Abstract
Nonsporulating molds (NSMs), especially basidiomycetes, have predominantly been reported as human pathogens responsible for allergic and invasive disease. Their conventional identification is problematic, as many isolates remain sterile in culture. Thus, inconclusive culture reports might adversely affect treatment decisions. The clinical significance of NSMs in pulmonary mycoses is poorly understood. We sequenced the internal transcribed spacer (ITS) region and D1/D2 domain of the larger subunit (LSU) of 52 NSMs isolated from respiratory specimens. The basidiomycetes were the predominant NSMs, of which Schizophyllum commune was the most common agent in allergic bronchopulmonary mycosis (ABPM), followed by Ceriporia lacerata in invasive fungal disease. Porostereum spadiceum, Phanaerochaete stereoides, Neosartorya fischeri, and Marasmiellus palmivorus were the other molds observed. Application of ITS and LSU region sequencing identified 92% of the isolates. The antifungal susceptibility data revealed that all basidiomycetes tested were susceptible to amphotericin B and resistant to caspofungin, fluconazole, and flucytosine. Except for 3 isolates of S. commune and a solitary isolate of M. palmivorus, all basidiomycetes had low MICs for itraconazole, posaconazole, and voriconazole. Basidiomycetes were isolated from patients with ABPM, invasive pulmonary mycosis/pneumonia, or fungal balls. In addition, the majority of the basidiomycetes were isolated from patients with chronic respiratory disorders who were sensitized to one of the basidiomycetous fungi and demonstrated precipitating antibodies against the incriminating fungi, indicating an indolent tissue reaction. Thus, isolation of basidiomycetes from the lower respiratory tract could be significant, and it is important to monitor these patients in order to prevent subsequent lung damage.
INTRODUCTION
Nonsporulating molds (NSMs) have recently been reported as emerging pathogens with a potential for invasive disease in susceptible patients. Identification of such fungi in specific clinical settings has a favorable impact on patient management and therapeutic outcomes (1, 2). The filamentous basidiomycetes include a substantial number of NSMs that may be resistant to antifungals (3–6). Nonsporulating filamentous basidiomycetes such as Hormographiella aspergillata and Volvariella volvacea are resistant to amphotericin B (AMB), caspofungin (CAS), itraconazole (ITC), voriconazole (VRC), and posaconazole (POS) and have been associated with high case fatality rates in the past (3–5, 7). Although filamentous basidiomycetes are being increasingly recognized in clinical specimens, their definitive identification using conventional methods can be problematic (8, 9). This is attributed to the fact that many basidiomycete isolates remain sterile and do not produce reproductive structures or conidia in culture (8). The spectrum of disease caused by basidiomycetous NSMs ranges from asymptomatic saprobic colonization, fungal balls, and allergic respiratory mycoses such as allergic fungal sinusitis and allergic bronchopulmonary mycosis (ABPM) to fatal invasive mycoses such as fungal pneumonias, brain abscesses, and fungemia (3–6, 8, 10–20).
In the past 2 decades, filamentous basidiomycetes have been reported as invasive pathogens in immunosuppressed patients, such as patients with hematological malignancies, patients with neutropenia, and solid-organ transplant recipients (3–6, 17–20). However, the true clinical significance of white cottony NSMs in pulmonary mycoses is poorly understood (8). The uncertainty regarding their clinical significance, coupled with lesser recognition among laboratories, has resulted in NSMs frequently being disregarded as environmental contaminants in the past. Furthermore, the reduced susceptibility to azoles and resistance to amphotericin B reported for some NSMs emphasize the need for correct species identification (3–6, 16, 17). In this study, our aim was to perform identification of white nonsporulating molds originating from the respiratory tracts of patients with chronic respiratory ailments, by phenotypic methods and combined sequencing of the internal transcribed spacer (ITS) and larger subunit (LSU) D1/D2 domain regions. Furthermore, the clinical significance of these molds was determined through follow-up findings and outcomes for the cases.
MATERIALS AND METHODS
Isolates and patient details.
A total of 4,948 clinical specimens (mucus plug, sputum, bronchoalveolar lavage fluid, bronchial aspirate, endotracheal aspirate, or fine-needle aspiration biopsy specimens) from patients with chronic respiratory ailments such as ABPM, fungal pneumonia, fungal balls, bronchial asthma, chronic obstructive pulmonary disease (COPD), bronchiectasis, or interstitial lung disease (ILD) who were attending the chest clinics of our institute were analyzed for the presence of NSMs during the period between January 2010 and May 2013. The clinical details for the enrolled patients were obtained retrospectively, through inspection of their medical records. All NSMs were subjected to phenotypic and molecular characterization.
Mycological investigations.
All specimens were processed for KOH wet mounting and histopathological examination of tissue specimens for the presence of hyaline septate hyphae. Specimens were cultured primarily on Sabouraud glucose agar with chloramphenicol, with or without cycloheximide, for 1 week at 28°C. The molds that failed to demonstrate sporulation after 1 week of incubation were taken for further characterization. Lactophenol cotton blue mounts of slide cultures on potato dextrose agar (PDA) were observed for microscopic characteristics. Also, for induction of sporulation, all isolates were cultured on PDA and malt extract agar plates for 3 to 4 weeks at 28°C, with periodic exposure to light. They were also cultured on autoclaved decayed wooden bark pieces from Syzygium cumini (blackberry tree, jambu) to provide them with their natural environment for sporulation, as described previously (15). All of the isolates were maintained on PDA slants at room temperature and also were stored in 40% glycerol and kept at −70°C. The sera of patients were tested for the presence of precipitating antibodies and specific IgE against basidiomycetes, Schizophyllum commune, and Ceriporia lacerata as described previously (21).
Molecular characterization by sequencing.
Molecular identification was performed by sequencing the ITS and D1/D2 regions of ribosomal DNA. DNA extraction and amplification were performed as described previously (16). Briefly, the extracted DNA was subjected to PCR amplification with the established primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) for ITS region amplification (22) and NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL4 (5′-GGTCCGTGTTTCAAGACGG-3′) for LSU region amplification (23). The amplicons of both regions were purified (Wizard SV Gel and PCR Clean-up System; Promega) and sequenced. The sequencing reactions were carried out by using a cycle sequencing kit (BigDye Terminator v3.1 cycle sequencing kit RR100; Applied Biosystems, Foster City, CA) with ITS1 and ITS4 as sequencing primers for ITS region amplicons and NL1 and NL4 as sequencing primers for D1/D2 region amplicons. The strands were sequenced with an ABI 3130XL genetic analyzer (Applied Biosystems, Foster City, CA).
Sequence analysis and species-level identification.
DNA sequences were analyzed with Sequencing Analysis 5.3.1 software (Applied Biosystems, Foster City, CA). Both ITS and D1/D2 domain consensus sequences were then subjected to Basic Local Alignment Search Tool (BLAST) searches at GenBank (http://www.ncbi.nlm.nih.gov/BLAST/Blast.cgi) and Centraalbureau voor Schimmelcultures (CBS) website analysis for species identification (http://www.cbs.knaw.nl/Collections/BioloMICSSequences.aspx?file=all). Sequence-based species identification was defined by ≥99% similarity. Query coverage of ≥95% was considered significant. Identities between 93% and 98% were considered for genus identification, and identities of <93% were inconclusive.
In vitro antifungal susceptibility testing.
The in vitro susceptibility profiles of NSM isolates were determined with slightly modified CLSI method M38-A2 (24). The modifications included growth of isolates on PDA for 2 to 3 weeks at 28°C and larger working inocula of 2.5 × 104 to 5.0 × 104 hyphal fragments/spores per ml. Microtiter plates were incubated at 35°C for 96 h. MIC endpoints were read visually. The two quality control strains (Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258) were used in each test, and their MIC ranges were according to CLSI guidelines. MICs for all strains were determined twice, on two different days, for reproducibility.
Nucleotide sequence accession numbers.
The sequences of the D1/D2 domains of 49 isolates have been submitted to GenBank under accession numbers KC414815 to KC414840, JX984627 to JX984630, KF291015 to KF291032, and JX292098. The ITS region sequences of 45 isolates have been submitted to GenBank under accession numbers KC414792 to KC414814, JX984623 to JX984626, KF290999 to KF291014, and JX271779 (Table 1).
Table 1.
Identification by GenBank BLAST searches of ITS and D1/D2 domain sequences of the 52 nonsporulating molds isolated from patients with bronchopulmonary disorders
| Isolate accession no. | ITS sequencing information |
D1/D2 domain sequencing information |
||||||
|---|---|---|---|---|---|---|---|---|
| Identification | Identity (%) | Query coverage (%) | GenBank Accession no. | Identification | Identity (%) | Query coverage (%) | GenBank Accession no. | |
| VPCI 1172/10 | Schizophyllum commune | 99 | 99 | KC414792 | Schizophyllum commune | 99 | 100 | KC414815 |
| VPCI 1354/10 | S. commune | 99 | 100 | KC414793 | S. commune | 99 | 100 | KC414816 |
| VPCI 1415/10 | S. commune | 99 | 100 | KC414794 | S. commune | 99 | 100 | KC414817 |
| VPCI 1417/10 | S. commune | 99 | 100 | KC414795 | S. commune | 100 | 100 | KC414818 |
| VPCI 1423/10 | S. commune | 99 | 98 | KC414796 | S. commune | 99 | 100 | KC414819 |
| VPCI 1515/10 | S. commune | 99 | 99 | KC414797 | S. commune | 99 | 100 | KC414820 |
| VPCI 461/11 | S. commune | 99 | 100 | KC414798 | S. commune | 99 | 100 | KC414821 |
| VPCI 789/11 | S. commune | 99 | 100 | KC414799 | S. commune | 99 | 100 | KC414822 |
| VPCI 1405/11 | S. commune | 100 | 100 | KC414800 | S. commune | 99 | 100 | KC414823 |
| VPCI 1838/11 | S. commune | 100 | 100 | KC414801 | S. commune | 99 | 97 | KC414824 |
| VPCI 1859/11 | S. commune | 100 | 100 | KC414802 | S. commune | 99 | 100 | KC414825 |
| VPCI 1935/11 | S. commune | 99 | 100 | KC414803 | S. commune | 99 | 97 | KC414826 |
| VPCI 1942/11 | S. commune | 100 | 100 | KC414804 | S. commune | 99 | 100 | KC414827 |
| VPCI 2020/11 | S. commune | 99 | 100 | KC414805 | S. commune | 99 | 100 | KC414828 |
| VPCI 2023/11 | S. commune | 99 | 100 | KC414806 | S. commune | 99 | 100 | KC414829 |
| VPCI 2024/11 | S. commune | 99 | 100 | KC414807 | S. commune | 99 | 99 | KC414830 |
| VPCI 2056/11 | S. commune | 100 | 100 | KC414808 | S. commune | 99 | 100 | KC414831 |
| VPCI 2149/11 | S. commune | 99 | 99 | KC414809 | S. commune | 100 | 100 | KC414832 |
| VPCI 278/P/11 | S. commune | 99 | 100 | KC414810 | S. commune | 99 | 100 | KC414833 |
| VPCI 279/P/11 | S. commune | 99 | 100 | KC414811 | S. commune | 99 | 100 | KC414834 |
| VPCI 400/P/12 | S. commune | 99 | 100 | KC414812 | S. commune | 99 | 100 | KC414835 |
| VPCI 425/P/12 | S. commune | 99 | 100 | KC414813 | S. commune | 99 | 100 | KC414836 |
| VPCI 427/P/12 | S. commune | 100 | 100 | KC414814 | S. commune | 99 | 100 | KC414837 |
| VPCI 1529/10 | Not amplified | S. commune | 100 | 100 | KC414838 | |||
| VPCI 1366/11 | Not amplified | S. commune | 99 | 100 | KC414839 | |||
| VPCI 1847/11 | Not amplified | S. commune | 99 | 100 | KC414840 | |||
| VPCI 240/P/13 | S. commune | 100 | 100 | KF291014 | S. commune | 100 | 100 | KF291030 |
| VPCI 1873/11 | Ceriporia lacerata | 99 | 97 | JX984623 | Ceriporia lacerata | 99 | 97 | JX984627 |
| VPCI 1921/11 | C. lacerata | 99 | 100 | JX984624 | C. lacerata | 99 | 100 | JX984628 |
| VPCI 1603/11 | C. lacerata | 99 | 95 | JX984625 | C. lacerata | 99 | 95 | JX984629 |
| VPCI 2549/11 | C. lacerata | 99 | 100 | JX984626 | C. lacerata | 99 | 100 | JX984630 |
| VPCI 1605/12 | C. lacerata | 99 | 100 | KF290999 | C. lacerata | 99 | 99 | KF291015 |
| VPCI 1624/12 | C. lacerata | 99 | 100 | KF291000 | C. lacerata | 99 | 100 | KF291016 |
| VPCI 1807/12 | C. lacerata | 99 | 100 | KF291001 | C. lacerata | 99 | 100 | KF291017 |
| VPCI 1829/12 | C. lacerata | 99 | 100 | KF291002 | C. lacerata | 99 | 100 | KF291018 |
| VPCI 1994/12 | C. lacerata | 99 | 100 | KF291003 | C. lacerata | 99 | 99 | KF291019 |
| VPCI 2006/12 | C. lacerata | 99 | 100 | KF291004 | C. lacerata | 99 | 100 | KF291020 |
| VPCI 425/P/12(1) | C. lacerata | 99 | 100 | KF291005 | C. lacerata | 99 | 99 | KF291021 |
| VPCI 1841/12 | Polyporales | 94 | 98 | KF291006 | Porostereum spadiceum | 99 | 100 | KF291022 |
| VPCI 1467/12 | Polyporales | 98 | 100 | KF291007 | P. spadiceum | 99 | 100 | KF291023 |
| VPCI 1485/12 | Polyporales | 95 | 99 | KF291008 | P. spadiceum | 99 | 100 | KF291024 |
| VPCI 407/P/12 | Polyporales | 96 | 99 | KF291009 | P. spadiceum | 99 | 100 | KF291025 |
| VPCI 1880/12 | Phanerochaete sordida | 95 | 100 | KF291010 | Phanerochaete stereoides | 99 | 100 | KF291026 |
| VPCI 2013/12 | Phanerochaete chrysosporium | 99 | 93 | KF291011 | P. stereoides | 99 | 100 | KF291027 |
| VPCI 2073/12 | P. chrysosporium | 99 | 94 | KF291012 | P. stereoides | 99 | 100 | KF291028 |
| VPCI 455/P/12 | Marasmiellus palmivorus | 99 | 100 | KF291013 | Marasmiellus palmivorus | 99 | 100 | KF291029 |
| VPCI 482/13 | Not amplified | Neosartorya fischeri | 99 | 99 | KF291031 | |||
| VPCI 800/13 | Not amplified | N. fischeri | 99 | 99 | KF291032 | |||
| VPCI 85/P/10 | Perenniporia sp. | JX271779 | Perenniporia sp. | JX292098 | ||||
| VPCI 501/P/12 | Basidiomycota | 99 | 86 | Basidiomycota | 99 | 92 | ||
| VPCI 2025/12 | Basidiomycota | 99 | 88 | Basidiomycota | 99 | 93 | ||
| VPCI 448/P/12 | Basidiomycota | 99 | 86 | Basidiomycota | 99 | 93 | ||
RESULTS
Characteristics of cultured isolates.
A total of 52 NSMs (1.05%) were cultured from 4,948 respiratory specimens tested. These 52 isolates originated from 47 patients. Forty-eight isolates were single isolates, whereas 2 isolates were recurrent isolates obtained from 2 different patients. NSMs were the predominant molds isolated as multiple colonies in at least 2 or 3 repeat respiratory specimens. In 10% of sputum specimens, however, patients had 2 to 4 coexisting colonies of transient Aspergillus species (Aspergillus niger and Aspergillus flavus) in any of the three specimens analyzed per patient. In addition, for these patients Aspergillus serological findings (Aspergillus-specific IgE and precipitins) were negative and basidiomycetes were repeatedly isolated. All of the specimens yielding basidiomycetes revealed direct KOH or histopathological findings positive for hyaline septate hyphae.
Of 52 white NSMs, 50 were presumptively identified as probable basidiomycetes based upon the presence of spicules, hyphal pegs, arthroconidia, and chlamydoconidia (Table 2). In addition, 39 of 50 NSMs showed clamp connections characteristic of basidiomycetes. Two of the 52 isolates showed only vesicles suggestive of Aspergillus species. ITS and D1/D2 domain sequencing of 49 NSMs identified 27 (52%) as Schizophyllum commune, 11 (21.1%) as Ceriporia lacerata, 4 (7.6%) as Porostereum spadiceum, 3 (5.7%) as Phanerochaete stereoides, 2 (3.8%) as Neosartorya fischeri, and 1 (1.9%) each as Marasmiellus palmivorus and Perenniporia species. Four C. lacerata isolates that were isolated from patients as colonizers and agents of fungal pneumonia (16) and one case of a fungal ball due to Perenniporia species (15) have already been reported. Induction of sporulation revealed basidia and basidiospores in 11 isolates (22%). Of these, 4 isolates (8%) showed fan-shaped basidiocarps consistent with S. commune fruiting bodies after 4 to 5 weeks of incubation on PDA at 28°C with periodic exposure to light. Interestingly, a solitary isolate of C. lacerata showed a brain coral-shaped basidiocarp on decayed wood of Syzygium cumini after 6 weeks of incubation at 28°C with periodic exposure to light (Fig. 1).
Table 2.
Characteristics of nonsporulating molds (n = 52) isolated from patients with bronchopulmonary disorders
| Fungus identified | Phenotypic characteristics | No. of isolates sporulated | Diagnosisa | Specimen type | Treatment | Patient outcomes |
|---|---|---|---|---|---|---|
| Schizophyllum commune (n = 27) | Spicules, n = 27; hyphal pegs, n = 27; clamp connections, n = 27 | 4b | Colonizer, n = 12 | Sputum, n = 12 | Oral steroids and inhaled corticosteroids with or without LABA, for COPD and ILD treatment | Lost to follow-up, n = 3; stable, n = 8; died, n = 1 |
| ABPM, n = 8 | Sputum plug, n = 8 | Inhaled corticosteroids with or without LABA and oral steroids, n = 6; itraconazole or voriconazole, n = 2 | Lost to follow-up, n = 1; stable/remission, n = 7 | |||
| Fungal pneumonia, n = 4 | BAL fluid, n = 2; FNAB, n = 1; TBLB, n = 1 | Voriconazole, n = 2 | Resolution, n = 1; died, n = 2; died before diagnosis, n = 1 | |||
| Fungal ball, n = 3 | BAL fluid, n = 2; sputum, n = 1 | Voriconazole or itraconazole, n = 2; observation | Stable, n = 3 | |||
| Ceriporia lacerata (n = 11) | Spicules, n = 11; hyphal pegs, n = 11; clamp connections, n = 7; basidia, n = 6; basidiospores, n = 6 | 7c | Fungal pneumonia, n = 6 | BAL fluid, n = 3; FNAB, n = 2; TBLB, n = 1 | Itraconazole or voriconazole, n = 2 | Lost to follow-up, n = 3; resolution, n = 2; died, n = 1 |
| Colonizer, n = 5 | Sputum, n = 4; sputum plug, n = 1 | Stable, n = 4; deterioration due to unrelated causes, n = 1 | ||||
| Porostereum spadiceum (n = 4) | Spicules, n = 4; hyphal pegs, n = 2; clamp connections, n = 2; chlamydoconidia, n = 1; arthroconidia, n = 3 | Colonizer, n = 2 | Sputum, n = 2 | Lost to follow-up, n = 2 | ||
| Phanerochaete stereoides (n = 3) | Spicules, n = 3; hyphal pegs, n = 3; chlamydoconidia, n = 2 | Colonizer, n = 2 | Sputum, n = 1; sputum plug, n = 1 | Inhaled corticosteroids with or without LABA, n = 1 | Stable, n = 2 | |
| Neosartorya fischeri (n = 2) | Only vesicular head | 2d | Fungal pneumonia, n = 2 | Endotracheal aspirate, n = 2; FNAB, n = 2 | Voriconazole, n = 2 | Died, n = 2 |
| Perenniporia sp. (n = 1) | Spicules, hyphal pegs | Fungal ball, n = 1 | BAL fluid, n = 1 | Intracavitary AMB, n = 1 | Stable | |
| Marasmiellus palmivorus (n = 1) | Only spicules | Colonizer, n = 1 | Sputum, n = 1 | Lost to follow-up | ||
| Unidentified (n = 3) | Spicules, hyphal pegs, clamp connections |
ABPM, allergic bronchopulmonary mycosis; BAL, bronchoalveolar lavage; FNAB, fine-needle aspiration biopsy; TBLB, transbronchial lung biopsy; LABA, long-acting beta agonist; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease.
Fig 1.
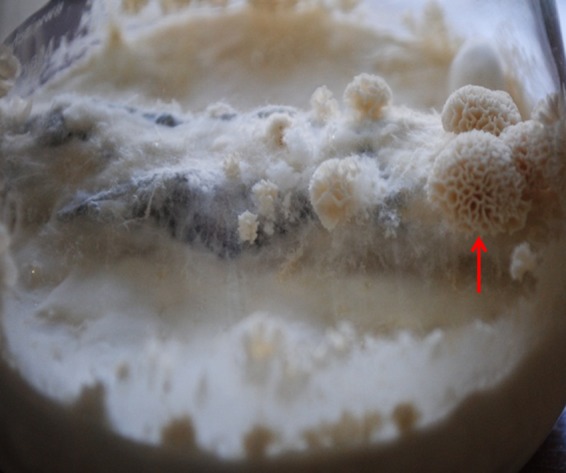
Culture flask showing a brain coral-shaped fruiting body (basidiocarp) (arrow) of Ceriporia lacerata VPCI 1921/11 inoculated on Syzygium cumini bark pieces after 6 weeks of incubation on PDA at 28°C with periodic exposure to light.
ITS and D1/D2 region BLAST analysis.
Nucleotide sequences with match lengths of ≥500 bp were analyzed. ITS and D1/D2 domain sequencing of 52 isolates revealed that 49 (94.2%) had a match in GenBank and could be identified to the species (n = 48) or genus (n = 1) level (Table 1). The remaining 3 isolates (5.7%) could be identified only to the taxon Basidiomycota. Of the 49 isolates, 48 (92%) were identified to the species level by D1/D2 region sequencing and only 36 (69.2%) by ITS sequencing alone. ITS region sequencing of the remaining 12 isolates revealed inconclusive results for 7 isolates (<95% query coverage or <99% identity); the ITS region could not be amplified for 5 isolates (9.6%) despite repeated attempts. The seven isolates that yielded inconclusive results by ITS sequencing were identified as Phanerochaete stereoides and Porostereum spadiceum by D1/D2 domain sequencing. The solitary Perenniporia isolate was identified to the genus level by both D1/D2 domain and ITS sequencing. BLAST search results of GenBank and the CBS database were in agreement for our basidiomycete isolates.
In vitro antifungal susceptibility.
In vitro susceptibility data for 40 NSM isolates tested are presented in Table 3. With the exception of POS and CAS findings, the susceptibility profiles of S. commune isolates have been presented previously (25). All of the tested species of basidiomycetes were susceptible to AMB and resistant to CAS, fluconazole, and flucytosine. They exhibited variable MICs for azoles, with POS and isavuconazole (ISA) showing the greatest activity against S. commune (POS geometric mean [GM], 0.11 μg/ml; ISA GM, 0.086 μg/ml) and C. lacerata (POS GM, 0.19 μg/ml; ISA GM, 0.094 μg/ml). However, three isolates of S. commune were resistant to azoles. One isolate was resistant to all of the azoles tested, i.e., ITC (MIC, 8 μg/ml), VRC (MIC, 2 μg/ml), ISA (MIC, 2 μg/ml), and POS (MIC, 2 μg/ml), another isolate was resistant to both ITC (MIC, 8 μg/ml) and VRC (MIC, 2 μg/ml), and the third isolate was resistant only to POS (MIC, 2 μg/ml). One isolate of M. palmivorus was resistant to all azoles tested except POS (MIC, 0.5 μg/ml). Porostereum spadiceum had low MICs for ITC and VRC but reduced susceptibility to POS and ISA. Notably, ISA had low MICs for all basidiomycetes tested except P. spadiceum and M. palmivorus. The ascomycete Neosartorya was susceptible to all of the antifungals tested except POS.
Table 3.
In vitro antifungal susceptibility profiles of nonsporulating molds (n = 40)
| Fungi (n) and parameters | MIC (μg/ml) fora: |
|||||||
|---|---|---|---|---|---|---|---|---|
| ITC | VRC | ISA | POS | AMB | CAS | FLU | FC | |
| Schizophyllum commune (27)b | ||||||||
| GM | 0.20 | 0.24 | 0.19 | 0.11 | 0.27 | 5.75 | 19.39 | 17.38 |
| Range | 0.03–8 | 0.06–2 | 0.015–2 | 0.015–2 | 0.03–2 | 2–8 | 2–64 | 2–64 |
| MIC50 | 0.125 | 0.25 | 0.125 | 0.125 | 0.5 | 8 | 16 | 32 |
| MIC90 | 1 | 0.5 | 0.5 | 1 | 1 | 8 | 64 | 64 |
| Ceriporia lacerata (8) | ||||||||
| GM | 0.147 | 0.229 | 0.094 | 0.086 | 0.5 | 8 | 10.3 | 10.3 |
| Range | 0.06–0.5 | 0.125–0.5 | 0.06–0.125 | 0.06–0.125 | 0.25–1 | 8 | 4–32 | 8–16 |
| MIC50 | 0.125 | 0.25 | 0.125 | 0.09 | 0.5 | 8 | 12 | 8 |
| MIC90 | 0.325 | 0.5 | 0.125 | 0.125 | 1 | 8 | 20.8 | 16 |
| Porostereum spadiceum (2), range | 0.03–0.25 | 0.06–0.5 | 2–8 | 1–4 | 0.03–0.125 | 1–8 | 1–32 | 2–64 |
| Marasmiellus palmivorus (1) | 16 | 8 | 8 | 0.5 | 0.06 | 8 | >64 | >64 |
| Neosartorya fischeri (2), range | 0.5–1 | 1 | 0.5 | 1–2 | 0.125–0.25 | 0.03–0.125 | >64 | >64 |
ITC, itraconazole; VRC, voriconazole; ISA, isavuconazole; POS, posaconazole; AMB, amphotericin B; CAS, caspofungin; FLU, fluconazole; FC, flucytosine; GM, geometric mean.
Data from reference 25, except for POS and CAS data.
Clinical summary.
The diagnoses of the enrolled patients for whom NSMs were isolated were categorized as follows: ABPM, asymptomatic colonization of the lung, invasive pulmonary fungal disease, and fungal balls (Table 2). Clinical details and treatment outcomes were available for 39 of the 47 cases. The basidiomycetes (96%) were the sole agents isolated in three of the groups, namely, ABPM, colonization, and fungal balls. In the invasive pulmonary mycosis group, in addition to basidiomycetes, 2 cases were due to the ascomycete N. fischeri. Schizophyllum commune was a colonizer in 54.5% of cases (12/22 cases), followed by C. lacerata (23%), P. spadiceum (9%), and M. palmivorus (4.5%). The next most frequently observed clinical diagnosis was of invasive pulmonary mycosis/pneumonia, with 50% of cases (6/12 cases) due to C. lacerata, 33% to S. commune, and 17% to N. fischeri. ABPM represented the third largest clinical group, with 8 cases all due to S. commune. Fungal balls due to S. commune were noted in 3 cases (75%) and a Perenniporia sp. in the remaining one case. It was observed that all patients with fungal balls and ABPM demonstrated precipitating antibodies against the incriminated basidiomycetes. Also, in cases in which S. commune and C. lacerata were presumed to be colonizers, precipitating antibodies against the isolated molds were demonstrated in 41% of cases.
Of 39 cases, 23 were diagnosed as fungal pneumonia (n = 11), ABPM (n = 8), or fungal balls (n = 4) (Table 2). In the remaining 16 cases, basidiomycetes were considered to be colonizers. The patients with basidiomycetes as colonizers had various clinical diagnoses involving both structurally damaged and intact lungs, such as COPD, ILD, posttubercular sequelae, and asthma. Asymptomatic colonization was defined as an absence of basidiomycetes in respiratory specimens during the follow-up period (2 to 6 months) for these patients in conjunction with no clinicoradiological worsening warranting any active intervention with antifungals.
Patients with fungal pneumonia, ABPM, or fungal balls were treated with standard therapy consisting of either VRC (loading dose of 400 mg twice a day on day 1, followed by one-half the dose) or ITC (200 mg twice a day). Other treatment modalities consisted of systemic and inhaled steroid therapy or observation for clinical deterioration in cases of ABPM or suspected colonization, respectively. Additionally, patients received standard treatment for their underlying pulmonary disorder. Of the 11 patients with fungal pneumonia, 6 received antifungal therapy. Of these, 4 received VRC while 2 received ITC. However, 4 (67%) of the treated patients died. The remaining 5 patients with invasive mycosis were either lost to follow-up or died before the diagnosis was established. Systemic steroid treatment formed the mainstay of therapy for 6 of the 8 ABPM patients; 2 patients received ITC. Five of the former patients and both of the latter patients achieved remission within 6 months and continue to maintain remission at the present time. Two patients with fungal balls due to S. commune received ITC or VRC, while another case was managed symptomatically. The patient with a fungal ball due to Perenniporia sp. received intracavitary AMB treatment (15). All of the patients with fungal balls continue to be asymptomatic despite persistence of the fungal ball.
DISCUSSION
The present study highlights the importance of filamentous basidiomycetes from respiratory specimens for 3 clinical groups of bronchopulmonary mycoses, namely, ABPM, invasive pulmonary mycosis, and fungal balls. It is known that basidiomycetous molds chronically colonizing the lung can precipitate invasive disease and/or sensitize individuals to fungal antigens, leading to allergic fungal cough, allergic sinusitis, asthma, and ABPM (13, 26–29). In the present study, these molds were most frequently identified as asymptomatic colonizers in patients with respiratory ailments. Since patients with chronic lung diseases are more prone to develop immunosuppression due to repeated courses of systemic/local steroid treatment, it is important to monitor such patients in order to detect early invasive disease due to basidiomycetes. In the present study, precipitating antibodies against the colonizing mold were demonstrated for 41% of patients by the Ouchterlony immunodiffusion method. Although immunoprecipitation testing remains the standard approach for diagnosing previous or continued exposure/sensitization to mold antigens, it fails to differentiate between previous exposure and the cause of disease (30–32).
Antifungal therapy could reduce the effects of antigen exposure by eradicating the colonizing mold (29, 32). Recently, the efficacy of antifungal drugs for treatment of atopic cough, fungus-associated chronic cough, and allergic fungal cough induced by basidiomycetous fungi has been reported (28, 29, 33, 34). Therefore, the need for identification and timely institution of antifungal therapy against environmental basidiomycetous molds in patients with symptomatic respiratory complaints can hardly be overemphasized. In this study, the basidiomycetes tested were susceptible to ITC, VRC, ISA, and POS and patients with invasive disease, allergic mycosis, or fungal balls received azole antifungals. Nevertheless, it may be argued that the high mortality rate (67%) for patients with invasive fungal disease despite the institution of antifungal therapy favors an alternative diagnosis. It should be noted that all such patients had advanced and irreversible pulmonary disease at baseline. Also, by the time the molds could be identified and treated, the patients had been admitted to the critical care unit for several days and were moribund. In addition, 2 patients had pneumonia due to N. fischeri. Previously, N. fischeri was reported for a solitary case of invasive fungal infection in a recipient of an allogeneic bone marrow transplant (35). As N. fischeri grows slowly in culture and fails to sporulate in routine media, the relevance of this pathogen in clinical contexts could be undetermined (35). Two of our patients with ABPM who received antifungal therapy and those who received standard steroid therapy responded similarly.
The clinical diagnoses such as invasive mycoses and colonization observed in our study were similar to the respiratory cases reported by Gonzalez et al. (36), who identified basidiomycetous fungi through phenotypic methods, with a large number of rare species remaining unidentified. In routine laboratories, the induction of sporulation for definitive identification usually requires about 3 weeks and is associated with high failure rates. As observed in the present study, specific identification through sporulation could be achieved for only 4 S. commune and 2 N. fischeri isolates. The use of both ITS and LSU region sequencing provided higher identification rates in this study as well as that of Romanelli et al. (37) (92% and 99.4%, respectively), which is in contrast to 79% reported by Pounder et al. (9), who used only ITS region sequencing. Among all of the isolates in the present study, 92% had existing matches for the ITS region and/or D1/D2 domain in the GenBank database. Thus, the combined use of ITS and D1/D2 region sequencing is a more powerful tool for the identification of basidiomycetes. Using this technique, two genera of basidiomycetes that had not been reported previously for human subjects, namely, M. palmivorus and P. spadiceum, were identified. This study demonstrates the relevance of NSMs in a clinical context. Previous series on the molecular characterization of NSMs did not consider the clinical backgrounds of patients (9, 37). However, the authors of both of those series indicated that NSMs isolated from otherwise sterile sites could be potential pathogens (9, 37). Finally, patients with chronic pulmonary diseases for whom basidiomycetes are isolated from the lower respiratory tract should be thoroughly evaluated to rule out an allergic or invasive disease, and the basidiomycetes should not just be ignored as possible contaminants. The importance of molecular techniques for unambiguous identification of fungi that fail to sporulate with conventional methods is highlighted.
ACKNOWLEDGMENTS
This work was carried out in part with financial assistance from the Department of Biotechnology (grant BT/39/NE/TBP/2010), Government of India, New Delhi, India. This work was supported in part by research grant 50647 from the Investigator Initiated Studies Program of Merck Sharp & Dohme Corp. (to J.F.M.).
The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp.
J.F.M. received grants from Astellas, Basilea, and Merck. He has been a consultant to Astellas, Basilea, and Merck and has received speaker's fees from Merck and Gilead. The other authors declare no potential conflicts of interest. We alone are responsible for the content and writing of the paper.
Footnotes
Published ahead of print 31 July 2013
REFERENCES
- 1.Guarro J, Kallas EG, Godoy P, Karenina A, Gené J, Stchigel A, Colombo AL. 2002. Cerebral aspergillosis caused by Neosartorya hiratsukae, Brazil. Emerg. Infect. Dis. 8:989–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Järv H, Lehtmaa J, Summerbell RC, Hoekstra ES, Samson RA, Naaber P. 2004. Isolation of Neosartorya pseudofischeri from blood: first hint of pulmonary aspergillosis. J. Clin. Microbiol. 42:925–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verweij PE, van Kasteren M, van de Nes J, de Hoog GS, de Pauw BE, Meis JF. 1997. Fatal pulmonary infection caused by the basidiomycete Hormographiella aspergillata. J. Clin. Microbiol. 35:2675–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salit RB, Shea YR, Gea-Banacloche J, Fahle GA, Abu-Asab M, Sugui JA, Carpenter AE, Quezado MM, Bishop MR, Kwon-Chung KJ. 2010. Death by edible mushroom: first report of Volvariella volvacea as an etiologic agent of invasive disease in a patient following double umbilical cord blood transplantation. J. Clin. Microbiol. 48:4329–4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suarez F, Olivier G, Garcia-Hermoso D, Randriamalala E, Ghez D, Bruneau J, Kauffmann-Lacroix C, Bougnoux ME, Lortholary O. 2011. Breakthrough Hormographiella aspergillata infections arising in neutropenic patients treated empirically with caspofungin. J. Clin. Microbiol. 49:461–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conen A, Weisser M, Hohler D, Frei R, Stern M. 2011. Hormographiella aspergillata: an emerging mould in acute leukaemia patients? Clin. Microbiol. Infect. 17:273–277 [DOI] [PubMed] [Google Scholar]
- 7.Gené J, Guillamón JM, Guarro J, Pujol I, Ulfig K. 1996. Molecular characterization, relatedness and antifungal susceptibility of the basidiomycetous Hormographiella species and Coprinus cinereus from clinical and environmental sources. Antonie Van Leeuwenhoek 70:49–57 [DOI] [PubMed] [Google Scholar]
- 8.Sigler L, de la Maza LM, Tan G, Egger KN, Sherburne RK. 1995. Diagnostic difficulties caused by a nonclamped Schizophyllum commune isolate in a case of fungus ball of the lung. J. Clin. Microbiol. 33:1979–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pounder JI, Simmon KE, Barton CA, Hohmann SL, Brandt ME, Petti CA. 2007. Discovering potential pathogens among fungi identified as nonsporulating molds. J. Clin. Microbiol. 45:568–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark S, Campbell CK, Sandison A, Choa DI. 1996. Schizophyllum commune: an unusual isolate from a patient with allergic fungal sinusitis. J. Infect. 32:147–150 [DOI] [PubMed] [Google Scholar]
- 11.Sigler L, Estrada S, Montealegre NA, Jaramillo E, Arango M, De Bedout C, Restrepo A. 1997. Maxillary sinusitis caused by Schizophyllum commune and experience with treatment. J. Med. Vet. Mycol. 35:365–370 [PubMed] [Google Scholar]
- 12.Rihs JD, Padhye AA, Good CB. 1996. Brain abscess caused by Schizophyllum commune: an emerging basidiomycete pathogen. J. Clin. Microbiol. 34:1628–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhary A, Randhawa HS, Gaur SN, Agarwal K, Kathuria S, Roy P, Klaassen CH, Meis JF. 2013. Schizophyllum commune as an emerging fungal pathogen: a review and report of two cases. Mycoses 56:1–10 [DOI] [PubMed] [Google Scholar]
- 14.Kamei K, Unno H, Nagao K, Kuriyama T, Nishimura K, Miyaji M. 1994. Allergic bronchopulmonary mycosis caused by the basidiomycetous fungus Schizophyllum commune. Clin. Infect. Dis. 18:305–309 [DOI] [PubMed] [Google Scholar]
- 15.Chowdhary A, Agarwal K, Kathuria S, Singh PK, Roy P, Gaur SN, Rodrigues AM, de Hoog GS, Meis JF. 2012. First human case of pulmonary fungal ball due to a Perenniporia species (a basidiomycete). J. Clin. Microbiol. 50:3786–3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdhary A, Agarwal K, Kathuria S, Singh PK, Roy P, Gaur SN, de Hoog GS, Meis JF. 2013. Clinical significance of filamentous basidiomycetes illustrated by isolates of the novel opportunist Ceriporia lacerata from the human respiratory tract. J. Clin. Microbiol. 51:585–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagrou K, Massonet C, Theunissen K, Meersseman W, Lontie M, Verbeken E, Van Eldere J, Maertens J. 2005. Fatal pulmonary infection in a leukaemic patient caused by Hormographiella aspergillata. J. Med. Microbiol. 54:685–688 [DOI] [PubMed] [Google Scholar]
- 18.Roan JN, Hsieh HY, Tsai HW, Wu CJ, Hsu CH, Wu SY, Yang YJ, Chang TC. 2009. Pulmonary nodules caused by Schizophyllum commune after cardiac transplantation. J. Infect. 58:164–167 [DOI] [PubMed] [Google Scholar]
- 19.Surmont I, van Aelst F, Verbanck J, de Hoog GS. 2002. A pulmonary infection caused by Coprinus cinereus (Hormographiella aspergillata) diagnosed after a neutropenic episode. Med. Mycol. 40:217–219 [DOI] [PubMed] [Google Scholar]
- 20.Buzina W, Lass-Flörl C, Kropshofer G, Freund MC, Marth E. 2005. The polypore mushroom Irpex lacteus, a new causative agent of fungal infections. J. Clin. Microbiol. 43:2009–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chowdhary A, Agarwal K, Randhawa HS, Kathuria S, Gaur SN, Najafzadeh MJ, Roy P, Arora N, Khanna G, Meis JF. 2012. A rare case of allergic bronchopulmonary mycosis caused by Alternaria alternata. Med. Mycol. 50:890–896 [DOI] [PubMed] [Google Scholar]
- 22.White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322 In Innis MA, Gelfand DH, Sninsky JJ, White TJ. (ed), PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA [Google Scholar]
- 23.Kurtzman CP, Robnett CJ. 1997. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 35:1216–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antimicrobial susceptibility testing of filamentous fungi; second edition Approved standard M38-A2 CLSI, Wayne, PA [Google Scholar]
- 25.Chowdhary A, Kathuria S, Singh PK, Agarwal K, Gaur SN, Roy P, Randhawa HS, Meis JF. 2013. Molecular characterization and in vitro antifungal susceptibility profile of Schizophyllum commune, an emerging basidiomycete in bronchopulmonary mycoses. Antimicrob. Agents Chemother. 57:2845–2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa H, Fujimura M, Takeuchi Y, Makimura K. 2009. The importance of basidiomyceteous fungi cultured from the sputum of chronic idiopathic cough: a study to determine the existence of recognizable clinical patterns to distinguish CIC from non-CIC. Respir. Med. 103:1492–1497 [DOI] [PubMed] [Google Scholar]
- 27.Ogawa H, Fujimura M, Takeuchi Y, Makimura K. 2011. Two cases of Schizophyllum asthma: is this a new clinical entity or a precursor of ABPM? Pulm. Pharmacol. Ther. 24:559–562 [DOI] [PubMed] [Google Scholar]
- 28.Ogawa H, Fujimura M, Takeuchi Y, Makimura K. 2009. Is Bjerkandera adusta important to fungus-associated chronic cough as an allergen? Eight cases' reports. J. Asthma 46:849–855 [DOI] [PubMed] [Google Scholar]
- 29.Ogawa H, Fujimura M, Tofuku Y. 2004. Treatment of atopic cough caused by Basidiomycetes antigen with low dose itraconazole. Lung 182:279–284 [DOI] [PubMed] [Google Scholar]
- 30.Bush RK, Portnoy JM, Saxon A, Terr AI, Wood RA. 2006. The medical effects of mold exposure. J. Allergy Clin. Immunol. 117:326–333 [DOI] [PubMed] [Google Scholar]
- 31.Trout DB, Seltzer JM, Page EH, Biagini RE, Schmechel D, Lewis DM, Boudreau AY. 2004. Clinical use of immunoassays in assessing exposure to fungi and potential health effects related to fungal exposure. Ann. Allergy Asthma Immunol. 92:483–492 [DOI] [PubMed] [Google Scholar]
- 32.Chowdhary A, Agarwal K, Kathuria S, Gaur SN, Randhawa HS, Meis JF. 2013. Allergic bronchopulmonary mycosis due to fungi other than Aspergillus: a global overview. Crit. Rev. Microbiol. [Epub ahead of print.] 10.3109/1040841X.2012.754401 [DOI] [PubMed] [Google Scholar]
- 33.Ogawa H, Fujimura M, Takeuchi Y, Makimura K. 2009. Efficacy of itraconazole in the treatment of patients with chronic cough whose sputa yield basidiomycetous fungi: fungus-associated chronic cough (FACC). J. Asthma 46:407–412 [DOI] [PubMed] [Google Scholar]
- 34.Ogawa H, Fujimura M, Tofuku Y. 2004. Two cases of atopic cough successfully treated by oral cleansing with amphotericin B: relationship with Basidiomycetes detected from pharyngeal swab. Allergol. Int. 53:193–196 [Google Scholar]
- 35.Lonial S, Williams L, Carrum G, Ostrowski M, McCarthy P., Jr 1997. Neosartorya fischeri: an invasive fungal pathogen in an allogeneic bone marrow transplant patient. Bone Marrow Transplant. 19:753–755 [DOI] [PubMed] [Google Scholar]
- 36.González GM, Sutton DA, Thompson E, Tijerina R, Rinaldi MG. 2001. In vitro activities of approved and investigational antifungal agents against 44 clinical isolates of basidiomycetous fungi. Antimicrob. Agents Chemother. 45:633–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romanelli AM, Sutton DA, Thompson EH, Rinaldi MG, Wickes BL. 2010. Sequence-based identification of filamentous basidiomycetous fungi from clinical specimens: a cautionary note. J. Clin. Microbiol. 48:741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]


