Abstract
Cyclic di-AMP (c-di-AMP) and cyclic di-GMP (c-di-GMP) are signaling molecules that play important roles in bacterial biology and pathogenesis. However, these nucleotides have not been explored in Streptococcus pneumoniae, an important bacterial pathogen. In this study, we characterized the c-di-AMP-associated genes of S. pneumoniae. The results showed that SPD_1392 (DacA) is a diadenylate cyclase that converts ATP to c-di-AMP. Both SPD_2032 (Pde1) and SPD_1153 (Pde2), which belong to the DHH subfamily 1 proteins, displayed c-di-AMP phosphodiesterase activity. Pde1 cleaved c-di-AMP into phosphoadenylyl adenosine (pApA), whereas Pde2 directly hydrolyzed c-di-AMP into AMP. Additionally, Pde2, but not Pde1, degraded pApA into AMP. Our results also demonstrated that both Pde1 and Pde2 played roles in bacterial growth, resistance to UV treatment, and virulence in a mouse pneumonia model. These results indicate that c-di-AMP homeostasis is essential for pneumococcal biology and disease.
INTRODUCTION
Streptococcus pneumoniae (the pneumococcus) is part of the commensal flora of the human upper respiratory tract. It is also a major cause of an array of infections, including pneumonia, otitis media (OM), sinusitis, meningitis, and bacteremia. The pathogenesis of the bacterium is still not fully understood. Adaptation to the host niche by the pneumococcus is essential for its successful transition from a commensal to an invasive pathogen. Thus, a better understanding of how the pathogen controls its gene expression and interacts with the host is essential to more effectively control pneumococcal infections.
Several cyclic nucleotides have been shown to play important roles in bacterial gene regulation and pathogenesis. These nucleotides include cyclic AMP (cAMP), cyclic GMP (cGMP), cyclic di-GMP (c-di-GMP), cyclic di-AMP (c-di-AMP), and cyclic AMP-GMP (1, 2). Both c-di-GMP and c-di-AMP were discovered recently and were recognized as second messengers utilized by many bacteria, including bacterial pathogens during infection.
c-di-GMP regulates many biological cascades in bacteria (3, 4). It is synthesized from two GTP molecules by diguanylate cyclase through a highly conserved GGDEF domain and hydrolyzed into phosphoguanylyl adenosine (pGpG) by phosphodiesterase enzymes containing a highly conserved EAL domain. Another c-di-GMP phosphodiesterase that contains an HD-GYP domain breaks down c-di-GMP directly into two GMPs (4). c-di-GMP plays a role in the pathogenesis of many bacteria (5–14). Vibrio cholerae vieA encodes a c-di-GMP phosphodiesterase, and deletion of this gene led to attenuation in an infant mouse model of cholera (13, 14). This is consistent with the overexpression of a diguanylate cyclase, which resulted in the inhibition of cholera toxin production. Salmonella enterica serovar Typhimurium ydiV (or cdgR) encodes an EAL domain protein, which is essential for bacterial survival during exposure to oxidase (15). In Pseudomonas aeruginosa, the mutation of either c-di-GMP phosphodiesterase gene, pvrR or rocR, also attenuated the virulence in a mouse infection model (7). In addition, c-di-GMP regulates bacterial pathogenesis through the control of motility or biofilm formation in P. aeruginosa, Yersinia pestis, V. cholerae, and S. Typhimurium (5, 10, 16–19).
Diadenylate cyclase was first identified in Bacillus subtilis and Thermotoga maritima (20). The protein was originally named DisA for DNA integrity scanning protein A and participates in a DNA damage checkpoint (21). DisA consists of a diadenylate cyclase domain, a linker domain, and a DNA-binding helix-hairpin-helix (HhH) domain and converts ATP to c-di-AMP but does not use GTP, ITP, CTP, or UTP as its substrate (20). The diadenylate cyclase domain exists across eubacteria and archaea (22, 23). Most of these bacteria possess one diadenylate cyclase; however, three diadenylate cyclases were identified in B. subtilis (23, 24). In B. subtilis and Staphylococcus aureus, c-di-AMP can be cleaved into phosphoadenylyl adenosine (pApA) by a phosphodiesterase, which has a PAS domain, a GGDEF domain, a DHH domain, and a DHHA1 domain. DHH and DHHA1 domains are essential for the phosphodiesterase activity (25, 26). Bacterial DNA damage in B. subtilis reduces the levels of c-di-AMP (27), which also serves as an essential signal molecule required for cell wall peptidoglycan architecture homeostasis of the bacterium (28). In S. aureus, c-di-AMP controls cell size and envelope stress, biofilm formation, drug resistance, and potassium uptake (25, 29–32). In addition, several reports indicate that c-di-AMP represents a putative bacterial secondary signaling molecule that triggers a cytosolic pathway of innate immunity (27, 33–37). Such response is mediated via STING (stimulator of interferon [IFN] genes), a transmembrane protein that functions as an essential signaling adaptor (34, 38, 39). Recent reports have revealed that DDX41, a pattern recognition receptor (PRR) with a DEAD (aspartate-glutamate-alanine-aspartate) motif, plays a critical role in cyclic dinucleotide-mediated activation of type I interferon and interferon-mediated signaling (33, 40).
The presence of c-di-AMP and a diadenylate cyclase in Streptococcus pyogenes has been reported (41). However, cyclic dinucleotide production and homeostasis have not been explored in S. pneumoniae, a Gram-positive pathogen. In the present study, we identify genes associated with c-di-AMP production and degradation in S. pneumoniae and show that these proteins modulate bacterial growth, UV resistance, and pathogenesis. Based on our findings, we named pneumococcal SPD_1392, SPD_2032, and SPD_1153 dacA, pde1, and pde2 and their encoded proteins DacA, Pde1, and Pde2, respectively. This is the first identification of an enzyme (Pde2) that cleaves c-di-AMP directly into AMP.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in the present study are listed in Table 1. ST581, a streptomycin-resistant D39 derivative equivalent to ST594 (42) that has a recessive rpsL allele, rpsL1, was used as the parental strain. The rpsL1 allele confers streptomycin resistance in the absence of the wild-type (WT) rpsL+ allele (42). Pneumococci were routinely grown in Todd-Hewitt broth containing 0.5% (wt/vol) yeast extract (THY) or on tryptic soy agar (TSA) plates containing 3% (vol/vol) sheep blood. The media were supplemented with kanamycin (200 μg/ml) or streptomycin (150 μg/ml) for selection of mutants, as specified. Escherichia coli DH5α was used for routine cloning, and BL21(DE3) was used for expression of recombinant proteins. The E. coli strains were grown in Luria-Bertani (LB) broth or LB agar plates, and all strains harboring expression plasmids were grown with kanamycin at a final concentration of 25 μg/ml.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| S. pneumoniae strains | ||
| D39 | S. pneumoniae serotype 2 | 63 |
| ST581 | D39 with rpsL1 allele; Strepr | 42 |
| ST2718 | Δpde1::Janus cassette in ST581; Kanr | This study |
| ST2719 | Δpde2::Janus cassette in ST581; Kanr | This study |
| ST2729 | In-frame deletion mutant of pde1 in ST581; Strepr | This study |
| ST2730 | In-frame deletion of pde1 and Δpde2::Janus cassette; Kanr | This study |
| ST2733 | In-frame deletion mutant of pde2 in ST581; Strepr | This study |
| ST2734 | In-frame deletion mutant of pde1 and pde2 in ST581; Strepr | This study |
| ST2847 | ST581(pVA838); Strepr Ermr | This study |
| ST2848 | ST2733(pST2846); Strepr Ermr | This study |
| ST2849 | ST2733(pVA838); Strepr Ermr | This study |
| ST2850 | ST2729(pST2846); Strepr Ermr | This study |
| ST2851 | ST2729(pVA838); Strepr Ermr | This study |
| E. coli strains | ||
| DH5α | E. coli strain used for cloning | Laboratory stock |
| BL21(DE3) | E. coli strain used for expression | Novagen |
| ST2714 | E. coli BL21(DE3)(pST2710); Kanr | This study |
| ST2715 | E. coli BL21(DE3)(pST2711); Kanr | This study |
| ST2761 | E. coli DH5α(pST2761); Apr | This study |
| ST2762 | E. coli DH5α(pST2762); Apr | This study |
| Plasmids | ||
| pET28a(+) | His tag expression vector; Kanr | Novagen |
| pProEXHTb | His tag expression vector; Apr | Invitrogen |
| pVA838 | E. coli-S. pneumoniae shuttle vector; Ermr | 64 |
| pST2710 | pET28a(+) carrying S. pneumoniae dacA ORF; Kanr | This study |
| pST2711 | pET28a(+) carrying S. pneumoniae pde2 ORF; Kanr | This study |
| pST2761 | pProEX HTb carrying S. pneumoniae pde1 aa 51–657 ORF; Apr | This study |
| pST2762 | pProEX HTb carrying S. pneumoniae pde1 aa 109–657 ORF; Apr | This study |
| pST2846 | pVA838 carrying S. pneumoniae pde2 promoter and ORF; Ermr | This study |
Strepr, streptomycin resistance; Kanr, kanamycin resistance; Ermr, erythromycin resistance; Apr, ampicillin resistance.
Construction of mutants in S. pneumoniae.
The mutants of S. pneumoniae were generated through homologous recombination in ST581 with a counterselectable Janus cassette, which consists of a kanamycin resistance gene and a dominant WT rpsL+ allele (43). To construct a single mutant, the upstream and downstream sequences flanking the target gene were amplified by PCR using D39 genomic DNA as the template. The upstream fragment has an XbaI site, and the downstream fragment has an XhoI site (Table 2). These fragments were digested and ligated with the XbaI- and XhoI-digested Janus cassette. The ligation product was transformed into ST581 to select a kanamycin-resistant and streptomycin-sensitive mutant.
Table 2.
Primers used in this study
| Primera | Oligonucleotide sequence (5′ to 3′)b | Purposec |
|---|---|---|
| Mutagenesis | ||
| Pr1097 | GAGATCTAGAACCGTTTGATTTTTAATGGATAATG | Janus cassette; For |
| Pr1098 | GAGACTCGAGCCTTTCCTTATGCTTTTGGAC | Janus cassette; Rev |
| Pr2874 | TGACGATAATCTTGTGACGG | dacA upstream; For |
| Pr2875 | TTTTCTAGATTGATAGCTATCGTCC | dacA upstream; Rev |
| Pr2876 | TTTCTCGAGTTTTAAAGAACGATTGC | dacA downstream; For |
| Pr2877 | CCCTGAAATACGTGTGTCAC | dacA downstream; rev |
| Pr2880 | TTTTGATCCTGATAATCACG | pde1 upstream; For |
| Pr2881 | TTTTCTAGATGAATGATAATGGTGTCG | pde1 upstream; Rev |
| Pr2882 | TTTCTCGAGTGGTGCTAATTCCTATAGC | pde1 downstream; For |
| Pr2883 | GTGGTTCGTGACAATAGTAC | pde1 downstream; Rev |
| Pr2900 | TTTCTCGAGATGATAATGGTGTCGTATTC | pde1 unmarked mutant; Rev |
| Pr2901 | CATTTTCCCTTGTAAGATGAGG | pde2 upstream; For |
| Pr2902 | TTTTCTAGACTTGTATAATGCACTCTCAG | pde2 upstream; Rev |
| Pr2903 | TTTCTCGAGATGTAACCTTGTCAGAAGC | pde2 downstream; For |
| Pr2904 | ACGCTTCGTAAGCTTGGTTG | pde2 downstream; Rev |
| Pr2905 | TTTCTCGAGAAGTGGATAAGACTCCAAACG | pde2 unmarked mutant; Rev |
| Overexpression | ||
| Pr2895 | TTTCATATGTTGGGAAGAGCGACAGATTTC | dacA 89-271 aa; For |
| Pr2879 | TTTGGATCCACAAGCAAAAAAGAGTGAGG | dacA 89-271 aa; Rev |
| Pr2884 | TTTCCATGGAGATTTGCCAACAAATTTTAG | pde2 ORF; For |
| Pr2885 | TTTAAGCTTGTTTTTAAGCAAGTTTTTTAAC | pde2 ORF; Rev |
| Pr2960 | GCGGATCCAAGAAACTGAGAGTGCAT | pde151-657; For |
| Pr2961 | GCCTCGAGTCATTCTTCTTTCTCCTTTTC | pde151-657; Rev |
| Pr2962 | GCGGATCCACCAAGGAAGATGGTGATTTTG | pde1109-657; For |
The reverse primer of pde1109-657 was identical to pde151-657 Rev.
The restriction site is underlined if present in an oligonucleotide.
For, forward primer; Rev, reverse primer.
In-frame deletion of each gene was generated by homologous recombination from a background of the kanamycin-resistant mutant. A new reverse primer with an XhoI site (Table 2) was designed to amplify the upstream sequence and ligated directly with the XhoI-digested downstream fragment. The ligation product was transformed into the kanamycin-resistant mutant to select a streptomycin-resistant but kanamycin-sensitive mutant due to loss of the Janus cassette. The in-frame deletion mutants for pde1 and pde2 were designated ST2729 and ST2733, respectively.
To construct a Δpde1 Δpde2 double mutant, pde2 was replaced with the Janus cassette in the background of ST2729 to generate ST2730. The same upstream and downstream sequences used for generating the pde2 in-frame deletion were ligated and transformed into ST2730 to obtain a Δpde1 Δpde2 in-frame deletion mutant (ST2734).
Growth curve, morphology, and UV treatment.
The S. pneumoniae WT (ST581) and the Δpde1 (ST2729), Δpde2 (ST2733), and Δpde1 Δpde2 (ST2734) mutants were used to determine bacterial growth, morphology, and survival after UV treatment. To generate the growth curves, approximately 106 CFU of each bacterial stock was inoculated into 10 ml THY broth and grown at 37°C with 5% CO2. The growth of each strain was measured hourly by determining the optical density of the culture at 620 nm (OD620).
To determine bacterial morphology, bacteria were inoculated into 10 ml THY broth and grown to an OD620 of 0.3 or 0.8. Samples at each time point were smeared on slides, heat fixed, and stained using a Gram-staining kit (Sigma). Bacterial morphology was then examined using a light microscope (Olympus) at ×400 magnification.
For UV treatment, 106 CFU of bacteria was inoculated into 10 ml THY broth and grown to an OD620 of 0.4 or 0.8. Samples at each time point were taken, and 10-fold serial dilutions were performed. Two microliters of each dilution was spotted onto duplicate blood agar plates. One set of plates was treated with 1 mJ/cm2 UV radiation using a UV cross-linker (Stratagene). The other set of plates remained untreated as controls. All the plates were then incubated at 37°C with 5% CO2 for 18 h to enumerate the CFU. The survival rate of each strain was calculated as the number of CFU detected from the UV-treated plates as a percentage of those quantified from the untreated plates.
Protein expression and purification.
Open reading frames (ORFs) of S. pneumoniae dacA, pde2, and the truncated fragments encoding amino acids (aa) 51 to 657 and 109 to 657 of Pde1 were PCR amplified using the primers listed in Table 2. S. pneumoniae D39 genomic DNA was used as the template. The PCR products for dacA and pde2 were individually cloned into the pET28a(+) vector (Novagen) between the NcoI and HindIII sites to generate pST2710 and pST2711, respectively. These plasmids were sequence verified and maintained in E. coli BL21(DE3) for expression. The PCR products for pde151-657 and pde1109-657 were cloned into the pProEX HTb vector (Invitrogen) between the BamHI and XhoI sites to generate pST2761 and pST2762, respectively. Both plasmids were sequence verified and maintained in E. coli DH5α for expression.
Expression of the proteins was induced with 0.05 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) at room temperature for 4 h for DacA and Pde2 or overnight at 16°C for truncated Pde1 proteins. The recombinant proteins were purified using an Ni-nitrilotriacetic acid (NTA) resin (Qiagen) with buffers, as we previously reported (44, 45). The protein concentrations were then determined using a BCA Protein Assay Kit (Thermo Scientific). The purified protein aliquots were stored at −80°C until use.
Gel filtration.
Size exclusion chromatography experiments were performed with either a HiLoad 16/60 Superdex 200-pg column (GE Healthcare) for Pde1109-657 or a Superdex 200 10/300 GL column (GE Healthcare) for Pde151-657 and Pde2 and connected to a Gradiphrac Automatic Sampler (Amersham Biosciences). The columns were equilibrated and eluted at a constant flow rate of 0.5 ml/min with running buffer containing 10% (vol/vol) glycerol in phosphate-buffered saline (PBS) at pH 7.4. The molecular masses of the proteins were determined by using a Gel Filtration Standard (Bio-Rad) according to the instructions for the Gel Filtration Principles and Methods (GE Healthcare).
Cleavage of BNPP.
Reaction mixtures (50 μl) consisted of 50 mM Tris-HCl at a specified pH, 10 mM NaCl, 5 μM 2-mercaptoethanol, 0.1 mM specified metal cation, and 2 mM bis-p-nitrophenyl phosphate (BNPP). The reaction was initiated by addition of 3 μM purified Pde1109-657 or 70 nM purified Pde2, and the reaction mixture was incubated at room temperature for 10 min for the samples with Pde2 or for 4 h for the samples with Pde1. Relative BNPP cleavage was determined by measuring the OD410 using a microplate reader (Spectrum Max 340PC; Molecular Devices). The assay mixtures used for metal ion screening contained 0.1 mM CaCl2, CoCl2, FeCl2, Fe(NO3)3, MgCl2, MnCl2, or ZnSO4. For pH analysis, reaction mixtures consisted of 50 mM Tris-HCl at pH 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, 9.0, or 9.5.
HPLC and mass spectrometry (MS).
Determination of the enzymatic activities of S. pneumoniae DacA using high-performance liquid chromatography (HPLC) was performed as reported previously (46, 47) with minor modification. Briefly, reaction mixtures (50 μl) contained 40 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 100 mM NaCl, and 0.1 mM ATP. The reaction was initiated by adding 10 μM pneumococcal DacA protein, incubated for 1 h at 37°C, and then terminated by adding 1 μl of 0.5 M EDTA. Finally, 20 μl of each sample was injected and separated by reverse-phase HPLC with a C18 column (250 by 4.6 mm; Vydac) using a Waters 625 LC system equipped with a 996 Photodiode Array Detector and a 717 Autosampler (Waters). Samples were eluted using the same buffers and program as previously reported (48). Nucleotides were monitored at 254 nm.
The reaction mixtures (10 μl) to determine the activity of Pde1 and Pde2 contained 50 mM Tris-HCl (pH 7.5), 1 mM MnCl2, and 0.5 mM the indicated nucleotide. The reaction was initiated by adding Pde1 to 10 μM and incubating for 4 h at 37°C or by adding Pde2 to 2.5 μM and incubating for 1 h at 37°C. Subsequently, each reaction was terminated by adding 1 μl of 0.5 M EDTA and diluting 1:5 with water. Finally, 20 μl of each sample was injected and separated by reverse-phase HPLC. c-di-AMP and pApA standards were purchased from Biolog. AMP was purchased from Sigma. The reaction of Pde2 was further analyzed by LC-tandem MS (MS-MS) using the same chromatography and mass spectrometry settings previously described (49) and monitoring in negative-ion mode at m/z 657 to 134 for c-di-AMP and m/z 346.18 to 78.69 for AMP. Chemically synthesized c-di-AMP and AMP ranging in concentration from 0.97 nM to 250 nM were used to generate a standard curve.
Kinetic measurement of enzymatic activities of Pde1 and Pde2.
The kinetic parameters were determined by monitoring the hydrolysis of c-di-AMP by Pde1 and Pde2 and the hydrolysis of pApA by Pde2 using HPLC. The assay conditions were the same as described above, except that Pde2 was incubated with pApA for 10 min. The kinetic parameters were obtained by fitting the enzymatic activities at various substrate concentrations to a Michaelis-Menten equation using Prism 5 version 5.0a (GraphPad Software).
Preparation and detection of bacterial c-di-AMP.
Bacteria were grown in 10 ml THY to an OD620 of 0.3 or 0.8. One milliliter of culture was harvested and resuspended in 500 μl of 50 mM Tris-HCl (pH 8.0). The suspension was sonicated for 20 s (10 s on with a 15-s interval) followed by boiling for 5 min, and the bacterial debris was removed by centrifugation for 5 min at 13,000 rpm. The supernatant was then used to detect c-di-AMP using an enzyme-linked immunosorbent assay (ELISA) that we developed at Albany Medical College (patent pending). This method was developed based on the identification of a c-di-AMP binding protein (CabP) in S. pneumoniae with high affinity and specificity in interaction with c-di-AMP (Y. Bai, J. Yang, T. Zarrella, Y. Zhang, D. W. Metzger, and G. Bai, unpublished data). Briefly, a 96-well plate was coated with CabP at 10 μg/ml at 4°C for at least 14 h. The plate was then washed and blocked with 1% bovine serum albumin (BSA) for 1 h. Samples and purified c-di-AMP (Biolog) standards were mixed with 25 nM biotin-labeled c-di-AMP (Biolog) and incubated for 2 h. After thorough washing, the plate was incubated with horseradish peroxidase-conjugated streptavidin (Thermo Scientific) for 1 h. The peroxidase was finally detected with o-phenylenediamine dihydrochloride (OPD) (Sigma) as a substrate and measured at OD492. The c-di-AMP levels in the samples were calculated based on the standard curve and were normalized by actual OD620 readings of each culture.
Preparation of polyclonal antibodies and Western blot analysis.
Antibodies were generated similarly to our previous description (46). Briefly, five female BALB/c mice (Taconic) were immunized subcutaneously with 50 μg of either Pde151-657 or Pde2 emulsified 1:1 with alum (Thermo Scientific) in 100 μl and boosted twice biweekly with the same amount of protein and adjuvant. The specificity of sera was confirmed by Western blotting with purified His-Pde151-657 and His-Pde2, respectively.
For Western blot analysis, purified proteins or bacterial lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto polyvinylidene difluoride (PVDF) membranes, and sequentially probed with antisera and with a peroxidase-conjugated goat anti-mouse IgG secondary antibody (Thermo Scientific). Peroxidase detection was carried out with the ECL Western blotting detection reagent and analysis system (Thermo Scientific).
Infection of mice.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Research Council. The protocol was approved by the Institutional Animal Care and Use Committee of Albany Medical College. The S. pneumoniae WT (ST581), Δpde1 (ST2729), Δpde2 (ST2733), and Δpde1 Δpde2 (ST2734) strains were grown to an OD620 of 0.4 in THY broth, washed, and diluted to 108 CFU/ml using PBS. Six- to 8-week old female BALB/c mice (Taconic) were inoculated intranasally with 50 μl (5 × 106 CFU) of bacteria (n = 8 per group). The mice were subsequently monitored for 9 days.
RESULTS
S. pneumoniae SPD_1392 is a diadenylate cyclase.
c-di-AMP and c-di-GMP are signaling molecules that have been recognized in a number of bacteria. However, these nucleotides and their associated genes have not been explored in S. pneumoniae. In this study, we analyzed the genome of the S. pneumoniae D39 strain (50) and were unable to identify a protein with a typical diguanylate cyclase GGDEF domain in the bacterium. It is likely that either S. pneumoniae cannot produce c-di-GMP or the nucleotide is produced by an atypical diguanylate cyclase. In contrast, SPD_1392 encodes a protein with a DisA_N domain (50) (Fig. 1A), suggesting that this protein might have diadenylate cyclase activity, similar to DisA, DacA (also referred to as CdaA or YbbP), and DacB (also referred to as CdaS or YoiJ) of B. subtilis. Therefore, we designated SPD_1392 DacA, for diadenylate cyclase. The amino acid sequence of S. pneumoniae DacA shares 46.8% identity with the DacA proteins of Listeria monocytogenes (36) and B. subtilis (23, 24) but shares only 15.6% and 13.3% identity with the DisA proteins of B. subtilis (20) and Mycobacterium tuberculosis (previously named DacA) (46), respectively. Genetically, S. pneumoniae dacA is the first gene of the dacA-cdaR-glmM locus, similar to the dacA genes of B. subtilis, S. aureus, and L. monocytogenes. Three transmembrane helices were identified at the N terminus in S. pneumoniae DacA by using the TMHMM server (http://www.cbs.dtu.dk/services/TMHMM-2.0/). We were unable to express full-length DacA in E. coli; thus, we expressed its C-terminal domain (Fig. 1A), purified it to homogeneity, and analyzed its activity using HPLC. Our results showed that the protein converted ATP to c-di-AMP, similar to B. subtilis DisA (Fig. 1B), indicating that it functions as a diadenylate cyclase.
Fig 1.
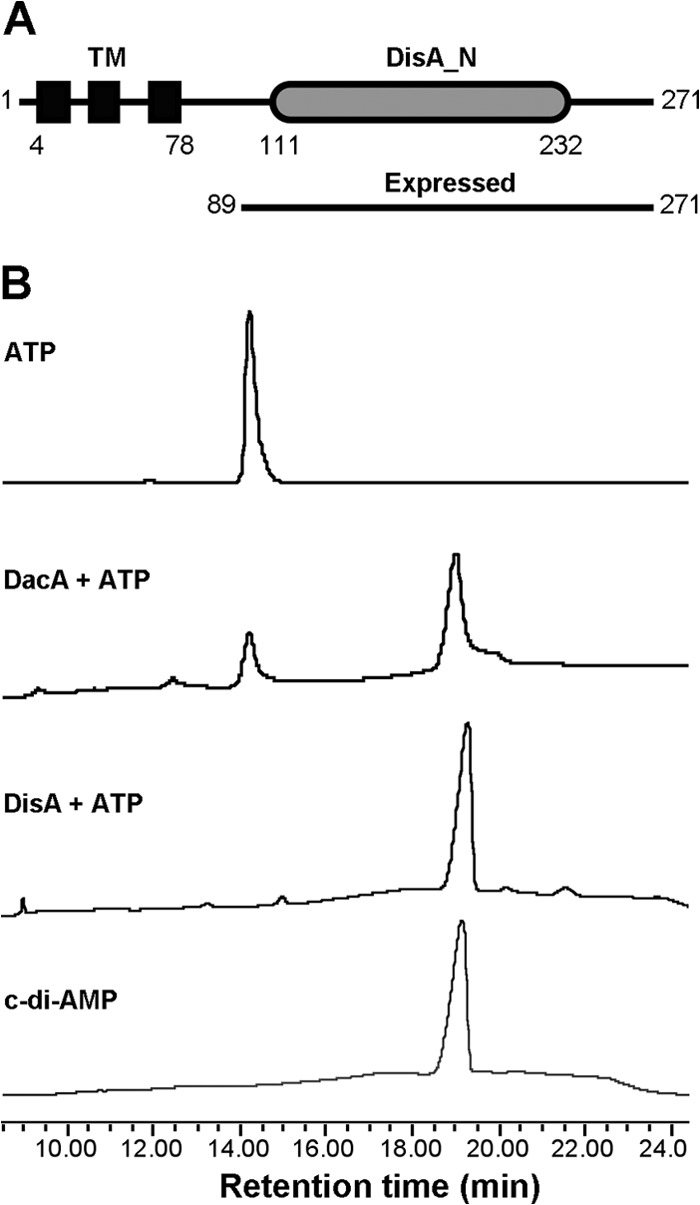
Domain architecture and activity of S. pneumoniae DacA. (A) Domain architecture of DacA. The numbers indicate the positions of amino acids in the full-length protein. TM, transmembrane domain. (B) Enzymatic activity of DacA. ATP was incubated with purified S. pneumoniae DacA, and the reaction mixture was separated using HPLC. ATP and c-di-AMP standards, as well as the reaction with purified B. subtilis DisA, were used as controls.
We tried five times to delete S. pneumoniae dacA by homologous recombination but failed to generate a ΔdacA mutant. This is consistent with the report that dacA is an essential gene in the pathogen (51).
S. pneumoniae SPD_2032 and SPD_1153 are c-di-AMP phosphodiesterases.
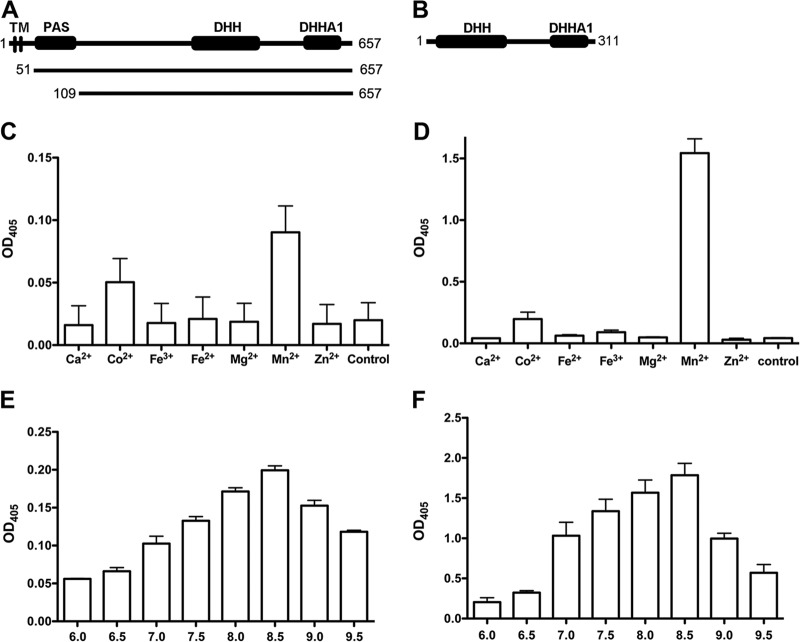
B. subtilis GdpP (formerly YybT) and S. aureus GdpP have been identified as c-di-AMP phosphodiesterases (25, 26, 53). Both proteins possess two transmembrane helices, a PAS domain, an atypical GGDEF domain, a DHH domain, and a DHHA1 domain. Experimental evidence showed that the C-terminal DHH and DHHA1 region is essential for c-di-AMP phosphodiesterase activity (25, 26). We analyzed the D39 genome and found that a protein encoded by SPD_2032 (pde1) has the same domain structures as GdpP (Fig. 2A). In addition, another protein encoded by SPD_1153 (pde2) also possesses DHH and DHHA1 domains (Fig. 2B), but not the other domains of Pde1, suggesting that this protein may also have c-di-AMP phosphodiesterase activity.
Fig 2.
Cleavage of BNPP by S. pneumoniae Pde1 and Pde2. (A and B) Domain architectures of Pde1 (A) and Pde2 (B). Two different constructs of Pde1, Pde151-657 and Pde1109-657 as indicated by the amino acid positions, were expressed and purified. (C and D) Cleavage of BNPP by Pde1109-657 (C) and Pde2 (D) in the presence of different metal cations (no cation was present in the control reaction). (E and F) Cleavage of BNPP by Pde1109-657 (E) and Pde2 (F) under different pH conditions. The data shown in panels C to F are the means of three independent experiments. The error bars denote the standard error of the mean (SEM).
We engineered two truncated Pde1 proteins to remove either the transmembrane domain (Pde151-657) or both the transmembrane and PAS domains (Pde1109-657). The proteins Pde151-657, Pde1109-657, and Pde2 were then purified to homogeneity. Both the Pde151-657 and Pde1109-657 proteins showed uniformly high-order oligomerization (not shown). A similar result has also been reported with B. subtilis GdpP (26). In contrast, Pde2 displayed a molecular mass of ∼70 kDa in solution (not shown), which is consistent with Pde2 existing as a dimer.
In order to optimize the catalytic activities of these proteins, we first used BNPP, a nonspecific substrate of phosphatase and phosphodiesterase, to determine the enzymatic activities of Pde109-657 and Pde2 under different conditions. Our results showed that both proteins preferred Mn2+ from the metal cations that we tested (Fig. 2C and D). In terms of pH, both Pde1109-657 and Pde2 proteins exhibited the highest enzymatic activity at pH 8.5 (Fig. 2E and F). The preference of Pde51-657 was similar to that of Pde1109-657 in all these assays (not shown).
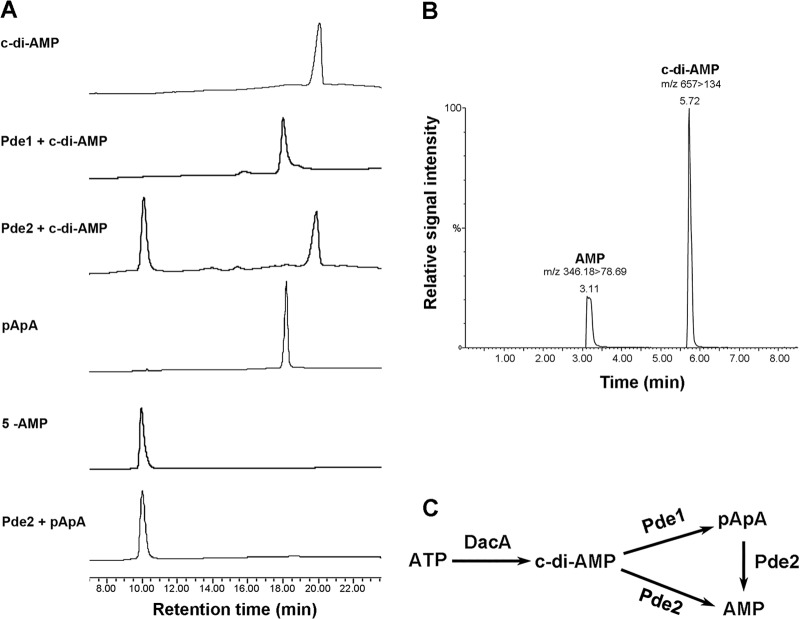
We determined the phosphodiesterase activities of the pneumococcal Pde1 and Pde2 proteins toward cleavage of c-di-AMP. Our results showed that Pde151-657 cleaved c-di-AMP exclusively into pApA (Fig. 3A). This result is consistent with the activity of B. subtilis GdpP (26). In contrast, Pde2 hydrolyzed c-di-AMP solely into AMP (Fig. 3A), which differs from the result with Pde1. Using LC–MS-MS, we confirmed that c-di-AMP was degraded to AMP by Pde2 (Fig. 3B). Additionally, Pde2 was also capable of converting pApA to AMP, whereas Pde1 showed no activity toward pApA (Fig. 3A). The activity of Pde1109-657 was similar to that of Pde151-657 in all the assays (not shown), suggesting that the PAS domain does not affect the enzymatic specificity against the tested nucleotides. These results indicate that both Pde1 and Pde2 possess c-di-AMP phosphodiesterase activity, but their cleavage products are different (Fig. 3C). Our kinetics result showed that c-di-AMP was cleaved comparably by Pde151-657 (Vmax = 101.6 ± 31.32 nmol mg−1 min−1; Km = 36.40 ± 17.43 μM) and Pde2 (Vmax = 48.92 ± 12.71 nmol mg−1 min−1; Km = 16.75 ± 8.70 μM) (Fig. 4A), whereas Pde2 preferred pApA (Vmax = 333.5 ± 61.21 μmol mg−1 min−1; Km = 23.96 ± 7.78 μM) (Fig. 4B) to c-di-AMP. Therefore, the cleavage product of c-di-AMP by Pde1 might be rapidly hydrolyzed into AMP by Pde2 (Fig. 3C).
Fig 3.
Phosphodiesterase activities of S. pneumoniae Pde1 and Pde2. (A) Pde151-657 or Pde2 protein was incubated with c-di-AMP or pApA, as indicated. Each sample was separated by HPLC and monitored at 254 nm. c-di-AMP, pApA, and AMP were used as standards. (B) Verification that AMP is the sole degradation product from c-di-AMP catalyzed by Pde2 using LC–MS-MS. AMP and c-di-AMP were eluted at 3.11 min and 5.72 min, respectively, which were identical to their standards (not shown). The m/z of each nucleotide is indicated. (C) Summary of the catalytic activities of pneumococcal DacA, Pde1, and Pde2 in homeostasis of c-di-AMP.
Fig 4.
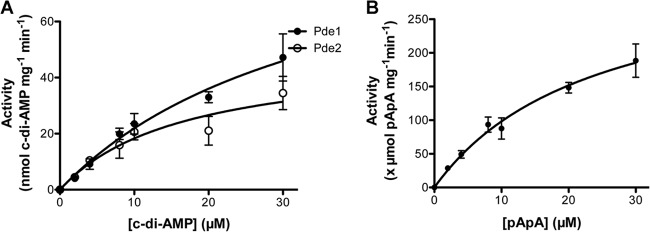
Cleavage of c-di-AMP and pApA by S. pneumoniae Pde1 and Pde2. (A) c-di-AMP at indicated concentrations was cleaved by Pde1 or Pde2 for 1 h. The enzymatic activity was determined as nmol c-di-AMP cleaved per mg protein per min. (B) pApA was cleaved by Pde2 for 10 min. Enzymatic activity was determined as μmol pApA cleaved by per mg protein per min. Note that different units are displayed for cleaved c-di-AMP (A) and pApA (B). The error bars indicate the SEM.
Both Pde1 and Pde2 play a role in controlling bacterial c-di-AMP levels.
In order to explore the biological functions of both Pde1 and Pde2 in S. pneumoniae, we generated mutant strains, including Δpde1 (ST2729), Δpde2 (ST2733), and Δpde1 Δpde2 (ST2734). From the genetic analysis, pde1 is the first gene in a putative operon of 4 or 5 genes (Fig. 5A), and pde2 may also be in an operon with SPD_1154 (Fig. 5B). Deletion of either pde1 or pde2 simply by replacement with a drug resistance marker may result in polar effects. Therefore, we constructed all these mutants with in-frame deletions to avoid polar effects on downstream genes (Fig. 5A and B). All three mutants were verified by PCR (not shown) and Western blot analysis using specific antibodies against Pde1 and Pde2, respectively (Fig. 5C).
Fig 5.
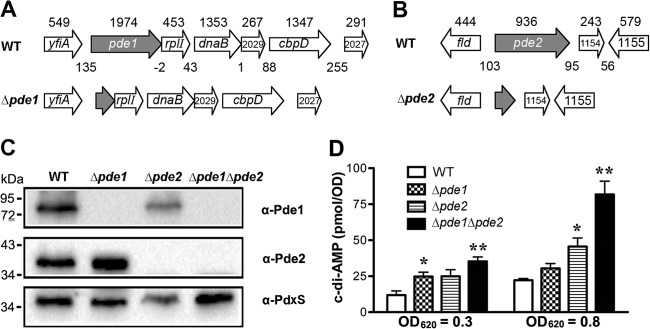
Construction of in-frame deletion mutants of pde1 and pde2 in S. pneumoniae. (A and B) The in-frame deletion mutants of S. pneumoniae pde1 (A) and pde2 (B) were generated using homologous recombination. The size (in bp) of each ORF is indicated above the gene. The length (in bp) of each intergenic region is also marked between adjacent genes. (C) Western blot analysis to verify the deletion of pde1 and pde2 using antibodies against Pde1 and Pde2, respectively. Subsequent blotting with a mouse anti-PdxS antibody (62) served as a loading control. (D) Detection of bacterial c-di-AMP levels. Bacteria were grown in THY broth to an OD620 of 0.3 or 0.8. Each sample was then harvested, resuspended in 50 mM Tris-HCl (pH 8.0), and sonicated. The bacterial debris was removed, and the supernatant was taken to detect c-di-AMP levels using ELISA. The concentration of each sample was normalized with the actual OD620. The data shown are the means of three independent experiments. The error bars denote the SEM. *, P < 0.05; **, P < 0.01 in a two-tailed t test using Prism 5.0a (GraphPad Software).
The c-di-AMP levels in all three mutants were compared to those in the parental strains. Deletion of either pde1 or pde2 resulted in only a moderate increase of the c-di-AMP levels compared to the parental strain (Fig. 5D). However, deletion of both genes exhibited up to a 4-fold increase in c-di-AMP levels compared to that of the parental strain (Fig. 5D). This result suggests that c-di-AMP is a biological substrate of both Pde1 and Pde2 and that the two enzymes both contribute to pneumococcal c-di-AMP homeostasis.
Both Pde1 and Pde2 play a role in pneumococcal growth.
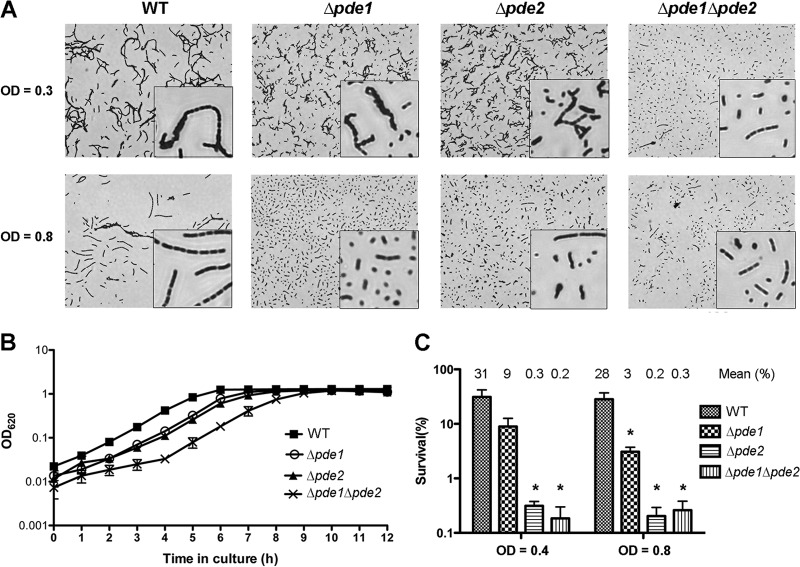
It has been reported that S. aureus GdpP plays a role in controlling bacterial cell size and envelope stress (25). In this study, we determined the roles of Pde1 and Pde2 in pneumococcal growth. All three mutants and the parental strain were grown to early and mid-log phases and were stained to determine their morphologies. Our results showed that in early log phase, the bacterial chains of both Δpde1 and Δpde2 strains were slightly shorter than that of the WT, and the change in the Δpde1 Δpde2 double mutant was more dramatic (Fig. 6A). In mid-log phase, the lengths of the chains of all three mutants were significantly shorter than that of the WT (Fig. 6A), indicating that both Pde1 and Pde2 play a role in pneumococcal chain formation. This result is consistent with the reports that increased c-di-AMP reduces the cell size of S. aureus and promotes sporulation of B. subtilis (25, 27).
Fig 6.
Growth phenotypes of the S. pneumoniae Δpde1 and Δpde2 mutants. (A) Morphology of the WT, Δpde1, Δpde2, and Δpde1 Δpde2 strains after Gram staining. The images were taken at a magnification of ×400. The insets are enlarged an additional 4-fold. The results shown are representative of three independent experiments. (B) Growth curves of the WT, Δpde1, Δpde2, and Δpde1 Δpde2 strains in THY broth. The same CFU from the bacterial stocks were inoculated, and bacterial growth was monitored hourly. The data shown are the means of three independent experiments. The error bars denote the SEM. (C) Response of WT, Δpde1, Δpde2, and Δpde1 Δpde2 strains to UV treatment. Bacterial serial dilutions were spotted onto TSA plates either with or without treatment with UV radiation. The plates were then incubated overnight, and the CFU were enumerated to determine the survival percentage of each bacterial strain. The data shown are the means of three independent experiments. The error bars denote the SEM. *, P < 0.05 in a two-tailed t test using Prism 5.0a (GraphPad Software).
The growth curves of the WT and the three mutants were determined at OD620. The results showed that both Δpde1 and Δpde2 reduced the growth rate slightly, and the double mutant synergized the reduction (Fig. 6B). This result differs from a previous report that no difference between the WT and the mutants was observed when they were grown in vitro (52). The difference between the two studies may have been produced by the methodology used to generate the mutants.
Pde1 orthologs in B. subtilis and Lactococcus lactis have been shown to play a role in stress response (26, 54). Additionally, it is also well established that both DisA and GdpP in B. subtilis are responsive to DNA damage (20, 27). In this study, we determined the pneumococcal response to UV treatment using S. pneumoniae WT (ST581), Δpde1 (ST2729), Δpde2 (ST2733), and Δpde1 Δpde2 (ST2734) strains. Our results showed that ∼30% of the WT bacteria survived after UV radiation at 1 mJ/cm2. However, approximately 8 to 9% of Δpde1 bacteria survived after the same UV treatment. The survival rates of both Δpde2 and Δpde1 Δpde2 bacteria were less than 0.5% (Fig. 6C). These results indicate that both Pde1 and Pde2 play a role in response to UV radiation, and Pde2 contributes more to response against UV damage than Pde1.
Both Pde1 and Pde2 are essential in pneumococcal pathogenesis.
The roles of Pde1 and Pde2 in pneumococcal pathogenesis have been well characterized, including adherence to human epithelial cells, as well as colonizing and causing OM, pneumonia, and bacteremia in various mouse infection models (52). In the mouse pneumonia competitive-infection model, bacterial loads in both the lung homogenate and the bronchoalveolar lavage fluid (BALF) of mice were numerated 48 h postinfection. Both Pde1 and Pde2 are essential for virulence (52).
In this study, we also determined the roles of Pde1 and Pde2 in pneumococcal virulence using a mouse pneumonia model. We compared the survival rates of mice infected with the WT, Δpde1, Δpde2, or Δpde1 Δpde2 strain. Our results showed that the median survival time of the WT-infected mice was 4.5 days, while the mice infected with the mutant strains, with the exception of one mouse infected with Δpde1, all survived until the end time point (Fig. 7). This result indicates that the virulence of all the three mutants was significantly attenuated compared to the parental strain, which is consistent with the observation of the bacterial loads (52). Therefore, both Pde1 and Pde2 are required during pneumococcal infection.
Fig 7.
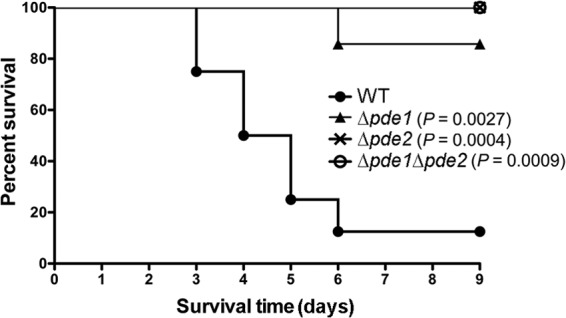
Infection of mice with the pneumococcal phosphodiesterase mutants. Approximately 5 × 106 CFU of the WT, Δpde1, Δpde2, or Δpde1 Δpde2 strain was inoculated intranasally in 50 μl PBS, and the mice were subsequently monitored for 9 days. The indicated P values are the statistical results for each mutant compared to the WT and analyzed by the log-rank (Mantel-Cox) test using Prism 5.0a (GraphPad Software).
DISCUSSION
In this study, S. pneumoniae dacA was identified as an ortholog of B. subtilis dacA (24). In B. subtilis, three diadenylate cyclases have been reported, DisA, DacA, and DacB (23, 24). Analysis using the TMHMM server revealed that the DacA proteins of B. subtilis, S. pneumoniae, L. monocytogenes, and S. aureus all possess three transmembrane helices, whereas B. subtilis DisA and DacB, as well as M. tuberculosis DisA, are cytoplasmic. Recent studies showed that disA, dacA, or dacB alone could be disrupted in B. subtilis, but a triple mutant of these genes could not be generated in the bacterium unless one of these cyclases was provided (24, 28). These observations clearly indicate that c-di-AMP, rather than any one particular diadenylate cyclase, is required for the viability of B. subtilis (24). Interestingly, dacA is an essential gene in S. pneumoniae (51), similar to those in L. monocytogenes and S. aureus (25, 36). Moreover, dacA encodes the only diadenylate cyclase identified in the three bacterial species. Therefore, the essentiality of dacA in these bacteria is very likely due to the need for c-di-AMP for bacterial viability.
It is interesting that the presence of c-di-AMP or c-di-GMP is relatively distinct between Gram-positive and Gram-negative bacteria (23). The majority of diguanylate cyclases have been identified in Gram-negative bacteria, whereas diadenylate cyclases have been reported mostly in Gram-positive bacteria. The presence of c-di-GMP signaling in Gram-positive bacteria has been directly demonstrated only in B. subtilis (55, 56) and the spore-forming, obligate anaerobe Clostridium difficile (57). We were unable to find any GGDEF motif in S. pneumoniae proteins. The Pde1 ortholog in Streptococcus mutans, AAN59731, has been implicated as a diguanylate cyclase and plays a role in biofilm formation (58). However, a typical GGDEF motif is absent in the protein, and its ortholog in B. subtilis is unable to generate c-di-GMP from GTP (26). Moreover, we showed that pneumococcal Pde1 functions as a phosphodiesterase, similar to its ortholog in B. subtilis (26). Therefore, there is no evidence that c-di-GMP is present in Streptococcus spp.
In a previous study, an ortholog of S. pneumoniae Pde2 in S. mutans was characterized as 3′-phosphoadenosine-5′-phosphate (pAp) phosphatase, which dephosphorylates pAp to AMP (59). Additionally, both Pde1 and Pde2 have been reported to contribute to pneumococcal virulence and to confer protection against pneumococcal disease (52). In this study, we explored whether these pneumococcal proteins were able to hydrolyze c-di-AMP and other nucleotides. Both of the DHH domain proteins in S. pneumoniae were shown to function as c-di-AMP phosphodiesterases. However, the cleavage products of Pde1 and Pde2 differ. Pde1 orthologs and similar enzymatic activities have been demonstrated in B. subtilis, S. aureus, and L. monocytogenes (25–27, 54, 60). However, cleavage of c-di-AMP by Pde2 has not been reported, but its orthologs can be found in all these bacteria, indicating that the bacteria all likely have more than one enzyme that cleaves c-di-AMP. Pde2 is an ortholog of B. subtilis YtqI (also named NrnA), which has been shown to degrade nanoRNA (RNA oligonucleotides of ≤5 nucleotides) and dephosphorylates pAp to AMP (61). In addition, the Pde2 ortholog in S. mutans degrades pAp (59), while B. subtilis GdpP is unable to hydrolyze pAp (26). Thus, the activities of these enzymes differ substantially in substrate preferences and catalytic products. Interestingly, in the c-di-GMP networks, when high initial levels of c-di-GMP were provided as the substrate, pGpG was detected as an intermediate degradation product by the HD_GYP domain protein. In contrast, we could not detect pApA in reactions with c-di-AMP and Pde2.
All the reported Pde1-like proteins have two transmembrane helices. In contrast, the Pde2-like proteins in these bacteria are all cytoplasmic, based on analysis using the TMHMM server (http://www.cbs.dtu.dk/services/TMHMM-2.0/). The presence of more than one c-di-AMP phosphodiesterase in a bacterium raises the following possibilities: (i) the two enzymes are responsible for local c-di-AMP pools within the bacterium due to their different distributions; (ii) their hydrolyzed products, pApA and AMP, have different biological roles; and (iii) these proteins may have other biological functions, in addition to cleaving c-di-AMP.
S. pneumoniae c-di-AMP homeostasis needs to be well maintained, since pneumococcal dacA is an essential gene and deletion of either pde1 or pde2 in the pathogen resulted in defective bacterial growth. Our observations are consistent with the report that both lack and high-level accumulation of c-di-AMP in B. subtilis are detrimental to bacterial growth (24). Surprisingly, deletion of pde1 and pde2 showed similar defects in bacterial growth but different levels of resistance to UV treatment, as the Δpde2 strain is more sensitive to UV than the Δpde1 strain. These observations further support the possibility that c-di-AMP microenvironments are generated by these phosphodiesterases.
Both Pde1 and Pde2 have been shown to be essential in different pneumococcal infection models, suggesting that c-di-AMP plays an important role during infections. It is likely that elevated bacterial c-di-AMP reduces the virulence of the pathogen. Nonetheless, it is important to understand what effector proteins and signal transduction pathways are involved in this response. Understanding the molecular basis of c-di-AMP signaling pathways will possibly provide new insights into controlling infections caused by S. pneumoniae.
ACKNOWLEDGMENTS
We are grateful for the technical assistance from Hongmin Li's laboratory and the Biochemistry Core at the Wadsworth Center, New York State Department of Health. We thank Gregor Witte for providing the DisA expression plasmid.
This work was supported by National Institutes of Health grant DC006917 (to D.W.M.).
Footnotes
Published ahead of print 6 September 2013
REFERENCES
- 1.Davies BW, Bogard RW, Young TS, Mekalanos JJ. 2012. Coordinated regulation of accessory genetic elements produces cyclic di-nucleotides for V. cholerae virulence. Cell 149:358–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomelsky M. 2011. cAMP, c-di-GMP, c-di-AMP and now cGMP: bacteria use them all! Mol. Microbiol. 79:562–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camilli A, Bassler BL. 2006. Bacterial small-molecule signaling pathways. Science 311:1113–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hengge R. 2009. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 7:263–273 [DOI] [PubMed] [Google Scholar]
- 5.Hammer BK, Bassler BL. 2009. Distinct sensory pathways in Vibrio cholerae El Tor and classical biotypes modulate cyclic dimeric GMP levels to control biofilm formation. J. Bacteriol. 191:169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazmierczak BI, Lebron MB, Murray TS. 2006. Analysis of FimX, a phosphodiesterase that governs twitching motility in Pseudomonas aeruginosa. Mol. Microbiol. 60:1026–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulasakara H, Lee V, Brencic A, Liberati N, Urbach J, Miyata S, Lee DG, Neely AN, Hyodo M, Hayakawa Y, Ausubel FM, Lory S. 2006. Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3′-5′)-cyclic-GMP in virulence. Proc. Natl. Acad. Sci. U. S. A. 103:2839–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamprokostopoulou A, Monteiro C, Rhen M, Romling U. 2010. Cyclic di-GMP signalling controls virulence properties of Salmonella enterica serovar Typhimurium at the mucosal lining. Environ. Microbiol. 12:40–53 [DOI] [PubMed] [Google Scholar]
- 9.Lim B, Beyhan S, Yildiz FH. 2007. Regulation of Vibrio polysaccharide synthesis and virulence factor production by CdgC, a GGDEF-EAL domain protein, in Vibrio cholerae. J. Bacteriol. 189:717–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Wilson HF, Tamayo R, Tischler AD, Lazinski DW, Camilli A. 2008. The Vibrio cholerae hybrid sensor kinase VieS contributes to motility and biofilm regulation by altering the cyclic diguanylate level. J. Bacteriol. 190:6439–6447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan RP, Lucey J, O'Donovan K, McCarthy Y, Yang L, Tolker-Nielsen T, Dow JM. 2009. HD-GYP domain proteins regulate biofilm formation and virulence in Pseudomonas aeruginosa. Environ. Microbiol. 11:1126–1136 [DOI] [PubMed] [Google Scholar]
- 12.Tamayo R, Schild S, Pratt JT, Camilli A. 2008. Role of cyclic di-GMP during El Tor biotype Vibrio cholerae infection: characterization of the in vivo-induced cyclic di-GMP phosphodiesterase CdpA. Infect. Immun. 76:1617–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tischler AD, Camilli A. 2004. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol. Microbiol. 53:857–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tischler AD, Camilli A. 2005. Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect. Immun. 73:5873–5882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hisert KB, MacCoss M, Shiloh MU, Darwin KH, Singh S, Jones RA, Ehrt S, Zhang Z, Gaffney BL, Gandotra S, Holden DW, Murray D, Nathan C. 2005. A glutamate-alanine-leucine (EAL) domain protein of Salmonella controls bacterial survival in mice, antioxidant defence and killing of macrophages: role of cyclic diGMP. Mol. Microbiol. 56:1234–1245 [DOI] [PubMed] [Google Scholar]
- 16.Bobrov AG, Kirillina O, Perry RD. 2007. Regulation of biofilm formation in Yersinia pestis. Adv. Exp. Med. Biol. 603:201–210 [DOI] [PubMed] [Google Scholar]
- 17.Borlee BR, Goldman AD, Murakami K, Samudrala R, Wozniak DJ, Parsek MR. 2010. Pseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Mol. Microbiol. 75:827–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grantcharova N, Peters V, Monteiro C, Zakikhany K, Romling U. 2010. Bistable expression of CsgD in biofilm development of Salmonella enterica serovar Typhimurium. J. Bacteriol. 192:456–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krasteva PV, Fong JC, Shikuma NJ, Beyhan S, Navarro MV, Yildiz FH, Sondermann H. 2010. Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science 327:866–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witte G, Hartung S, Buttner K, Hopfner KP. 2008. Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol. Cell 30:167–178 [DOI] [PubMed] [Google Scholar]
- 21.Bejerano-Sagie M, Oppenheimer-Shaanan Y, Berlatzky I, Rouvinski A, Meyerovich M, Ben-Yehuda S. 2006. A checkpoint protein that scans the chromosome for damage at the start of sporulation in Bacillus subtilis. Cell 125:679–690 [DOI] [PubMed] [Google Scholar]
- 22.Corrigan RM, Grundling A. 2013. Cyclic di-AMP: another second messenger enters the fray. Nat. Rev. Microbiol. 11:513–524 [DOI] [PubMed] [Google Scholar]
- 23.Romling U. 2008. Great times for small molecules: c-di-AMP, a second messenger candidate in Bacteria and Archaea. Sci. Signal. 1:pe39. 10.1126/scisignal.133pe39 [DOI] [PubMed] [Google Scholar]
- 24.Mehne FM, Gunka K, Eilers H, Herzberg C, Kaever V, Stulke J. 2013. Cyclic di-AMP homeostasis in Bacillus subtilis: both lack and high-level accumulation of the nucleotide are detrimental for cell growth. J. Biol. Chem. 288:2004–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corrigan RM, Abbott JC, Burhenne H, Kaever V, Grundling A. 2011. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog. 7:e1002217. 10.1371/journal.ppat.1002217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao F, See RY, Zhang D, Toh DC, Ji Q, Liang ZX. 2010. YybT is a signaling protein that contains a cyclic dinucleotide phosphodiesterase domain and a GGDEF domain with ATPase activity. J. Biol. Chem. 285:473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oppenheimer-Shaanan Y, Wexselblatt E, Katzhendler J, Yavin E, Ben-Yehuda S. 2011. c-di-AMP reports DNA integrity during sporulation in Bacillus subtilis. EMBO Rep. 12:594–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo Y, Helmann JD. 2012. Analysis of the role of Bacillus subtilis sigma(M) in beta-lactam resistance reveals an essential role for c-di-AMP in peptidoglycan homeostasis. Mol. Microbiol. 83:623–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerjee R, Gretes M, Harlem C, Basuino L, Chambers HF. 2010. A mecA-negative strain of methicillin-resistant Staphylococcus aureus with high-level beta-lactam resistance contains mutations in three genes. Antimicrob. Agents Chemother. 54:4900–4902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corrigan RM, Campeotto I, Jeganathan T, Roelofs KG, Lee VT, Grundling A. 2013. Systematic identification of conserved bacterial c-di-AMP receptor proteins. Proc. Natl. Acad. Sci. U. S. A. 110:9084–9089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffiths JM, O'Neill AJ. 2012. Loss of function of the GdpP protein leads to joint beta-lactam/glycopeptide tolerance in Staphylococcus aureus. Antimicrob. Agents Chemother. 56:579–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland LM, O'Donnell ST, Ryjenkov DA, Gomelsky L, Slater SR, Fey PD, Gomelsky M, O'Gara JP. 2008. A staphylococcal GGDEF domain protein regulates biofilm formation independently of cyclic dimeric GMP. J. Bacteriol. 190:5178–5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parvatiyar K, Zhang Z, Teles RM, Ouyang S, Jiang Y, Iyer SS, Zaver SA, Schenk M, Zeng S, Zhong W, Liu ZJ, Modlin RL, Liu YJ, Cheng G. 2012. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat. Immunol. 13:1155–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sauer JD, Sotelo-Troha K, von Moltke J, Monroe KM, Rae CS, Brubaker SW, Hyodo M, Hayakawa Y, Woodward JJ, Portnoy DA, Vance RE. 2011. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect. Immun. 79:688–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz KT, Carleton JD, Quillin SJ, Rollins SD, Portnoy DA, Leber JH. 2012. Hyperinduction of host beta interferon by a Listeria monocytogenes strain naturally overexpressing the multidrug efflux pump MdrT. Infect. Immun. 80:1537–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woodward JJ, Iavarone AT, Portnoy DA. 2010. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328:1703–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto T, Hara H, Tsuchiya K, Sakai S, Fang R, Matsuura M, Nomura T, Sato F, Mitsuyama M, Kawamura I. 2012. Listeria monocytogenes strain-specific impairment of the TetR regulator underlies the drastic increase in cyclic di-AMP secretion and beta interferon-inducing ability. Infect. Immun. 80:2323–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. 2011. STING is a direct innate immune sensor of cyclic di-GMP. Nature 478:515–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin L, Hill KK, Filak H, Mogan J, Knowles H, Zhang B, Perraud AL, Cambier JC, Lenz LL. 2011. MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP. J. Immunol. 187:2595–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowie AG. 2012. Innate sensing of bacterial cyclic dinucleotides: more than just STING. Nat. Immunol. 13:1137–1139 [DOI] [PubMed] [Google Scholar]
- 41.Kamegaya T, Kuroda K, Hayakawa Y. 2011. Identification of a Streptococcus pyogenes SF370 gene involved in production of c-di-AMP. Nagoya J. Med. Sci. 73:49–57 [PMC free article] [PubMed] [Google Scholar]
- 42.Lu L, Ma Y, Zhang J-R. 2006. Streptococcus pneumoniae recruits complement factor H through the amino terminus of CbpA. J. Biol. Chem. 281:15464–15474 [DOI] [PubMed] [Google Scholar]
- 43.Sung CK, Li H, Claverys JP, Morrison DA. 2001. An rpsL cassette, janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 67:5190–5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bai G, Gazdik MA, Schaak DD, McDonough KA. 2007. The Mycobacterium bovis BCG cyclic AMP receptor-like protein is a functional DNA binding protein in vitro and in vivo, but its activity differs from that of its M. tuberculosis ortholog, Rv3676. Infect. Immun. 75:5509–5517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai G, McCue LA, McDonough KA. 2005. Characterization of Mycobacterium tuberculosis Rv3676 (CRPMt), a cyclic AMP receptor protein-like DNA binding protein. J. Bacteriol. 187:7795–7804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bai Y, Yang J, Zhou X, Ding X, Eisele LE, Bai G. 2012. Mycobacterium tuberculosis Rv3586 (DacA) is a diadenylate cyclase that converts ATP or ADP into c-di-AMP. PLoS One 7:e35206. 10.1371/journal.pone.0035206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christen M, Christen B, Folcher M, Schauerte A, Jenal U. 2005. Identification and characterization of a cyclic di-GMP-specific phosphodiesterase and its allosteric control by GTP. J. Biol. Chem. 280:30829–30837 [DOI] [PubMed] [Google Scholar]
- 48.Ryjenkov DA, Tarutina M, Moskvin OV, Gomelsky M. 2005. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J. Bacteriol. 187:1792–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Massie JP, Reynolds EL, Koestler BJ, Cong JP, Agostoni M, Waters CM. 2012. Quantification of high-specificity cyclic diguanylate signaling. Proc. Natl. Acad. Sci. U. S. A. 109:12746–12751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lanie JA, Ng WL, Kazmierczak KM, Andrzejewski TM, Davidsen TM, Wayne KJ, Tettelin H, Glass JI, Winkler ME. 2007. Genome sequence of Avery's virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J. Bacteriol. 189:38–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song JH, Ko KS, Lee JY, Baek JY, Oh WS, Yoon HS, Jeong JY, Chun J. 2005. Identification of essential genes in Streptococcus pneumoniae by allelic replacement mutagenesis. Mol. Cells 19:365–374 [PubMed] [Google Scholar]
- 52.Cron LE, Stol K, Burghout P, van Selm S, Simonetti ER, Bootsma HJ, Hermans PW. 2011. Two DHH subfamily 1 proteins contribute to pneumococcal virulence and confer protection against pneumococcal disease. Infect. Immun. 79:3697–3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rao F, Ji Q, Soehano I, Liang ZX. 2011. Unusual heme-binding PAS domain from YybT family proteins. J. Bacteriol. 193:1543–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith WM, Pham TH, Lei L, Dou J, Soomro AH, Beatson SA, Dykes GA, Turner MS. 2012. Heat resistance and salt hypersensitivity in Lactococcus lactis due to spontaneous mutation of llmg_1816 (gdpP) induced by high-temperature growth. Appl. Environ. Microbiol. 78:7753–7759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y, Chai Y, Guo JH, Losick R. 2012. Evidence for cyclic di-GMP-mediated signaling in Bacillus subtilis. J. Bacteriol. 194:5080–5090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao X, Mukherjee S, Matthews PM, Hammad LA, Kearns DB, Dann CE., III 26 July 2013. Functional characterization of core components of the Bacillus subtilis c-di-GMP signaling pathway. J. Bacteriol. 10.1128/JB.00373-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Purcell EB, McKee RW, McBride SM, Waters CM, Tamayo R. 2012. Cyclic diguanylate inversely regulates motility and aggregation in Clostridium difficile. J. Bacteriol. 194:3307–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan W, Qu T, Zhao H, Su L, Yu Q, Gao J, Wu B. 2010. The effect of c-di-GMP (3′-5′-cyclic diguanylic acid) on the biofilm formation and adherence of Streptococcus mutans. Microbiol. Res. 165:87–96 [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Biswas I. 2009. 3′-Phosphoadenosine-5′-phosphate phosphatase activity is required for superoxide stress tolerance in Streptococcus mutans. J. Bacteriol. 191:4330–4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pozzi C, Waters EM, Rudkin JK, Schaeffer CR, Lohan AJ, Tong P, Loftus BJ, Pier GB, Fey PD, Massey RC, O'Gara JP. 2012. Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog. 8:e1002626. 10.1371/journal.ppat.1002626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mechold U, Fang G, Ngo S, Ogryzko V, Danchin A. 2007. YtqI from Bacillus subtilis has both oligoribonuclease and pAp-phosphatase activity. Nucleic Acids Res. 35:4552–4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El Qaidi S, Yang J, Zhang JR, Metzger DW, Bai G. 2013. The vitamin B6 biosynthesis pathway in Streptococcus pneumoniae is controlled by pyridoxal 5′-phosphate and the transcription factor PdxR and has an impact on ear infection. J. Bacteriol. 195:2187–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Avery OT, Macleod CM, McCarty M. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 79:137–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Macrina FL, Evans RP, Tobian JA, Hartley DL, Clewell DB, Jones KR. 1983. Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene 25:145–150 [DOI] [PubMed] [Google Scholar]