Abstract
The risk of infections and the appearance of symptoms (e.g., odors) represent the main troubles resulting from malignant wounds. The aim of this study was to characterize the balance of bacterial floras and the relationships between biofilms and bacteria and the emergence of symptoms. Experimental research was carried out for 42 days on malignant wounds associated with breast cancer. Investigations of bacterial floras (aerobes, aero-anaerobes, and anaerobes), detection of the presence of biofilms by microscopic epifluorescence, and clinical assessment were performed. We characterized biofilms in 32 malignant wounds associated with breast cancer and bacterial floras in 25 such wounds. A mixed group of floras, composed of 54 different bacterial types, was identified, with an average number per patient of 3.6 aerobic species and 1.7 anaerobic species; the presence of strict anaerobic bacterial strains was evidenced in 70% of the wounds; biofilm was observed in 35% of the cases. Odor was a reliable indicator of colonization by anaerobes, even when this symptom was not directly linked to any of the identified anaerobic bacteria. Bacteria are more likely to be present during myelosuppression and significantly increase the emergence of odors and pain when present at amounts of >105 · g−1. The presence of biofilms was not associated with clinical signs or with precise types of bacteria. No infections occurred during the 42-day evaluation period. This study provides a dynamic description of the bacterial floras of tumoral wounds. The study results highlight the absolute need for new therapeutic options that are effective for use on circulating bacteria as well as on bacteria organized in biofilm.
INTRODUCTION
Malignant wounds consist of the infiltration of cutaneous tissue by tumoral cells (1). Such wounds may take on various appearances. They are often associated with the noncurable phase of the disease and are likely to cause discomfort to patients, such as obnoxious odors, exudates, and pain (2, 3).
In France, a national survey showed that malignant wounds represent 1.6% of all types of wounds for outpatients and 1.7% for nonambulatory patients (4). In a Swiss study, the prevalence of malignant wounds among patients with metastatic cancer was 6.6% (5), in the 5 to 10% range previously determined (6, 7). Malignant breast wounds represent the most frequent kinds of tumor wounds (8, 9, 10).
Local infections of malignant wounds are difficult to diagnose, particularly when patients are treated with chemotherapy. Indeed, whereas chemotherapy can help tumoral wound healing it also increases the risk of infections and slows the healing process. The diagnosis of infection is all the more difficult as malignant wounds are often foul smelling, necrotic, and exudative, and the region is often inflammatory due to the tumor mass. Unpleasant smells are one of the most common complaints from patients (11). Mixed bacterial floras develop on chronic wounds, composed of interacting aerobic and anaerobic bacteria (12) that come from the skin, the cavities located near the lesion, and the environment.
Biofilms represent a complex but frequent type of organization of bacteria. In contact with an interface, bacteria organize themselves and synthetize an extracellular three-dimensional matrix which protects them from external aggression such as antibiotics and antiseptics. Bacteria organized into biofilms must be distinguished from circulating bacteria (or planktonic bacteria). Biofilms can develop on both inert and living surfaces in a three-phase cycle, adhesion, maturation, and dispersion (13). Biofilms are clinically invisible, except when particularly thick. Then, they are described as a coating of viscous liquid, called slime, located on the surface of a wound with delayed healing. They seem to be associated with abundant exudates and with a lengthened inflammatory phase (13, 14). Identifying and characterizing biofilms is not possible with the usual collection and analysis techniques (15). The role of biofilms in infectious processes and/or in the evolution of wounds has been a matter of debate (16, 17). The presence of biofilms affects up to 60% of chronic wounds, and they may be less frequent in acute wounds (18). It seems that biofilms will develop in individuals with weakened immune systems or with poor nutritional status and in the presence of ischemic tissues.
The aims of this study were to characterize the bacterial floras and the biofilms of malignant wounds associated with breast cancer and to study their correlations with smells, infections, and tumor evolution.
MATERIALS AND METHODS
Inclusion criteria.
For this study of malignant wounds associated with breast cancer, the inclusion criteria were wound surface area >10 cm2, patient age >18 years, patient participation in a follow-up program of at least 1 month supervised by the medical and nursing staff, and availability of patient objective information and freely written informed consent.
Exclusion criteria.
Patients were excluded if medical follow-up was not practically possible for geographical, social, or psychological reasons and if the patients were deprived of freedom and/or were under guardianship.
Sample size and duration of the study.
Given the exploratory nature of the research, and since little is known about this patient population, calculating the sample size of the patients' test group was both impossible and unnecessary before initiating the study. Considering the frequency of malignant wounds associated with breast cancer, patient recruitment by the Institut Curie, and the results of a pilot study carried out from March 2007 to March 2008, a 40-patient cohort was initially agreed upon.
The pilot study showed that the evaluation period had to be short in order to deter too many patients from dropping out. The duration of the evaluations was then set at 42 days, with an evaluation every 21 days, in keeping with the rhythm of most chemotherapy cycles.
Clinical assessment.
The information that was collected during the wound-healing consultation, either as outpatient follow-up or in the hospital, was logged in a data file identified by a study number. This information included the type of wound, histology, site, degree of evolution and age, aspects of the wound and the skin around the lesion, descriptions of the local and systemic treatments, and evaluations of the local symptoms, risks, and complications.
The tool used for the clinical evaluation of pain was the simple verbal scale (SVS), and the other symptoms (odor, bleeding, and exudates) were graded on 4 levels (none, slight, moderate, and intense).
Colonization was defined as the presence of germs on the wound with no inflammatory response or sign of infection. Infection was defined as the multiplication of germs on the wound with the presence of local and/or general signs of infection.
Microbiological sampling and testing.
The detection of aerobic, facultative anaerobic, and strict anaerobic bacteria was carried out in a quantitative way. Microbiological samples were obtained with a single-use Volkmann microliter curette calibrated on the deepest and/or most necrotic zone and were brought to the laboratory without delay.
On arrival at the laboratory, each calibrated sample was immediately discharged in brain heart infusion (BHI) broth and diluted 1:10 and 1:100 in BHI. Seeding of 1 μl from each broth was performed on different agar media, which included 1 chocolate, 2 Columbia ANC (nalidixic acid plus colimycin), 1 Drigalski, 1 chromID CPS, 1 Chapman, 1 Pseudomonas, 1 chromogenic selective agar for Staphylococcus aureus ID (SAID), 1 Schaedler, 1 Schaedler neomycin plus vancomycin, and 1 Sabouraud gentamicin plus chloramphenicol agar plate.
The agar plates were incubated at 37°C for 1 to 5 days under different atmospheric conditions, aerobic and/or CO2 and/or anaerobic. Anaerobic conditions were obtained with an Oxoid AnaeroGen system. All the agar plates were analyzed from day 1 to day 5 and all the bacteria were listed and identified with API identification galleries from bioMérieux (API E, API 20A, API Candida, API 20 STREP, API 20 STAPH, API CORYNE, API 20NE) and Oxoid (Rap-ID ANA II).
Biofilm analysis.
The presence of biofilm was defined by the observation with an epifluorescence microscope (enlargement, ×1,000) of patterns of bacteria organized in colonies and of the biofilm matrix composed of exopolymers. The samplings for biofilm detection were collected at the same time and in the same way as those for microbiological tests. They were immediately fixed in paraformaldehyde (PFA) 3% and conserved in a moist chamber before being processed. Processing included labeling of the extracellular matrix with lectins (Arachis hypogaea agglutinin, Lycopersicon esculentum agglutinin, Bandeiraea simplicifolia agglutinin, or concanavalin A agglutinin; Sigma-Aldrich) coupled with a fluorophore (fluorescein or rhodamine) and labeling of microorganisms with a DNA intercalator (4′,6-diamidino-2-phenylindole [DAPI]; Sigma-Aldrich).
Statistical analysis.
Results of clinical, microbiological, and biofilm data were described in percentages for qualitative variables and in average, standard deviation, median, and interquartile range for quantitative variables. The distribution of quantitative variables was studied. The intersection of the variables was carried out using a χ2 test, variance analysis, or Pearson's correlation coefficient, depending on the variables. Nonparametric analysis was employed when necessary, using Fisher, Kruskal-Wallis, and Spearman's coefficient tests. Analyses were made with 5% α and β risks. Clinical wound variables were cross-tabulated with the microbiological results and with the biofilm analyses. Bacteria were tested for their type, presence or absence, and quantity (e.g., ≤105 · g−1 and >105 · g−1).
Patient consent and ethical aspects.
After being informed of the conditions of the study, patients who met the inclusion criteria were required to sign a written informed consent and were then assigned a study number. All data and results were compiled into a unique database. The study was carried out in full compliance with the ethics rules that apply in this area and the current regulations. The study protocol was accepted by the People's Protection Committee.
RESULTS
Over a 7-month period, 32 patients were enrolled in this study. The characteristics of the population are listed in Table 1.
Table 1.
Characteristics of the population
| Patient characteristics at baseline (n = 32) | Results |
|---|---|
| Age (yr) | |
| Median | 59 |
| Range | 30–96 |
| Gender (no. [%]) | |
| Female | 32 (100) |
| Male | 0 |
| Duration of wound (mo) | |
| Median | 8 |
| Range | 1–63 |
| Histological type (no. [%]) | |
| Ductal invasive carcinoma | 32 (100) |
| Other | 0 |
| Wound origin (no. [%]) | |
| Primary tumor | 9 (28) |
| Cutaneous metastasis | 23 (72) |
| Wound location (no. [%]) | |
| Thorax | 31 (97) |
| Other | 1 (3) |
| General antitumor treatment (no. [%]) | |
| Chemotherapy | 19 (60) |
| Hormonal therapy | 3 (9) |
| None | 10 (31) |
| Local antitumor treatment (no. [%]) | |
| Radiation therapy | 2 (6) |
| Chemotherapy (Miltexa) | 1 (3) |
| None | 29 (91) |
| Supportive care (no. [%]) | |
| Corticosteroid therapy | 5 (16) |
| Antibiotic therapy | 6 (19) |
| None | 21 (65) |
Miltex, topical treatment with Miltefosine.
Number of evaluations.
Eighteen patients (56%) participated in the three evaluations at 21-day intervals. Ten patients (31%) were evaluated twice and 4 patients (13%) only once. Three patients died, 1 patient was referred to a palliative care unit, and 4 patients failed to attend the planned evaluations. Four patients presented wound healing with partial reepithelialization, and therefore sampling could not be completed. On the whole, the study is based on 78 clinical evaluations with microbiological and biofilm analyses; strict anaerobes were sought in the first 25 patients (61 samples).
Description of wounds.
The lengths and widths of the wounds were measured at day 0, day 21, and day 42. Day 0 sizes were compared to day 42 sizes to exclude reepithelialized wounds. The medians of wound length were 11 cm at day 0 and 12.5 cm at day 42; the medians of wound width were 7 cm at day 0 and 9 cm at day 42. There was a slight although hardly significant trend toward an increase of the wound surface areas (P = 0.894).
The evolution of the aspect and color of the wounds was clinically evaluated (with a colorimetric four-color scale) between days 0 and 42. Most wounds showed a trend toward cavitation. Colors did not show much variation. The most prevalent colors were yellow (fibrin, tumor necrosis) and red (tumor buds). The surrounding skin showed signs of inflammation in 52%, which reflected the tumor mass rather than an infectious process.
Microbiological analyses.
As expected, the wound microbiological floras were predominantly polymicrobial. We identified 54 different bacterial strains, 37 of which were aerobic and facultative anaerobic bacteria and 17 of which were strict anaerobic bacteria.
Concerning aerobic and facultative anaerobic bacteria (Table 2), the most frequently identified were, in descending order of frequency, Staphylococcus aureus, Pseudomonas aeruginosa, Corynebacterium striatum, Proteus mirabilis, and Escherichia coli. Concerning strict anaerobic bacteria, the most frequently identified, with no prevalence of a specific species, were Fusobacterium necrophorum, Parvimonas micra, Peptoniphilus asaccharolyticus, and Porphyromonas asaccharolytica.
Table 2.
Aerobic and facultative anaerobic bacteria identified on wounds at each evaluation (days 0, 21, and 42)
| Genus or type of bacteria | Dominant species | % identified for total genus (% dominant species) on day: |
||
|---|---|---|---|---|
| 0 | 21 | 42 | ||
| Staphylococcus | S. aureus | 88 (64) | 82 (62) | 86 (73) |
| Pseudomonas | P. aeruginosa | 52 (40) | 57 (52) | 40 (33) |
| Corynebacterium | C. striatum | 40 (28) | 48 (43) | 40 (40) |
| Streptococcus | Group B streptococcus | 36 (16) | 44 (24) | 33 (13) |
| Proteus | 28 (24) | 43 (33) | 40 (27) | |
| Escherichia | E. coli | 20 | 24 | 27 |
| Enterococcus | E. faecalis | 20 (16) | 15 (10) | 20 (13) |
| Klebsiella | K. oxytoca | 8 | 14 | 20 |
| Others (15 different strains) | ≤10 | ≤10 | ≤10 | |
| Anaerobes | 68 | 62 | ||
Most of the patients presented multispecies floras (Fig. 1). The average numbers per patient were 3.6 aerobic species and 1.7 strict anaerobic species.
Fig 1.
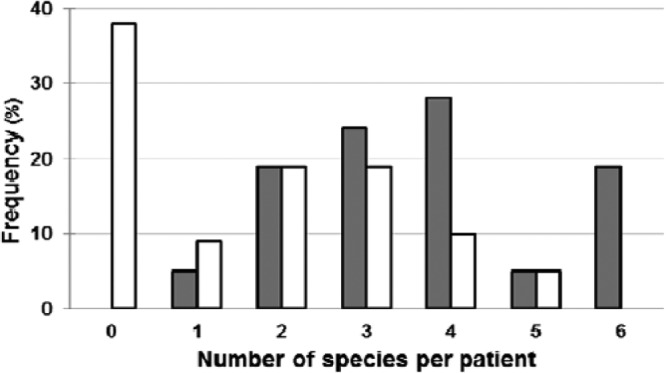
Relative frequencies of the numbers of different aerobic ( ) and anaerobic (□) species per patient as observed on day 21.
) and anaerobic (□) species per patient as observed on day 21.
Predominant bacteria remained mostly the same between days 0 and 42 (Table 2). Moreover, in 20 patients, if 1 or more of the 5 aerobic or aero-anaerobic bacteria mentioned above were present at day 0, they were also found at day 42, except in 2 patients, in whom C. striatum and P. aeruginosa were identified at only day 42. For strict anaerobic germs found on 15 patients, the floras seemed more variable even if the majority of the bacteria identified at day 0 were found at day 42.
Despite the significant number of bacteria on the wounds, no infectious episodes happened during the study. However, 56 to 60% of the patients presented risks of adjacent infections (central line), and 60% were undergoing a chemotherapy-based treatment.
Diversity of the wound floras and consequences.
It is thought that the presence and the quantity of anaerobic bacteria on a wound can increase risks of infections, and that the synergy between certain aerobic and anaerobic bacteria might be more dangerous than the presence of some specific microbial agents. For the 25 patients, all wounds were colonized with at least one aerobic or one facultative anaerobic bacterial strain. Moreover, 70% of the wounds presented one or more strict anaerobic bacterial strains. Strict anaerobic species constituted approximately 33% of all identified bacterial species.
In the absence of infection, we studied the balance between microorganisms and its correlation with symptoms to determine under what conditions those symptoms develop, which may indicate the likely evolution of the wound toward infection. The presence of strict anaerobic bacteria was significantly associated with the presence of odors (P = 0.009) (Fig. 2A) and exudates (P = 0.05) without incidence of pain. However, it was not possible to correlate these symptoms with the presence of specific strict anaerobic species. These results were probably due to the synergistic effects which occur in mixed and polymicrobial floras.
Fig 2.
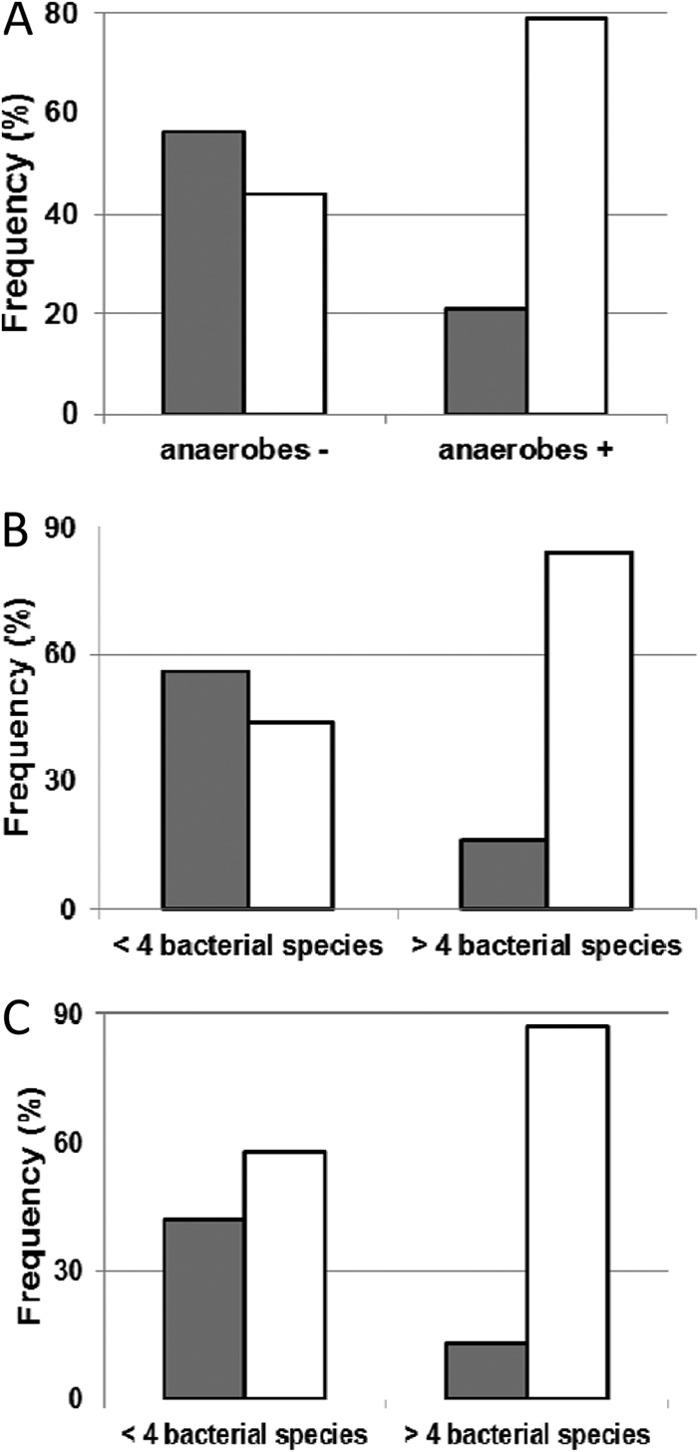
Impact of the presence of strict anaerobes (A) and the numbers of bacterial species (B, C) on various symptoms. (A) Impact of the presence of strict anaerobic species on odors (no odor,  ; odor from weak to strong, □). (B) Impact of bacterial species number on odors (no odor,
; odor from weak to strong, □). (B) Impact of bacterial species number on odors (no odor,  ; odor from weak to strong, □). (C) Impact of bacterial species number on exudates (no/weak exudate,
; odor from weak to strong, □). (C) Impact of bacterial species number on exudates (no/weak exudate,  ; moderate/strong exudate, □).
; moderate/strong exudate, □).
Regardless of the bacterial species, the presence of more than 4 different bacterial species increased the risk of odors from 43.5% to 84.2% (P = 0.0008) (Fig. 2B) and of exudates from 56.5% to 86.8% (P = 0.007) (Fig. 2C).
Quantitative assessment of wound floras and risks of infection.
Most of the time, wound infection is microbiologically defined by the presence of >105 · g−1 bacteria on the wound. We decided to compare the intensity of symptoms with the quantity of bacteria isolated from the wounds (Table 3, Fig. 3).
Table 3.
Relationships between the number of bacterial species (and strict anaerobic bacterial species) identified on wounds and the quantitative assessment of bacteria
| Species data | <105 · g−1 bacteria | >105 · g−1 bacteria | Total | P |
|---|---|---|---|---|
| Species (no.) | ||||
| Median | 4 | 7 | 6 | 0.0003 |
| Range (minimum to maximum) | 1–11 | 1–10 | 1–11 | |
| Strict anaerobes (no.) | ||||
| Median | 1 | 3 | 1 | 0.0006 |
| Range (minimum to maximum) | 0–5 | 0–6 | 0–6 | |
| Strict anaerobes (%) | ||||
| Median | 0.16 | 0.37 | 0.25 | 0.004 |
| Range (minimum to maximum) | 0–0.6 | 0–0.6 | 0–0.6 |
Fig 3.
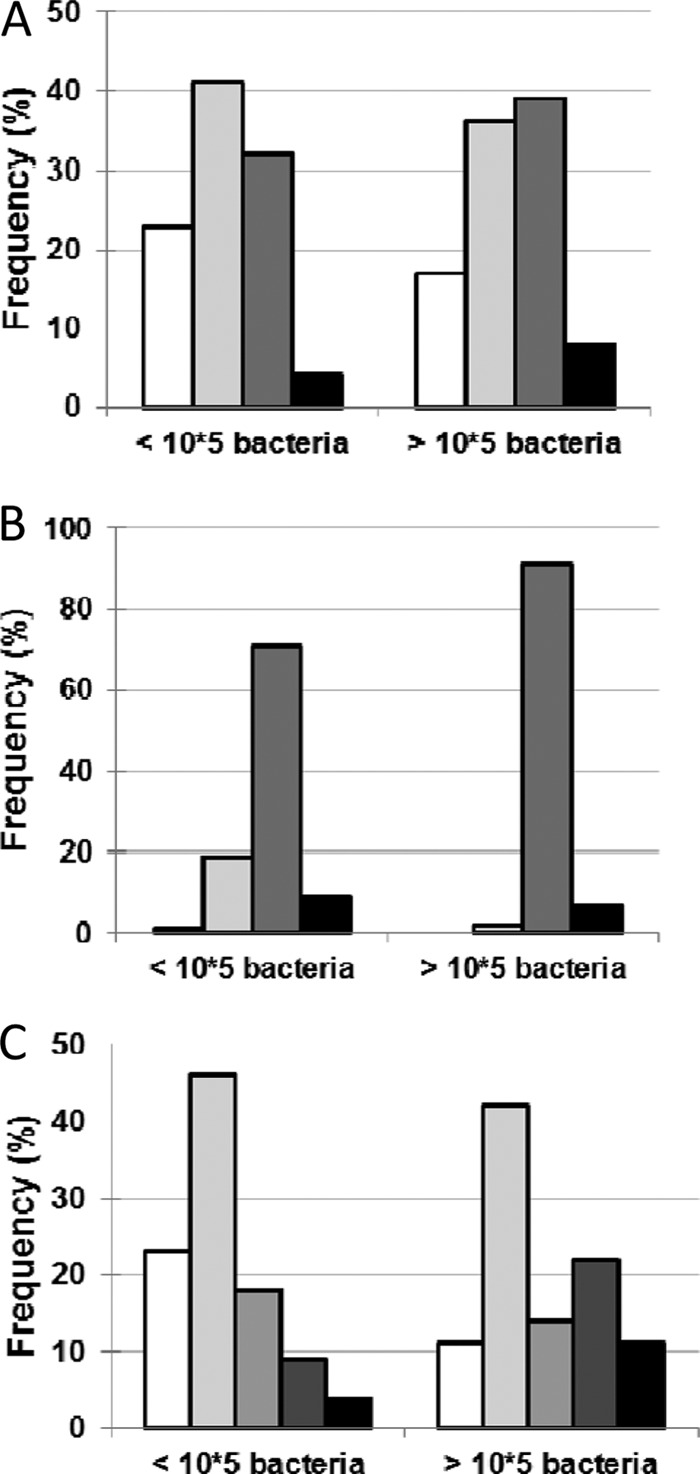
Impact of bacterial load on symptoms. (A) Impact of bacterial load on odor intensity (no odor, □; weak odor,  ; moderate odor,
; moderate odor,  ; strong odor, ■) (P = 0.56). (B) Impact of bacterial load on exudates (no exudate, □; weak exudate,
; strong odor, ■) (P = 0.56). (B) Impact of bacterial load on exudates (no exudate, □; weak exudate,  ; moderate exudate,
; moderate exudate,  ; strong exudate, ■) (P = 0.07). (C) Impact of bacterial load on pain (no pain, □; weak pain,
; strong exudate, ■) (P = 0.07). (C) Impact of bacterial load on pain (no pain, □; weak pain,  ; moderate pain, ▩; strong pain,
; moderate pain, ▩; strong pain,  ; intense pain, ■) (P = 0.04).
; intense pain, ■) (P = 0.04).
Results showed that, in the presence of ≥105 · g−1 bacteria, pain was significantly more present (P = 0.04), with a tendency toward an increase of exudates (P = 0.07). The threshold of >104 · g−1 bacteria was statistically significant for the emergence of odors (P = 0.02), while 105 CFU · g−1 was not (P = 0.56). The total concentration of bacteria is an important parameter for the development of odors; conversely, the number of various species on the wound is not bound to the emergence of volatile compounds (7 compounds for 1 bacterial species; 10 compounds for 2, 4, or 5 different species).
Qualitative assessment of bacterial floras showed that the numbers of strict anaerobe species and the ratios of anaerobes to aerobes were higher when the total floras had developed (>105 · g−1 bacteria) (Table 3).
Analyses showed that large amounts of bacteria (>105 · g−1) and high numbers of identified bacterial species were more frequent in the wounds of the 24% to 29% patients presenting with chemotherapy-induced myelosuppression (<500 polynuclear neutrophils [PNN]/mm3) at each evaluation.
Biofilm analysis.
Biofilms were evaluated on all wounds (78 samples). Twelve samples could not be analyzed due to technical failures. To perform biofilm analysis, all samples were stained by DAPI (Fig. 4, blue-highlighted cells) and lectins (Fig. 4, green- and red-highlighted saccharides) and observed by microscopy (e.g., Fig. 4). Nucleic acid staining combined with lectin labeling demonstrates the location of bacteria and saccharides relative to cells and microcolonies in the biofilm structure. For patient 1 at day 21 (P1D21), DAPI staining showed the presence of eukaryotic cell nuclei, and lectin staining was not intense. No bacteria were detected in this sample. For other samples, DAPI staining revealed bacteria organized in aggregates. These aggregates represented either a fraction of the sample or complete invasion. For samples P1D42 and P6D0, a few nuclei of eukaryotic cells, medium-size bacterial aggregates, and the presence of low exopolysaccharide levels were observed. When the sample contained only bacteria, as in samples P2D0 and P7D21, lectin staining was intense and indicated the presence of exopolysaccharides around bacteria. Biofilm was identified in 23 (35%) of the 66 samples available for analysis.
Fig 4.
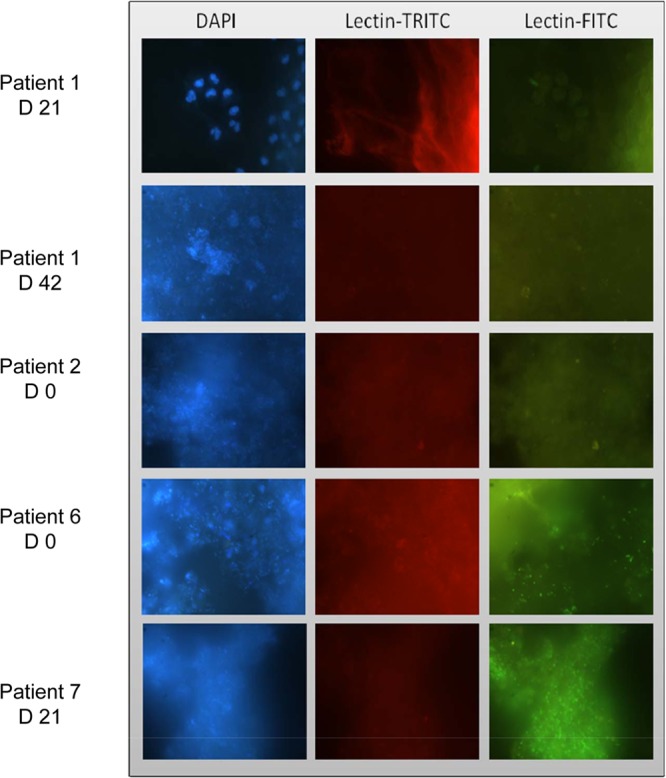
Biofilm identification on tumoral wounds using epifluorescence microscopy. Samples were stained with lectins coupled to fluorochromes and DAPI. Samples of patients 1 and 2, lectins Bandeiraea simplicifolia-tetramethylrhodamine isothiocyanate (TRITC) and Lycopersicon esculentum-fluorescein isothiocyanate (FITC); samples of patients 6 and 7, lectins Arachis hypogaea-TRITC and Lycopersicon esculentum-FITC.
In order to identify predictive factors for the development of biofilm, clinical and microbiological data were analyzed depending on the presence or not of biofilm.
The presence of biofilm was not associated with a particular clinical aspect of wounds or with the length of wound evolution (Table 4). No biofilm was identified on the 4 wounds that showed reepithelialization during the study, suggesting that the presence of biofilm impairs wound healing.
Table 4.
Relationships between biofilm, clinical characteristics of patients, and symptomatic data
| Characteristics and symptomatic data | No. (%) with biofilm: |
Total (no. [%]) (n = 66 [100%]) | P | |
|---|---|---|---|---|
| Present (n = 23 [35%]) | Absent (n = 43 [65%]) | |||
| Length of evolution | ||||
| <6 wk | 2 (50) | 2 (50) | 4 | 0.57 |
| 6 wk to 8 mo | 9 (42.9) | 12 (57.1) | 21 | |
| >8 mo | 11 (31.4) | 24 (68.6) | 35 | |
| Chemotherapy | ||||
| No | 0 | 6 (100) | 6 | 0.16 |
| Yes | 16 (33.3) | 32 (66.7) | 48 | |
| Aplasia | ||||
| No | 11 (28.9) | 27 (71.1) | 38 | 0.8 |
| Yes | 5 (31.2) | 11 (68.8) | 16 | |
| Odors | ||||
| Absence | 5 (21.7) | 10 (23.3) | 15 (22.7) | 0.88 |
| Presence | 18 (78.3) | 33 (76.7) | 51 (77.3) | |
| Exudates | ||||
| Weak/absent | 3 (13) | 9 (20.9) | 12 (18.2) | 0.64 |
| Moderate/strong | 20 (87) | 34 (79.1) | 54 (81.8) | |
| Pain | ||||
| Absence | 6 (26.1) | 14 (32.6) | 20 (30.3) | |
| Weak | 8 (34.8) | 17 (39.5) | 25 (37.9) | 0.58 |
| Moderate | 3 (13) | 9 (20.9) | 12 (18.2) | |
| Strong | 3 (13) | 2 (4.7) | 5 (7.6) | |
| Intense | 3 (13) | 1 (2.3) | 4 (6.1) | |
| Provoked bleeding | ||||
| Absence | 11 (47.8) | 11 (25.6) | 22 (33.3) | 0.06 |
| Presence | 12 (52.2) | 32 (74.4) | 44 (66.7) | |
Moreover, the presence of biofilms was not associated with the identification of a particular bacterial species, suggesting that the presence of biofilm could be the consequence of diverse floras rather than a particular bacterium. Nor was the presence of biofilms associated with the quantitative number of bacteria (> or <105 · g−1).
Comparison of clinical symptoms in the absence or presence of biofilms showed that the biofilms did not significantly increase their emergence. There was merely a tendency toward a decreasing risk of provoked bleedings in the presence of biofilms (P = 0.06.) The type of local nursing care did not influence the development of biofilms, and neither did the presence of general medical treatment.
DISCUSSION
In this study, microbiological analyses showed polymicrobial floras with a predominance of S. aureus, P. aeruginosa, C. striatum, and P. mirabilis. In the literature, the polymicrobial colonization of chronic wounds has been characterized in various conditions, and the predominance of S. aureus and P. aeruginosa has already been observed (19, 20, 21, 22). In a study of tumoral wounds, 25 different bacterial species were identified, with the predominance of S. aureus, followed by enterobacteria, S. haemolyticus, and P. aeruginosa. Strict anaerobes were identified in only 16% of samples (23). In another study of 70 malignant wounds (30 breast cancers), bacteroides and E. coli were predominant and the samples showed more anaerobes than aerobes (24). In our study, particular attention was paid to an immediate analysis of the samples that were kept in an anaerobic atmosphere. This can explain the differences with the results of other studies.
We show that odors and exudates are more frequent in the presence of strict anaerobic bacterial strains. The role of anaerobes in the emergence of symptoms has already been characterized. That is why the use of specific antibiotics is a common practice, such as metronidazole, which could also have an impact on facultative anaerobic floras because its action is not restricted to strict anaerobes (7, 25, 26, 27). Proliferation of anaerobes in tumoral wounds is directly linked to the presence of superficial or deep tumor-induced ischemic tissues.
The presence of biofilms was identified in 35% of samples. This number is much lower than what has been described in the literature for chronic wounds (13, 18). Nevertheless, previous studies on biofilms were carried out in small populations, and other studies involving more patients are needed to determine if the prevalence of biofilms in tumoral wounds is specifically low or was overestimated in the past.
Our study shows that biofilms are not associated with the presence of a precise bacterial strain and do not increase symptoms. On tumoral wounds, for which wound healing is not the main objective, biofilm withdrawal may then simply serve to delay infectious processes. Local strategies combining wound debridement and antibiofilm solutions are potentially traumatic and should thus be applied with due consideration given to patients' general condition (28, 29, 30).
It seems that bacterial colonization can result in the development of biofilms and the emergence of symptoms and may be the starting point of infectious processes. A major problem is that whereas management of malignant wounds relies mostly on clinical supervision, symptoms and clinical signs do not seem to be appropriate warning signals for bacterial imbalance. To help clinicians, it is necessary to develop diagnostic tools for detecting the presence of biofilms as well as significant amounts of bacteria and for identifying all bacterial species. This would facilitate the resolution of the present controversy regarding biofilms in clinical diagnosis (31, 32, 33).
In conclusion, this study provides a dynamic description of the bacterial floras of tumoral wounds, which contrasts with the usual idea of the stability of chronic wounds. When symptoms emerge, the number and the type of bacterial species as well as the total bacterial load have already created a bacterial imbalance. Then, returning to baseline conditions seems difficult. This underlines the definite need for new therapeutic options that are active on circulating bacteria as well as on bacteria organized in biofilms and could be effective before the emergence of symptoms.
ACKNOWLEDGMENTS
This work was supported by the Hospitalization and Care Organization Division of the Minister of Health (France).
The authors declare no conflicts of interest.
Footnotes
Published ahead of print 7 August 2013
REFERENCES
- 1.Grocott P. 1999. The management of fungating wounds. J. Wound Care 8:232–234 [DOI] [PubMed] [Google Scholar]
- 2.McMurray V. 2003. Managing patients with fungating malignant wounds. Nurs. Times 99:55–57 [PubMed] [Google Scholar]
- 3.Langemo DK, Anderson J, Hanson D, Hunter S, Thompson P. 2007. Managing fungating wounds. Adv. Skin Wound Care 20:312–314 [DOI] [PubMed] [Google Scholar]
- 4.Meaume S, Kerihuel JC, Fromantin I, Teot L. 2012. Workload and prevalence of open wounds in the community: French Vulnus initiative. J. Wound Care 21:62–73 [DOI] [PubMed] [Google Scholar]
- 5.Probst S, Arber A, Faithfull S. 2009. Malignant fungating wounds: a survey of nurses' clinical practice in Switzerland. Eur. J. Oncol. Nurs. 13:295–298 [DOI] [PubMed] [Google Scholar]
- 6.Lookingbill DP, Spangler N, Helm KF. 1993. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J. Am. Acad. Dermatol. 29(2 Pt 1):228–236 [DOI] [PubMed] [Google Scholar]
- 7.Clark J. 2002. Metronidazole gel in managing malodorous fungating wounds. Br. J. Nurs. 11(6 Suppl):S54–S60 [DOI] [PubMed] [Google Scholar]
- 8.Stotter A, Kroll S, McNeese M, Holmes F, Oswald MJ, Romsdahl M. 1991. Salvage treatment for loco-regional recurrence following breast conservation therapy for early breast cancer. Eur. J. Surg. Oncol. 17:231–236 [PubMed] [Google Scholar]
- 9.Lund-Nielsen B, Müller K, Adamsen L. 2005. Malignant wounds in women with breast cancer: feminine and sexual perspectives. J. Clin. Nurs. 14:56–64 [DOI] [PubMed] [Google Scholar]
- 10.Dowsett C. 2002. Malignant fungating wounds: assessment and management. Br. J. Community Nurs. 7:394–400 [DOI] [PubMed] [Google Scholar]
- 11.Lazelle-Ali C. 2007. Psychological and physical care of malodorous fungating wounds. Br. J. Nurs. 16:S16–S24 [DOI] [PubMed] [Google Scholar]
- 12.Bowler PG, Davies BJ. 1999. The microbiology of infected and noninfected leg ulcers. Int. J. Dermatol. 38:573–578 [DOI] [PubMed] [Google Scholar]
- 13.Black CE, Costerton JW. 2010. Current concepts regarding the effect of wound microbial ecology and biofilms on wound healing. Surg. Clin. North Am. 90:1147–1160 [DOI] [PubMed] [Google Scholar]
- 14.Philips P, Sampson E, Yang Q, Antonelli P, Progulske-Fox A, Schultz G. 2008. Bacterial biofilms in wound. Wound Heal. S. Afr. 1:10–12 [DOI] [PubMed] [Google Scholar]
- 15.Kirketerp-Moller K, Jensen P, Fazli M, Madsen KG, Pedersen J, Moser C, Tolker-Nielsen T, Hoiby N, Givskov M, Bjarnsholt T. 2008. Distribution, organisation, and ecology of bacteria in chronic wounds. J. Clin. Microbiol. 46:2717–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ammons MC. 2010. Anti-biofilm strategies and the need for innovations in wound care. Recent Pat. Antiinfect. Drug Discov. 5:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson M. 2001. Bacterial biofilms and human disease. Sci. Prog. 84(Pt 3):235–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James GA, Swogger E, Wolcott R, Pulcini ED, Secor P, Sestrich J, Costerton JW, Stewart PS. 2008. Biofilms in chronic wounds. Wounds Repair Regen. 16:37–44 [DOI] [PubMed] [Google Scholar]
- 19.Rhoads DD, Cox SB, Rees EJ, Sun Y, Wolcott RD. 2012. Clinical identification of bacteria in human chronic wound infections: culturing vs. 16S ribosomal DNA sequencing. BMC Infect. Dis. 12:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price LB, Liu CM, Melendez JH, Frankel YM, Engelthaler D, Aziz M, Bowers J, Rattray R, Ravel J, Kingsley C, Keim PS, Lazarus GS, Zenilman JM. 2009. Community analysis of chronic wound bacteria using 16S rRNA gene-based pyrosequencing: impact of diabetes and antibiotics on chronic wound microbiota. PLoS One 4:e6462. 10.1371/journal.pone.0006462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowler PG, Duerden B, Armstrong DG. 2001. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 14:244–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansson C, Andersson E, Swanbeck G. 1988. Leg ulcer epidemiology in Gothenburg. Acta Chir. Scand. Suppl. 544:12–16 [PubMed] [Google Scholar]
- 23.Lund-Nielsen B, Adamsen L, Gottrup F, Rorth M, Tolver A, Kolmos JK. 2011. Qualitative bacteriology in malignant wounds–a prospective, randomized, clinical study to compare the effect of honey and silver dressing. Ostomy Wound Manage. 57:28–36 [PubMed] [Google Scholar]
- 24.Rotimi VO, Durosinmi Etti FA. 1984. The bacteriology of infected malignant ulcers. J. Clin. Pathol. 37:592–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ingham HR, Sisson PR, Tharagonnet D, Selkon JB, Codd AA. 1977. Inhibition of phagocytosis in vitro by obligate anaerobes. Lancet ii:1252–1254 [DOI] [PubMed] [Google Scholar]
- 26.Ashford RFU, Plant GT, Maher J, Pickering D, Coe MA, Drury A, Goold MA, Teare EL. 1980. Metrodinazole in smelly tumours. Lancet i:874–875 [DOI] [PubMed] [Google Scholar]
- 27.Seaman S. 2006. Management of malignant fungating wounds in advanced cancer. Semin. Oncol. Nurs. 22:185–193 [DOI] [PubMed] [Google Scholar]
- 28.Andriessen AE, Eberlein T. 2008. Assessment of a wound cleansing solution in the treatment of problem wounds. Wounds 20:171–175 [PubMed] [Google Scholar]
- 29.Kaerhn K, Eberlein T. 2009. In-vitro test for comparing the efficacy of wound rinsing solutions. Br. J. Nurs. 8:S4, S6–S8, S10 [DOI] [PubMed] [Google Scholar]
- 30.Wolcott RD, Rumbaugh KP, James G, Schultz G, Yang Q, Watters C, Stewart PS, Dowd SE. 2010. Biofilm maturity studies indicate sharp debridement opens a time-dependent therapeutic window. J. Wound Care 19:320–328 [DOI] [PubMed] [Google Scholar]
- 31.Cowan T. 2012. Visible biofilms–a controversial issue! J. Wound Care 21:106. [DOI] [PubMed] [Google Scholar]
- 32.Hurlow J. 2012. Response to White and Cutting critique. J. Wound Care 21:198. [DOI] [PubMed] [Google Scholar]
- 33.White RJ, Cutting KF. 2012. Wound biofilms–are they visible? J. Wound Care 21:140–141 [DOI] [PubMed] [Google Scholar]


