Abstract
Flavin-based electron bifurcation has recently been characterized as an essential energy conservation mechanism that is utilized by hydrogenotrophic methanogenic Archaea to generate low-potential electrons in an ATP-independent manner. Electron bifurcation likely takes place at the flavin associated with the α subunit of heterodisulfide reductase (HdrA). In Methanococcus maripaludis the electrons for this reaction come from either formate or H2 via formate dehydrogenase (Fdh) or Hdr-associated hydrogenase (Vhu). However, how these enzymes bind to HdrA to deliver electrons is unknown. Here, we present evidence that the δ subunit of hydrogenase (VhuD) is central to the interaction of both enzymes with HdrA. When M. maripaludis is grown under conditions where both Fdh and Vhu are expressed, these enzymes compete for binding to VhuD, which in turn binds to HdrA. Under these conditions, both enzymes are fully functional and are bound to VhuD in substoichiometric quantities. We also show that Fdh copurifies specifically with VhuD in the absence of other hydrogenase subunits. Surprisingly, in the absence of Vhu, growth on hydrogen still occurs; we show that this involves F420-reducing hydrogenase. The data presented here represent an initial characterization of specific protein interactions centered on Hdr in a hydrogenotrophic methanogen that utilizes multiple electron donors for growth.
INTRODUCTION
Energy conservation in hydrogenotrophic methanogenic Archaea is interesting in that hydrogenotrophs lack cytochromes yet still rely on the generation of chemiosmotic membrane potential for ATP synthesis. One critical step that makes this possible is the coupling of the exergonic reduction of a terminal heterodisulfide (CoM-S-S-CoB) to the endergonic reduction of CO2 to formyl-methanofuran (formyl-MFR), rendering methanogenesis a cycle (1–5). Central to these coupled reactions is the recent discovery of flavin-based electron bifurcation in heterodisulfide reductase (Hdr), an energy conservation mechanism that generates low-potential electrons in an ATP-independent manner (2, 3). Although it has become apparent that electron flow is bifurcated at Hdr, how electrons arrive there is still unclear.
Hdr from Methanothermobacter spp. copurifies with a three-subunit hydrogenase known as the methyl-viologen reducing hydrogenase (Mvh) (6, 7). In the model species Methanococcus maripaludis, two methyl-viologen reducing hydrogenases are present, one containing selenocysteine (Vhu) and one cysteine (Vhc), encoded by two separate operons (8). It has long been assumed that the δ subunit (VhuD or VhcD in M. maripaludis) is the site of binding to Hdr. This assumption is based on the presence of HdrA-MvhD fusion proteins encoded by the genomes of Methanosarcina spp. and Archaeoglobus fulgidus (6). Additionally, methanogens that lack genes for the active site of Mvh, such as Methanospirillum hungatei, still encode MvhD, suggesting that although Mvh is not used for heterodisulfide reduction in these organisms, MvhD is still essential for electron flow to Hdr (9). MvhD contains a 2Fe-2S cluster, further implicating it as an electron transfer protein (3).
We have shown that, in the hydrogenotroph M. maripaludis, H2 oxidation by Vhu or Vhc is not essential for heterodisulfide reduction with cultures grown on formate and that both formate dehydrogenase (Fdh) and Vhu bind Hdr (1). A ΔvhuAU ΔvhcA mutant retains wild-type growth rates with formate as an electron donor but has a growth defect specifically on H2. However, in this mutant background, the vhuD and vhcD genes were left intact; therefore, it is still unclear whether this subunit is essential for electron flow to Hdr when cultures are grown with formate. Additionally, how Vhu and Fdh bind to Hdr remains unclear. We investigated the interactions of these proteins by analyzing the subunit composition of the Hdr protein complex in cells grown under conditions where formate dehydrogenase (Fdh) is either expressed or repressed. Our results suggest that the active-site subunits of Vhu (VhuAGU) and Fdh (FdhAB) compete for binding to VhuD under conditions where Fdh is present. Additionally, we show evidence that in the ΔvhuAU ΔvhcA strain, slow growth on H2 involves a cryptic electron flow pathway involving F420-reducing hydrogenase.
MATERIALS AND METHODS
Growth conditions.
Strains used in this study are listed in Table 1. All strains are derivatives of M. maripaludis strain S2 (10). For batch culture, strains were grown with McCas medium with 276-kPa H2:CO2 (80:20) in the headspace or with formate medium with 207-kPa N2:CO2 (80:20) (11, 12). Growth curves were done in triplicate, and optical density (OD) was monitored at 660 nm. For continuous culture under H2 limitation/phosphate excess or H2 excess/phosphate limitation, a previously described chemostat system was employed (1, 13, 14).
Table 1.
Strains used in this study
Protein purification.
Cell material from strains containing oligo-His-tagged proteins grown under defined nutrient limitation was used for protein purification. All procedures were done in a Coy anaerobic chamber. Solutions were degassed and then allowed to equilibrate with the chamber atmosphere (5% H2, 95% N2). Frozen cell paste was thawed on ice and sonicated (Misonix Sonicator model XL-2000) at a setting of 11 in short 3-s bursts. Unbroken cell debris was pelleted, and the cell extract was subjected to nickel affinity purification. A modified version of our previous protocol was used for purification (1); binding buffer contained 25 mM HEPES (pH 7.5), 12.5 mM MgCl2, 100 mM NaCl, 10 mM imidazole, and 0.5 mM dithionite. Wash and elution buffers were the same as the binding buffer, except that they contained 30 and 100 mM imidazole, respectively. The eluted protein was concentrated with a Vivaspin 500 centrifugal concentrator (polyethersulfone membrane, 5-kDa molecular mass cutoff) before further purification by fast performance liquid chromatography (FPLC; AKTA purifier UPC10 system with a UV and conductivity cell from GE Life Sciences) using a Hiprep 16-60 Sephacryl S300HR sizing column run at a flow rate of 0.5 ml min−1. The FPLC running buffer was the same as the binding buffer but without the imidazole. The retention time of protein molecular size standards for the Hiprep column provided in the literature was used as a reference (15). Specific 1-ml fractions were identified, pooled, and concentrated.
Subunit composition analysis of purified proteins.
Pooled and concentrated fractions from FPLC purification were subjected to SDS-PAGE using 4 to 20% Precise protein gels and stained with GelCode blue safe protein stain (Thermo Scientific). Gels were subjected to densitometry analysis using the ImageJ suite (http://imagej.nih.gov/ij/) (16), and band intensity was normalized to predicted protein mass. Stoichiometry of subunits was determined by calculating the ratio of normalized band intensity between two proteins.
In vitro methane production assays.
Methane production was assayed using cell material from strain MM901 grown with either formate or H2 (1). For each assay, 10 to 20 ml of culture was collected and anaerobically lysed by sonication (Misonix sonicator model XL-2000) on setting 2 in 10-s bursts. Insoluble cell material was removed by centrifugation, and the supernatant was mixed in methane assay buffer composed of 100 mM trizma base (pH 7.1), 15 mM MgCl2·6H2O, 5 mM ATP, 2 mM 2-mercaptoethanol, and 500 μM flavin adenine dinucleotide (FAD). Formic acid (50 mM) was included, or H2 was added to the headspace. The headspace was pressurized to 138 kPa with N2:CO2 (80:20) for assays performed with formic acid or H2:CO2 (80:20) for assays with H2 (4). Methane production was initiated by addition of 1 mM (final concentration) CoM-S-S-CoB (17). Methane production was monitored using a Buck Scientific model 910 gas chromatograph (GC) equipped with a flame ionization detector (4). Protein concentration was determined using Bradford assays (18).
CoM-S-S-CoB was synthesized as described previously using thiol exchange chemistry with CoB-S-S-CoB homodisulfide and HS-CoM (17), and it was purified by high-performance liquid chromatography (HPLC). CoB-S-S-CoB homodisulfide was a gift from William B. Whitman. HPLC was performed using a Magic 2002 HPLC with a Magic C18AQ 5-μ, 100-Å (2.0 by 150 mm) column equipped with a C1 (silica) reverse-phase micro guard column (Michrom Bioresources Inc.) at a 0.3 ml min−1 flow rate and with buffer containing 25 mM ammonium bicarbonate with a methanol gradient (0 to 80%).
RESULTS
Size-exclusion chromatography of purified His-tagged heterodisulfide reductase.
In previous work, we found that in cells of M. maripaludis grown under H2 excess, Hdr, Vhu, and formylmethanofuran dehydrogenase (Fwd) formed a supercomplex that presumably catalyzes the electron bifurcation-based reductions of CO2 and CoM-S-S-CoB (1). (In the presence of selenium, vhc is repressed, so only Vhu is observed [1, 19]). In the present work, we used a 6× His tag on the β subunit of Hdr to purify the protein by nickel affinity chromatography as before, but we additionally subjected the protein to gel filtration chromatography by FPLC. (M. maripaludis encodes two HdrB subunits, which appear to be interchangeable [1]; here, we tagged HdrB2.) A peak eluted at approximately 500 kDa (Fig. 1A), consistent with a dimer of HdrABC-VhuAUGD (predicted mass of ∼450 kDa). This size is consistent with the Hdr-Mvh complex from Methanothermobacter marburgensis, which was reported to have an apparent molecular mass of 500 kDa with a 1:1 stoichiometry of hydrogenase to Hdr (7). This subunit composition was confirmed by SDS-PAGE (Fig. 1B). In contrast to our previous results (1), Fwd was not present. Fwd may have dissociated during gel filtration chromatography, or it may have been destabilized due to the use of a much lower concentration of sodium dithionite, which at high concentration interfered with UV detection in the FPLC.
Fig 1.
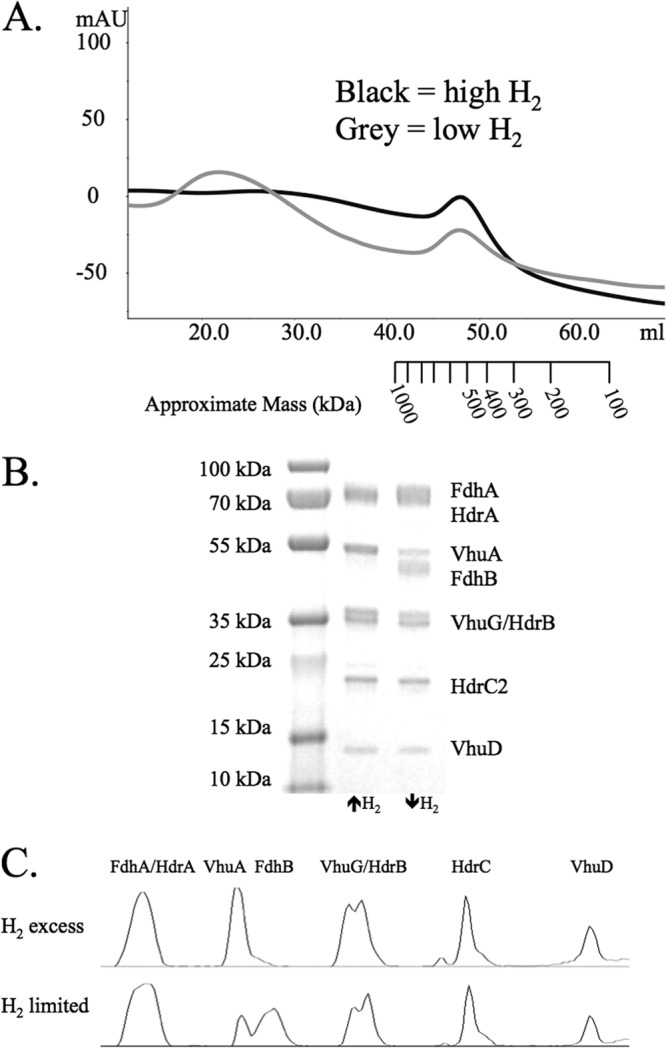
Purification of His-tagged HdrB2. (A) Gel filtration of the Hdr protein complex from M. maripaludis grown under H2 excess (black) or H2 limitation (gray). The approximate mass ladder is based on the retention times of molecular mass standards as described in Materials and Methods. (B) SDS-PAGE gel of stained protein collected from the peaks shown in panel A. (C) Densitometry plots for the lanes from the SDS-PAGE gel.
Fdh and hydrogenase compete for binding to the protein complex.
When M. maripaludis is grown under H2 limitation, fdh is derepressed and Fdh is incorporated into the Hdr-Vhu protein complex (1). We sought to determine if Fdh binds to a separate site on the complex or if Fdh displaces Vhu as the electron-donating enzyme. Nickel affinity purification followed by size-exclusion chromatography of an HdrB-His-expressing strain grown with H2 limitation revealed that the apparent molecular mass of the Vhu-Fdh-Hdr complex was similar to the mass of a Vhu-Hdr complex (Fig. 1A) despite the additional presence of Fdh (Fig. 1B). This suggested that Fdh displaced Vhu, as additional binding of two molecules of Fdh would increase the apparent mass of the protein complex by 234 kDa.
Densitometry analysis confirmed that Fdh binding to the protein complex displaced the hydrogenase (Fig. 1C and Table 2). The relative subunit stoichiometry of VhuA to HdrC decreased from 1:1 in cells grown with H2 excess to 0.37:1 in cells grown with H2 limitation, while the relative stoichiometry of FdhB to HdrC increased from 0.10:1 to 0.77:1 (Table 2). In contrast, the VhuD subunit remained bound to the protein complex at an approximate ratio of 1:1 under both growth conditions. Furthermore, the ratio of VhuA to VhuD decreased from 1.01:1 with H2 excess to 0.39:1 with H2 limitation, while the ratio of FdhB to VhuD increased from 0.10:1 to 0.82:1. As VhuD is hypothesized to interact with HdrA (6), we hypothesized that Fdh bound the same site of VhuD as VhuAGU, leading to displacement of the hydrogenase. This would make vhuD an essential gene for cultures grown on formate. Consistent with this, we have been unable to generate a vhuD deletion in M. maripaludis grown with formate (data not shown).
Table 2.
Ratios of proteins identified in purified complexesa
| Condition | VhuA-HdrC | FdhB-HdrC | VhuD-HdrC | VhuA-VhuD | FhdB-VhuD |
|---|---|---|---|---|---|
| High H2 | 1.0:1 | 0.10:1 | 0.99:1 | 1.0:1 | 0.10:1 |
| Low H2 | 0.37:1 | 0.77:1 | 0.94:1 | 0.39:1 | 0.82:1 |
Data are based on densitometry plots from Fig. 1C.
VhuD copurifies with Fdh.
To assess the binding associations of Fdh, a His-tagged version of this protein was also purified. (M. maripaludis encodes two Fdh proteins; we tagged Fdh1, since the genes for the alternative form of the enzyme [Fdh2] are only expressed when cells are grown with limiting concentrations of formate in the absence of H2 [20].) Cell extracts of an FdhA-His-expressing strain were subjected to nickel affinity purification followed by FPLC. A large peak eluted consistent with the size of the Hdr protein complex (Fig. 2A, P1), and it was found to be comprised of proteins from Hdr and Fdh (Fig. 2B). VhuD was also present, consistent with a role in binding Fdh. Bands for other subunits of the hydrogenase were not visible despite their purification with Fdh-His in previous studies (1). This is likely due to a more stringent wash (30 mM imidazole) than what was used previously (10 mM) and the fact that an Hdr dimer can bind either one Fdh and one VhuAGU or two Fdh molecules. Two Fdh molecules with two His tags would remain bound more tightly to the nickel resin, resulting in an enrichment in 2:2:2 Fdh-VhuD-Hdr complexes over 1:1:2:2 Fdh-VhuAGU-VhuD-Hdr complexes.
Fig 2.
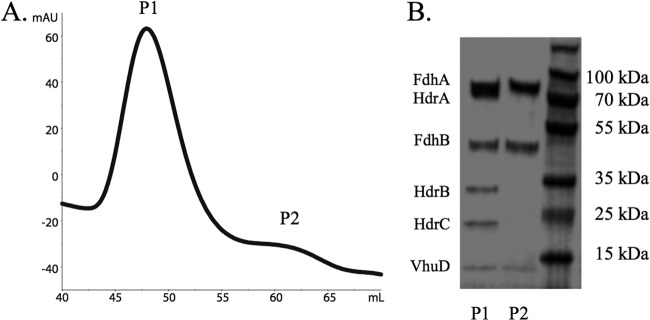
Purification of His-tagged FdhA1. (A) Gel filtration of the purified protein complex from M. maripaludis grown under H2 limitation. Peaks labeled P1 and P2 were collected and subjected to SDS-PAGE. (B) SDS-PAGE gel of protein from P1 and P2.
Interestingly, a small peak was also visible after FPLC purification (Fig. 2A, P2) that was composed of FdhA, FdhB, and VhuD (Fig. 2B). The calculated mass of this peak was ∼125 kDa, consistent with a 1:1:1 stoichiometry of the subunits. P2 comprised a very small fraction of the total protein purified; therefore, it was likely a dissociation product generated during the purification. However, the fact that VhuD remained bound to Fdh upon dissociation verifies that Fdh binds VhuD while competing with VhuAGU for binding to the complex.
M. maripaludis maintains functional hydrogenase and Fdh when both are present in the Hdr protein complex.
The presence of both hydrogenase and Fdh bound to Hdr when cells are grown on formate or under H2 limitation (1) suggests that M. maripaludis is poised to rapidly switch between these two electron donors. Batch culture M. maripaludis experiences H2 limitation when the culture optical density (OD) reaches values greater than ∼0.4, as H2 utilization becomes limited by the rate of H2 transfer into the aqueous phase (21). Therefore, we tested the ability of cell extracts from batch-grown M. maripaludis to switch electron donors using a previously established in vitro methane production assay (4, 17, 22). Cultures were grown to a maximum OD on H2 (OD of ∼1.0) or formate (OD of ∼0.6), and whole-cell protein was assayed for the ability to generate methane from the alternative electron donor. Thus, H2-grown cell extract was assayed with formate and formate-grown extract was assayed with H2. Cell extracts generated methane with both electron donors regardless of which was used for growth (Fig. 3A and B), suggesting that both hydrogenase and Fdh bound to Hdr are functional regardless of the actual electron donor being used for growth.
Fig 3.
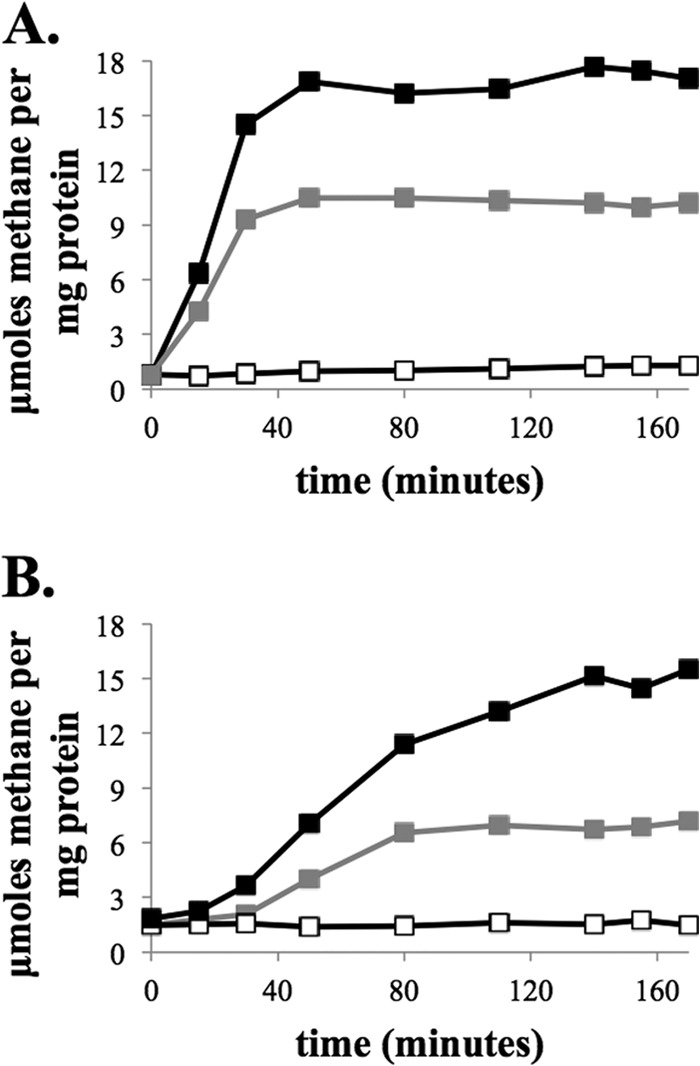
CH4 production from cell extracts of M. maripaludis strain MM901 grown with either H2 (A) or formate (B). Methane production was stimulated by addition of CoM-S-S-CoB. Controls where CoM-S-S-CoB was excluded are shown (white symbols). Black symbols, CH4 production by cell extracts using H2 as the electron donor. Gray symbols, CH4 production by cell extracts using formate as the electron donor.
F420-reducing hydrogenase plays a role in heterodisulfide reduction in the ΔvhuAU ΔvhcA mutant background.
Surprisingly, a ΔvhuAU ΔvhcA mutant of M. maripaludis grows on H2, albeit slowly (1) (Fig. 4). In addition to utilizing H2 as an electron donor for heterodisulfide reduction, Methanococcus voltae was reported to possess an inefficient, membrane-associated F420H2-heterodisulfide oxidoreductase activity that was dependent on F420-reducing hydrogenase (23). We hypothesized that the growth of the M. maripaludis ΔvhuAU ΔvhcA mutant on H2 depends on F420-reducing hydrogenase. To test this hypothesis, we assayed the ability of a ΔvhuAU ΔvhcA ΔfruA ΔfrcA mutant to grow with H2 as an electron donor. This mutant showed little or no growth on H2 but had kinetics similar to those of ΔvhuAU ΔvhcA on formate (Fig. 4).
Fig 4.
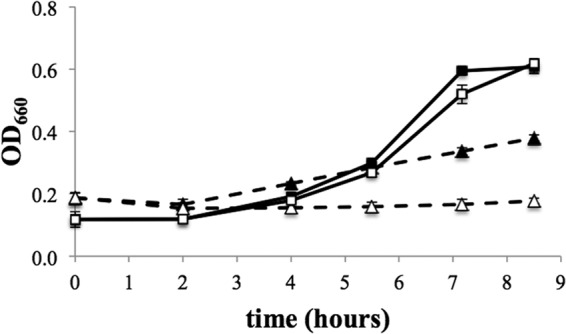
Growth of a ΔvhuAU ΔvhcA strain (black symbols) and a ΔvhuAU ΔvhcA ΔfruA ΔfrcA strain (white symbols). Solid lines, growth with formate. Dashed lines, growth with H2. Triplicate cultures grown with a 10% inoculum are shown. Error bars represent one standard deviation of the means.
DISCUSSION
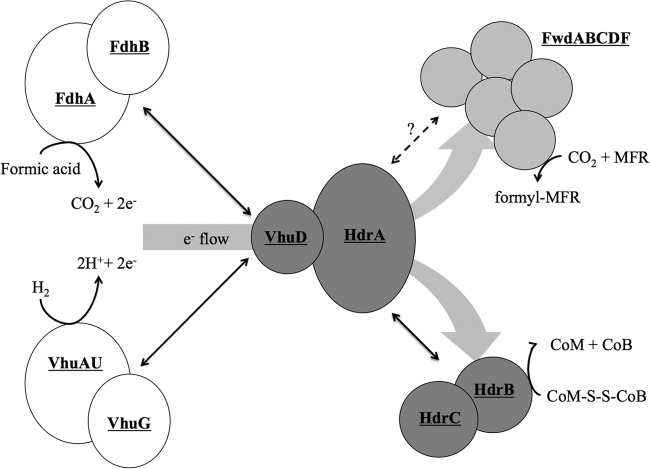
A picture of how the proteins in the Hdr complex interact is starting to emerge (Fig. 5). Results presented here show that VhuD is the primary electron conduit to Hdr from either Fdh or hydrogenase. Thus, VhuD sits at the intersection of the substrate-oxidizing enzymes and the active site of electron bifurcation, regardless of whether formate or hydrogen is the electron donor. Additional interactions can be inferred. Contact between VhuD and Hdr probably occurs at HdrA, since the homologous proteins are fused in some organisms (6). However, which subunit of Fdh or VhuAGU interact directly with VhuD is still unknown. From HdrA, electron flow is bifurcated to the active sites of CO2 reduction and heterodisulfide reduction. Fwd, the five-subunit enzyme that reduces CO2 to formyl-MFR, has been purified alone or as part of the Hdr-Vhu-Fdh supercomplex (1, 24) and likely binds near the electron-bifurcating center of HdrA. Although HdrA, HdrB, and HdrC interact (25), it is unclear whether HdrB or HdrC binds to HdrA. HdrC likely binds to HdrB, the site of heterodisulfide reduction, since these are fusion proteins in Methanosarcina spp. (26).
Fig 5.
Protein interactions in the Hdr supercomplex. Formate dehydrogenase (FdhAB) and hydrogenase (VhuAGU) compete for binding to VhuD. VhuA and VhuU are drawn together, since they form the catalytic site of H2 oxidation (30, 31). Electrons flow to HdrA, where their path is bifurcated to both FwdABCDF and HdrBC. Black double arrows indicate known interactions between subcomplexes. The dashed line between HdrA and Fwd signifies a hypothesized site of interaction between these subcomplexes. Reactions catalyzed by the individual enzymes are shown. The model only shows one of each enzyme, despite the fact that two of each are in the M. maripaludis genome (8). This reflects that, for the most part, the alternative forms are not expressed under the growth conditions used. HdrA, Vhu, and Fwd (as well as Fru) contain selenocysteine. M. maripaludis also encodes cysteine-containing versions, but these are repressed by selenium (19, 32). Additionally, Fwd contains tungsten, and its paralog, the molybdenum-containing formylmethanofuran dehydrogenase Fmd, requires high molybdenum for expression (33). In the case of Fdh, the genes for the alternative form of the enzyme are only expressed when cells are grown with limiting concentrations of formate in the absence of H2 (20). Analysis of the M. maripaludis proteome verifies that only one form of these enzymes is highly expressed under our culture conditions (28, 34).
Our results show that either Fdh or hydrogenase can bind to VhuD, and the interaction of Fdh and VhuD occurs in the absence of other hydrogenase subunits (Fig. 2B, P2). Cultures grown with H2 excess do not maintain high levels of Fdh (20, 27, 28), but when H2 is growth limiting, Fdh is abundant and competes with the Hdr-associated hydrogenase for binding to VhuD. When H2 is growth limiting there is evidently an advantage for M. maripaludis to be poised to alternate between H2 and formate as substrates for methanogenesis, and indeed, both substrates were functional in cell extracts (Fig. 3A and B). Mvh from M. marburgensis was found to exist in both Hdr-bound and free forms, suggesting that hydrogenase displaced from the Hdr complex by Fdh is maintained by the cell rather than degraded (6).
We also found that the F420-reducing hydrogenase (Fru/Frc) plays a role in heterodisulfide reduction when Vhu and Vhc are not functional, since a ΔvhuAU ΔvhcA mutant grows slowly on H2 but a ΔvhuAU ΔvhcA ΔfruA ΔfrcA mutant did not grow (Fig. 4). This suggests three possibilities. First, F420H2 generated by Fru/Frc could be an electron donor to Hdr via an F420H2 dehydrogenase. This is the case for the methylotrophic methanogens from the order Methanosarcinales, where electron flow from F420 to heterodisulfide is coupled via an electron transport chain to a chemiosmotic membrane gradient (3). However, neither a homologous F420 dehydrogenase nor components of the electron transport chain are present in hydrogenotrophic methanogens. Second, F420H2 generated by Fru/Frc could donate electrons to Hdr via Fdh, which uses F420 as an electron acceptor. However, the existence of the Hmd-Mtd cycle, an alternative pathway for F420 reduction with H2, argues against the first two possibilities, both of which rely on the known role of Fru/Frc to generate free F420H2. In the Hmd-Mtd cycle, a combination of the H2-dependent methylene-H4MPT dehydrogenase (Hmd) and the F420-dependent methylene-H4MPT dehydrogenase (Mtd) acting in reverse results in F420 reduction with H2 (11, 29). The Hmd-Mtd cycle is adequate for F420 reduction, since a ΔfruA ΔfrcA mutant has no growth defect on H2 (11, 29). Therefore, F420H2 should be abundant even in a ΔfruA ΔfrcA mutant. These data suggest a third possibility: electron flow occurs from Fru/Frc to Hdr without free F420H2 as an intermediate. This may occur with H2 donating electrons directly or acting through bound F420. However, there is as of yet no experimental evidence to indicate that Fru or Frc interacts directly with Hdr (1).
ACKNOWLEDGMENTS
We thank William B. Whitman for providing CoB-S-S-CoB homodisulfide and Murray Hackett for use of the HPLC.
This research was funded by grant DE-FG02-05ER15709 from the Chemical Sciences, Geosciences, and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy. K.C.C. was supported in part by a Public Health Service National Research Service Award, T32 GM07270, from the National Institute of General Medical Sciences.
Footnotes
Published ahead of print 13 September 2013
REFERENCES
- 1.Costa KC, Wong PM, Wang T, Lie TJ, Dodsworth JA, Swanson I, Burn JA, Hackett M, Leigh JA. 2010. Protein complexing in a methanogen suggests electron bifurcation and electron delivery from formate to heterodisulfide reductase. Proc. Natl. Acad. Sci. U. S. A. 107:11050–11055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaster AK, Moll J, Parey K, Thauer RK. 2011. Coupling of ferredoxin and heterodisulfide reduction via electron bifurcation in hydrogenotrophic methanogenic archaea. Proc. Natl. Acad. Sci. U. S. A. 108:2981–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thauer RK, Kaster AK, Seedorf H, Buckel W, Hedderich R. 2008. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 6:579–591 [DOI] [PubMed] [Google Scholar]
- 4.Lie TJ, Costa KC, Lupa B, Korpole S, Whitman WB, Leigh JA. 2012. Essential anaplerotic role for the energy-converting hydrogenase Eha in hydrogenotrophic methanogenesis. Proc. Natl. Acad. Sci. U. S. A. 109:15473–15478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thauer RK. 2012. The Wolfe cycle comes full circle. Proc. Natl. Acad. Sci. U. S. A. 109:15084–15085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stojanowic A, Mander GJ, Duin EC, Hedderich R. 2003. Physiological role of the F420-non-reducing hydrogenase (Mvh) from Methanothermobacter marburgensis. Arch. Microbiol. 180:194–203 [DOI] [PubMed] [Google Scholar]
- 7.Setzke E, Hedderich R, Heiden S, Thauer RK. 1994. H2: heterodisulfide oxidoreductase complex from Methanobacterium thermoautotrophicum. Composition and properties. Eur. J. Biochem. 220:139–148 [DOI] [PubMed] [Google Scholar]
- 8.Hendrickson EL, Kaul R, Zhou Y, Bovee D, Chapman P, Chung J, Conway de Macario E, Dodsworth JA, Gillett W, Graham DE, Hackett M, Haydock AK, Kang A, Land ML, Levy R, Lie TJ, Major TA, Moore BC, Porat I, Palmeiri A, Rouse G, Saenphimmachak C, Soll D, Van Dien S, Wang T, Whitman WB, Xia Q, Zhang Y, Larimer FW, Olson MV, Leigh JA. 2004. Complete genome sequence of the genetically tractable hydrogenotrophic methanogen Methanococcus maripaludis. J. Bacteriol. 186:6956–6969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thauer RK, Kaster AK, Goenrich M, Schick M, Hiromoto T, Shima S. 2010. Hydrogenases from methanogenic archaea, nickel, a novel cofactor, and H2 storage. Annu. Rev. Biochem. 79:507–536 [DOI] [PubMed] [Google Scholar]
- 10.Whitman WB, Shieh J, Sohn S, Caras DS, Premachandran U. 1986. Isolation and characterization of 22 mesophilic methanococci. Syst. Appl. Microbiol. 7:235–240 [Google Scholar]
- 11.Hendrickson EL, Leigh JA. 2008. Roles of coenzyme F420-reducing hydrogenases and hydrogen- and F420-dependent methylenetetrahydromethanopterin dehydrogenases in reduction of F420 and production of hydrogen during methanogenesis. J. Bacteriol. 190:4818–4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore BC, Leigh JA. 2005. Markerless mutagenesis in Methanococcus maripaludis demonstrates roles for alanine dehydrogenase, alanine racemase, and alanine permease. J. Bacteriol. 187:972–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haydock AK, Porat I, Whitman WB, Leigh JA. 2004. Continuous culture of Methanococcus maripaludis under defined nutrient conditions. FEMS Microbiol. Lett. 238:85–91 [DOI] [PubMed] [Google Scholar]
- 14.Leigh JA. 2011. Growth of methanogens under defined hydrogen conditions. Methods Enzymol. 494:111–118 [DOI] [PubMed] [Google Scholar]
- 15.Dodsworth JA, Leigh JA. 2006. Regulation of nitrogenase by 2-oxoglutarate-reversible, direct binding of a PII-like nitrogen sensor protein to dinitrogenase. Proc. Natl. Acad. Sci. U. S. A. 103:9779–9784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9:671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bobik TA, Wolfe RS. 1988. Physiological importance of the heterodisulfide of coenzyme M and 7-mercaptoheptanoylthreonine phosphate in the reduction of carbon dioxide to methane in Methanobacterium. Proc. Natl. Acad. Sci. U. S. A. 85:60–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 19.Noll I, Muller S, Klein A. 1999. Transcriptional regulation of genes encoding the selenium-free [NiFe]-hydrogenases in the archaeon Methanococcus voltae involves positive and negative control elements. Genetics 152:1335–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa KC, Yoon SH, Pan M, Burn JA, Baliga NS, Leigh JA. 2013. Effects of H2 and formate on growth yield and regulation of methanogenesis in Methanococcus maripaludis. J. Bacteriol. 195:1456–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lupa B, Hendrickson EL, Leigh JA, Whitman WB. 2008. Formate-dependent H2 production by the mesophilic methanogen Methanococcus maripaludis. Appl. Environ. Microbiol. 74:6584–6590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunsalus RP, Wolfe RS. 1977. Stimulation of CO2 reduction to methane by methylcoenzyme M in extracts Methanobacterium. Biochem. Biophys. Res. Commun. 76:790–795 [DOI] [PubMed] [Google Scholar]
- 23.Brodersen J, Gottschalk G, Deppenmeier U. 1999. Membrane-bound F420H2-dependent heterodisulfide reduction in Methanococcus voltae. Arch. Microbiol. 171:115–121 [DOI] [PubMed] [Google Scholar]
- 24.Wasserfallen A. 1994. Formylmethanofuran synthesis by formylmethanofuran dehydrogenase from Methanobacterium thermoautotrophicum Marburg. Biochem. Biophys. Res. Commun. 199:1256–1261 [DOI] [PubMed] [Google Scholar]
- 25.Hedderich R, Berkessel A, Thauer RK. 1990. Purification and properties of heterodisulfide reductase from Methanobacterium thermoautotrophicum (strain Marburg). Eur. J. Biochem. 193:255–261 [DOI] [PubMed] [Google Scholar]
- 26.Hamann N, Mander GJ, Shokes JE, Scott RA, Bennati M, Hedderich R. 2007. A cysteine-rich CCG domain contains a novel [4Fe-4S] cluster binding motif as deduced from studies with subunit B of heterodisulfide reductase from Methanothermobacter marburgensis. Biochemistry 46:12875–12885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hendrickson EL, Haydock AK, Moore BC, Whitman WB, Leigh JA. 2007. Functionally distinct genes regulated by hydrogen limitation and growth rate in methanogenic Archaea. Proc. Natl. Acad. Sci. U. S. A. 104:8930–8934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia Q, Wang T, Hendrickson EL, Lie TJ, Hackett M, Leigh JA. 2009. Quantitative proteomics of nutrient limitation in the hydrogenotrophic methanogen Methanococcus maripaludis. BMC Microbiol. 9:149. 10.1186/1471-2180-9-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Afting C, Kremmer E, Brucker C, Hochheimer A, Thauer RK. 2000. Regulation of the synthesis of H2-forming methylenetetrahydromethanopterin dehydrogenase (Hmd) and of HmdII and HmdIII in Methanothermobacter marburgensis. Arch. Microbiol. 174:225–232 [DOI] [PubMed] [Google Scholar]
- 30.Pfeiffer M, Bestgen H, Burger A, Klein A. 1998. The vhuU gene encoding a small subunit of a selenium-containing [NiFe]-hydrogenase in Methanococcus voltae appears to be essential for the cell. Arch. Microbiol. 170:418–426 [DOI] [PubMed] [Google Scholar]
- 31.Pfeiffer M, Bingemann R, Klein A. 1998. Fusion of two subunits does not impair the function of a [NiFeSe]-hydrogenase in the archaeon Methanococcus voltae. Eur. J. Biochem. 256:447–452 [DOI] [PubMed] [Google Scholar]
- 32.Berghofer Y, Agha-Amiri K, Klein A. 1994. Selenium is involved in the negative regulation of the expression of selenium-free [NiFe] hydrogenases in Methanococcus voltae. Mol. Gen. Genet. 242:369–373 [DOI] [PubMed] [Google Scholar]
- 33.Hochheimer A, Hedderich R, Thauer RK. 1998. The formylmethanofuran dehydrogenase isoenzymes in Methanobacterium wolfei and Methanobacterium thermoautotrophicum: induction of the molybdenum isoenzyme by molybdate and constitutive synthesis of the tungsten isoenzyme. Arch. Microbiol. 170:389–393 [DOI] [PubMed] [Google Scholar]
- 34.Xia Q, Hendrickson EL, Zhang Y, Wang T, Taub F, Moore BC, Porat I, Whitman WB, Hackett M, Leigh JA. 2006. Quantitative proteomics of the archaeon Methanococcus maripaludis validated by microarray analysis and real-time PCR. Mol. Cell. Proteomics 5:868–881 [DOI] [PMC free article] [PubMed] [Google Scholar]