Abstract
This case of imported refractory schistosomiasis has highlighted the usefulness of cell-free parasite DNA as a diagnostic marker to assess active schistosome infection. In contrast to the rapid disappearance of ova in urine, parasite DNA remained persistent in several other specimen types even after the fourth treatment with praziquantel. This result was consistent with the presence of morphologically intact ova in bladder biopsy samples and with the corresponding symptoms.
CASE REPORT
In January 2009, a previously healthy Japanese man, 21 years of age, sought medical attention after an approximately 2-month history of hematuria, discomfort during urination, and hematospermia. He had returned from a 5-month trip (May to October 2008) around various African countries. During the trip, he bathed in Jinja (Uganda), Lake Malawi (Malawi), and the Niger River and specifically developed itchy skin after swimming in the Dogon region of Mali. Urinalysis revealed the presence of Schistosoma haematobium ova. The patient was treated with praziquantel (PZQ; 40 mg/kg of body weight twice a day [b.i.d.] orally [p.o.] for two consecutive days). After the first PZQ treatment, the patient had a fever of 38°C, a relatively high white blood cell (WBC) count (10,000/mm3), and eosinophilia (30%).
For detection of ova, sediment from 10 ml of urine and 250 μl of semen was examined by microscope. Parasite ova were detected in urine on day 1 of the first PZQ treatment course, and detection results were negative after that. On the other hand, ova in semen were detected until 101 days after the first PZQ treatment.
Cell-free schistosome DNA in bodily fluids was detected by conventional PCR and/or sequence capture-PCR. The genetic examination for parasite DNA in the patient's specimen was approved by the Bioethics Committee of Dokkyo Medical University (approval no. 1969), and the patient's consent was obtained. Prior to DNA extraction, 3.5 ml of urine was concentrated to 140 μl using an Amicon Ultra-15 centrifugal filter system with an Ultracel-100K membrane (Millipore Ireland Ltd., Cork, Ireland). DNA was extracted from concentrated urine, serum, and supernatant of semen (140 μl each) using a QIAamp viral RNA minikit (Qiagen Sciences), and DNA from the sediment of 500 μl of semen was extracted using a NucleoSpin tissue system (Macherey-Nagel GmbH & Co. KG, Düren, Germany) according to the manufacturers' instructions. Approximately 2 ml of the saliva sample was collected using an Oragene·DNA self-collection kit (DNA Genotek Inc., Canada); DNA was extracted according to the manufacturer's instructions. The primer pair CF (5′-GATCGTAAATTTGGA/TACTGC) and CR (5′-CCAACCATAAACATATGATG) was designed to amplify a part of the schistosome mitochondrial cytochrome c oxidase subunit 1 (CO1) gene, which is common to at least 4 human schistosome species (S. mansoni [253 bp] and S. haematobium, S. japonicum, and S. mekongi [254 bp]). The primer pair ShF (5′-AGTCGTGTCGATTTTAAGAC) and CR was designed to amplify S. haematobium CO1 (365 bp), and the primer pair SmF (5′-TCCTTTATCAATTTGAGAGG) and CR was designed to amplify S. mansoni CO1 (479 bp) (1). Sequence capture-PCR is the combination of purifying and concentrating methods for target nucleic acid in clinical samples and PCR. As described in the literature (2–5), crude DNA samples were purified and concentrated using both 5′-biotinylated CF and CR and magnetic beads with streptavidin, and then PCR was performed. We were able to amplify S. haematobium DNA in the patient's bodily fluids; however, S. mansoni DNA was not amplified. Parasite DNA in bodily fluids was detected even 1 month after the fourth PZQ treatment (303 days after the first treatment).
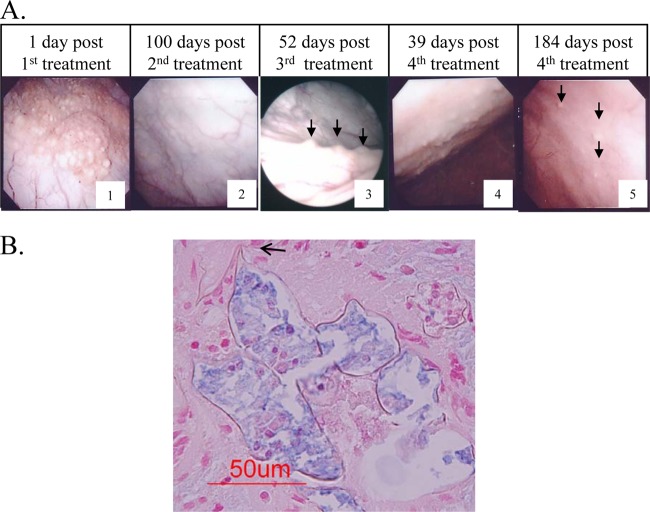
Using cystoscopy, we identified massive polypoid lesions in the urinary bladder wall, which gradually decreased in number with repeated PZQ treatments (Fig. 1A). However, histopathological findings from examinations of bladder biopsy sites of polypoid lesions with petechiae revealed parasite ova with intact cells even 2 months after the third treatment (255 days after the first treatment) (Fig. 1B). Finally, 184 days after the fourth PZQ treatment (457 days after the first treatment), most of the lesions diminished to trace levels and the subjective symptoms were resolved; thereafter, the patient was routinely examined in follow-up checks. The therapeutic process and examination results are summarized in Table 1.
Fig 1.
Cystoscopic images of the urinary bladder wall (A) and microscopic images of the biopsy sites of the polypoid lesion (B) after praziquantel treatments. (A) Massive polypoid lesions with petechiae (arrows) gradually decreased in number with PZQ treatments. (B) One of the representative pathological specimens of a biopsy site at 2 months after the third PZQ treatment. Note the Schistosoma haematobium ovum with a typical posterior spine (arrow) (Alucian blue stain, pH 2.5).
Table 1.
The therapeutic process and examination resultsa
| Days post-PZQ treatmentb |
Detection of ova |
Detection of occult blood (urine) | Detection of DNA (PCR/sc-PCR) |
Corresponding cystoscopy panel in Fig. 1A | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | Urine | Semen | Bladder biopsy specimen | Serum | Urine | Semen | Saliva | ||
| 1 | (+) | ND | ND | (+) | ND | (+)/ND | ND | (+)/ND | 1 | |||
| 8 | (−) | (+) | ND | (−) | ND | ND | ND | ND | ||||
| 60 | (−) | ND | ND | (−) | (−)/(+) | (−)/(+) | ND | (−)/(+) | ||||
| 101 | (−) | (+)c | ND | (−) | (−)/(+) | (−)/(+) | (−)/(+) | (−)/(+) | ||||
| 102 | 1 | |||||||||||
| 158 | 56 | (−) | (−) | ND | (−) | (−)/(+) | (−)/(+) | (+)/(+) | (−)/(−) | |||
| 203 | 100 | (−) | (−) | ND | (−) | (−)/(+) | (−)/(+) | (−)/(+) | ND | 2 | ||
| 203 | 100 | 1 | ||||||||||
| 233 | 131 | 30 | (−) | (−) | ND | (−) | (−)/(+) | (−)/(−) | (−)/(−) | (−)/(−) | ||
| 255 | 153 | 52 | ND | ND | (+) | ND | ND | ND | ND | ND | 3 | |
| 273 | 171 | 70 | 1 | |||||||||
| 303 | 201 | 100 | 30 | (−) | (−) | ND | (−) | (−)/(+) | (+)/(+) | (−)/(+) | (−)/(−) | |
| 312 | 210 | 109 | 39 | (−) | ND | ND | ND | ND | ND | ND | ND | 4 |
| 457 | 355 | 254 | 184 | (−) | ND | ND | ND | ND | ND | ND | ND | 5 |
(−), not detected; (+); detected; PZQ, praziquantel; sc-PCR, sequence capture-PCR; ND, not done.
PZQ treatment 1 consisted of 40 mg/kg of body weight/day for 2 days; PZQ treatment 2 consisted of 40 mg/kg/day for 2 days; PZQ treatment 3 consisted of 40 mg/kg/day for 2 days; PZQ treatment 4 consisted of 60 mg/kg/day for 2 days.
Results for ova in semen at day 101 were first negative, however, later, when reexamined retrospectively, the results were positive.
The current diagnostic methods of schistosomiases rely on an ovum-related phenomenon: detection of ovum- and parasite-specific antibodies and ovum-induced pathology. Although detection of ova is still the gold standard for diagnosing schistosomiasis, it is difficult to detect long-standing/chronic infection and low-level infection in patients after repeated treatments; moreover, detection of ova is ineffective in the early stage of infection (prepatent period). The cell-free circulating schistosome DNA consists of the fragments of parasite-derived DNA that exist in the host's bodily fluids. It was detected in plasma/serum and urine of schistosomiasis patients (6, 7, 8, 9). In our study, the primer pairs (CF and CR, ShF and CR, and SmF and CR) had been originally designed to amplify the mitochondrial CO1 gene, which enabled us to differentiate four species of human schistosomes (1). It was confirmed that the patient was infected with S. haematobium and was not infected with S. mansoni.
Because schistosomiasis (japonica) has been eliminated from Japan, it is unlikely that the patient will get reinfected now that he has returned home. Parasite ova in urine, a common testing material for schistosomiasis hematobia, were detected for only 1 week after the first PZQ treatment. On the other hand, ovum was detected in semen even 101 days after the first PZQ treatment and indicates the significance of semen as a testing material for schistosomiasis hematobia. We had expected the cell-free parasite DNA to disappear soon after PZQ treatment along with the ova. However, it was detected in the bodily fluids even after 1 month after the fourth PZQ treatment (303 days after the first treatment). The idea of the reliability of persistent parasite DNA was supported by the presence of morphologically intact parasite ova in the bladder biopsy sample, pathology, symptoms, and other signs; the patient reported subjective symptoms of discomfort with urination even 2 months after the third PZQ treatment (255 days after the first treatment) and sometimes noticed hematuria during therapy.
It has been reported that schistosome DNA was detected in serum for as long as 10 to 19.3 weeks after a single PZQ treatment (8, 10). One possible explanation for the lingering worm DNA detection may have been unsatisfactory treatment. Immature ova/worms are refractory to PZQ treatment (11–14). Multiple treatments with PZQ are needed with intervals between the treatments to allow maturation of immature worms/ova (2 to 4 weeks) (13–15). In the present case, the persistent parasite DNA was detected even after repeated PZQ treatments and it indicates that this was a refractory case.
To date, there have been no reports on PZQ resistance in areas of schistosomiasis hematobia endemicity (15–17). On the other hand, several cases refractory to repeated PZQ administration have been reported in countries where the disease is nonendemic, i.e., imported cases (18–20). It is speculated that schistosomiasis patients, who are rare in countries where the disease is nonendemic, tend to be followed up by detailed examinations, such as cystoscopy and bladder biopsy sampling, in addition to detection of ova, thus enabling identification of refractory cases. By the conventional gold standard, the examination of ova in urine, the present case would have been considered cured soon after the first PZQ treatment. Chronic infection with S. haematobium is carcinogenic to humans (21, 22). In the present study, we have demonstrated the limitations of detection of ova as a method of therapeutic evaluation. Cell-free schistosome DNA may be a potential tool to monitor active worms/ova to reduce the risk of cancer triggered by prolonged S. haematobium infection because of inaccurate diagnosis. Therefore, further studies of novel diagnostic techniques such as cell-free schistosome DNA detection to determine/assess active infection are required.
ACKNOWLEDGMENTS
This work was supported in part by a Health Labor Sciences Research grant from the Ministry of Health, Labor and Welfare (H20-Shinkosaiko-Ippan-016) and by Japan Society for the Promotion of Science (JSPS) KAKENHI (no. 24590510).
Sincere appreciation is expressed to Ayako Yoshida of the Parasitic Disease Unit, Faculty of Medicine, University of Miyazaki, for her valuable advice and support. We thank Noriko Suzuki and Ken-ichi Manaka of the Research Support Center, Dokkyo Medical University, for preparing histopathological specimens and for technical support, respectively. We also thank our laboratory coworkers, Mayumi Oshita and Mayu Tanaka, at the Laboratory of Tropical Medicine and Parasitology, Dokkyo Medical University, for their assistance. We must also thank William Hassett, Division of Language Education, Dokkyo Medical University, for polishing the English manuscript.
We declare no competing financial interests.
Footnotes
Published ahead of print 24 July 2013
REFERENCES
- 1.Kato-Hayashi N, Kirinoki M, Iwamura Y, Kanazawa T, Kitikoon V, Matsuda H, Chigusa Y. 2010. Identification and differentiation of human schistosomes by polymerase chain reaction. Exp. Parasitol. 124:325–329 [DOI] [PubMed] [Google Scholar]
- 2.Mangiapan G, Vokurka M, Schoules L, Cadranel J, Lecossier D, van Embden J, Hance AJ. 1996. Sequence capture-PCR improves detection of mycobacterial DNA in clinical specimens. J. Clin. Microbiol. 34:1209–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsh I, Whittington R, Millar D. 2000. Quality control and optimized procedure of hybridization capture-PCR for the identification of Mycobacterium avium subsp. paratuberculosis in faeces. Mol. Cell. Probes 14:219–232 [DOI] [PubMed] [Google Scholar]
- 4.Taylor MJ, Hughes MS, Skuce RA, Neill SD. 2001. Detection of Mycobacterium bovis in bovine clinical specimens using real-time fluorescence and fluorescence resonance energy transfer probe rapid-cycle PCR. J. Clin. Microbiol. 39:1272–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maibach RC, Dutly F, Altwegg M. 2002. Detection of Tropheryma whipplei DNA in feces by PCR using target capture method. J. Clin. Microbiol. 40:2466–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pontes LA, Dias-Neto E, Rabello A. 2002. Detection by polymerase chain reaction of Schistosoma mansoni DNA in human serum and feces. Am. J. Trop. Med. Hyg. 66:157–162 [DOI] [PubMed] [Google Scholar]
- 7.Sandoval N, Siles-Lucas M, Pérez-Arellano JL, Carranza C, Puente S, López-Abán J, Muro A. 2006. A new PCR-based approach for the specific amplification of DNA from different Schistosoma species applicable to human urine samples. Parasitology 133:581–587 [DOI] [PubMed] [Google Scholar]
- 8.Wichmann D, Panning M, Quack T, Kramme S, Burchard GD, Grevelding C, Drosten C. 2009. Diagnosing schistosomiasis by detection of cell-free parasite DNA in human plasma. PLoS Negl. Trop. Dis. 3:e422. 10.1371/journal.pntd.0000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wichmann D, Poppert S, Von Thien H, Clerix J, Dieckmann S, Jensenius M, Parola P, Richter J, Schunk M, Stich A, Zanger P, Burchard GD, Tannich E. 2013. Prospective European-wide multicentre study on a blood based real-time PCR for the diagnosis of acute schistosomiasis. BMC Infect. Dis. 13:55. 10.1186/1471-2334-13-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia CM, Rong R, Lu ZX, Shi CJ, Xu J, Zang HQ, Gong W, Luo W. 2009. Schistosoma japonicum: a PCR assay for the early detection and evaluation of treatment in a rabbit model. Exp. Parasitol. 121:175–179 [DOI] [PubMed] [Google Scholar]
- 11.Xiao SH, Catto BA, Webster LT., Jr 1985. Effects of praziquantel on different developmental stages of Schistosoma mansoni in vitro and in vivo. J. Infect. Dis. 151:1130–1137 [DOI] [PubMed] [Google Scholar]
- 12.Sabah AA, Fletcher C, Webbe G, Doenhoff MJ. 1986. Schissosoma mansoni: chemotherapy of infections of different ages. Exp. Parasitol. 61:294–303 [DOI] [PubMed] [Google Scholar]
- 13.Hirose Y, Kirinoki M, Matsuda H. 2003. Efficacy of administration of praziquantel on 2 days 2 weeks apart against Schistosoma japonicum eggs in mice. Parasitol. Int. 52:141–146 [DOI] [PubMed] [Google Scholar]
- 14.Pica-Mattoccia L, Cioli D. 2004. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int. J. Parasitol. 34:527–533 [DOI] [PubMed] [Google Scholar]
- 15.N′Goran EK, Gnaka HN, Tanner M, Utzinger J. 2003. Efficacy and side-effects of two praziquantel treatments against Schistosoma haematobium infection, among schoolchildren from Côte d'Ivotre. Ann. Trop. Med. Parasitol. 97:37–51 [DOI] [PubMed] [Google Scholar]
- 16.Tchuenté LA, Shaw DJ, Polla L, Cioli D, Vercruysse J. 2004. Efficacy of praziquantel against Schistosoma haematobium infection in children. Am. J. Trop. Med. Hyg. 71:778–782 [PubMed] [Google Scholar]
- 17.Guidi A, Andolina C, Makame Ame S, Albonico M, Cioli D, Juma Haji H. 2010. Praziquantel efficacy and long-term appraisal of schistosomiasis control in Pemba Island. Trop. Med. Int. Health 15:614–618 [DOI] [PubMed] [Google Scholar]
- 18.Silva IM, Thiengo R, Conceição MJ, Rey L, Lenzi HL, Pereira Filho E, Ribeiro CR. 2005. Therapeutic failure of praziquantel in the treatment of Schistosoma haematobium infection in Brazilians returning from Africa. Mem. Inst. Oswaldo Cruz 100:445–449 [DOI] [PubMed] [Google Scholar]
- 19.Silva IM, Pereira Filho E, Thiengo R, Ribeiro PC, Conceição MJ, Panasco M, Lenzi HL. 2008. Schistosomiasis haematobia: histopathological course determined by cyctoscopy in a patient in whom praziquantel treatment failed. Rev. Inst. Med. Trop. Sao Paulo 50:343–346 [DOI] [PubMed] [Google Scholar]
- 20.Alonso D, Muñoz J, Gascón J, Valls ME, Corachan M. 2006. Failure of standard treatment with praziquantel in two returned travelers with Schistosoma haematobium infection. Am. J. Trop. Med. Hyg. 74:342–344 [PubMed] [Google Scholar]
- 21.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans 1994. Infection with schistosomes (Schistosoma haematobium, Schistosoma mansoni and Schistosoma japonicum). IARC Monogr. Eval. Carcinog. Risks Hum. 61:45–119 [PMC free article] [PubMed] [Google Scholar]
- 22.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans 2012. Biological agents: a review of human carcinogens. IARC Monogr. Eval. Carcinog. Risks Hum. 100B:1–441 [PMC free article] [PubMed] [Google Scholar]