Abstract
Contact precautions are recommended in hospitals to prevent the transmission of vancomycin-resistant enterococci (VRE); however, there is no clear policy for how long patients should be under contact precautions due to a lack of information on the duration of carriage of these organisms. We conducted a retrospective cohort study to understand the duration of carriage of VRE (by screening of a single stool culture) and associated factors among patients who had been identified with VRE infection and/or colonization since the year 2000 at our health facilities. Of the 345 eligible participants, 136 did not respond, 90 declined to participate, and 16 did not send in the required specimens. Of the 103 remaining participants, 13 were found to have current VRE fecal carriage. The proportion of colonized patients fell from 40% (2/5) in the first year to 23.3% (7/30) in year 4. None of the 40 patients who had VRE detected >4 years prior were found to be colonized at the time of the study. The longest duration of detected VRE positivity was 46.5 months. Univariate analysis revealed that recent exposure to any antibiotics (P = 0.016), multiple antibiotics (P = 0.001), amoxicillin-clavulanic acid (P = 0.021), piperacillin-tazobactam (P = 0.007), glycopeptides (P < 0.001), meropenem (P = 0.007), aminoglycosides (P = 0.021), or fluoroquinolones (P = 0.021), being the index case in a clinical specimen (P = 0.016), and recent hospitalization (P < 0.001) were significantly associated with continued carriage on follow-up. In the surviving outpatients, a significant proportion appeared to clear VRE carriage. Our results suggest that in the absence of recent risk factors, such as hospitalization or antibiotic use, patients with a remote history of colonization (>4 years) may no longer require contact isolation precautions.
INTRODUCTION
Contact precautions are recommended in hospitals to prevent and control the transmission of vancomycin-resistant enterococci (VRE). There is controversy as to how long patients with VRE should be kept under contact precautions. Currently, in some health facilities, colonization is assumed to be lifelong, and many hospitals therefore presume “once colonized, always colonized.” No treatments have been shown to eradicate VRE carriage. Although prolonged fecal colonization has been documented for up to 709 days, the proportion of patients carrying VRE over time is uncertain (1). Most follow-up studies have been performed over periods of, at most, 2 to 3 years (1–4). The reemergence of carriage after apparent clearance has been demonstrated to be particularly associated with antibiotic selection pressure (1, 5). Relapse or reacquisition of VRE has been reported in 7.8 to 27% of patients who had 3 prior consecutive negative cultures of stool or rectal swab specimens (3, 4, 6).
The increasing cumulative number of colonized patients requiring contact precautions poses difficulties for patient flow and management due to varied availability of and competing priorities for single rooms in hospitals. In Australia, where the vanB genotype VRE predominates, we are aware of a diversity in infection control practices that are adopted by various hospitals, owing to limited evidence for a standard protocol. In this study, we aimed to measure the duration of fecal VRE carriage by surveying patients who had been detected to have VRE colonization and/or infection since the year 2000 and to identify the factors associated with long-term persistent colonization or recolonization in this patient group.
MATERIALS AND METHODS
Study design and setting.
We performed a retrospective cohort study of patients who had previously attended Alfred Health and were found to have colonization and/or infection with VRE. Alfred Health consists of three hospitals, the Alfred Hospital, Caulfield Hospital, and Sandringham Hospital, in Melbourne, Australia. Our hospitals primarily serve the southern region of Melbourne and metropolitan Melbourne but receive patients from throughout the state of Victoria for specialty services. Since 1997, approximately 1,600 patients have been detected with VRE colonization at these facilities. The majority of cases were detected at Alfred Hospital, which is a 350-bed tertiary referral hospital with specialist services in solid organ transplantation, hematology and bone marrow transplantation, HIV/AIDS, burns, and trauma, with a 36-bed intensive care unit.
Participants and data collection.
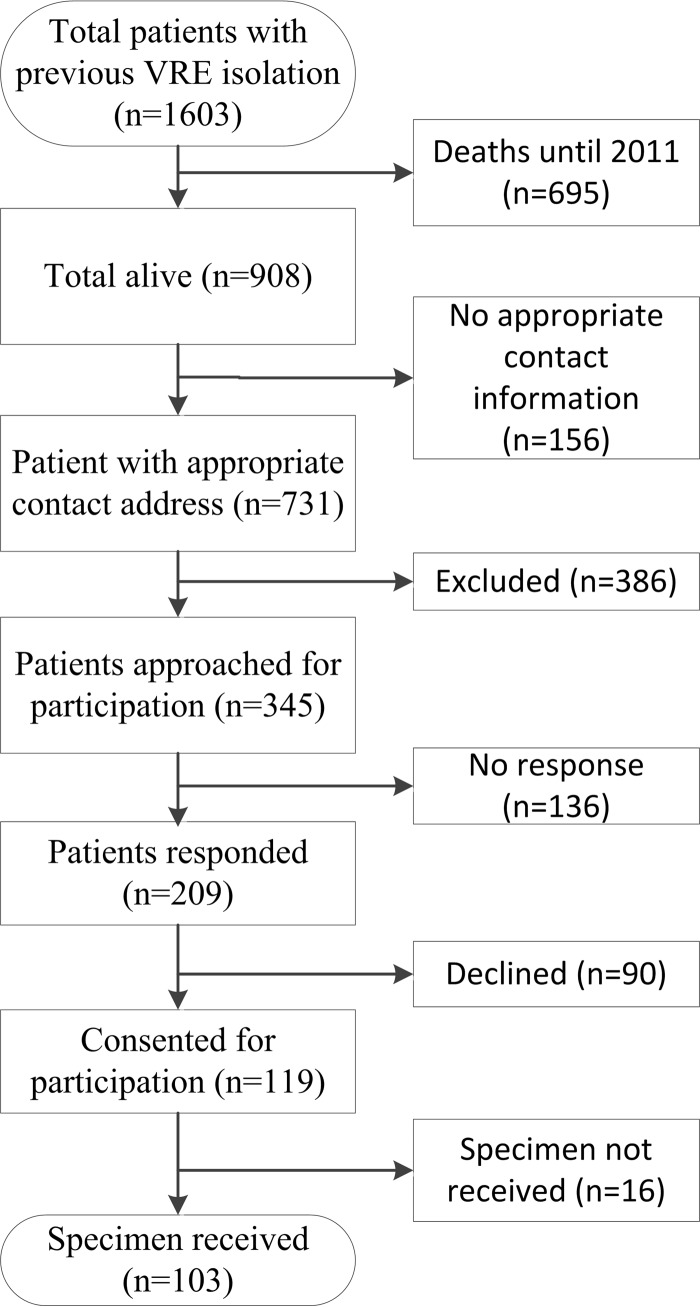
Patients known to have colonization and/or infection with VRE between January 2000 and December 2011, who were not known to have died, and were aged ≥18 years at the start of study (July 2012) were further assessed for possible inclusion in the study. Patients were excluded if known to have terminal illness when they were last reviewed, had cognitive or psychiatric issues precluding consent, were discharged for palliative or high-level nursing home care, were registered on a “do not call” registry, were ≥90 years of age at the time of follow-up, or had no known address, a temporary address, or were an overseas resident (Fig. 1).
Fig 1.
Flowchart showing recruitment for participation in study. The numbers of each patient are indicated in parentheses.
An information folder containing the details of the study outcomes, risks and benefits, and consent forms with reply-paid return envelopes for study participation were mailed to the eligible participants. Further information on their queries was offered via telephone by a research nurse. Reply-paid envelopes containing a specimen collection kit, a written description of the appropriate feces specimen collection protocol, and a questionnaire asking for information on recent hospitalizations, medical procedures, and medications were sent to the consenting patients.
Specimen collection and microbiology.
We adopted the fecal specimen collection strategy as described previously for the Australian National Bowel Cancer Screening Program using fecal occult blood testing (7). A single stool specimen was obtained by participants and returned to the Infection Prevention Unit via the mail in Australia Post-approved packaging. Specimens were sent to the laboratory at Alfred Hospital after the research nurse ensured that the sample was adequate. The specimens were then plated onto bile esculin medium (BBL Enterococcosel agar; BBL, Cockeysville, MD) with 6 μg/ml vancomycin and incubated at 37°C for up to 72 h. Enterococcus species were identified using the Vitek 2 system (bioMérieux). A PCR was used to detect the vanA or vanB genes, as described previously (8). VRE colonization was considered positive if an isolate of Enterococcus faecalis or Enterococcus faecium with a vanA or vanB gene was detected. VRE isolates from the same participants, which were previously isolated and stored at −80°C, were revived on blood agar culture plates. Paired, first-time, and current isolates of E. faecium were assessed for genetic relatedness by high-resolution melting (HRM) genotyping, as previously described (9).
We defined the index strain as the strain that was isolated when the patient was first detected with VRE and the follow-up strain as the isolate that was detected as part of this study. In those patients found to be colonized at the time of the study, we defined persistence as when the index and follow-up strains were of the same HRM type and recolonization as when the index and follow-up strains were different on HRM genotyping.
Statistical analysis.
We compared categorical variables with chi-square test or Fisher's exact test and continuous variables with the Wilcoxon rank-sum test or Student's t test among participants with and without current VRE carriage. Odds ratios for current VRE carriage were calculated with 95% confidence intervals. A P value of <0.05 was considered to indicate statistical significance. All the statistical analyses were conducted in Stata 12.1 (StataCorp, College Station, TX).
This study was reviewed and approved by the Alfred Health Human Research Ethics and Monash University Human Research Ethics committees.
RESULTS
Of the 908 patients that were not known to have died, contact details were available for 731 (80.5%). Of the 386 excluded patients, 25.1% had a terminal illness or were in palliative care, 28.5% were >90 years of age, 15.5% had known cognitive disorders, 8.8% had psychological issues precluding consent, 7.8% were unable to communicate in English, 9.3% were in high-level nursing home care, and 13.5% had other reasons for exclusion, including being aged <18 years.
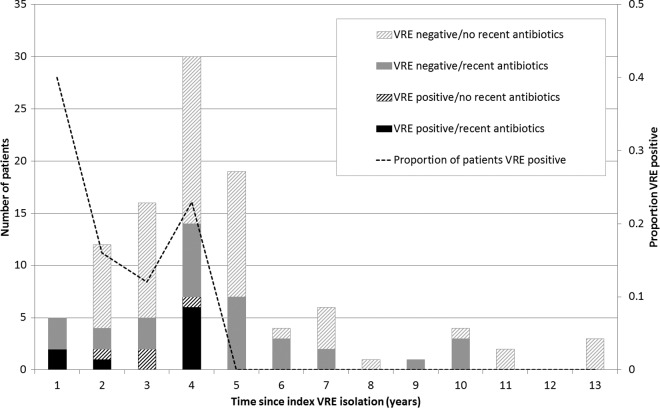
A total of 345 patients were invited to participate, of whom 136 did not respond, 90 declined to participate, and 16 did not return a specimen (Fig. 1). Of the 103 participating patients known to have had previous VRE colonization or infection, 13 were found to have VRE fecal carriage on follow-up. All index isolates of VRE were of the vanB genotype from E. faecium, except one that was of the vanB genotype from E. faecalis, whereas all follow-up VRE isolates were vanB E. faecium. The proportion of colonized patients fell from 40% (2/5) in the first year to 23.3% (7/30) in year 4 (Fig. 2). None of the 40 patients whose VRE detection was >4 years prior were found to be colonized on follow-up. The longest detected duration of VRE carriage was after 46.5 months. In the 63 patients where follow-up was for <4 years since the initial VRE detection, 24 (38.1%) had recent exposure to antibiotics (Fig. 2), and 14 (22.2%) had been hospitalized within the previous 3 months. Among the 40 patients with index VRE colonization for >4 years, 16 (40%) had a recent exposure to antibiotics; however, only 4 (11.1%) had a recent inpatient hospital stay.
Fig 2.
Current VRE colonization status over time according to recent exposure to antibiotics. Dotted line (secondary axis), proportion of VRE-positive patients by year since the index isolation of VRE.
Patient characteristics among the current VRE-positive and VRE-negative participants were similar (Table 1). However, a higher odds of current VRE carriage was observed if the first-time VRE detection was from a clinical specimen rather than from rectal swab screening (P < 0.016).
Table 1.
Demographic and clinical characteristics of VRE-positive and VRE-negative participants
| Variablesa | VRE statusb (n) |
Odds ratio (95% CI)c | P | |
|---|---|---|---|---|
| Positive (13) | Negative (90) | |||
| Patient characteristics (at time of study) | ||||
| Median age (yr) (Q1, Q3) | 60.9 (58.5, 62.5) | 62.6 (53.1, 71.4) | 0.8 | |
| Charlson comorbidity index (mean [SD]) | 0.85 (1.14) | 0.64 (0.98) | 0.5 | |
| Male | 8 (61.5) | 45 (50.5) | 1.6 (0.4–6.5) | 0.5 |
| Chronic abdominal conditions | 1 (7.7) | 15 (16.7) | 0.4 (0.02–2.7) | 0.5 |
| Previous organ transplant | 4 (30.7) | 23 (25.6) | 1.3 (0.3–4.6) | 0.6 |
| Diabetes | 3 (23.1) | 8 (8.9) | 3.1 (0.6–13.2) | 0.08 |
| Patient characteristics (index case) | ||||
| Clinical culture vs. surveillance rectal swab | 9 (69.2) | 31 (34.4) | 4.3 (1.1–20.3) | 0.016 |
| Patient categories | ||||
| Respiratory/transplants | 3 (23.1) | 7 (7.7) | 3.6 (0.6–15.8) | 0.06 |
| Cardiac/transplants | 1 (7.7) | 15 (16.7) | 0.4 (0.1–2.7) | 0.5 |
| Burns/trauma | 4 (30.7) | 10 (11.1) | 3.6 (0.7–15.8) | 0.06 |
| Hematology | 0 (0.0) | 13 (14.4) | 0.2 | |
| Infectious diseases | 1 (7.7) | 4 (4.4) | 1.8 (0.1–15.6) | 0.3 |
| Gastrointestinal/colorectal | 0 (0.0) | 14 (15.6) | 0.1 | |
| Plastic surgery | 2 (15.4) | 4 (4.4) | 3.9 (0.4–24.3) | 0.09 |
| Others | 2 (15.4) | 21 (23.3) | 0.6 (0.1–2.7) | 0.6 |
| Recent exposures (<3 mo) | ||||
| Hospitalization | 7 (53.8) | 11 (12.2) | 8.4 (1.9–35.6) | <0.001 |
| Unplanned or urgent hospitalization | 4 (30.1) | 4 (4.4) | 5.3 (2.1–13.4) | <0.001 |
| Planned or elective hospitalization | 3 (23.1) | 5 (55.5) | 5.1 (0.7–30.5) | 0.03 |
| Any antibiotic | 9 (69.2) | 31(34.4) | 4.3 (1.1–20.3) | 0.016 |
| Antibiotic treatment (oral) | 8 (61.5) | 30 (33.3) | 3.2 (0.9–11.5) | 0.04 |
| Antibiotic treatment (intravenous) | 5 (38.5) | 4 (4.4) | 13.4 (2.7–64.0) | 0.001 |
| Multiple antibiotics | 6 (46.2) | 7 (7.8) | 10.2 (2.5–39.4) | 0.001 |
| Hospitalization plus antibiotics | 5 (38.5) | 3 (3.33) | 18.1 (3.4–101.4) | 0.001 |
| Oral steroids | 5 (38.5) | 15 (16.7) | 3.1 (0.8–10.9) | 0.09 |
| Overseas travel | 0 (0.0) | 10 (11.1) | 0.2 | |
| Farm animals | 1 (7.7) | 10 (11.1) | 0.7 (0.03–4.6) | 0.8 |
| Endoscopy within 12 mo | 1 (7.7) | 9 (10.0) | 0.7 (0.01–6.4) | 0.8 |
| Abdominal surgery within 12 mo | 0 (0.0) | 4 (4.4) | 0.7 | |
Q1, 1st quartile; Q3, 3rd quartile; SD, standard deviation.
Data are presented as no. (%) of patients, unless indicated otherwise.
CI, confidence interval.
Factors associated with colonization on follow-up.
Recent hospitalization (any type) (P < 0.001), unplanned or urgent hospitalization (P < 0.001), planned or elective hospitalization (P = 0.03), and the use of any antibiotics (P = 0.016), oral antibiotics (P = 0.04), intravenous antibiotics (P = 0.001), and multiple antibiotics (P = 0.001) were all associated with VRE carriage on follow-up (Table 1). Regarding specific antibiotics, recent exposure to amoxicillin-clavulanic acid (P = 0.021), piperacillin-tazobactam (P = 0.007), glycopeptides (P < 0.001), meropenem (P = 0.007), fluoroquinolones (P = 0.021), and aminoglycosides (0.021) was associated with VRE carriage on follow-up (Table 2).
Table 2.
Recent exposure to antibiotics in VRE-positive and VRE-negative participants
| Antibiotics | No. (%) with VRE status: |
Odds ratio (95% CI) | P | |
|---|---|---|---|---|
| Positive | Negative | |||
| n | 13 | 90 | ||
| Penicillins | 3 (23.1) | 9 (10.0) | 2.7 (0.5–11.4) | 0.1 |
| Cephalosporins | 1 (7.7) | 6 (6.7) | 1.2 (0.1–8.8) | 0.8 |
| Trimethoprim | 1 (7.7) | 2 (2.2) | 3.7 (0.1–50.4) | 0.2 |
| Trimethoprim-sulfamethoxazole | 3 (23.1) | 9 (10.0) | 2.7 (0.5–11.4) | 0.1 |
| Amoxicillin-clavulanic acid | 2 (15.4) | 1 (1.1) | 16.2 (1.1–481.2) | 0.021 |
| Piperacillin-tazobactam | 3 (23.1) | 0 (0.0) | 0.007 | |
| Glycopeptides | 4 (30.7) | 1 (1.1) | 39.6 (4.1–985.5) | <0.001 |
| Macrolides | 2 (15.4) | 7 (7.7) | 2.2 (0.3–11.1) | 0.2 |
| Meropenem | 2 (15.4) | 0 (0.0) | 0.007 | |
| Aminoglycosides | 2 (15.4) | 1 (1.1) | 16.2 (1.1–481.2) | 0.021 |
| Tetracyclines | 0 (0.0) | 1 (1.1) | 0.6 | |
| Fluoroquinolones | 2 (15.4) | 1 (1.1) | 16.2 (1.1–481.2) | 0.021 |
| Fosfomycin | 1 (1.1) | 0 (0.0) | 0.6 | |
HRM genotyping of E. faecium.
Of the 11 patients with available paired isolates, the index and follow-up isolates were the same melting type (MelT) in 6 patients. Five patients had different MelTs for the index and follow-up isolates. One patient had E. faecalis initially and E. faecium carriage on follow-up. All 6 with different strain types or species had recent exposure to antibiotics compared with 2 of 6 with persistence of the original strain type (P = 0.06, Fisher's exact test). The longest persistence of the same strain was 3.5 years (Table 3). In addition, 4 out of 6 patients with a discordant strain or species type had recent exposure to both antibiotics and hospital admission compared to only 1 out of 6 with a concordant strain or species type.
Table 3.
Genotyping of isolates
| Patient no. | Melting type of VRE isolate |
Time since first isolation (yr) | Recent hospitalization | Recent antibiotic use | Type of antibiotic(s) received | |
|---|---|---|---|---|---|---|
| Previous | Current | |||||
| 1 | 121 | 121 | 2.16 | No | No | None |
| 2 | 121 | 121 | 2.13 | No | No | None |
| 3 | 121 | 121 | 1.89 | No | No | None |
| 4 | 121 | 121 | 3.49 | Yes | No | None |
| 5 | 121 | 121 | 3.2 | Yes | Yes | Vancomycin, teicoplanin, meropenem, piperacillin-tazobactam, amoxicillin, norfloxacin, fosfomycin, Bactrim, gentamicin |
| 6 | 121 | 11 | 3.65 | No | Yes | Flucloxacillin |
| 7 | 121 | 11 | 3.78 | No | Yes | Cephalexin |
| 8 | 121 | 10 | 0.84 | Yes | Yes | Vancomycin, meropenem |
| 9 | 121 | 10 | 3.82 | Yes | Yes | Vancomycin, piperacillin-tazobactam, amoxicillin-clavulanate |
| 10 | 116 | 184 | 0.94 | Yes | Yes | Vancomycin, piperacillin-tazobactam, ciprofloxacin |
| 11 | 11 | 11 | 3.29 | No | Yes | Azithromycin, amoxicillin-clavulanate, Bactrim |
| 12 | Enterococcus faecalis | Enterococcus faeciuma | 3.22 | Yes | Yes | Vancomycin, tobramycin, Bactrim, azithromycin, trimethoprim |
| 13 | E. faeciuma | E. faecium (116) | 1.43 | Yes | Yes | Amoxicillin |
Isolate not available for high-resolution melting (HRM) analysis.
DISCUSSION
In this study, we found that although the duration of carriage of detectable colonization can be as long as 4 years, a significant proportion of discharged patients either clear or cease shedding VRE in their feces after 1 year. This is a significant finding that favors a risk-based use of contact precautions rather than a universal application of lifelong contact precautions in all previously identified VRE-positive patients. While it remains unclear whether there is low-level colonization below the limit of detection that might intermittently reemerge, these results suggest that patients with a remote history of colonization (>4 years prior) may not necessarily require contact precautions on subsequent admission to a health care facility. The use of glycopeptides, piperacillin-tazobactam, and meropenem was strongly associated with persistent VRE carriage, and the use of such antibiotics in colonized patients seems to be an important factor in maintaining endemicity.
Our findings are consistent with those of a recent study that looked at discharged hospital patients and estimated that approximately 95% of patients cleared VRE after 30 weeks from the initial acquisition (10). However, others have reported that prolonged fecal VRE carriage is dependent on several factors, including recent exposure to antibiotics, longer length of hospital stay, repeated hospital admissions, surgery, and high-risk patient groups, such as those on dialysis (10, 11).
The HRM genotyping results suggest that at least half of the patients who were VRE positive at the time of the study were recolonized with new strains, as was suggested by the genetic discordance between the index and follow-up strains. Recent exposure to antibiotics was much higher in patients with discordant strains, suggesting that frequent and/or long-term antibiotic administration may have led to recolonization. Other studies have also reported different strains in the same patients when the two colonization and/or infection events are separated by relatively long time intervals (12). In addition, the dominant strain of VRE involved in outbreaks appears to be changing over time, as evidenced in another Australian hospital (13). This suggests that prolonged colonization may not be a primary driver of endemicity in hospitals.
We observed that long-term carriage was associated with index isolation of VRE from a clinical culture rather than a surveillance rectal culture (P = 0.016). This may be because the patients with positive clinical cultures are more severely ill, require treatment with more antibiotics, and have more frequent hospitalizations. However, we did not find any association between the type of index case (clinical specimen versus surveillance rectal swab) and recent exposure to antibiotics, hospitalization, or comorbidity index at the time of study. Of note, we are not able to exclude the possibility that patients with index clinical cultures had more frequent hospitalizations, more exposure to antibiotics, and differences in comorbidities at the time of first acquisition, which may be associated with a higher colonization density. Similar associations have also been observed in patients with carbapenem-resistant Enterobacteriaceae spp., where repeat hospitalization and detection of the index case in a clinical specimen (as opposed to a surveillance culture) were associated with longer duration of carriage following hospital discharge (14).
We used a relatively new approach for genotyping based on a high-resolution melting curve analysis of 8 single nucleotide polymorphisms (SNPs) within 4 of the 7 housekeeping genes employed in multilocus sequence typing (MLST) of E. faecium and that is referred to as HRM genotyping. This method has been reported to be highly concordant with MLST or pulsed-field gel electrophoresis (9). However, we cannot exclude the possibility of discordant strains in follow-up due to colonization with multiple genotypes during the index colonization period, or because of inter- or intraspecies lateral vanB gene transfer to susceptible strains of E. faecium within the human intestines (15, 16).
This study has several limitations. First, a large proportion of patients with prior VRE colonization were excluded before invitation to participate in the study. This was inevitable given that age, palliation status, and cognitive and psychological issues precluded informed consent. Additionally, only 30% of the eligible patients participated in this study, despite secondary contact with those who did not initially decline to participate. Although this is similar to the participation rates in fecal occult blood test bowel cancer screening using specimen collection from outpatients via the postal service (7, 17), we cannot exclude the possibility of selection bias due to nonresponse. Second, we do not have information on the potential patient risk factors during the time of index strain isolation and over subsequent years, which might have contributed to sustained colonization. Third, the number of patients with current VRE colonization was low, and thus we have limited statistical power to detect other factors that are potentially associated with persistent colonization. Finally, the identification of current VRE colonization in our study was based on the detection of VRE in a single stool culture. Although stool culture is more sensitive than other methods of screening, such as rectal swabs, some studies suggest that it is not as sensitive as multiple stool specimens, and therefore we may have underestimated the proportion with persistent VRE carriage (5). However, it is also likely that participants whose cultures were falsely negative were likely to have VRE present in low densities (18). Low-density carriage is probably of marginal clinical significance, as there is evidence that patients with low-level carriage are at a lower risk of contaminating patient skin or the hospital environment (18, 19).
In conclusion, a significant proportion of patients initially found to have positive cultures for VRE had negative cultures on follow-up. It remains unclear whether this represents a level of colonization below the limit of detection that has the potential to reemerge, but it nevertheless suggests that patients without recent exposure to antibiotics or hospitalization and with a remote history of colonization (>4 years prior) may not require continued contact isolation precautions. VRE endemicity seems to be sustained by a combination of prolonged colonization and recolonization events in patients with recent antibiotic exposure and/or hospital inpatient stay. Prudent use of antimicrobials and improvements in standard infection prevention and control measures, including risk assessment, should be the mainstay of hospital control programs where VRE has become established.
ACKNOWLEDGMENTS
We thank Gemma Klintworth, Penny Leszkiewicz, Deborah Rhodes, and Pauline Bass for their assistance, and Kerrie Watson, Lana McCartney, and Nandini Jothin for assistance with data management.
The author responsibilities were as follows: study concept and design, S.K. and A.C.C.; data collection, G.L. and S.K.; analysis and interpretation of data, S.K., A.C.C., K.L., J.K., S.A., P.D.R.J., and S.A.B.; drafting of the manuscript, S.K.; critical revision, all authors; statistical analysis, S.K. and A.C.C.; and study supervision, A.C.C. and J.K.
The authors declare no conflicts of interest.
Funding for this study was received from Alfred Health.
Footnotes
Published ahead of print 7 August 2013
REFERENCES
- 1.Byers KE, Anglim AM, Anneski CJ, Farr BM. 2002. Duration of colonization with vancomycin-resistant Enterococcus. Infect. Control. Hosp. Epidemiol. 23:207–211 [DOI] [PubMed] [Google Scholar]
- 2.Henning KJ, Delencastre H, Eagan J, Boone N, Brown A, Chung M, Wollner N, Armstrong D. 1996. Vancomycin-resistant Enterococcus faecium on a pediatric oncology ward: duration of stool shedding and incidence of clinical infection. Pediatr. Infect. Dis. J. 15:848–854 [DOI] [PubMed] [Google Scholar]
- 3.Lai KK, Fontecchio SA, Kelley AL, Melvin ZS, Baker S. 1997. The epidemiology of fecal carriage of vancomycin-resistant enterococci. Infect. Control Hosp. Epidemiol. 18:762–765 [PubMed] [Google Scholar]
- 4.Henard S, Lozniewski A, Aissa N, Jouzeau N, Rabaud C. 2011. Evaluation of the duration of vanA vancomycin-resistant Enterococcus faecium carriage and clearance during a large-scale outbreak in a region of eastern France. Am. J. Infect. Control 39:169–171 [DOI] [PubMed] [Google Scholar]
- 5.Baden LR, Thiemke W, Skolnik A, Chambers R, Strymish J, Gold HS, Moellering RC, Jr, Eliopoulos GM. 2001. Prolonged colonization with vancomycin-resistant Enterococcus faecium in long-term care patients and the significance of “clearance.” Clin. Infect. Dis. 33:1654–1660 [DOI] [PubMed] [Google Scholar]
- 6.Patel R, Allen SL, Manahan JM, Wright AJ, Krom RA, Wiesner RH, Persing DH, Cockerill FR, Thompson RL. 2001. Natural history of vancomycin-resistant enterococcal colonization in liver and kidney transplant recipients. Liver Transpl. 7:27–31 [DOI] [PubMed] [Google Scholar]
- 7.Multicentre Australian Colorectal-Neoplasia Screening (MACS) Group 2006. A comparison of colorectal neoplasia screening tests: a multicentre community-based study of the impact of consumer choice. Med. J. Aust. 184:546–550 [DOI] [PubMed] [Google Scholar]
- 8.Dutka-Malen S, Evers S, Courvalin P. 1995. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 33:1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong SYC, Xie S, Richardson LJ, Ballard SA, Dakh F, Grabsch EA, Grayson ML, Howden BP, Johnson PDR, Giffard PM. 2011. High-resolution melting genotyping of Enterococcus faecium based on multilocus sequence typing derived single nucleotide polymorphisms. PLoS One 6:e29189. 10.1371/journal.pone.0029189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sohn KM, Peck KR, Joo EJ, Ha YE, Kang CI, Chung DR, Lee NY, Song JH. 2012. Duration of colonization and risk factors for prolonged carriage of vancomycin-resistant enterococci after discharge from the hospital. Int. J. Infect. Dis. 17:e240–e246. 10.1016/j.ijid.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 11.Park I, Park RW, Lim SK, Lee W, Shin JS, Yu S, Shin GT, Kim H. 2011. Rectal culture screening for vancomycin-resistant enterococcus in chronic haemodialysis patients: false-negative rates and duration of colonisation. J. Hosp. Infect. 79:147–150 [DOI] [PubMed] [Google Scholar]
- 12.Baran J, Jr, Riederer KM, Ramanathan J, Khatib R. 2001. Recurrent vancomycin-resistant Enterococcus bacteremia: prevalence, predisposing factors, and strain relatedness. Clin. Infect. Dis. 32:1381–1383 [DOI] [PubMed] [Google Scholar]
- 13.Johnson PD, Ballard SA, Grabsch EA, Stinear TP, Seemann T, Young HL, Grayson ML, Howden BP. 2010. A sustained hospital outbreak of vancomycin-resistant Enterococcus faecium bacteremia due to emergence of vanB E. faecium sequence type 203. J. Infect. Dis. 202:1278–1286 [DOI] [PubMed] [Google Scholar]
- 14.Zimmerman FS, Assous MV, Bdolah-Abram T, Lachish T, Yinnon AM, Wiener-Well Y. 2013. Duration of carriage of carbapenem-resistant Enterobacteriaceae following hospital discharge. Am. J. Infect. Control 41:190–194 [DOI] [PubMed] [Google Scholar]
- 15.Graham M, Ballard SA, Grabsch EA, Johnson PDR, Grayson ML. 2008. High rates of fecal carriage of nonenterococcal vanB in both children and adults. Antimicrob. Agents Chemother. 52:1195–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Launay A, Ballard SA, Johnson PD, Grayson ML, Lambert T. 2006. Transfer of vancomycin resistance transposon Tn1549 from Clostridium symbiosum to Enterococcus spp. in the gut of gnotobiotic mice. Antimicrob. Agents Chemother. 50:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segnan N, Senore C, Andreoni B, Arrigoni A, Bisanti L, Cardelli A, Castiglione G, Crosta C, DiPlacido R, Ferrari A, Ferraris R, Ferrero F, Fracchia M, Gasperoni S, Malfitana G, Recchia S, Risio M, Rizzetto M, Saracco G, Spandre M, Turco D, Turco P, Zappa M, SCORE2 Working Group-Italy 2005. Randomized trial of different screening strategies for colorectal cancer: patient response and detection rates. J. Natl. Cancer Inst. 97:347–357 [DOI] [PubMed] [Google Scholar]
- 18.Donskey CJ, Chowdhry TK, Hecker MT, Hoyen CK, Hanrahan JA, Hujer AM, Hutton-Thomas RA, Whalen CC, Bonomo RA, Rice LB. 2000. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N. Engl. J. Med. 343:1925–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Agata EMCD, Gautam S, Green WK, Tang YW. 2001. High rate of false-negative results of the rectal swab culture method in detection of gastrointestinal colonization with vancomycin-resistant enterococci. Clin. Infect. Dis. 34:167–172 [DOI] [PubMed] [Google Scholar]