Abstract
Clostridium difficile infection is almost unrecognized in mainland China. We have undertaken a study in a large Chinese teaching hospital in Changsha, Hunan, China, to identify cases of C. difficile, record patient characteristics, and define the molecular epidemiology with respect to ribotype distribution and cross-infection. Between April 2009 and February 2010, we examined fecal samples from 70 hospitalized patients with diarrhea who were receiving or had received antibiotics within the previous 6 weeks. Clinical information was collected and the samples were cultured for C. difficile retrospectively. Isolates were ribotyped, and multiple-locus variable-number tandem-repeat assay (MLVA) subtyping was performed on clusters of the same ribotype. The mean age of patients from whom C. difficile was cultured was 58 years, with only 4/21 patients aged >65 years. All patients, with a single exception, had received a third-generation cephalosporin and/or a quinolone antibiotic. Twenty-one isolates of C. difficile were recovered, and seven different ribotypes were identified, the dominant types being 017 (48%), 046 (14%), and 012 (14%). We identified two clusters of cross-infection with indistinguishable isolates of ribotype 017, with evidence of spread both within and between wards. We have identified C. difficile as a possibly significant problem, with cross-infection and a distinct ribotype distribution, in a large Chinese hospital. C. difficile may be underrecognized in China, and further epidemiological studies across the country together with the introduction of routine diagnostic testing are needed to ascertain the size of this potentially significant problem.
INTRODUCTION
Since the recognition over 30 years ago that Clostridium difficile infection presents as a spectrum of intestinal disease ranging from mild self-limiting diarrhea to life-threatening pseudomembranous colitis (PMC), most reports and studies have come from Europe and North America (1, 2). Other parts of the world, including South America, appear to be spared from C. difficile infections, although the very small number of reported cases from South America may be partially due to lack of diagnostic testing (3). In the Far East, some countries, such as Japan and Korea, have recognized for some time that C. difficile is a significant cause of nosocomial infections (4, 5). In the case of the region's most populous country, China, the situation is less clear. A recent literature review of Chinese language-based journals concluded that although a number of case reports and short series were identified there were only a very small number of cases (6). The reports mainly concentrated on compromised patients, thus giving the impression that C. difficile is both unusual and not endemic in hospitals (6). Patients with diarrhea are not normally tested for C. difficile, and in consequence the incidence is unknown. The authors were able to identify only a single study, undertaken at Huashan Hospital in Shanghai in 2008, for which data on ribotype, toxin production, risk factors, clinical features, and antimicrobial resistance were reported (7, 8). In that study, over the course of a year 75 isolates of Clostridium difficile were recovered, for which only 38 isolates had a ribotype number assigned according to the internationally recognized scheme of Stubbs and colleagues (9). No evidence of nosocomial infection was presented, although the clinical characteristics of the patients were subsequently reported (8). The ribotyping data showed the most common ribotype to be 017 (14/38 isolates), which has been frequently reported from Korea and Japan, followed by 012 (13/38 isolates); the data are shown in detail in Fig. 1 (7). Recently, multilocus sequence type (MLST) data have been published on isolates largely from Beijing, for which the dominant MLST type was found to be ST37, which the authors stated was representative of ribotype 017 (10). In contrast to mainland China, in Hong Kong C. difficile has been recognized for some time, the earliest report being from 1991 to 1993 in bone marrow transplant patients (11). A survey at Queen Mary Hospital, Hong Kong, in 1996 to 1997 reported 100/3,112 feces samples from patients with diarrhea to be positive for culture of C. difficile (12). A more recent survey at the same hospital using a tissue culture cytotoxin assay between September and December 2008 detected 37/723 positive samples (13). The investigators observed that the number of patients diagnosed in 2003 was 82, compared to 66 in 2008, suggesting there had not been a substantial increase in C. difficile infections. Studies in Hong Kong of the occurrence of C. difficile in patients receiving enteral feeding identified 12 cases in 38 patients treated for tuberculosis between 1999 and 2005 (14, 15). There have been only two published epidemiological studies from Hong Kong, both of which identified hospital outbreaks of ribotype 002 (16, 17). Interestingly C. difficile has also been recognized in Taiwan for the last 20 years (18).
Fig 1.
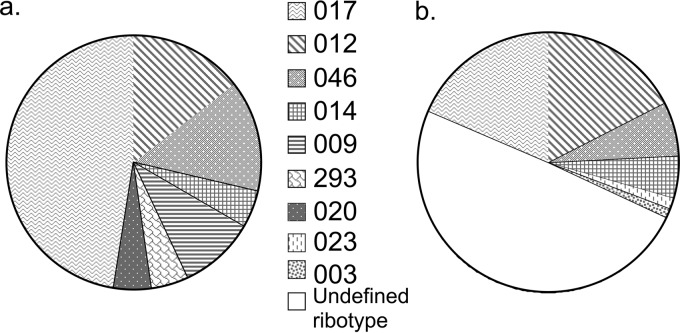
Comparison of the distribution of PCR ribotypes of Clostridium difficile from 21 patients at Xiangya Hospital, Changsha (a), and 75 patients at Fudan University Hospital, Shanghai (b) (9).
In order to expand our understanding of C. difficile in mainland China and identify the extent of hospital cross-infection, a prospective study was undertaken between April 2009 and February 2010 at Xiangya Hospital Central South University, Changsha, Hunan Province. Presumptive cases of C. difficile infection were identified among patients for whom a feces sample had been submitted to the laboratory for evaluation of diarrhea. Clinical data were collected and feces cultured for C. difficile with ribotyping and multiple-locus variable-number tandem-repeat assay (MLVA) to identify cross-infection within common ribotypes retrospectively.
MATERIALS AND METHODS
Bacterial isolates.
The study was undertaken at Xiangya Hospital South Central University, which is a 1,500-bed teaching hospital in central South China. Feces specimens were obtained from 70 patients from whom a sample had been submitted to the laboratory between April 2009 and February 2010 for the investigation of diarrhea. These specimens were negative for bacterial/viral pathogens but were identified as presumptively positive for C. difficile and were cultured for C. difficile bacteria. There was no hospital protocol for the diagnosis or treatment of C. difficile. Presumptive C. difficile was defined in patients who produced two or more loose stools (Bristol stool type 5 to 7) for at least two consecutive days, who were receiving or had received antibiotics in the preceding 6 weeks, and from whom toxigenic C. difficile was cultured. Patient demographics, antibiotic history, and clinical details were obtained together with the fecal specimen, which was frozen at −70°C prior to culture. A portion of feces (approximately 1 g) was mixed with an equal volume of 100% alcohol. The suspension was mixed by vortexing and was left to settle at room temperature for 30 min. The fecal deposit was inoculated onto cefoxitin-cycloserine fructose agar (Oxoid, Basingstoke, United Kingdom) and incubated in an anaerobic chamber for 48 to 72 h. Presumptive identification of C. difficile colonies was based on their typical morphology on agar plates, Gram stain, and the characteristic horse manure odor. Suspected colonies were subcultured to egg yolk agar (EYA) and nonselective anaerobic blood agar plates. Identification was confirmed by a negative lecithinase test for colonies on EYA; a positive C. difficile somatic antigen latex agglutination test (Microgen, Surrey, United Kingdom), and yellow-green fluorescence under long-wave UV light for colonies on blood agar. The collection of clinical data and culture for C. difficile was approved by the Xiangya University Hospital Institutional Review Board.
PCR ribotyping and tcdA/tcdB detection.
PCR ribotyping was performed as previously described, except that amplification reactions were performed in a final volume of 50 μl containing 2.5 mM MgCl2 and 5 μl DNA template (9). Strains with novel ribotype patterns were sent to the Anaerobe Reference Unit, Cardiff, for identification of new PCR ribotypes. Strains were tested for the presence of tcdA and tcdB as described by Lemee and colleagues (19).
Multilocus variable-number tandem-repeat analysis.
Multilocus variable-number tandem-repeat analysis (MLVA) using the set of 15 loci described by Manzoor and colleagues was performed as previously described (20). Isolates with ≥4 summed tandem-repeat differences (STRDs) were classified as being different strains (20).
RESULTS
C. difficile strain characteristics.
Feces samples were obtained from 70 patients identified as having presumptive C. difficile, of whom 21 were culture positive for C. difficile, although the medium available lacked 0.1% pure taurocholic acid, which may have reduced its sensitivity. Seven different PCR ribotypes were identified; the dominant PCR ribotype was 017 (48%), followed by 046 (14%) and 012 (14%); strains of the epidemic PCR ribotypes 027 and 078 were not observed (Fig. 1). The PCR ribotypes other than 017 were randomly spread over the time period and did not cluster. The new ribotype 293 was identified. PCR toxin gene testing confirmed all PCR ribotype 017 isolates to be negative for tcdA and positive for tcdB. All other isolates were positive for both genes with the exception of the two ribotype 009 isolates, which were negative for both genes.
MLVA was performed on isolates that could potentially form a cluster (two or more isolates of the same ribotype); a total of 18 isolates were subtyped. Isolates of PCR ribotype 012 were all distinct MLVA types, as were the three isolates of PCR ribotype 046 and the two isolates of PCR ribotype 009; the smallest number of STRDs was 16. Within the 10 PCR ribotype 017 isolates, 4 separate MLVA types were identified. Two clusters were found, each containing four isolates; the remaining two isolates were completely unrelated. In order to identify potential transmission, the wards which patients carrying ribotype 017 occupied at the time they developed presumptive C. difficile were identified (Fig. 2). Isolates from spinal surgery and neurology wards clustered in one complex, while isolates from spinal surgery, respiratory, and hematology wards clustered in the second complex. Presumptive transmission was observed between two patients (50 and 55) in the spinal surgery ward who overlapped in stay; each carried an indistinguishable MLVA type (Table 1; Fig. 2). Indistinguishable MLVA types were also found in two patients (numbers 3 and 67) on the respiratory ward from diverse time points.
Fig 2.
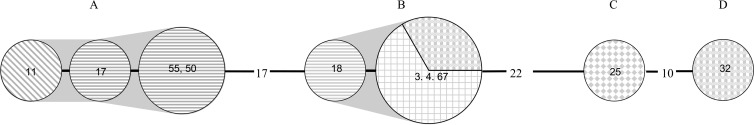
Minimum spanning tree (Manhattan) produced using 15 loci on 10 PCR ribotype 017 isolates from Xiangya Hospital, China. Each node represents a single MLVA profile and the number of isolates with that profile is indicated by the node size. Nodes are coded by ward as follows: neurology (diagonal lines), spinal surgery (horizontal lines), respiratory (grid lines), hematology (shaded and checkerboard lines), and cardiology (checkerboard). Numbers between the circles are summed tandem repeat differences (STRDs); the unnumbered lines represent 1 STRD and numbers within the circles are the patient numbers. Gray shading identifies isolates belonging to a cluster with STRDs of <4.
Table 1.
Clinical data collected from 21 patients with C. difficile infection from Xiangya Hospital, China
| Patient no. | Age/sex | Ward | Antibiotics administered (length of treatment [days]) | Date admitted (length of stay [days]) | Diarrhea onset date (mo/day/yr) | Sample date | PCR ribotype |
|---|---|---|---|---|---|---|---|
| 1 | 40/M | A&E/CM | Cefepime (2), cefoperazone-tazobactam (11), cefpiramide (5) | 4/3/2009 (27) | 4/5/2009 | 4/7/2009 | 293 |
| 3 | 54/M | R | Moxifloxacin (3), cefuroxime (8) | 3/20/2009 (20) | 4/2/2009 | 4/3/2009 | 017 |
| 4 | 52/M | H | Piperacillin-tazobactam (8), gatifloxacin (28), vancomycin (6), meropenem (6), imipenem-cilastatin (6) | 3/10/2009 (54) | 8/4/2009 | 4/13/2009 | 017 |
| 6 | 54/M | VS/ICUb | Piperacillin-sulbactam (16) | 5/13/2009 (22) | 6/1/2009 | 6/2/2009 | 046 |
| 9 | 72/M | O/ICU | Ceftizoxime (6), cefoperazone-sulbactam (1), levofloxacin (3), azithromycin (5), meropenem (11), cefepime (2), vancomycin (3) | 2/16/2009 (68) | 2/27/2009 | 4/5/2009 | 009 |
| 11 | 79/F | N | Cefoperazone-tazobactam (6), pazufloxacin (3), azithromycin (6), teicoplanin (9), vancomycin (8) | 4/25/2009 (17) | 5/1/2009 | 5/2/2009 | 017 |
| 13 | 55/M | S | Pazufloxacin (5), cefamandole (3), gentamicin (3) | 3/26/2009 (19) | 4/2/2009 | 4/4/2009 | 012 |
| 17 | 57/M | S | Pazufloxacin (13), cefamandole (13), gentamicin (1) | 4/10/2009 (24) | 5/4/09 | 5/6/2009 | 017 |
| 18 | 75/F | S | Ceftriaxone (8), clindamycin (14), cefonicid (4), cefodizime (6), meropenem (6) | 5/20/2009 (23) | 6/2/09 | 6/2/2009 | 017 |
| 25 | 56/F | C | Cefotaxime (6), ciprofloxacin (5), clindamycin (6) | 4/30/2009 (15) | 5/10/2009 | 5/12/2009 | 017 |
| 32 | 56/M | H | Levofloxacin (3), teicoplanin (3), meropenem (4) | 5/15/2009 (7) | 5/18/2009 | 19/05/2009 | 017 |
| 35 | 57/M | S/ICUa | Pazufloxacin (8), cefamandole (3) | 5/14/2009 (46) | 5/22/2009 | 5/24/2009 | 012 |
| 36 | 49/M | GS | Cefamandole (7), levofloxacin (8) | 5/20/2009 (16) | 5/20/2009 | 5/21/2009 | 046 |
| 43 | 53/F | GI | Cefamandole (4), levofloxacin (6) | 12/25/2009 (14) | 1/1/2010 | 1/2/2010 | 014 |
| 44 | 73/F | N | Cefmenoxime (6), moxifloxacin (10), piperacillin-tazobactam (3) | 12/26/2009 (20) | 1/7/2010 | 1/8/2010 | 020 |
| 49 | 50/F | O | Levofloxacin (3), ceftriaxone (3), cefoperazone-tazobactam (7) | 8/5/2009 (15) | 8/13/2009 | 8/13/2009 | 046 |
| 50 | 53/F | S | Cefamandole (6), pazufloxacin (6), gentamicin (6) | 8/8/2009 (37) | 9/16/2009 | 9/19/2009 | 017 |
| 55 | 54/M | CM/S | Cefamandole (1), gentamicin (20), cefoperazone-sulbactam (10), ceftriaxone (8), pazufloxacin (20), vancomycin (10) | 7/25/2009 (40) | 10/16/2009 | 10/17/2009 | 017 |
| 59 | 45/F | O | Ceftriaxone (6), azithromycin (3) | 12/15/2009 (13) | 12/23/2009 | 12/25/2009 | 009 |
| 60 | 81/M | GI | Pazufloxacin (8), cefmenoxime (15) | 3/10/2009 (61) | 4/5/2009 | 5/7/2009 | 012 |
| 67 | 54/F | R | Levofloxacin (5), ceftriaxone (3) | 2/1/2010 (18) | 2/8/2010 | 2/8/2010 | 017 |
Hospital wards: A&E, emergency department; CM, traditional Chinese medicine; R, respiratory; H, hematology; VS, vascular surgery; ICU, intensive care unit; O, oncology; N, neurology; S, spinal surgery; C, cardiology; GS, general surgery; GI, gastrointestinal.
These patients required ICU admission for C. difficile infection.
Clinical data.
Clinical data were collected from the 21 patients from whom C. difficile was cultured during this study (Table 1). All patients, with a single exception (no. 6), received either a quinolone or a third-generation cephalosporin antibiotic (sometimes both) prior to the onset of diarrhea; quinolones were administered to 81% and third-generation cephalosporins to 52% of patients, respectively. Two patients were admitted to the intensive care unit due to diarrhea; however, there were no cases of toxic megacolon or pseudomembranous colitis and no deaths. This study was retrospective, so none of the patients were treated for C. difficile. Within mainland China, C. difficile infection is not currently diagnosed because it is not included in diagnostic testing. The mean age of patients was 58 years and only 3/21 were aged >65 years. The mean time to onset of diarrhea in patients following admission was 14.0 days, with a mode of 12 days. Only a single patient was admitted with diarrhea.
DISCUSSION
Although the People's Republic of China is the most populous country in the world and has a large and rapidly developing secondary and tertiary care hospital system, reports and studies of C. difficile there are almost entirely absent from the literature. The only English-language publication of a prospective study of C. difficile in a hospital was a report of a study from a single hospital site in Shanghai performed over a 1-year period in 2007 to 2008 (8). The hospital had a lot of tertiary referrals, so follow-up was difficult and no evidence of cross-infection was identified (8). A recent review of the Chinese-language publications on C. difficile identified only local case reports and case series mainly from immunocompromised patients, with no epidemiological studies and no clinical studies (6). The authors suggested that C. difficile may be rarer than in the United States and Europe, but in the absence of studies this conclusion was tentative at best.
In mainland China there is a low submission rate of samples from patients for examination in microbiology laboratories. In our experience it is not a normal practice to diagnose C. difficile infection or therefore treat patients, as it is not regarded as a problem. The aim of our study was to seek cases of C. difficile infection in a large hospital in mainland China to test the assertion that C. difficile is rare. We identified C. difficile infection by culture to enable molecular typing of isolates to see if cross-infection was occurring. We identified 70 possible cases of C. difficile infection, of which 21 patients had C. difficile cultured. Of the cultured isolates, only two lacked tcdA and tcdB. A positive sample rate of 30% was observed, which is higher than the rates found in the only other Chinese studies reported, from mainland China (12.6%) and Hong Kong (5.1%) (7, 13). However, our samples were submitted for a diagnosis of diarrhea, and because submission rates of microbiology samples in Chinese hospitals are known to be low, in consequence there may have been unrecognized collection/diagnosis biases. The mean age of the patients was low, at 58 years, with only 19% >65 years old, which contrasts markedly with the findings of a recent European study in which 63% were >65 years old (1). Interestingly, a study using a commercial PCR test on 55 feces samples from patients in Wuhan found 14 samples to be positive and the mean age of the patients to be 60 years, supporting our findings (21). The population structure of China is young, with only 100 million of the 1,307.6 million population in 2005 aged >65 years (Centre of Policy Studies [COPS] report of future population trends in China [see www.monash.edu.au/policy/ftp/workpapr/g-191.pdf]). However, this will change rapidly, as population models suggest the >65-year-old group will grow to 200 million in 2026, with a significant change occurring by 2020 (COPS report of future population trends in China). The elderly population will continue to grow, so that by 2029 it will be 233 million, exceeding the child population (232 million) in 2029 for the first time in the history of China (COPS report of future population trends in China).
The quantity and range of antibiotics prescribed at the Xiangya Central South University Hospital were much greater (all but one patient received either a quinolone or third-generation cephalosporin, with 14/21 receiving both) (Table 1) than those prescribed in the United Kingdom, according to our experience, particularly since the introduction of antibiotic stewardship to control C. difficile (United Kingdom Department of Health report on C. difficile infection [see www.hpa.org.uk/webc/HPAwebFile/HPAwebC/1232006607827]). China has the right setting for C. difficile infection, with heavy antibiotic prescribing, with the production of antibiotics being recently quoted as 210,000 tons per annum, approximately half being used in human medicine (22). The per capita use in China based on this production figure is approximately 10 times that of England, according to data from 2009 (23). The frequency of use of nonprescribed antibiotics in the community in China has been reported as 36% of individuals per annum, compared to 3% in the United Kingdom (24). We believe that the heavy use we observed of quinolones and cephalosporins, which are highly selective for C. difficile, was a major driver for the cases of presumptive C. difficile we observed.
The predominant C. difficile ribotype found at Xiangya Hospital South Central University was 017 (48%), followed by 012 (14%) and 046 (14%). Interestingly, this was the same order and approximate frequency distribution reported from the Shanghai study, which was performed 750 miles away (Fig. 1) (7). Clusters of these three ribotypes were observed, so we used MLVA subtyping for the first time on Chinese isolates to test whether cross-infection had occurred.
The application of MLVA subtyping to the isolates from Changsha identified a probable outbreak of PCR ribotype 017; two clusters of related isolates were identified, indicating presumed transmission of strains between patients. This was highlighted by two patients on the spinal surgery ward who were inpatients during the same period and were both infected by C. difficile of the same MLVA type. Identical MLVA types were identified in patients admitted to the respiratory ward in April 2009 and February 2010. This suggests a possible persistence of C. difficile spores in the environment, which continue to be a source for new infections, and highlights the requirement for more stringent infection control measures. Alternatively, this particular MLVA may be common in patients attending the hospital.
Our finding that ribotype 017 was dominant is supported by limited data from the only other study in mainland China (Fig. 1). The only survey of ribotypes from Hong Kong was from a single hospital and used a limited number of standard ribotypes for comparison (but did include 017, 046, and 012). Unusually, 70% were of an unknown pattern, with a further 11.6% being nontypeable (16). Only 0.6% of isolates were 017 and 2.3% were 012, with no 046 identified, which is markedly different from our findings in mainland China. However, 10.1% of isolates were 002, representing a local endemic strain which was presumed to be causing cross-infection (16). The distribution of ribotypes in European countries varies quite markedly between countries and is very different from that of mainland China (1). When ribotypes dominant in mainland China were compared with the European distribution of ribotypes, ribotype 012 made up 4.0% of isolates, 017 made up 4.0%, and 046 made up 2.0% (1). Interestingly, 012 and 017 were mainly seen in Southeast Europe.
Ribotype 017 C. difficile strains have long been recognized to cause pseudomembranous colitis (PMC), although they invariably are toxin A− B+ (25). A large-scale study of hospital-associated C. difficile in Korea identified toxin A− B+ as the most common strain with a strong statistical association with PMC (26). It has been suggested that altered virulence properties of the typically toxin A-negative 017 ribotype are responsible for its dominance and ability to cause outbreaks of clinical disease (27). This may not be the case, however, because previous studies found that the toxin A-negative 017 ribotype does not contain binary toxin genes and that tcdC is intact and functional, unlike PCR ribotype 027 (28). A high proportion of PCR ribotype 017 isolates have been shown to harbor ermB, which confers resistance to macrolides and lincosamides (28, 29). In view of the heavy use of antibiotics in Southeast Asia, selection due to resistance could be the explanation for its dominance. The virulent PCR ribotype 027 that has caused multiple outbreaks throughout Europe and North America was not observed in our study. To date, PCR ribotype 027 has been reported from Hong Kong, Japan, and Korea but not from China (13, 30, 31). Similarly, another virulent strain, PCR ribotype 078, which has caused problems in the Netherlands, was not seen.
This study has provided evidence of probable cross-transmission of C. difficile within a major Chinese teaching hospital. We present evidence of both cross-infection caused by ribotype 017 and endemicity of this strain in a number of wards. Risk factors in China, such as widespread overprescription of antibiotics and the growing elderly hospitalized population, means there is potential for C. difficile to become a serious problem. Our data, together with limited data from a limited previous study, suggest that China has a characteristic pattern of ribotypes in hospitals, possibly across most of the country, which suggests a well-established problem. Intensification of medical treatment in large modern hospitals is creating a vulnerable population susceptible to C. difficile. Currently in China, patients with nosocomially acquired diarrhea are very rarely, if ever, investigated for C. difficile infection. Routine laboratory diagnosis would make the development of infection control strategies possible, including early identification of symptomatic cases, subsequent isolation of patients, and directed environmental cleaning. An antibiotic stewardship scheme targeted at broad-spectrum antibiotics, particularly fluoroquinolones and cephalosporins, would be useful in reducing superfluous usage and controlling C. difficile infections. Future recognition of C. difficile infection as an important nosocomial infection in China and further epidemiological studies are essential to avoid increases in morbidity and mortality associated with C. difficile. The annual cost of C. difficile infection in the European Union (population 457 million) has been estimated to be 4,000 million US$ per annum (2); such a heavy financial cost would have a significant adverse impact on the Chinese health economy.
ACKNOWLEDGMENTS
P.M.H., W.E.L., R.C., T.K.W.L., and K.H. conceived and designed the experiments; C.M., E.S., and S.M. performed the experimental work; Z.J.J., Q.G., and V.C. collected and analyzed the clinical data; C.M., S.M., K.H., and P.M.H. analyzed the microbiological data; P.M.H., C.M., and L.X. performed the literature search; P.M.H., C.M., and L.X. wrote the paper; and W.E.L., T.K.W.L., R.C., V.C., S.M., and K.H. performed the critical revision of the paper.
The authors declare that they have no conflicts of interest.
This work was supported by grants from the Hunan Provincial Health Bureau (project B2011-006), the Heart of England NHS Foundation Trust (SD006), and RFCID Hong Kong.
Footnotes
Published ahead of print 31 July 2013
REFERENCES
- 1.Bauer MP, Notermans DW, van Benthem BH, Brazier JS, Wilcox MH, Rupnik M, Monnet DL, van Dissel JT, Kuijper EJ; ECDIS Study Group 2011. Clostridium difficile infection in Europe: a hospital-based survey. Lancet 377:63–73 [DOI] [PubMed] [Google Scholar]
- 2.Kuijper EJ, Coignard B, Tull P; ESCMID Study Group for Clostridium difficile; EU Member States; European Centre for Disease Prevention and Control 2006. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin. Microbiol. Infect. 12(Suppl 6):2–18 [DOI] [PubMed] [Google Scholar]
- 3.Balassiano IT, Yates EA, Domingues RM, Ferreira EO. 2012. Clostridium difficile: a problem of concern in developed countries and still a mystery in Latin America. J. Med. Microbiol. 61:169–179 [DOI] [PubMed] [Google Scholar]
- 4.Iwashima Y, Nakamura A, Kato H, Kato H, Wakimoto Y, Wakiyama N, Kaji C, Ueda R. 2010. A retrospective study of the epidemiology of Clostridium difficile infection at a University Hospital in Japan: genotypic features of the isolates and clinical characteristics of the patients. J. Infect. Chemother. 16:329–333 [DOI] [PubMed] [Google Scholar]
- 5.Kim H, Jeong SH, Roh KH, Hong SG, Kim JW, Shin MG, Kim MN, Shin HB, Uh Y, Lee H, Lee K. 2010. Investigation of toxin gene diversity, molecular epidemiology, and antimicrobial resistance of Clostridium difficile isolated from 12 hospitals in South Korea. Korean J. Lab. Med. 30:491–497 [DOI] [PubMed] [Google Scholar]
- 6.Jin K, Wang S, Huang Z, Lu S. 2010. Clostridium difficile infections in China. J. Biomed. Res. 24:411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang H, Fang H, Weintraub A, Nord CE. 2009. Distinct ribotypes and rates of antimicrobial drug resistance in Clostridium difficile from Shanghai and Stockholm. Clin. Microbiol. Infect. 15:1170–1173 [DOI] [PubMed] [Google Scholar]
- 8.Huang H, Wu S, Wang M, Zhang Y, Fang H, Palmgren AC, Weintraub A, Nord CE. 2008. Molecular and clinical characteristics of Clostridium difficile infection in a University Hospital in Shanghai, China. Clin. Infect. Dis. 47:1606–1608 [DOI] [PubMed] [Google Scholar]
- 9.Stubbs SL, Brazier JS, O'Neill GL, Duerden BI. 1999. PCR targeted to the 16S-23S rRNA gene intergenic spacer region of Clostridium difficile and construction of a library consisting of 116 different PCR ribotypes. J. Clin. Microbiol. 37:461–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan Q, Zhang J, Chen C, Zhou H, DU P, Cui Z, Cen R, Liu L, Li W, Cao B, Lu J, Cheng Y. 2013. Multilocus sequence typing (MLST) analysis of 104 Clostridium difficile strains isolated from China. Epidemiol. Infect. 141:195–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuen KY, Woo PC, Liang RH, Chiu EK, Chen FF, Wong SS, Lau YL, Ha SY, Peiris JS, Siau H, Chan TK. 1998. Clinical significance of alimentary tract microbes in bone marrow transplant recipients. Diagn. Microbiol. Infect. Dis. 30:75–81 [DOI] [PubMed] [Google Scholar]
- 12.Wong SS, Ho PL, Woo PC, Yuen KY. 1999. Bacteremia caused by staphylococci with inducible vancomycin heteroresistance. Clin. Infect. Dis. 29:760–767 [DOI] [PubMed] [Google Scholar]
- 13.Cheng VC, Yam WC, Chan JF, To KK, Ho PL, Yuen KY. 2009. Clostridium difficile ribotype 027 arrives in Hong Kong. Int. J. Antimicrob. Agents 34:492–493 [DOI] [PubMed] [Google Scholar]
- 14.Chang KC, Leung CC, Yew WW, Lam FM, Ho PL, Chau CH, Cheng VC, Yuen KY. 2009. Analyses of fluoroquinolones and Clostridium difficile-associated diarrhoea in tuberculosis patients. Int. J. Tuberc. Lung Dis. 13:341–346 [PubMed] [Google Scholar]
- 15.Lee JS, Auyeung TW. 2003. A comparison of two feeding methods in the alleviation of diarrhoea in older tube-fed patients: a randomised controlled trial. Age Ageing 32:388–393 [DOI] [PubMed] [Google Scholar]
- 16.Cheng VC, Yam WC, Lam OT, Tsang JL, Tse EY, Siu GK, Chan JF, Tse H, To KK, Tai JW, Ho PL, Yuen KY. 2011. Clostridium difficile isolates with increased sporulation: emergence of PCR ribotype 002 in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 30:1371–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam T, Yuk M, Tsang N, Wong M, Chuang S. 2012. Clostridium difficile infection outbreak in a male rehabilitation ward, Hong Kong (China), 2011. Western Pac. Surveill. Response J. 3:59–60. 10.5365/wpsar.2012.3.4.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin YC, Huang YT, Tsai PJ, Lee TF, Lee NY, Liao CH, Lin SY, Ko WC, Hsueh PR. 2011. Antimicrobial susceptibilities and molecular epidemiology of clinical isolates of Clostridium difficile in Taiwan. Antimicrob. Agents Chemother. 55:1701–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemee L, Dhalluin A, Testelin S, Mattrat MA, Maillard K, Lemeland JF, Pons JL. 2004. Multiplex PCR targeting tpi (triose phosphate isomerase), tcdA (toxin A), and tcdB (toxin B) genes for toxigenic culture of Clostridium difficile. J. Clin. Microbiol. 42:5710–5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manzoor SE, Tanner HE, Marriott CL, Brazier JS, Hardy KJ, Platt S, Hawkey PM. 2011. Extended multilocus variable-number tandem-repeat analysis of Clostridium difficile correlates exactly with ribotyping and enables identification of hospital transmission. J. Clin. Microbiol. 49:3523–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galaydick J, Landon E, Weber SG, Xu Y, Sun D, Zhou J, Sun L, Sherer R. 2012. The prevalence of Clostridium difficile in Wuhan China. International Conference on Emerging and Infectious Diseases (ICEID), poster 348. [Google Scholar]
- 22.Hvistendahl M. 2012. Public health. China takes aim at rampant antibiotic resistance. Science 336:795. [DOI] [PubMed] [Google Scholar]
- 23.Ashiru-Oredope D, Sharland M, Charani E, McNulty C, Cooke J; ARHAI Antimicrobial Stewardship Group 2012. Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: Start Smart–Then Focus. J. Antimicrob. Chemother. 67(Suppl 1):i51–i63 [DOI] [PubMed] [Google Scholar]
- 24.Morgan DJ, Okeke IN, Laxminarayan R, Perencevich EN, Weisenberg S. 2011. Non-prescription antimicrobial use worldwide: a systematic review. Lancet Infect. Dis. 11:692–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cairns MD, Stabler RA, Shetty N, Wren BW. 2012. The continually evolving Clostridium difficile species. Future Microbiol. 7:945–957 [DOI] [PubMed] [Google Scholar]
- 26.Shin BM, Kuak EY, Yoo SJ, Shin WC, Yoo HM. 2008. Emerging toxin A-B+ variant strain of Clostridium difficile responsible for pseudomembranous colitis at a tertiary care hospital in Korea. Diagn. Microbiol. Infect. Dis. 60:333–337 [DOI] [PubMed] [Google Scholar]
- 27.Drudy D, Harnedy N, Fanning S, Hannan M, Kyne L. 2007. Emergence and control of fluoroquinolone-resistant, toxin A-negative, toxin B-positive Clostridium difficile. Infect. Control Hosp. Epidemiol. 28:932–940 [DOI] [PubMed] [Google Scholar]
- 28.Goorhuis A, Legaria MC, van den Berg RJ, Harmanus C, Klaassen CH, Brazier JS, Lumelsky G, Kuijper EJ. 2009. Application of multiple-locus variable-number tandem-repeat analysis to determine clonal spread of toxin A-negative Clostridium difficile in a general hospital in Buenos Aires, Argentina. Clin. Microbiol. Infect. 15:1080–1086 [DOI] [PubMed] [Google Scholar]
- 29.Pituch H, Brazier JS, Obuch-Woszczatynski P, Wultanska D, Meisel-Mikolajczyk F, Luczak M. 2006. Prevalence and association of PCR ribotypes of Clostridium difficile isolated from symptomatic patients from Warsaw with macrolide-lincosamide-streptogramin B (MLSB) type resistance. J. Med. Microbiol. 55:207–213 [DOI] [PubMed] [Google Scholar]
- 30.Kato H, Ito Y, van den Berg RJ, Kuijper EJ, Arakawa Y. 2007. First isolation of Clostridium difficile 027 in Japan. Euro Surveill. 12:E070111.3 [DOI] [PubMed] [Google Scholar]
- 31.Tae CH, Jung SA, Song HJ, Kim SE, Choi HJ, Lee M, Hwang Y, Kim H, Lee K. 2009. The first case of antibiotic-associated colitis by Clostridium difficile PCR ribotype 027 in Korea. J. Korean Med. Sci. 24:520–524 [DOI] [PMC free article] [PubMed] [Google Scholar]