Abstract
PCR is very effective in diagnosing acute Q fever in the early stages of infection, when bacterial DNA is present in the bloodstream but antibodies have not yet developed. The objective of this study was to further analyze the diagnostic value of semiquantitative real-time PCR (qPCR) in diagnosing acute Q fever in an outbreak situation. At the Jeroen Bosch Hospital, in 2009, qPCR testing for Coxiella burnetii DNA was performed for 2,715 patients suspected of having acute Q fever (positive, n = 385; negative, n = 2,330). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the qPCR assay were calculated for patients with negative qPCR results with a follow-up sample obtained within 14 days (n = 305) and qPCR-positive patients with at least one follow-up sample (n = 369). The correctness of the qPCR result was based on immunofluorescence assay results for samples submitted for qPCR and follow-up testing. The sensitivity of the Q fever qPCR assay was 92.2%, specificity 98.9%, PPV 99.2%, and NPV 89.8%. Patients who later developed serologic profiles indicative of chronic Q fever infection had significantly higher C. burnetii DNA loads during the acute phase than did patients who did not (P < 0.001). qPCR testing is a valuable tool for the diagnosis of acute Q fever and should be used in outbreak situations when the onset of symptoms is <15 days earlier. Special attention is needed in the follow-up monitoring of patients with high C. burnetii DNA loads during the acute phase, as this might be an indicator for the development of a serologic profile indicative of chronic infection.
INTRODUCTION
Infection by the intracellular bacterium Coxiella burnetii, which is transmitted to humans from infected animals (in particular, small ruminants), causes Q fever (1). During four consecutive years starting in 2007, the largest Q fever epidemic recorded to date occurred in the south of the Netherlands (>4,000 notified cases) (2, 3). The affected area has a high density of dairy goats, which were considered the primary source of infection (3–6).
The gold standard for serologic diagnosis of an infectious disease is either seroconversion or a 4-fold increase in antibody titers. The reference test for serologic diagnosis of Q fever is the immunofluorescence assay (IFA) (1, 7). Antibodies against phase II antigens are expressed during acute infections, whereas high levels of antibodies against phase I antigens are detected in patients with chronic Q fever (8–10). IgM antibodies against phase II antigens (IgM phase II) are the first antibodies to appear after infection, followed by IgG antibodies against phase II antigens (IgG phase II) and IgM antibodies against phase I antigens (IgM phase I) and finally IgG antibodies against phase I antigens (IgG phase I) (8). However, there is a lag in antibody responses of 7 to 15 days after the onset of symptoms, which is an important disadvantage to note for serology, as it is the primary method of diagnosis for acute Q fever (1). Another difficulty in diagnosing acute Q fever is the persistence of IgM phase II antibodies (11).
C. burnetii DNA-specific PCR can be an effective tool to diagnose acute Q fever before seroconversion takes place, as C. burnetii DNA has been found in blood samples from patients in that stage of the disease (12). It is unclear whether cell-free DNA or intact bacteria are generally present in blood. The latter has been demonstrated by a well-documented case of Q fever transmitted by blood transfusion (13). The sensitivities of various C. burnetii PCR assays to detect Q fever within 2 weeks after disease onset range from 26% to 98% (12, 14, 15).
Acute Q fever infections can remain asymptomatic or present as a flu-like illness, pneumonia, or hepatitis (1, 10, 16). Chronic Q fever develops in approximately 2% of symptomatic acute Q fever cases (17). Patients with preexisting valvular disease, aneurysms, or vascular grafts, immunocompromised patients, and pregnant women are most at risk for chronic infection (1). The diagnosis of chronic Q fever is difficult and relies mainly on serologic findings, blood or tissue PCR assay results, clinical findings, the presence of risk factors, and the results of diagnostic imaging techniques (18). Nevertheless, as chronic Q fever has high morbidity and mortality rates if left untreated, early detection and treatment are important (19).
Delays in treatment and failure to diagnose acute Q fever may lead to prolonged morbidity and increased hospital admission rates (3, 20–22). In 2007 in the Netherlands, the percentage of hospitalized patients was 46% and there was a considerable period of time between the actual onset of the epidemic and the public health department knowing of its presence (3). Routine laboratory diagnostic tests based on seroconversion in convalescent-phase serum samples and the unawareness of medical staff members resulted in relatively late diagnoses; both of those factors improved in subsequent years, which reduced the diagnostic delays (3, 23, 24).
The majority of diagnostic samples from the Dutch epidemic were submitted to our laboratory, which is located in the center of the epidemic area (Department of Medical Microbiology and Infection Control, Jeroen Bosch Hospital [JBH], 's-Hertogenbosch, the Netherlands). The laboratory serves a catchment area of approximately 500,000 persons in a semirural district, supporting two hospitals and surrounding general practitioners (GPs). In 2009, the JBH received over 18,000 requests for Q fever diagnostic tests, with a maximum of 182 requests on 1 day, and diagnosed more than 1,300 Q fever cases. In order to improve laboratory logistics during the outbreak situation, an algorithm was developed to cope with the large number of submitted samples. IgM phase II tests, enzyme-linked immunosorbent assays (ELISAs), and PCR assays were included as routine procedures within this algorithm (see Materials and Methods for more details) (25).
The objective of this study was to further analyze the diagnostic value of semiquantitative real-time PCR (qPCR) in the diagnosis of acute Q fever in an outbreak situation. Additionally, cycle threshold (CT) values (i.e., bacterial DNA load) in the acute phase were studied.
MATERIALS AND METHODS
In this study, all samples submitted to our laboratory in 2009 by GPs and hospital physicians requesting acute Q fever diagnostic tests for which qPCR testing was performed were investigated. The C. burnetii qPCR test, together with its analytical sensitivity and specificity, has been described previously (12, 26).
Samples were excluded if an external laboratory requested the test, if qPCR was performed to check for chronic Q fever infection, or if acute Q fever had been diagnosed previously. The main reason for not including samples from external laboratories was that, in most cases, only one sample per patient was received for additional qPCR testing to identify chronic Q fever.
GPs and hospital physicians submitting samples either supplied or did not supply the requested date of disease onset. Diagnostic evaluations for patients suspected of having acute Q fever were performed according to the diagnostic algorithm that was established in our laboratory in 2009 (25). In brief, all serum samples were initially screened with a qualitative ELISA for IgM phase II (MII screen), according to the manufacturer's instructions (Institut Virion/Serion GmbH, Würzburg, Germany), with a DSX automated ELISA processing system (Dynex Technologies, Chantilly, VA). qPCR was performed on all MII screen-negative samples from GP-referred patients with a period of ≤14 days between the onset of illness and the acquisition of the serum sample, as well as all MII screen-negative samples from hospital-admitted patients. When the date of disease onset was missing from requests from GPs, qPCR was not performed and a second sample was requested. In cases in which qPCR was performed and the result was negative, a second sample (after 14 days) also was requested (25). IFAs for IgM and IgG phase I and phase II (Focus Diagnostics, Inc., Cypress, CA) were performed on all MII screen-positive and borderline-positive serum samples, according to the manufacturer's instructions. Titers of 1:32 or greater were considered positive (25). After a patient was diagnosed with acute Q fever, follow-up samples were requested at 3, 6, and 12 months, to monitor the development of chronic Q fever (27).
C. burnetii qPCR targeting the multicopy IS1111a gene (26) was performed in duplicate as described (the input volume of serum was 500 μl and the elution volume was 60 μl; for retesting, the input volume of serum was 500 μl and the elution volume was 25 μl) (12). qPCR results were considered positive when at least one CT value was <45. To avoid contamination of qPCR assays with genomic C. burnetii DNA or amplicons from previous reactions, the preparation of the PCR reagents, the isolation of DNA, and the amplification by qPCR (closed system) were carried out in three separate rooms designated for those activities. A mock isolation (negative control [NC], i.e., simulated isolation that mimics the process of sample handling) and a water control (no-template control [NTC]) were included in each run. Among the 347 NC plus 315 NTC samples that were analyzed in 2009, we once detected a C. burnetii signal with a CT of 36.22 in an NC sample that was in the qPCR plate next to a relatively high-level positive sample (CT of 28.58); all other 661 control samples tested negative.
To assess whether qPCR-positive patients actually had acute Q fever, IFA results for one or more follow-up samples were evaluated. To classify a positive qPCR result as correct, IgG phase I and/or phase II seroconversion with a titer of ≥1:64 or a ≥4-fold increase in the IgG phase II titer needed to be detected in a follow-up sample. Due to the enormous number of samples that were submitted to our laboratory for Q fever diagnostic tests (over 18,000 samples in 2009), IFA results sometimes were not titrated but were recorded only as “positive.” In addition, IgG phase I and II levels were not always determined in samples submitted for qPCR testing with a suspicion of acute Q fever (when ELISA/IFA IgM phase II results were negative [25]). Therefore, to establish seroconversion rates, we assumed that the IgM phase II-negative qPCR samples were also negative for IgG phase I and II. To test this assumption, we analyzed the IgG phase I and II status of 16 qPCR-positive IgM phase II-negative patient samples. All 16 samples tested negative for IgG phase I and II. Patients with inconclusive serologic results, patients with nonspecific antibody responses, and patients with detectable IgG antibodies for whom IgG seroconversion or a 4-fold IgG phase II titer increase could not be confirmed were excluded from this analysis. To evaluate whether negative qPCR results correctly identified patients without acute Q fever, we included all qPCR-negative samples from patients who submitted a second serum sample within 14 days after the first sample. Thus, we excluded qPCR-negative patients with no follow-up sample, patients with IgG phase II antibodies present in the diagnostic qPCR-negative sample without a 4-fold IgG phase II titer increase, and patients with a follow-up sample submitted ≥15 days after the first sample, as we were unable to assess whether the qPCR results were correct for those patients. To illustrate the latter case, a patient with a follow-up sample submitted ≥15 days after the first sample could in theory be infected after the first sample was obtained, as qPCR results are positive for only approximately 14 days after the onset of symptoms.
Additionally, CT values obtained during the acute phase of infection for qPCR-positive patients were investigated in relation to age, time after onset of symptoms, IgG phase I titer at 12 months after diagnosis, and development of a serologic profile for chronic Q fever. A serologic profile indicative of chronic Q fever was defined as an IgG phase I titer of ≥1:1,024 in serum obtained approximately 12 months after the qPCR-positive sample. Proven chronic Q fever was defined as an IgG phase I titer of ≥1:1,024 and a positive qPCR result observed within approximately 12 months after the acute Q fever diagnosis. To estimate the C. burnetii DNA load (genome equivalents [GEq] per milliliter) in serum from duplicate CT values, the following formula was used: C. burnetii DNA load (GEq/ml) = × 2 × 6 × (100/57). The first part of the formula is based on the dilution experiment described previously (12), the factor 2 on 500 μl serum input for DNA isolation, the factor 6 on qPCR input of 10 μl of a 60-μl elution volume, and the factor 100/57 on correction for the easyMAG isolation efficiency for bacterial DNA of 57% (28).
Descriptive characteristics were investigated by calculating relative frequencies, medians, and interquartile ranges (IQRs). Chi-square tests were performed to assess significant differences in gender distribution, and Mann-Whitney U tests were used for age distribution and CT values. To investigate the diagnostic value of qPCR in diagnosing acute Q fever, we calculated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) and corresponding 95% confidence intervals (Wilson score intervals). Furthermore, the cumulative percentage of patients developing a chronic Q fever serologic profile for different C. burnetii DNA loads in the acute phase was calculated. Spearman's rank correlation (rs) coefficients were calculated to determine potential correlations between age, time after symptom onset, and IgG phase I titer at 12 months after diagnosis. Data were analyzed using SPSS Statistics version 19.0.0 (SPSS Inc.) and Microsoft Excel (Microsoft Corp.).
RESULTS
In 2009, qPCR was performed at the JBH for 3,040 patients. After the exclusion of 325 patients because of requests by external laboratories (n = 267), checking for chronic infection (n = 36), and previously diagnosed acute Q fever infections (n = 22), 2,715 patients remained. Table 1 presents characteristics of these patients. The date of symptom onset provided by GPs and hospital physicians submitting samples was available for 68% (n = 1,836). Negative qPCR responses were observed for 2,330 patients, and 385 patients had positive qPCR results. A statistically significant difference was observed in gender distribution between qPCR-positive and -negative patients (P < 0.001) (Table 1).
Table 1.
Characteristics of patients with Coxiella burnetii real-time PCR results obtained in 2009 to assess the presence of acute Q fever infection
| qPCR result | No. (%) male | Median age (IQR) (yr) |
|---|---|---|
| Total (n = 2,715) | 1,412 (52) | 49 (34–64) |
| Positive (n = 385) | 264 (69)a | 50 (38.5–60)b |
| Negative (n = 2,330) | 1,151 (49)a | 49 (33–64)b |
Positive qPCR result versus negative qPCR result: P < 0.001, chi-square test.
Positive qPCR result versus negative qPCR result: P = 0.563, Mann-Whitney U test. IQR, interquartile range.
Of the 2,330 patients with negative qPCR results, 1,299 (56%) did not have a follow-up serum sample taken and 714 (31%) had their follow-up sample taken ≥15 days after the first sample. Moreover, 12 patients were excluded from the diagnostic value analysis because they had inconclusive serologic results (n = 3), nonspecific serologic responses (n = 3), already evident antibodies in a previous sample (i.e., qPCR was unnecessary [n = 2]), IgG phase II present in the qPCR-negative sample without a 4-fold IgG phase II titer increase (n = 2), or previously resolved Q fever infection (n = 2). In the end, 305 qPCR-negative patients remained available for analysis (male, n = 154 [51%]; median age, 52 years [IQR, 39 to 62 years]). Nine (2%) of the 385 qPCR-positive patients did not provide a follow-up sample, six patients had detectable IgG phase II antibodies although IgG seroconversion or a 4-fold IgG phase II titer increase could not be confirmed with the data available, and one qPCR-positive patient was excluded because of inconclusive serologic results. Subsequently, 369 qPCR-positive patients remained available for the analysis (male, n = 250 [68%]; median age, 50 years [IQR, 38.5 to 60 years]), including 366 based on seroconversion and 3 based on a 4-fold increase in antibody titer.
Table 2 presents the outcome of the diagnostic value analysis for the qPCR assay for the diagnosis of acute Q fever. Seventy-two (20%) of the 369 qPCR-positive patients had detectable IgM phase II measured by IFA and/or ELISA in their qPCR-positive samples. Table 3 presents the diagnostic values found with the numbers presented in Table 2. The sensitivity was 92.2%, specificity 98.9%, PPV 99.2%, and NPV 89.8%. The three false-positive samples (CT values [duplicate] of 36.90 and 37.39, 36.38 and undetectable, and 34.32 and 35.65) were retested; the first two showed inhibition of the qPCR and the third was again positive (CT values of 33.61 and 31.60). The follow-up samples for the patient who repeatedly tested positive showed no IgG phase I and low IgG phase II levels (1:32) up to 1 year after the qPCR-positive sample and thus did not meet our definition of confirmed acute Q fever infection.
Table 2.
Numbers of patients included in diagnostic value analysis for the Coxiella burnetii real-time PCR assay for the diagnosis of acute Q fever
| qPCR result | No. of patients |
||
|---|---|---|---|
| Infection in follow-up samplea | No infection in follow-up sampleb | Total | |
| Positive | 366 | 3 | 369 |
| Negative | 31 | 274 | 305 |
| Total | 397 | 277 | 674 |
IgG phase I and/or phase II seroconversion with a titer of ≥1:64 in a follow-up sample or a ≥4-fold increase in the IgG phase II titer in a follow-up sample.
No IgG phase II antibodies (<1:64) detected in one or more follow-up samples.
Table 3.
Diagnostic value of the Coxiella burnetii real-time PCR assay for the diagnosis of acute Q fever
| Diagnostic value | % (95% confidence interval)a |
|---|---|
| Sensitivity | 92.2 (89.1–94.4) |
| Specificity | 98.9 (96.9–99.6) |
| Positive predictive value | 99.2 (97.6–99.7) |
| Negative predictive value | 89.8 (85.9–92.8) |
| Confirmed acute Q fever infection rate | 58.9 (55.2–62.6) |
Wilson score interval.
Of the 31 patients with false-negative qPCR test results (all based on seroconversion), 19 patients had negative MII screen results, 10 patients had positive or ambiguous MII screen results and positive IgM phase II IFA results, and 1 patient had a positive IgM phase II IFA result; for 1 patient, only qPCR was performed on the first sample but the second sample, submitted 5 days later, was IFA IgM phase II, IgM phase I, and IgG phase II positive. Four of the 12 patients for whom IgM phase II antibodies (ELISA and/or IFA) were detected also had detectable IgM phase I antibodies, while IgG phase II results were negative for all 12 patients. Four of the 31 patients never developed IgG phase I antibodies during the follow-up period.
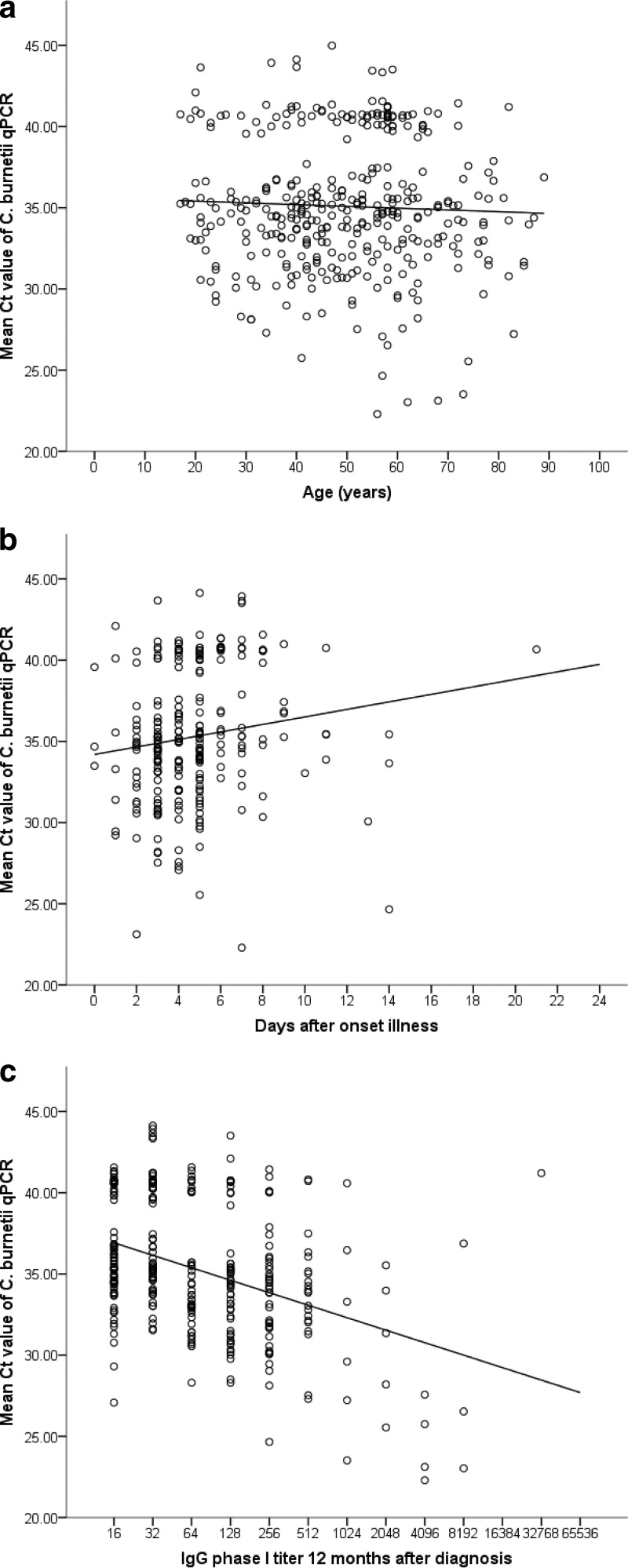
Table 4 and Fig. 1 show the relationships observed between CT values and age, time after illness onset, and IgG phase I titers approximately 12 months after diagnosis. Significant correlations were found for the number of days after the onset of illness and IgG phase I titers at 12 months after diagnosis (Table 4). Significant weak negative correlations also were observed for IgG phase I and II antibody levels at 3 and 6 months after diagnosis and for IgG phase II antibody levels at 12 months after diagnosis (data not shown). Additionally, it was observed that 107/369 (30%) of qPCR-positive patients had low circulating C. burnetii DNA loads (<10 GEq/ml) at the time of diagnostic sampling.
Table 4.
Spearman rank correlation coefficients for mean CT values for duplicate Coxiella burnetii real-time PCR tests in relation to age, number of days after illness onset, and IgG phase I titers 12 months after diagnosis
| Parameter analyzed | No. of patients | Spearman rank correlation coefficient (rs)a | Pb |
|---|---|---|---|
| Age | 366 | −0.005 | 0.924 |
| Days after illness onset | 249 | 0.232 | <0.001 |
| IgG phase I titer 12 mo after diagnosis | 312 | −0.338 | <0.001 |
Spearman rank correlation coefficient for the relationship between CT values and the parameter indicated.
Two-tailed test.
Fig 1.
Relationships between the mean cycle threshold (CT) values of duplicate Coxiella burnetii real-time PCR tests and age (n = 366) (a), days after the onset of illness (n = 249) (b), and IgG phase I titers at 12 months after diagnosis (n = 312) (c).
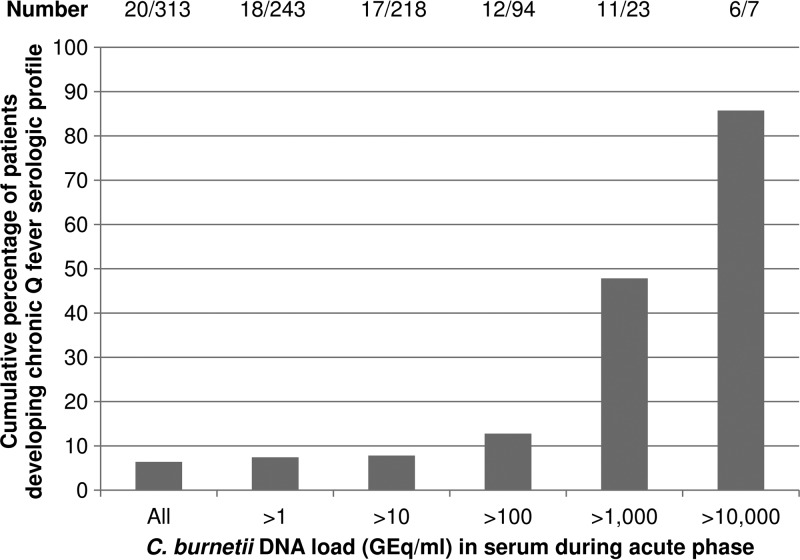
Follow-up serum samples obtained approximately 12 months after diagnosis were available for 85% (312/369) of the patients with true-positive qPCR results. Nineteen of those patients developed serologic profiles indicative of chronic Q fever. One additional patient developed proven chronic Q fever without a 12-month follow-up sample (deceased). Overall, 20/313 (6.4%) had serologic profiles indicative of chronic Q fever, and one additional patient with a serologic profile indicative of chronic Q fever was observed among the patients with false-negative qPCR results, with IgM phase I and II antibodies present in the diagnostic qPCR-negative sample (Table 5). The median CT value for the 20 patients diagnosed with positive qPCR results was 28.90 (IQR, 25.60 to 35.55), which was significantly different from the median CT value for the 293 patients without serologic profiles for chronic Q fever (35.01 [IQR, 33.00 to 39.56]; P < 0.001). Furthermore, 10 of those 20 patients were among the patients with the 17 lowest CT values found (CT ≤ 28.20) among all 366 patients (median CT value for those 10 patients, 25.65 [IQR, 23.10 to 27.31]). Figure 2 presents the cumulative percentages of patients who developed serologic profiles indicative of chronic Q fever with different C. burnetii DNA loads in the acute-phase serum samples. Eighty-six percent of patients with C. burnetii DNA loads above 10,000 GEq/ml in the acute phase developed serologic profiles indicative of chronic Q fever.
Table 5.
Clinical and serologic findings for the 21 patients with serologic profiles indicative of chronic Q fever 12 months after acute Q fever infection
| Patient no. | Mean CT value at acute Q fever diagnosis | Serologic results 12 mo after diagnosisa |
Clinical findings | Chronic Q fever categoryb | ||
|---|---|---|---|---|---|---|
| IgG phase I titer | IgG phase II titer | qPCR result | ||||
| 1 | 22.30 | 4,096 | 8,192 | Negative | No risk factorsc | C |
| 2 | 23.03 | >4,096 | >4,096 | Negative | No risk factorsc | C |
| 3 | 23.12 | 4,096 | 4,096 | Positive | Focus unknown | A |
| 4 | 23.52 | 1,024 | 1,024 | Negative | Granulomatous abnormality in lung | B |
| 5 | 25.55 | 2,048 | 2,048 | Negative | No risk factorsc | C |
| 6 | 25.75 | 4,096 | 4,096 | Negative | No risk factorsc | C |
| 7 | 26.53 | >4,096 | >4,096 | Negative | Infected vascular prosthesis | A |
| 8 | 27.23 | 1,024 | >4,096 | Negatived | Endocarditis | A |
| 9 | 27.57 | 4,096 | 16,384 | Negative | Endocarditis | B |
| 10 | 28.20 | 2,048 | >4,096 | Negative | No risk factorsc | C |
| 11 | 29.60 | 1,024 | >4,096 | Negative | No risk factorsc | C |
| 12 | 31.36 | 2,048 | 4,096 | Negative | No risk factorsc | C |
| 13 | 33.29 | 1,024 | 1,024 | Negative | No risk factorsc | C |
| 14 | 33.98 | 2,048 | >4,096 | Negative | Deceased with several comorbidities | Unknown |
| 15 | 35.53 | 2,048 | 4,096 | Negative | Endocarditis | B |
| 16 | 35.56 | NA | NA | NA | Kidney transplant; deceased within 12 mo after diagnosis, had positive qPCR result for follow-up serum sample | A |
| 17 | 36.46 | 1,024 | 2,048 | Negative | No risk factorsc | C |
| 18 | 36.88 | >4,096 | >4,096 | Positive | Infected aneurysm | A |
| 19 | 40.59 | 1,024 | >4,096 | Negative | No risk factorsc | C |
| 20 | 41.21 | 32,768 | 65,536 | Positive | Endocarditis | A |
| 21 | False-negative qPCR result, positive IgM phase I and II results for diagnostic qPCR-negative sample | 1,024 | 1,024 | Negative | No risk factorsc | C |
NA, not applicable.
A, proven chronic Q fever; B, probable chronic Q fever; C, possible chronic Q fever, according to Wegdam-Blans et al. (18).
No cardiac or vascular risk factors.
A follow-up serum sample obtained >12 months after the acute Q fever diagnosis was qPCR positive.
Fig 2.
Cumulative percentages of patients who developed serologic profiles indicative of chronic Q fever infection, classified according to the estimated C. burnetii DNA load in the acute-phase serum samples. Patients were included only when a serum sample obtained approximately 12 months after the qPCR-positive sample was available (n = 312) or when a proven chronic Q fever infection was observed <12 months after the acute Q fever diagnosis (n = 1). Chronic Q fever infection was determined on the basis of an IgG phase I titer of ≥1:1,024 in a serum sample obtained approximately 12 months after the qPCR-positive sample or observation of a proven chronic Q fever infection (IgG phase I titer of ≥1:1,024 and a positive qPCR result) <12 months after the acute Q fever diagnosis.
DISCUSSION
This study presents data for a large number of patients who were tested with qPCR for C. burnetii DNA during an acute Q fever outbreak. Previously, we showed that the qPCR assay used in our department detects C. burnetii DNA in virtually all sera of seronegative patients with acute Q fever but results rapidly become negative when the antibody response develops (12). However, the positive and negative predictive values of the C. burnetii qPCR have not been examined thus far.
For the Q fever diagnostic work-up, we followed the algorithm described previously (25). IgG phase I and II levels were not determined in the samples submitted for qPCR testing when ELISA/IFA IgM phase II results were negative. Therefore, to establish seroconversion rates, we assumed that the IgM phase II-negative qPCR samples were also negative for IgG phase I and II. This assumption was reasonable since we were dealing with a high-incidence outbreak in a low-prevalence area; therefore, the chances for chronic Q fever in this patient cohort were very small. IFA retesting of the qPCR-positive samples from patients who had IgG phase I titers of ≥1:1,024 in the first follow-up sample (n = 18; 14 samples were available, and 4 samples were unavailable) indeed showed that none had detectable IgG phase I or II antibodies in the initial qPCR-positive sample.
In this study, we have been quite conservative in assigning patients to the true-positive and true-negative categories, and the presented diagnostic values might be underestimations. For example, patients with negative qPCR and positive MII screen and/or IFA IgM phase I and II results were assigned to the false-negative qPCR category, although the qPCR finding probably was a true-negative result as IgM antibodies were already present. If we had investigated the diagnostic value of the qPCR assay before the presence of antibodies, then the 12 patients with detectable IgM phase II antibodies (IFA and/or ELISA) in their qPCR-negative samples would not have been classified in the false-negative category and the reported sensitivity would be an underestimation.
We were not able to include all qPCR-negative patients as the majority of qPCR-negative patients did not submit a follow-up sample or it was submitted too late. Usually, general practitioners and patients are less likely to send in requested follow-up samples when symptoms have resolved or another cause of illness has been found. They are more likely to send in requested follow-up samples when undiagnosed symptoms persist. Nevertheless, some bias might have occurred in this study as we missed some cases with false-negative qPCR results. Thus, the sensitivity and, to a lesser extent, the negative predictive value presented here are overestimations.
We found three patients with false-positive qPCR results, one of whom also had positive retest results. The other two samples unfortunately showed inhibition of the qPCR in the retest, probably due to the smaller elution volume (25 μl in the retest versus 60 μl in the first test) and therefore higher concentrations of inhibiting substances after DNA isolation. Although no clear seroconversion was observed for the patient with the consistent positive qPCR test results, a low positive IgG phase II titer (1:32) was found in two follow-up samples.
Positive qPCR results for blood donors who do not develop a clear antibody response were observed previously by Hogema et al. (29). Those authors found that three of the six blood donors with qPCR-positive results did not have seroconversion measured by IFA (29). Since qPCR is an extremely sensitive technique for the detection of DNA, contamination of patient samples during processing always poses a risk for false-positive test results. This might have been the case for our three false-positive patients.
It was observed that often low circulating C. burnetii DNA loads were present in qPCR-positive samples (CT values of >36.50 correspond to loads of <10 GEq/ml). This indicates that high test sensitivity is important for detection of such low C. burnetii loads. These low DNA loads may well contribute to the differences in the reported sensitivities of various PCR assays (26% to 98% [12, 14, 15]).
We excluded patients for whom qPCR was performed in relation to chronic Q fever infection, as we assessed performance of the qPCR assay in the acute phase. qPCR results can be positive for Q fever patients in the acute phase, which occurs in the first 2 weeks after disease onset (12), and for patients with chronic Q fever (18). After the acute phase, qPCR results become negative at the time of antibody development. qPCR results can again become positive for chronic Q fever patients when IgG phase I antibodies are present (18). It is not known whether all patients who develop chronic Q fever have a period between the acute phase and the chronic phase during which qPCR results are negative. Some patients may not be able to control the acute infection at all, directly progressing to a chronic stage with persistence of qPCR positivity.
Although weak, a significant positive correlation was observed between CT values and days after the onset of illness, as well as a significant negative correlation between CT values and IgG phase I titers at 12 months after diagnosis. A statistically significantly lower CT value (i.e., a higher bacterial DNA load) during acute infection was found for patients who later developed serologic profiles indicative of chronic Q fever infection than for patients who did not. Only four of the 10 patients with CT values of ≤28.20 (corresponding to approximately 2,900 GEq/ml) had clear risk factors for chronic Q fever (Table 5). The 21 patients with serologic profiles indicative of chronic Q fever (including the patient with a false-negative qPCR result) were classified as described previously (18), which resulted in six cases of proven chronic Q fever, three probable cases, and 11 possible cases, while one case could not be classified (the patient was deceased with several comorbidities, with negative qPCR results for the 12-month follow-up sample). Of patients with C. burnetii DNA loads of >1,000 GEq/ml, 48% developed serologic profiles indicative of chronic infection, which increased to 86% for patients with loads of >10,000 GEq/ml. Thus, a low CT value/high bacterial DNA load, which unfortunately is measurable only in the first 2 weeks after disease onset, might be an additional risk factor for developing a serologic profile indicative of chronic infection, independent of already known risk factors. Additional patients with low CT values might be identified in new outbreak situations or by retrospective testing of stored serum samples obtained from acute Q fever patients <15 days after symptom onset.
Molecular tests are generally sensitive to carryover and cross-contamination, which may lead to false-positive test results. Although the three false-positive test results in our study were probably due to contamination, there are several indications that cross-contamination was not a common phenomenon in the C. burnetii qPCR testing in our laboratory. First, as mentioned in Materials and Methods, we used separate rooms for the preparation of the PCR reagents, the isolation of DNA, and the amplification by qPCR (closed system). Second, the simulated isolations that mimicked the process of sample handling and the water controls included in each run systematically tested negative. Third, our laboratory participated in an interlaboratory evaluation/quality check of the qPCR assay for C. burnetii (26). No contamination was seen for the qPCR assay with serum samples. Further, if cross-contamination had occurred, then the samples with the lowest C. burnetii burdens would be most suspected of being contaminated. Of the 369 patients with positive qPCR results in this study, 281 patients had duplicate CT values of <40, 78 patients had one CT value of <40 and one negative qPCR result, two patients had one CT value of <40 and one between 40 and 45, and eight patients had one CT value between 40 and 45 and one negative CT value. All except 1 of the 88 patients with the lowest C. burnetii loads developed antibodies against C. burnetii during the follow-up period. The single patient who did not develop a serologic response was considered to have a false-positive result. If our high sensitivity reflected contamination, then we would have had more false-positive findings.
In conclusion, qPCR is an effective and valuable tool for the diagnosis of acute Q fever in outbreak situations. C. burnetii qPCR testing with serum samples should be included routinely in the diagnostic work-up for patients with suspected acute Q fever in outbreak situations when the onset of symptoms is <15 days earlier. Special attention is needed in the follow-up monitoring of patients with high C. burnetii DNA loads (i.e., low CT values) during the acute phase of Q fever infection, as this might be an indicator for the development of a serologic profile indicative of chronic Q fever.
ACKNOWLEDGMENTS
Financial support for this research study was provided by the Netherlands Organization for Health Research and Development (ZonMW) (grant 205520006).
We thank Wim van der Hoek, Marianne van der Sande, and Roel Coutinho of the National Institute of Public Health and the Environment for their valuable contributions to this article. Also, we thank the laboratory technicians of the serology unit of the Department of Medical Microbiology and Infection Control and the Laboratory for Molecular Diagnostics of Jeroen Bosch Hospital for their efforts in analyzing all serum samples.
No conflicts of interest are declared.
Footnotes
Published ahead of print 17 July 2013
REFERENCES
- 1.Maurin M, Raoult D. 1999. Q fever. Clin. Microbiol. Rev. 12:518–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Hoek W, Schneeberger PM, Oomen T, Wegdam-Blans MC, Dijkstra F, Notermans DW, Bijlmer HA, Groeneveld K, Wijkmans CJ, Rietveld A, Kampschreur LM, van Duynhoven Y. 2012. Shifting priorities in the aftermath of a Q fever epidemic in 2007 to 2009 in the Netherlands: from acute to chronic infection. Euro Surveill. 17:pii=20059 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20059 [PubMed] [Google Scholar]
- 3.Dijkstra F, van der Hoek W, Wijers N, Schimmer B, Rietveld A, Wijkmans CJ, Vellema P, Schneeberger PM. 2012. The 2007–2010 Q fever epidemic in The Netherlands: characteristics of notified acute Q fever patients and the association with dairy goat farming. FEMS Immunol. Med. Microbiol. 64:3–12 [DOI] [PubMed] [Google Scholar]
- 4.Karagiannis I, Schimmer B, Van Lier A, Timen A, Schneeberger P, Van Rotterdam B, De Bruin A, Wijkmans C, Rietveld A, Van Duynhoven Y. 2009. Investigation of a Q fever outbreak in a rural area of The Netherlands. Epidemiol. Infect. 137:1283–1294 [DOI] [PubMed] [Google Scholar]
- 5.Schimmer B, ter Schegget R, Wegdam M, Zuchner L, de Bruin A, Schneeberger PM, Veenstra T, Vellema P, van der Hoek W. 2010. The use of a geographic information system to identify a dairy goat farm as the most likely source of an urban Q-fever outbreak. BMC Infect. Dis. 10:69. 10.1186/1471-2334-10-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veenstra T, Snijders BE, Schimmer B, Rietveld A, Van Dam S, Schneeberger PM, Dijkstra F, Van Der Sande MA, Van Der Hoek W. 2011. Risk factors for Q fever in the Netherlands. WebmedCentral Infect. Dis. 2:WMC002006 [Google Scholar]
- 7.Dupont HT, Thirion X, Raoult D. 1994. Q fever serology: cutoff determination for microimmunofluorescence. Clin. Diagn. Lab. Immunol. 1:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupuis G, Péter O, Peacock M, Burgdorfer W, Haller E. 1985. Immunoglobulin responses in acute Q fever. J. Clin. Microbiol. 22:484–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fournier PE, Marrie TJ, Raoult D. 1998. Diagnosis of Q fever. J. Clin. Microbiol. 36:1823–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raoult D, Marrie T, Mege J. 2005. Natural history and pathophysiology of Q fever. Lancet Infect. Dis. 5:219–226 [DOI] [PubMed] [Google Scholar]
- 11.Wegdam-Blans MCA, Wielders CCH, Meekelenkamp J, Korbeeck JM, Herremans T, Tjhie HT, Bijlmer HA, Koopmans MPG, Schneeberger PM. 2012. Evaluation of commonly used serological tests for detection of Coxiella burnetii antibodies in well-defined acute and follow-up sera. Clin. Vaccine Immunol. 19:1110–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneeberger PM, Hermans MHA, van Hannen EJ, Schellekens JJA, Leenders ACAP, Wever PC. 2010. Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever. Clin. Vaccine Immunol. 17:286–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canadian Diseases Weekly Report 1977. Comment on Q fever transmitted by blood transfusion—United States. Can. Dis. Wkly. Rep. 3:210 (Editorial.) [Google Scholar]
- 14.Fournier PE, Raoult D. 2003. Comparison of PCR and serology assays for early diagnosis of acute Q fever. J. Clin. Microbiol. 41:5094–5098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turra M, Chang G, Whybrow D, Higgins G, Qiao M. 2006. Diagnosis of acute Q fever by PCR on sera during a recent outbreak in rural South Australia. Ann. N. Y. Acad. Sci. 1078:566–569 [DOI] [PubMed] [Google Scholar]
- 16.Parker NR, Barralet JH, Bell AM. 2006. Q fever. Lancet 367:679–688 [DOI] [PubMed] [Google Scholar]
- 17.European Centre for Disease Prevention and Control 2010. Risk assessment on Q fever. European Centre for Disease Prevention and Control, Stockholm, Sweden [Google Scholar]
- 18.Wegdam-Blans MCA, Kampschreur LM, Delsing CE, Bleeker-Rovers CP, Sprong T, van Kasteren MEE, Notermans DW, Renders NHM, Bijlmer HA, Lestrade PJ, Koopmans MPG, Nabuurs-Franssen MH, Oosterheert JJ, Dutch Q Fever Consensus Group 2012. Chronic Q fever: review of the literature and a proposal of new diagnostic criteria. J. Infect. 64:247–259 [DOI] [PubMed] [Google Scholar]
- 19.Million M, Thuny F, Richet H, Raoult D. 2010. Long-term outcome of Q fever endocarditis: a 26-year personal survey. Lancet Infect. Dis. 10:527–535 [DOI] [PubMed] [Google Scholar]
- 20.Schimmer B, Morroy G, Dijkstra F, Schneeberger PM, Weers-Pothoff G, Timen A, Wijkmans C, van der Hoek W. 2008. Large ongoing Q fever outbreak in the south of The Netherlands, 2008. Euro Surveill. 13:pii=18939 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18939 [PubMed] [Google Scholar]
- 21.Gikas A, Kofteridis DP, Manios A, Pediaditis J, Tselentis Y. 2001. Newer macrolides as empiric treatment for acute Q fever infection. Antimicrob. Agents Chemother. 45:3644–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marrie TJ. 2003. Coxiella burnetii pneumonia. Eur. Respir. J. 21:713–719 [DOI] [PubMed] [Google Scholar]
- 23.van der Hoek W, Dijkstra F, Schimmer B, Schneeberger PM, Vellema P, Wijkmans C, ter Schegget R, Hackert V, van Duynhoven Y. 2010. Q fever in the Netherlands: an update on the epidemiology and control measures. Euro Surveill. 15:pii=19520 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19520 [PubMed] [Google Scholar]
- 24.van der Hoek W, Dijkstra F, Wijers N, Rietveld A, Wijkmans CJ, van Steenbergen JE, Notermans DW, Schneeberger PM. 2010. Three years of Q fever in the Netherlands: faster diagnosis. Ned. Tijdschr. Geneeskd. 154:A1845 (In Dutch.) [PubMed] [Google Scholar]
- 25.Jager MM, Weers-Pothoff G, Hermans MHA, Meekelenkamp JCE, Schellekens JJA, Renders NHM, Leenders ACAP, Schneeberger PM, Wever PC. 2011. Evaluation of a diagnostic algorithm for acute Q fever in an outbreak setting. Clin. Vaccine Immunol. 18:963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tilburg JJ, Melchers WJ, Pettersson AM, Rossen JWA, Hermans MHA, van Hannen EJ, Nabuurs-Franssen MH, de Vries MC, Horrevorts AM, Klaassen CHW. 2010. Interlaboratory evaluation of different extraction and real-time PCR methods for detection of Coxiella burnetii DNA in serum. J. Clin. Microbiol. 48:3923–3927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Hoek W, Versteeg B, Meekelenkamp JCE, Renders NHM, Leenders ACAP, Weers-Pothoff I, Hermans MHA, Zaaijer HL, Wever PC, Schneeberger PM. 2011. Follow-up of 686 patients with acute Q fever and detection of chronic infection. Clin. Infect. Dis. 52:1431–1436 [DOI] [PubMed] [Google Scholar]
- 28.Schuurman T, de Boer R, Patty R, Kooistra-Smid M, van Zwet A. 2007. Comparative evaluation of in-house manual, and commercial semi-automated and automated DNA extraction platforms in the sample preparation of human stool specimens for a Salmonella enterica 5′-nuclease assay. J. Microbiol. Methods 71:238–245 [DOI] [PubMed] [Google Scholar]
- 29.Hogema BM, Slot E, Molier M, Schneeberger PM, Hermans MH, van Hannen EJ, van der Hoek W, Cuijpers HT, Zaaijer HL. 2012. Coxiella burnetii infection among blood donors during the 2009 Q-fever outbreak in The Netherlands. Transfusion 52:144–150 [DOI] [PubMed] [Google Scholar]