Abstract
Among 55 children with cultures positive for acute otitis media with spontaneous otorrhea, 28 (51%) had cultures positive for aural Streptococcus pneumoniae, and in 10 of these, two distinct strains were detected, in which 5 had pairs of strains that were both capsule-bearing serotypes. Such cases were more likely to have cultures positive for other otopathogens than those with only one pneumococcus present.
TEXT
The most common bacteria causing acute otitis media (AOM) in children are Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pyogenes. Spontaneous otorrhea can complicate AOM, and S. pyogenes may be found in higher percentages and H. influenzae and M. catarrhalis in lower percentages among such patients than among those with AOM and intact tympanic membranes (1).
Nasopharyngeal colonization by potential middle-ear pathogens is presumed to precede AOM. There is an association between nasal bacterial load and the presence and severity of ear disease (2), and aural S. pneumoniae shows a close genetic relatedness with its nasopharyngeal counterpart (3).
Understanding of the etiopathogenesis of AOM is increasing. Viral and bacterial causation are no longer seen as alternatives. Intercurrent respiratory viral infections may render the middle ear susceptible to symptomatic infection with bacteria that normally colonize the nasopharynx. Ruohola et al. suggest that the majority of acute middle-ear infections in children are due to bacterial and viral coinfection (4).
More than one bacterial species can simultaneously infect the middle ear in AOM (1, 4). Multibacterial species biofilm formation may be involved in chronic recurrent otitis media pathogenesis, perhaps explaining the demonstrated effectiveness of conjugate pneumococcal vaccines against AOM but not recurrent disease (5).
Conventional S. pneumoniae culture and serotyping methodologies underestimate multiple-serotype carriage. Molecular serotyping improves detection of multiple serotypes and determines the relative abundance of each (6, 7) but has not previously been applied to the middle ear.
The pneumococcal conjugate vaccine (PCV) became available in Portugal in 2001 but has not been included in the national immunization program. Coverage from private market sales data was around 65% in 2011, following a peak of around 79% in 2007 (oral communication, Pfizer). Since 2010, 13-valent PCV (PCV13) has been used predominantly.
Tympanocentesis is not routinely performed in the investigation and management of AOM in Portugal. To obtain data on the etiology of AOM, we studied children with AOM with spontaneous otorrhea (AOMSO). We hypothesized that just as simultaneous nasal colonization with multiple pneumococcal serotypes and strains occurs (7), this may also be the case in the ear.
The study was conducted at Coimbra Children's Hospital, a 120-bed tertiary care center in central Portugal, with more than 60,000 emergency service (ES) visits each year. It was approved by the hospital ethics committee. Parents or guardians provided written informed consent.
Children (0 to 13 years old) with AOMSO, defined as a history of acute onset signs and symptoms of middle-ear inflammation, with the presence of spontaneous otorrhea not due to acute otitis externa, who visited ES between December 2010 and July 2011 were studied prospectively. Disease onset was defined as the time of first symptom (fever and/or ear pain and/or otorrhea). Children with recurrent AOM or previous ear, nose, and throat surgery were included. Demographic and clinical data were recorded, and paired swabs were taken from the nasopharynx and aural discharge. No prior external ear canal toilet or aspiration through the perforation was performed. Swabs were stored at −80°C in skim-milk tryptone glucose glycerol (STGG) (Oxoid, Basingstoke, United Kingdom) broth until batched analysis by semiquantitative bacterial culture within 18 months. No routine cultures were performed.
S. pneumoniae, H. influenzae, M. catarrhalis, and S. pyogenes were considered true AOM pathogens. Staphylococcus aureus was excluded, as it may be a contaminant from the skin or external ear canal. Standard microbiological techniques were used for isolation and identification of S. pneumoniae and H. influenzae, as described previously (8). Additionally M. catarrhalis and S. pyogenes were cultured and identified using standard procedures (briefly, using Columbia blood agar supplemented with 5% defibrinated horse blood and streptococcal selective plates with colistin sulfate and oxolinic acid [COBA; Oxoid Limited, Basingstoke, United Kingdom], respectively). M. catarrhalis identity was confirmed by the cytochrome c oxidase test (Pro-Lab Diagnostics, Merseyside, United Kingdom) and the presence of acetate esterase activity (Indoxyl strip test; Sigma-Aldrich, Dorset, United Kingdom). S. pyogenes identity was based on β-hemolysis and detection of pyrrolidonyl peptidase activity (Pyrase strip test; Sigma-Aldrich).
Molecular serotyping was undertaken on all S. pneumoniae culture-positive aural samples and nasal samples from the same patients, using a microarray-based method to determine the serotype from cps gene content from genomic DNA hybridization (6). Detection of nontypeables, in the presence of one or more other S. pneumoniae serotypes, represented either true unencapsulated S. pneumoniae and/or closely related Streptococcus spp.
We used a chi-square test to check for significance of associations with STATA 12.0.
Over 5 months, 113 children with AOMSO were studied (113 aural swabs and 108 nasal swabs). The median age was 27 months (range, 3 to 158 months), and 62 (54.8%) were boys. Fourteen (12.4%) were receiving antibiotics at the time of swabbing, and 52 (46.0%) had received them in the previous month. Forty (35.4%) had smoking parents, and 100 (88.5%) attended nursery or school. The median/mean duration of disease was 1/2.5 days (range, 0 to 14): 0 to 3 days in 89 children (78.8%), 4 to 7 days in 15 children (13.3%), and 8 to 14 days in 9 children (8.0%). Previous history of AOM and/or ear surgery was recorded in 17 (14.9%) cases. Regarding vaccination history, 85/112 (75.9%) had received at least one dose of S. pneumoniae conjugate vaccine (Prevenar 7 or 13 and/or Synflorix).
Fifty-five (48.7%) children were culture positive for bacteria from aural discharge, and among these cultures, S. pneumoniae was present in 50.9% (28) (Fig. 1), S. pyogenes in 30.9% (17), M. catarrhalis in 27.3% (15), and H. influenzae in 20.0% (11). Fourteen children (25.4%) had two or more otopathogen species: S. pneumoniae and M. catarrhalis in 5; S. pneumoniae and H. influenzae in 4; M. catarrhalis and S. pyogenes in 2; M. catarrhalis and H. influenzae in 1; S. pneumoniae, H. influenzae, and S. pyogenes in 1; and S. pneumoniae, H. influenzae, S. pyogenes, and M. catarrhalis in 1.
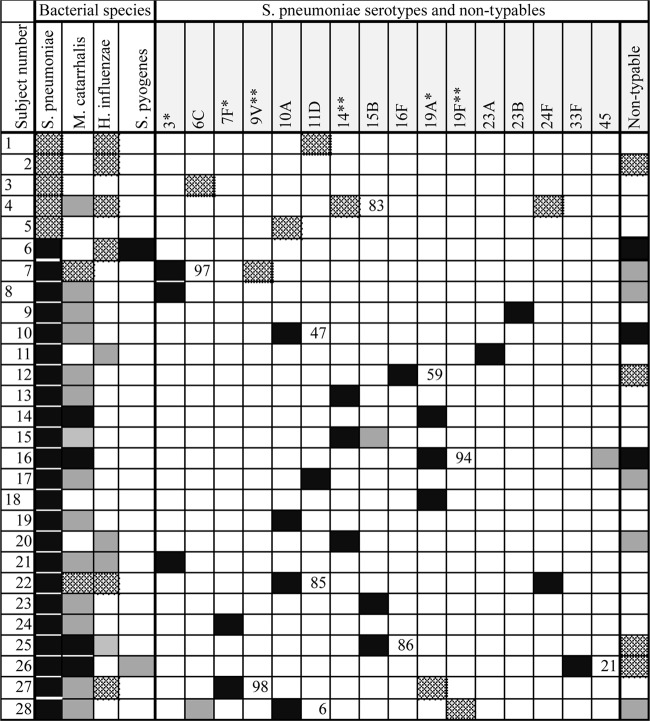
Fig 1.
Microbiological characteristics of 28 children with acute otitis media with spontaneous otorrhea that was culture positive for pneumococcus. Subject numbers of children on antibiotics are left justified. Culture results are shown on the left and microarray serotyping results on the right. The percentage of capsular locus DNA in the ear is shown to the right of the first of each pair of aural strains in subjects where two were detected. PCV13 serotypes are indicated with an asterisk and PCV7/13 serotypes with two. Black box, ear and nose; patterned box, ear; gray box, nose.
Of the 28 children with S. pneumoniae-positive culture from aural discharge (median age of 32.5 months, range of 5 to 125 months), five had S. pneumoniae in the ear only and 23 had it both in the ear and nose (one had nontypeable S. pneumoniae detected in the nose by PCR and array only and not by culture). In 10 (36%) cases, two distinct streptococcal strains were identified in the aural sample, often with one predominating (Fig. 1). Five of these were pairs of capsule-bearing serotypes (Fig. 1). Sixteen different serotypes were found in the ear: 6 PCV13 vaccine types (the most frequent being 19A and 14), and 10 nonvaccine types (the most frequent being 10A) (Fig. 1).
In the 23 children who had S. pneumoniae in both sites, individual serotypes could be found either in the ear or in the nose, but all 23 had at least one serotype that was found simultaneously in both places (Fig. 1). Cases with multiple aural streptococcal strains did not differ noticeably in age from the group with a single strain (median/mean ages of 37.5/35.9 and 32.5/38.6 months, respectively).
Seven of the 10 cases who had multiple aural streptococcal strains also had other bacterial species isolated from the ear: M. catarrhalis in five and H. influenzae in three. This occurred in only four of the 18 cases, with only a single aural streptococcal strain (chi-square = 6.23, P = 0.0125) (Fig. 1).
This is the first report of the microbial etiology of AOMSO in Portugal. Among this group of children, the duration of disease from the first acute symptom or symptoms to enrollment in the study lasted less than 3 days for the majority, and only four children with aural S. pneumoniae had more than 7 days of illness. S. pneumoniae was the predominant bacterium and, as reported by others (1), we found that S. pyogenes was identified in a higher percentage than usually reported in patients with AOM with intact tympanic membranes.
The proportion of cases from whom aural bacteria were successfully cultured in this study was relatively low. Brook and Gober reported that by culturing both the otorrhea fluid and middle-ear fluid obtained by needle aspiration, 28% additional pathogens were identified (9). Use of PCR detection may also increase bacterial detection rates (4), particularly in children who have received antibiotics. We did not include S. aureus in our analysis, as it is often assumed to be a contaminant in studies of AOMSO. Nevertheless, it was frequently cultured both from ear and nose in these children.
Although detection of more than one bacterial species in aural samples was as frequent in this AOMSO series as in others, by using molecular serotyping, we were also able to show that multiple S. pneumoniae serotypes were sometimes present in the ear. Although the clinical significance is uncertain, in such cases, serotypes that are at relatively low density may be underrecognized as otopathogens when conventional serotyping methods are used.
Children with multiple aural S. pneumoniae serotypes were more likely to have multiple bacterial species present as well. There is increasing evidence that AOM and recurrent AOM lie on a spectrum of disease whose pathogenesis varies according to the microenvironment that has developed in the middle ear with increasing chronicity of disease. Initially AOM may represent penetration of the middle ear by a single bacterial strain, facilitated by preceding viral infection. However, this may evolve into a more complex picture as other nose-colonizing bacterial strains and species join the process, perhaps no longer necessarily virus driven and more closely resembling the multibacterial environment of the nasopharynx. One study suggests evolution over time with different species detected in successive samples (10). Contributions from the flora of the external auditory canal may also become relevant once the tympanic membrane has been breached. If combinations of pneumococcal strains persist for longer in the ear than the nose, this might provide additional opportunities for horizontal gene exchange. Some of these hypotheses can be tested in future studies in this patient group.
ACKNOWLEDGMENTS
We thank children and their families for their participation. We also thank the study nurses.
Associação de Saúde Infantil de Coimbra—Portugal (ASIC), a charity for the hospital which employs F.R., but not F.R., has received funding for consultancy, postgraduate speaking, and travel bursaries to attend educational meetings from Sanofi Pasteur MSD, GSK, and Pfizer. J.H. has received funding for consultancy from GSK Biologicals and grants from GSK Biologicals, Pfizer, and Sanofi Pasteur. A.F. is employed by the University of Bristol and University Hospitals Bristol NHS Foundation Trust. Both institutions, but not A.F., have received funding from Pfizer, Sanofi Pasteur MSD, and GSK for research conducted by A.F. and for consultancy and lectures. B.M.-A., P.S., K.M.E.T., K.G., G.G., and L.J. have no interests to declare.
Footnotes
Published ahead of print 24 July 2013
REFERENCES
- 1.Leibovitz E, Serebro M, Givon-Lavi N, Greenberg D, Broides A, Leiberman A, Dagan R. 2009. Epidemiologic and microbiologic characteristics of culture-positive spontaneous otorrhea in children with acute otitis media. Pediatr. Infect. Dis. J. 28:381–384 [DOI] [PubMed] [Google Scholar]
- 2.Smith-Vaughan H, Byun R, Nadkarni M, Jacques NA, Hunter N, Halpin S, Morris PS, Leach AJ. 2006. Measuring nasal bacterial load and its association with otitis media. BMC Ear Nose Throat Disord. 6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tonnaer EL, Rijkers GT, Meis JF, Klaassen CH, Bogaert D, Hermans PW, Curfs JH. 2005. Genetic relatedness between pneumococcal populations originating from the nasopharynx, adenoid, and tympanic cavity of children with otitis media. J. Clin. Microbiol. 43:3140–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruohola A, Meurman O, Nikkari S, Skottman T, Salmi A, Waris M, Osterback R, Eerola E, Allander T, Niesters H, Heikkinen T, Ruuskanen O. 2006. Microbiology of acute otitis media in children with tympanostomy tubes: prevalences of bacteria and viruses. Clin. Infect. Dis. 43:1417–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veenhoven R, Bogaert D, Uiterwaal C, Brouwer C, Kiezebrink H, Bruin J, IJzerman E, Hermans P, de Groot R, Zegers B, Kuis W, Rijkers G, Schilder A, Sanders E. 2003. Effect of conjugate pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media: a randomised study. Lancet 361:2189–2195 [DOI] [PubMed] [Google Scholar]
- 6.Turner P, Hinds J, Turner C, Jankhot A, Gould K, Bentley SD, Nosten F, Goldblatt D. 2011. Improved detection of nasopharyngeal cocolonization by multiple pneumococcal serotypes by use of latex agglutination or molecular serotyping by microarray. J. Clin. Microbiol. 49:1784–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brugger SD, Frey P, Aebi S, Hinds J, Muhlemann K. 2010. Multiple colonization with S. pneumoniae before and after introduction of the seven-valent conjugated pneumococcal polysaccharide vaccine. PLoS One 5:e11638. 10.1371/journal.pone.0011638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues F, Foster D, Nicoli E, Trotter C, Vipond B, Muir P, Goncalves G, Januario L, Finn A. 2013. Relationships between rhinitis symptoms, respiratory viral infections and nasopharyngeal colonization with Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus in children attending daycare. Pediatr. Infect. Dis. J. 32:227–232 [DOI] [PubMed] [Google Scholar]
- 9.Brook I, Gober AE. 2000. Reliability of the microbiology of spontaneously draining acute otitis media in children. Pediatr. Infect. Dis. J. 19:571–573 [DOI] [PubMed] [Google Scholar]
- 10.Ruohola A, Meurman O, Nikkari S, Skottman T, Heikkinen T, Ruuskanen O. 2007. The dynamics of bacteria in the middle ear during the course of acute otitis media with tympanostomy tube otorrhea. Pediatr. Infect. Dis. J. 26:892–896 [DOI] [PubMed] [Google Scholar]