinerea, a facultative phytopathogen able to infect green organs or fruits from a large diversity of plants, including Vitis vinifera grapes. The mechanism of action of resveratrol against B. cinerea is not well understood, but it has been suggested to interfere with respiration or to cause membrane peroxidation. The toxicity is due to the formation of the resveratrol dimer ε-viniferin, resulting from the activity of a specific secreted laccase, rather than to resveratrol itself (53). The effects are particularly visible on the morphology and germination of B. cinerea spores (63), the starting stage of the pathogenesis process. Deep morphological alterations were also observed in resveratrol-exposed cells of U. maydis (36).
Here, we show that resveratrol is poorly effective on Neurospora, where it hardly affects conidia germination and only produces very slight effects on mycelial growth and development. After different assays, we could only observe a minor reduction of wild-type growth by resveratrol under growth-restricted conditions, obtained by using sorbose as the carbon source. The slight reduction in colony growth was not apparent in the Δcao-1 mutants, suggesting that the effect was due to resveratrol degradation to 3,4-dihydroxybenzaldehyde and 4-hydroxybenzaldehyde rather than to direct resveratrol toxicity. This effect is unrelated to the laccase-dependent resveratrol toxicity in B. cinerea, interpreted as a result of ε-viniferin formation (53). Resveratrol resistance in other fungi has been attributed to the multidrug efflux pump activity of ABC transporter systems (64, 65). Several such systems also operate in Neurospora (66), providing an explanation for the low toxicity of this compound on this fungus.
In our experiments, resveratrol was added to the medium at high concentrations, actually exceeding the solubility limit in water of ca. 30 mg liter−1. Since it is only partly dissolved, precipitation particles can be observed under the microscope. In B. cinerea, a degradation halo surrounding the colony borders reveals degradation of external resveratrol (53). Such an effect was not observed in the borders of wild-type Neurospora colonies, suggesting that CAO-1 is only active in the cytoplasm. The low concentration of dissolved resveratrol available to enter the cell, probably reduced through the activity of an ABC transporter system, is sufficient for the effective induction of the cao-1 gene, presumably an additional mechanism to keep resveratrol concentrations under control.
The poor knowledge of Neurospora life conditions in nature hinders the postulation of plausible hypotheses to explain the occurrence of resveratrol-cleaving activity in this fungus. Neurospora is well known as a primary colonizer of burned trees and shrubs (see, e.g., references 67 and 68). The complex chemistry of burned vegetation might include the occurrence of toxic biphenolic compounds that could be degraded by CAO-1. Some endophytic fungi, including species from widespread genera such as Aspergillus, Penicillium, or Mucor, are able to produce resveratrol (69). This biosynthetic capacity might possibly enhance its survival by counteracting other competing microorganisms. The ability of Neurospora to cleave resveratrol might imply an evolutionary advantage for competition with such microorganisms, either by removing this defensive compound or by the generation of toxic cleavage products.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Spanish Government (Ministerio de Ciencia y Tecnología, projects BIO2006-01323 and BIO2009-11131) and the Andalusian Government (project P07-CVI-02813), Deutsche Forschungsgemeinschaft (DFG) grant AL 892/1-4, and the DFG Graduiertenkolleg 1305 Signalsysteme in pflanzlichen Modelorganismen. Spanish grants included support from the European Union (European Regional Development Fund [ERDF]).
Footnotes
Published ahead of print 26 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00084-13.
REFERENCES
- 1.Auldridge ME, McCarty DR, Klee HJ. 2006. Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr. Opin. Plant Biol. 9:315–321 [DOI] [PubMed] [Google Scholar]
- 2.Bouvier F, Isner JC, Dogbo O, Camara B. 2005. Oxidative tailoring of carotenoids: a prospect towards novel functions in plants. Trends Plant Sci. 10:187–194 [DOI] [PubMed] [Google Scholar]
- 3.Moise AR, von Lintig J, Palczewski K. 2005. Related enzymes solve evolutionarily recurrent problems in the metabolism of carotenoids. Trends Plant Sci. 10:178–186 [DOI] [PubMed] [Google Scholar]
- 4.Wyss A. 2004. Carotene oxygenases: a new family of double bond cleavage enzymes. J. Nutr. 134:246S–250S [DOI] [PubMed] [Google Scholar]
- 5.Hirschberg J. 2001. Carotenoid biosynthesis in flowering plants. Curr. Opin. Plant Biol. 4:210–218 [DOI] [PubMed] [Google Scholar]
- 6.Britton G, Liaaen-Jensen S, Pfander HP. 2004. Carotenoids: Handbook. Birkhauser, Boston, MA [Google Scholar]
- 7.Marasco EK, Schmidt-Dannert C. 2008. Identification of bacterial carotenoid cleavage dioxygenase homologues that cleave the interphenyl α,β double bond of stilbene derivatives via a monooxygenase reaction. Chembiochem 9:1450–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamoda S, Saburi Y. 1993. Structural and enzymatical comparison of lignostilbene-α,β-dioxygenase isozymes, I, II, and III, from Pseudomonas paucimobilis TMY1009. Biosci. Biotechnol. Biochem. 57:931–934 [DOI] [PubMed] [Google Scholar]
- 9.Kiselev KV. 2011. Perspectives for production and application of resveratrol. Appl. Microbiol. Biotechnol. 90:417–425 [DOI] [PubMed] [Google Scholar]
- 10.Yu W, Fu YC, Wang W. 2012. Cellular and molecular effects of resveratrol in health and disease. J. Cell. Biochem. 113:752–759 [DOI] [PubMed] [Google Scholar]
- 11.Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR. 1997. Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276:1872–1874 [DOI] [PubMed] [Google Scholar]
- 12.Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S. 2012. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 335:1348–1351 [DOI] [PubMed] [Google Scholar]
- 13.von Lintig J, Vogt K. 2004. Vitamin A formation in animals: molecular identification and functional characterization of carotene cleaving enzymes. J. Nutr. 134:251S–256S [DOI] [PubMed] [Google Scholar]
- 14.Sharma AK, Spudich JL, Doolittle WF. 2006. Microbial rhodopsins: functional versatility and genetic mobility. Trends Microbiol. 14:463–469 [DOI] [PubMed] [Google Scholar]
- 15.Spudich JL. 2006. The multitalented microbial sensory rhodopsins. Trends Microbiol. 14:480–487 [DOI] [PubMed] [Google Scholar]
- 16.Spudich JL. 1998. Variations on a molecular switch: transport and sensory signalling by archaeal rhodopsins. Mol. Microbiol. 28:1051–1058 [DOI] [PubMed] [Google Scholar]
- 17.Menon ST, Han M, Sakmar TP. 2001. Rhodopsin: structural basis of molecular physiology. Physiol. Rev. 81:1659–1688 [DOI] [PubMed] [Google Scholar]
- 18.Ruch S, Beyer P, Ernst H, Al-Babili S. 2005. Retinal biosynthesis in Eubacteria: in vitro characterization of a novel carotenoid oxygenase from Synechocystis sp. PCC 6803. Mol. Microbiol. 55:1015–1024 [DOI] [PubMed] [Google Scholar]
- 19.Scherzinger D, Ruch S, Kloer DP, Wilde A, Al-Babili S. 2006. Retinal is formed from apo-carotenoids in Nostoc sp. PCC7120: in vitro characterization of an apo-carotenoid oxygenase. Biochem. J. 398:361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medina HR, Cerdá-Olmedo E, Al-Babili S. 2011. Cleavage oxygenases for the biosynthesis of trisporoids and other apocarotenoids in Phycomyces. Mol. Microbiol. 82:199–208 [DOI] [PubMed] [Google Scholar]
- 21.Tagua VG, Medina HR, Martín-Domínguez R, Eslava AP, Corrochano LM, Cerdá-Olmedo E, Idnurm A. 2012. A gene for carotene cleavage required for pheromone biosynthesis and carotene regulation in the fungus Phycomyces blakesleeanus. Fungal Genet. Biol. 49:398–404 [DOI] [PubMed] [Google Scholar]
- 22.Avalos J, Corrochano LM. 2013. Carotenoid biosynthesis in Neurospora, p 227–241 In Kasbekar DP, McCluskey K. (ed), Neurospora: genomics and molecular biology. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 23.Avalos J, Estrada AF. 2010. Regulation by light in Fusarium. Fungal Genet. Biol. 47:930–938 [DOI] [PubMed] [Google Scholar]
- 24.Saelices L, Youssar L, Holdermann I, Al-Babili S, Avalos J. 2007. Identification of the gene responsible for torulene cleavage in the Neurospora carotenoid pathway. Mol. Genet. Genomics 278:527–537 [DOI] [PubMed] [Google Scholar]
- 25.Prado-Cabrero A, Estrada AF, Al-Babili S, Avalos J. 2007. Identification and biochemical characterization of a novel carotenoid oxygenase: elucidation of the cleavage step in the Fusarium carotenoid pathway. Mol. Microbiol. 64:448–460 [DOI] [PubMed] [Google Scholar]
- 26.Estrada AF, Youssar L, Scherzinger D, Al-Babili S, Avalos J. 2008. The ylo-1 gene encodes an aldehyde dehydrogenase responsible for the last reaction in the Neurospora carotenoid pathway. Mol. Microbiol. 69:1207–1220 [DOI] [PubMed] [Google Scholar]
- 27.Díaz-Sánchez V, Estrada AF, Trautmann D, Al-Babili S, Avalos J. 2011. The gene carD encodes the aldehyde dehydrogenase responsible for neurosporaxanthin biosynthesis in Fusarium fujikuroi. FEBS J. 278:3164–3176 [DOI] [PubMed] [Google Scholar]
- 28.Chen CH, Dunlap JC, Loros JJ. 2010. Neurospora illuminates fungal photoreception. Fungal Genet. Biol. 47:922–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bieszke JA, Braun EL, Bean LE, Kang S, Natvig DO, Borkovich KA. 1999. The nop-1 gene of Neurospora crassa encodes a seven transmembrane helix retinal-binding protein homologous to archaeal rhodopsins. Proc. Natl. Acad. Sci. U. S. A. 96:8034–8039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thewes S, Prado-Cabrero A, Prado MM, Tudzynski B, Avalos J. 2005. Characterization of a gene in the car cluster of Fusarium fujikuroi that codes for a protein of the carotenoid oxygenase family. Mol. Genet. Genomics 274:217–228 [DOI] [PubMed] [Google Scholar]
- 31.Prado-Cabrero A, Scherzinger D, Avalos J, Al-Babili S. 2007. Retinal biosynthesis in fungi: characterization of the carotenoid oxygenase CarX from Fusarium fujikuroi. Eukaryot. Cell 6:650–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Estrada AF, Avalos J. 2009. Regulation and targeted mutation of opsA, coding for the NOP-1 opsin orthologue in Fusarium fujikuroi. J. Mol. Biol. 387:59–73 [DOI] [PubMed] [Google Scholar]
- 33.Prado MM, Prado-Cabrero A, Fernández-Martín R, Avalos J. 2004. A gene of the opsin family in the carotenoid gene cluster of Fusarium fujikuroi. Curr. Genet. 46:47–58 [DOI] [PubMed] [Google Scholar]
- 34.Brown LS. 2004. Fungal rhodopsins and opsin-related proteins: eukaryotic homologues of bacteriorhodopsin with unknown functions. Photochem. Photobiol. Sci. 3:555–565 [DOI] [PubMed] [Google Scholar]
- 35.Estrada AF, Brefort T, Mengel C, Díaz-Sánchez V, Alder A, Al-Babili S, Avalos J. 2009. Ustilago maydis accumulates β-carotene at levels determined by a retinal-forming carotenoid oxygenase. Fungal Genet. Biol. 46:803–813 [DOI] [PubMed] [Google Scholar]
- 36.Brefort T, Scherzinger D, Limón MC, Estrada AF, Trautmann D, Mengel C, Avalos J, Al-Babili S. 2011. Cleavage of resveratrol in fungi: characterization of the enzyme Rco1 from Ustilago maydis. Fungal Genet. Biol. 48:132–143 [DOI] [PubMed] [Google Scholar]
- 37.Bieszke JA, Spudich EN, Scott KL, Borkovich KA, Spudich JL. 1999. A eukaryotic protein, NOP-1, binds retinal to form an archaeal rhodopsin-like photochemically reactive pigment. Biochemistry 38:14138–14145 [DOI] [PubMed] [Google Scholar]
- 38.Brown LS, Dioumaev AK, Lanyi JK, Spudich EN, Spudich JL. 2001. Photochemical reaction cycle and proton transfers in Neurospora rhodopsin. J. Biol. Chem. 276:32495–32505 [DOI] [PubMed] [Google Scholar]
- 39.McCluskey K. 2003. The Fungal Genetics Stock Center: from molds to molecules. Adv. Appl. Microbiol. 52:245–262 [DOI] [PubMed] [Google Scholar]
- 40.Davis RH, de Serres FJ. 1970. Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol. 71A:79–143 [Google Scholar]
- 41.Colot HV, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, Weiss RL, Borkovich KA, Dunlap JC. 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. U. S. A. 103:10352–10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gritz L, Davies J. 1983. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene 25:179–188 [DOI] [PubMed] [Google Scholar]
- 43.Staben C, Jensen B, Singer M, Pollock J, Schechtman M, Kinsey J, Selker E. 1989. Use of a bacterial hygromycin B resistance gene as a dominant selectable marker in Neurospora crassa transformation. Fungal Genet. Newsl. 36:79–81 [Google Scholar]
- 44.Winston F, Dollard C, Ricupero-Hovasse SL. 1995. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11:53–55 [DOI] [PubMed] [Google Scholar]
- 45.Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P. 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110:119–122 [DOI] [PubMed] [Google Scholar]
- 46.Weinkove D, Poyatos JA, Greiner H, Oltra E, Avalos J, Fukshansky L, Barrero AF, Cerdá-Olmedo E. 1998. Mutants of Phycomyces with decreased gallic acid content. Fungal Genet. Biol. 25:196–203 [DOI] [PubMed] [Google Scholar]
- 47.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 48.Scherzinger D, Scheffer E, Bar C, Ernst H, Al-Babili S. 2010. The Mycobacterium tuberculosis ORF Rv0654 encodes a carotenoid oxygenase mediating central and excentric cleavage of conventional and aromatic carotenoids. FEBS J. 277:4662–4673 [DOI] [PubMed] [Google Scholar]
- 49.Barua AB, Olson JA. 2000. β-Carotene is converted primarily to retinoids in rats in vivo. J. Nutr. 130:1996–2001 [DOI] [PubMed] [Google Scholar]
- 50.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ninomiya Y, Suzuki K, Ishii C, Inoue H. 2004. Highly efficient gene replacements in Neurospora strains deficient for nonhomologous end-joining. Proc. Natl. Acad. Sci. U. S. A. 101:12248–12253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee K. 2012. Asexual and sexual developments of Neurospora crassa on natural substrata. Fungal Ecol. 5:223–229 [Google Scholar]
- 53.Schouten A, Wagemakers L, Stefanato FL, van der Kaaij RM, van Kan JA. 2002. Resveratrol acts as a natural profungicide and induces self-intoxication by a specific laccase. Mol. Microbiol. 43:883–894 [DOI] [PubMed] [Google Scholar]
- 54.Zorn H, Langhoff S, Scheibner M, Nimtz M, Berger RG. 2003. A peroxidase from Lepista irina cleaves β,β-carotene to flavor compounds. Biol. Chem. 384:1049–1056 [DOI] [PubMed] [Google Scholar]
- 55.Zorn H, Langhoff S, Scheibner M, Berger RG. 2003. Cleavage of β,β-carotene to flavor compounds by fungi. Appl. Microbiol. Biotechnol. 62:331–336 [DOI] [PubMed] [Google Scholar]
- 56.Estrada AF, Maier D, Scherzinger D, Avalos J, Al-Babili S. 2008. Novel apocarotenoid intermediates in Neurospora crassa mutants imply a new biosynthetic reaction sequence leading to neurosporaxanthin formation. Fungal Genet. Biol. 45:1497–1505 [DOI] [PubMed] [Google Scholar]
- 57.Corson DW, Crouch RK. 1996. Physiological activity of retinoids in natural and artificial visual pigments. Photochem. Photobiol. 63:595–600 [DOI] [PubMed] [Google Scholar]
- 58.Saranak J, Foster KW. 1997. Rhodopsin guides fungal phototaxis. Nature 387:465–466 [DOI] [PubMed] [Google Scholar]
- 59.Paulo L, Oleastro M, Gallardo E, Queiroz JA, Domingues F. 2011. Antimicrobial properties of resveratrol: a review, p 1225–1235 In Méndez-Vilas A. (ed), Science against microbial pathogens: communicating current research and technological advances, vol 2 Formatex, Badajoz, Spain [Google Scholar]
- 60.Jung HJ, Seu YB, Lee DG. 2007. Candicidal action of resveratrol isolated from grapes on human pathogenic yeast C. albicans. J. Microbiol. Biotechnol. 17:1324–1329 [PubMed] [Google Scholar]
- 61.Filip A, Plocková M, Smidrkal J, Spicková Z, Melzoch K, Schmidt S. 2003. Resveratrol and its antioxidant and antimicrobial effectiveness. Food Chem. 83:585–593 [Google Scholar]
- 62.Jung HJ, Hwang IA, Sung WS, Kang H, Kang BS, Seu YB, Lee DG. 2005. Fungicidal effect of resveratrol on human infectious fungi. Arch. Pharm. Res. 28:557–560 [DOI] [PubMed] [Google Scholar]
- 63.Adrian M, Jeandet P. 2012. Effects of resveratrol on the ultrastructure of Botrytis cinerea conidia and biological significance in plant/pathogen interactions. Fitoterapia 83:1345–1350 [DOI] [PubMed] [Google Scholar]
- 64.Andrade AC, Del Sorbo G, Van Nistelrooy JG, Waard MA. 2000. The ABC transporter AtrB from Aspergillus nidulans mediates resistance to all major classes of fungicides and some natural toxic compounds. Microbiology 146:1987–1997 [DOI] [PubMed] [Google Scholar]
- 65.Zwiers LH, Stergiopoulos I, Gielkens MM, Goodall SD, De Waard MA. 2003. ABC transporters of the wheat pathogen Mycosphaerella graminicola function as protectants against biotic and xenobiotic toxic compounds. Mol. Genet. Genomics 269:499–507 [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Zhang Z, Zhang X, Zhang H, Sun X, Hu C, Li S. 2012. CDR4 is the major contributor to azole resistance among four Pdr5p-like ABC transporters in Neurospora crassa. Fungal Biol. 116:848–854 [DOI] [PubMed] [Google Scholar]
- 67.Jacobson DJ, Powell AJ, Dettman JR, Saenz GS, Barton MM, Hiltz MD, Dvorachek WH, Jr, Glass NL, Taylor JW, Natvig DO. 2004. Neurospora in temperate forests of western North America. Mycologia 96:66–74 [PubMed] [Google Scholar]
- 68.Luque EM, Gutiérrez G, Navarro-Sampedro L, Olmedo M, Rodríguez-Romero J, Ruger-Herreros C, Tagua VG, Corrochano LM. 2012. A relationship between carotenoid accumulation and the distribution of species of the fungus Neurospora in Spain. PLoS One 7:e33658. 10.1371/journal.pone.0033658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi J, Zeng Q, Liu Y, Pan Z. 2012. Alternaria sp. MG1, a resveratrol-producing fungus: isolation, identification, and optimal cultivation conditions for resveratrol production. Appl. Microbiol. Biotechnol. 95:369–379 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.